Abstract
Identification of a small population of neuronal action potentials (APs) firing is considered essential to discover the operating principles of neuronal circuits. A promising method is to indirectly monitor the AP discharges in neurons from the recordings their intracellular calcium fluorescence transients. However, it is hard to reveal the nonlinear relationship between neuronal calcium fluorescence transients and the corresponding AP burst discharging. We propose a method to reconstruct the neuronal AP train from calcium fluorescence diversifications based on a multiscale filter and a convolution operation. Results of experimental data processing show that the false-positive rate and the event detection rate are about 10 and 90%, respectively. Meanwhile, the APs firing at a high frequency up to 40 Hz can also be successfully identified. From the results, it can be concluded that the method has strong power to reconstruct a neuronal AP train from a burst firing.
Keywords: action potential firing, multiscale filter, calcium fluorescence transient, template convolution
Introduction
Spatiotemporal dynamic patterns of local neural circuits are essential for discovering the mechanisms of the nervous system.1, 2, 3, 4 Recently, with the advances in calcium indictors and the two-photon microscopy technique, reconstructions of the action potential (AP) discharge in local neural circuits from their simultaneously recorded calcium fluorescence transients in vivo and in vitro were carried out.5, 6, 7, 8, 9 Furthermore, two-photon fluorescence random scanning microscopy has been designed and constructed, through which calcium fluorescence transients sampled at about a 1-kHz frequency from neuronal local circuits can be recorded synchronously.10, 11, 12 These data at a high sampling rate enable us to reconstruct AP firing of local neuronal circuits at a millisecond-scale precision.13 Although the neuronal burst discharge carries more coding information than a single AP firing,14, 15, 16 revealing neuronal AP burst trains from the corresponding calcium waves is well-known as hard work.17, 18, 19, 20 This is so not only because burst firing is caused by the large amount of depolarization15, 16 and has not been clearly defined, but also because neuronal calcium fluorescence transients always have a nonlinear relationship with their APs firing, causing the diversities and complexities of calcium fluorescence waves.21 Practically,14, 15, 16 some burst firing sequences consist of APs with intervals of less than 20 ms.
Driven by the necessity to reveal the principles of neuronal circuits, many valuable methods to reconstruct AP sequences from their corresponding calcium fluorescence transients have been designed. For example, the template-matching method was developed to detect a single AP discharge from a calcium fluorescence transient, which works well whenever neurons discharge sparsely.22, 23 The uncorrected24 and corrected first-derivative method including deconvolution7, 25, 26 can provide reliable neuronal firing rates, but are limited to inferring neuronal AP trains from calcium fluorescence transients with a low signal-to-noise ratio (SNR), defined as the ratio of the amplitude of the template to the standard deviation of noise in the initial calcium waves. Some statistical methods such as clustering27 and a combination of principal component analysis and a support vector machine,28 can only partially describe the calcium wave diversities that derive from the neuronal irregularly discharging at a high frequency rate. Some sophisticated methods9, 29, 30 are suitable for reconstructing the AP train from a burst firing. However, compared to the methods just mentioned, more setting parameters or hypotheses may confine their applications. Recently, a method termed “peeling” was proposed for this purpose, but a fixed template matching the rising duration is still necessary.13
For the calcium fluorescence transients at a high frequency (1 kHz), the nonlinear calcium dynamics triggered by neuronal burst firing have become increasingly obvious and are difficult to describe using a single model or several patterns. In addition, the SNR of fluorescence signals is a decisive factor for the accuracy of the reconstruction. Therefore, aimed to reconstruct the AP burst firing trains from calcium fluorescence transient with relative low SNRs, a method based on multiscale filter combined with template convolution (MSF_TC) is proposed. First, the parts of calcium fluorescence transients corresponding to neuronal AP firing are identified using a multiscale filter, and the AP train candidates are generated. Then, we combine the AP train candidates with the template function, depending on the fitting degrees with the original calcium waves, to confirm AP firing timing sequences.
To illustrate the MSF_TC algorithm performance, this method was applied in processing real data sets from our optical system.11, 12 The reconstruction results of the proposed method and that of two typical methods, the template-matching method and the deconvolution method, were compared. The reconstruction results were further analyzed to emphasize some important features of the MSF_TC algorithm.
Methods
Recording Calcium Fluorescence Transients
Sample preparing and fluorescence labeling
Slices were made from postnatal (P)14-P20 Wistar rats. The animals were anesthetized with 1% sodium phentobarbital (50 mg∕kg). After fast decapitation, the brain was dissected rapidly and placed in ice-cold oxygenated (95% O2 and 5% CO2) ACSF (artificial cerebrospinal fluid) containing (in mM) 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 1 NaH2PO4, 26 NaHCO3, and 11 glucose. Cortical and hippocampal slices, 350- to 400-μm thick, were cut with a vibratome (VTS1000, Leica) and maintained in an incubation chamber for at least 1 h at room temperature (22 to 25°C) before recording. During the experiments, the slice was transferred to a submersion-recording chamber and incubated in oxygenated ACSF (1 to 3.0 ml∕min) at room temperature.
After 1 h in the incubation chamber, the slices were transferred to a recording chamber, where they were stained with calcium indicators. As in whole-cell patch recording, all local population cells were performed sequentially by the glass micropipette and maintained about 20 min to result in reliable and rapid loading of somata and subsequent complete labeling of dendritic and axonal arborizations. Bath-applied 4-AP at a final concentration of 100 μM was used to induce epileptiform discharging.
Imaging system for calcium fluorescence transients
A custom-constructed random access two-photon fluorescence microscope system was used to image the calcium fluorescence transients. The setup of the system has been described in detail by Lv et al.11 Briefly, a Ti:sapphire laser (Tsunami, Spectra Physics) was pumped by a solid state cw laser (Millennia Xs, Spectra Physics) to produce a mode-locked laser output31 (800 nm, ∼100-fs pulse width at 80 MHz repetitive frequency). Laser intensity was adjusted with an electro-optic modulator (EOM). After the dispersion compensation prism (tilted at 45 deg), two orthogonal acousto-optic deflectors (AODs) were used for x–y deflection of the laser beam. Then, the laser beam was coupled to an upright microscope (BX61WI, Olympus). A water immersion objective 40× (Olympus) with a working distance of 3.3 mm, and a numerical aperture 0.80 was used for imaging. The imaging process was controlled by software custom-written in LabVIEW.
Simultaneous recording of calcium fluorescence transients and AP train
To compare the real with the reconstructed AP train of a single neuron, neuronal calcium fluorescence transients and AP firing were recorded simultaneously. Calcium fluorescence transients was expressed as ΔF∕F = (F–F0)∕F0, where F0 is the resting calcium fluorescence signal, and F are the calcium fluorescence intensities. In this paper, the dwelling time of each region of interest (ROI) is 250 μs, and four ROIs were preselected, so the random access sampling rate is 1 kHz. Whole-cell patch recording was obtained to monitor membrane voltage of neurons using a patch clamp amplifier (EPC-9, HEKA). Recording pipettes were routinely filled with a solution containing (in mM) 125 K-gluconate, 15 KCl, 10 Hepes, 4 MgCl2, 3 Mg-ATP, 0.3 Na-GTP, 10 Na2-phosphocreatine, 0.2 EGTA; and 100 μM Fluo-5F (pH 7.3 with KOH, 290 to 300 mOsm). Signals were sampled at 10 kHz by the amplifier system.
Reconstructing Neuronal AP Train
The MSF_TC algorithm contains three steps. The first step is the extraction of the template, which is then used in the following two steps. The AP train candidates are generated in the second step. Last, through template convolution, the optimal AP train is confirmed and regarded as the reconstruction results.
Extraction of the template
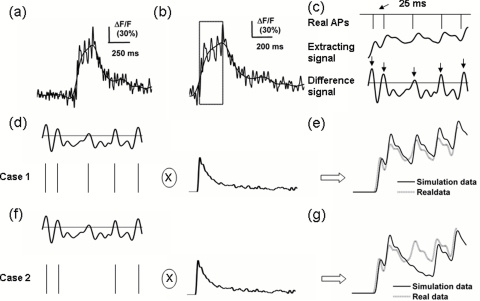
The template extraction is primarily based on the fact that the calcium wave triggered by single AP remains almost unchanged.32, 33 Thus, 10 to 20 calcium waves from individual calcium fluorescence transients are aligned at the time when they are ascending most rapidly, and then averaged. The averaged trace is then regarded as the template. For demonstration in Figs. 1d to 1g a template is used to be convoluted with the AP train. Note that the value of the template baseline should be rectified to zero.
Figure 1.
MSF_TC algorithm for AP train reconstruction. (a) calcium fluorescence transients after 10- and 100-Hz low-pass filtering, respectively. (b) Identify the valid calcium fluorescence transient and extract its rising duration [the box in (b)]. (c) The extracted rising duration (middle) corresponds to 5 APs (top), and the predicted APs firing at the peak of the differential of the rising duration of the calcium waves, as indicated by the arrows (bottom). Note that the peak should be more than the given threshold (horizontal line in the bottom panel). Five predicted APs can generate 32 AP train candidates according to Eq. 4. (d) and (f) Two of 32 AP train candidates that convolved with the template and simulated signals were generated. The AP train is regarded as a reconstructed AP train, which corresponds to the simulated signal that has the most similar real signal. Based on this, the AP train in (d) is the reconstructed AP train rather than that in (f).
AP train candidates generation
In this step, identification of a valid calcium fluorescence transient and the extraction of its rising duration are necessary. The 10- and 100-Hz low-pass Butterworth filtered calcium fluorescence transients sampled at 1 kHz were used to identify a valid signal and reconstruct the AP train, respectively, as shown in Figs. 1a, 1b. The number of zeros and poles in the Butterworth filter were set to be 3 and 10, respectively, at all times. The rising and decay duration triggered by a single AP firing usually last hundreds of milliseconds. Using the 10-Hz low-pass filter, the AP firing and corresponding rising durations are easily identified. The identified arising duration, the adjacent decay duration, and the following 500-ms duration consist of a part of the calcium fluorescence transients [Fig. 1b], the time interval of which corresponds to the 100-Hz filtered signal. The corresponding signal is used to reconstruct the AP train, which is mostly based on the consideration that the rising duration trigged by two APs firing at a 25-ms interval can still be completely separated [Fig. 1c] using a 100-Hz low-pass filter. Accordingly, MSF_TC can successfully identify APs firing at frequencies up to 40 Hz from the calcium fluorescence transients.
As indicated in the box in Fig. 1b and the middle panel of Fig. 1c, the extracted rising duration p(t) t ∈ [ti,ti+1+2k] from the identified valid signal could meet the following criteria:
| (1) |
| (2) |
| (3) |
where ti is the time corresponding to the peak or valley value p(ti), and thr denotes the threshold determined by clustering for the amplitudes of the noise and the calcium waves triggered by a single AP. A suitable setting is 0.6 to 0.7 times the amplitude of the template. The times [t1, t2,…, tk], indicated by the arrows in the bottom panel of Fig. 1c, correspond to the peaks of the differential of the extracted rising duration [Fig. 1c, middle], and the peak values are larger than the standard deviation of the differential signal [horizontal line in Fig. 1c, bottom panel]. These times are used to generate AP train candidates, as follows:
| (4) |
where ℓs(t) is a candidate of AP train, s = 1, 2,…, 2k. Here, ℓs(ti) = 1 means that single AP fires at time ti. Note that ℓs(t1) = 2 represents a special AP burst firing pattern that two AP fire at an about 10-ms time interval and their waves are not completely separated. This usually happens at the beginning of the burst.34, 35
Template convolution
The AP candidate train ℓs(t) was convolved with the template to get the corresponding simulating signal fs(t). As shown in Figs. 1d, 1e, the simulated signal [solid line in the Fig. 1e] was obtained by convoluting the AP train candidate containing five APs [Fig. 1d] with the template. The difference between the simulated signal fs(t) and the extracted rising duration p(t) t ∈ [ti, ti+1+2k] is measured by the Euclidean distance shown in
| (5) |
The minimum value ks* indicates that the simulating signal fs*(t) is the most similar to the rising duration. Thus, the AP train corresponding to fs*(t) is regarded as reconstructed AP train. As illustrated in Figs. 1d through 1g, the AP train candidate in Fig. 1d is more suitable to represent the real AP train than that in Fig. 1f.
Results
The MSF_TC algorithm was applied to eight pieces of experimental data from four different cells. They are 300 s long and sampled at a 1-kHz frequency. The template of any trace was extracted from the individual data sets. The difference between the reconstructed and real AP trains reflecting the efficiency of the MSF_TC algorithm was weighed with false positive rates and event detection rates.
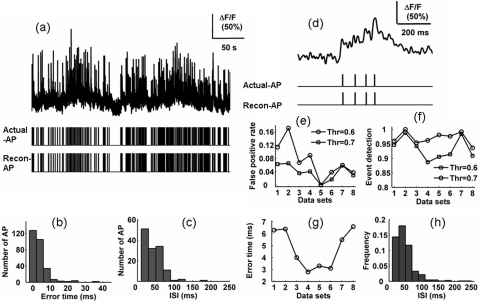
In Fig. 2a, the data set 1 (top) contains 301 APs, and the visual comparison of real (middle) and reconstruction (bottom) AP train indicated that no difference between them exists. In this case, the false positive rate and the event detection rate were 6 to 11 and 94 to 95%, respectively, when the threshold was set to be 0.6 to 0.7 times the amplitude of the template, as indicted in Figs. 2e, 2f (labeled the number 1 on the x axis). As shown in Figs. 2e, 2f, the reconstruction results from the other seven pieces of data were similar with that already considered.
Figure 2.
Capability of MSF_TC algorithm in AP train reconstruction: (a) calcium fluorescence transients (top), the corresponding real (Middle) and reconstructed (bottom) AP trains; (b) histogram of the difference between AP firing reconstruction and real timing obtained by analyzing data set in (a); (c) ISI distribution from the actual AP train [middle panel in (a)]; and (d) burst AP firing corresponding calcium fluorescence, real and reconstructed AP train. For an eight-data-set analysis, a 10% change of the threshold influenced by (e) the false positive rate and (f) the event detection. (g) plot of the standard deviation of the absolute error between real and reconstructed AP timings and (h) ISI distribution for eight real AP trains.
We also measured the time precision of the AP train reconstruction by comparing it with the real AP train. The absolute error of AP firing estimated timing and real timing distributed over the 0- to 10-ms interval was more than 90% [Fig. 2b]. The standard deviation of the absolute errors was less than 7 ms for data set 1, and was also suitable for the other seven data sets [Fig. 2g].
To show the ability of the MSF_TC algorithm to reconstruct the burst firing, a histogram of the inter-AP interval (ISI) less than 1000 ms was given by analyzing data set 1 [Fig. 2c]. From the histogram, we can conclude that high-frequency and short-duration firing are common patterns in our data set. For further verification, ISI distribution from eight AP trains was also given and ISIs less than 50 ms held 30% [Fig. 2h]. In addition, a part of calcium fluorescence transient triggered by a burst firing is shown in Fig. 2d. During the AP burst firing, the rising duration generated by individual AP obviously changes, which heavily hinders the reconstruction of AP train using some characteristics of a calcium fluorescence transient, such as its rising amplitude and the amplitude variance. Considered from the reconstruction results and our data set features, the MSF_TC algorithm can effectively identify an individual AP from a burst.
To test the robustness of the MSF_TC algorithm against the threshold, a 10% change in the threshold was applied, namely, the threshold increasing from 0.6 to 0.7 times of the amplitude of the template. The false positive rate (FPR) and the event detection rate (EVR) were increased and decreased by an average of about 3%, respectively [Figs. 2e, 2f].
We compared the reconstruction performance of the MSF_TC algorithm with that of two other typical methods, template matching22, 23 and the deconvolution method7 (Table 1). The calculations, including parameter selection, were carried out in accordance with previous work.7, 22, 23 For MSF_TC, the threshold value was set to 0.7 times the amplitude of the template. Low EDRs demonstrate that the template matching is unsuitable for reconstructing the neuronal burst firing. For the deconvolution method, the effective results are strongly dependent on the high SNR. For example, the SNRs of data sets 1 to 4 are about a range of from 2 to 2.5; in contrast, the data set 6 reaches 4. Therefore, compared with the deconvolution method, the MSF_TC algorithm could provide effective results from the calcium transients even with a relatively low SNR.
Table 1.
MSF_TC algorithm compared with template matching and the deconvolution method.
| DataSet | MSF_TC Algorithm | Template Matching | Deconvolution | |||
|---|---|---|---|---|---|---|
| FPR (%) | EDR (%) | FPR (%) | EDR (%) | FPR (%) | EDR (%) | |
| 1 | 6 | 95 | 18 | 38 | 2 | 65 |
| 2 | 7 | 99 | 28 | 70 | 6 | 67 |
| 3 | 4 | 94 | 30 | 70 | 7 | 70 |
| 4 | 4 | 89 | 37 | 75 | 8 | 83 |
| 5 | 0 | 91 | 25 | 74 | 17 | 100 |
| 6 | 2 | 92 | 19 | 33 | 5 | 98 |
| 7 | 6 | 98 | 12 | 44 | 3 | 93 |
| 8 | 3 | 91 | 18 | 43 | 20 | 100 |
Discussion and Conclusions
As already noted, the neuronal bursts firings are extremely important for information encoding. We proposed a simple method to reconstruct an AP train from the calcium fluorescence transients that is suitable for even neuronal burst firing. By the multiscale filter application and valid calcium identification, the influence of long-duration noise on the reconstruction result is eliminated to a large extent. Furthermore, the simulation signals generated by the convolution of AP train candidates with the template can provide many patterns to match neuronal burst firing. These procedures guarantee the reliability and effectiveness of the MSF_TC algorithm in the analysis of the data sets.
Note that the method requires a proper template, so acquiring the template is an important procedure. Calcium fluorescence transients induced by a single AP is an all or nothing process.36 In addition, the model for computing calcium dynamics demonstrates that the amplitude of the calcium fluorescence transient is almost constant.21 Therefore, the template can be successfully obtained by extracting and averaging the calcium traces generated by a single AP.
In the MSF_TC algorithm, multiscale filters are adopted. The scale selection is determined by the sum of the rise and decay duration formed by single AP firing and the noise type. There is a significant difference between the AP firing and no AP firing, corresponding to a 10-Hz low-pass-filtered calcium fluorescence transient. Thus, identifying valid calcium fluorescence is easy and the threshold is not discussed. In addition, properly increasing the length of the valid calcium fluorescence transient prevents missing the APs, whose firings lead to calcium wave part separations. Obviously, identifying an individual AP from a burst is impossible using the 10-Hz filtered signal. To resolve the rising durations triggered by two APs with 25-ms firing intervals, that is, the two rising durations are completely separate—100-Hz low-pass filters were properly selected, as verified by our results. Note that, in part of our data sets, the occurrence of two APs with an interval of 20 ms can cause the complete separation of the rising duration. As a result, the APs firing at a 50-Hz frequency from a burst were successfully identified.
Compared with other typical methods, i.e., template matching22 and the deconvolution method,7 the results illustrate that MSF_TC algorithm is superior to these two methods. The reason may be as follows: many of the burst firings corresponding to the calcium fluorescence transients lead the template matching to have a low efficacy and an outstanding performance of the deconvolution method is strongly dependent on a high SNR. For sparse AP firing, the MSF_TC algorithm is still inferior to template matching, because it chooses only the characteristics of the rising duration that identify an individual AP from a burst firing. Furthermore, like the other methods,9, 29, 30 the MSF_TC algorithm can not provide the AP firing rate when the rising durations are not separated. Properly use of decay duration may enhance the efficiency of identifying individual APs from a burst firing.
In summary, according to the characteristics of the data sets from our optical system, a reliable method was designed to reconstruct AP trains from calcium fluorescence transients. The results showed that the MSF_TC algorithm has a perfect performance in revealing an individual AP from calcium fluorescence transients corresponding to a burst firing, which should be beneficial for further deciphering neuronal circuits with a higher temporal resolution.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 30927001 and 30925013). WRC was supported by National Institutes of Health (NIH) Grants DC009666 and DC003918.
Reference
- Stosiek C., Garaschuk O., Holthoff K., and Konnerth A., “In vivo two-photon calcium imaging of neuronal networks,” Proc. Natl. Acad. Sci. U.S.A. 100(12), 7319–7324 (2003). 10.1073/pnas.1232232100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Santamaria F., and Tanaka K., “Local calcium signaling in neurons,” Neuron 40(2), 331–346 (2003). 10.1016/S0896-6273(03)00639-1 [DOI] [PubMed] [Google Scholar]
- Ohki K., Chung S., Ch’Ng Y. H., Kara P., and Reid R. C., “Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex,” Nature 433(7026), 597–603 (2005). 10.1038/nature03274 [DOI] [PubMed] [Google Scholar]
- Rochefort N. L., Jia H., and Konnerth A., “Calcium imaging in the living brain: prospects for molecular medicine,” Trends Mol. Med. 14(9), 389–399 (2008). 10.1016/j.molmed.2008.07.005 [DOI] [PubMed] [Google Scholar]
- Denk W., Strickler J. H., and Webb W. W., “Two-photon laser scanning fluorescence microscopy,” Science 248(4951), 73–76 (1990). 10.1126/science.2321027 [DOI] [PubMed] [Google Scholar]
- Liu X., Gong H., Li X., and Zhou W., “Monitoring calcium concentration in neurons with cameleon,” J. Biosci. Bioeng. 105(2), 106–109 (2008). 10.1263/jbb.105.106 [DOI] [PubMed] [Google Scholar]
- Yaksi E. and Friedrich R. W., “Reconstruction of firing rate changes across neuronal populations by temporally deconvolved Ca2+ imaging,” Nature Meth. 3(5), 377–383 (2006). 10.1038/nmeth874 [DOI] [PubMed] [Google Scholar]
- Dombeck D. A., Khabbaz A. N., Collman F., Adelman T. L., and Tank D. W., “Imaging large-scale neural activity with cellular resolution in awake, mobile mice,” Neuron 56(1), 43–57 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg D. S., Houweling A. R., and Kerr J., “Population imaging of ongoing neuronal activity in the visual cortex of awake rats,” Nature Neurosci. 11(7), 749–751 (2008). 10.1038/nn.2140 [DOI] [PubMed] [Google Scholar]
- Salome R., Kremer Y., Dieudonne S., Leger J. F., Krichevsky O., Wyart C., Chatenay D., and Bourdieu L., “Ultrafast random-access scanning in two-photon microscopy using acousto-optic deflectors,” J. Neurosci. Meth. 154(1–2), 161–174 (2006). [DOI] [PubMed] [Google Scholar]
- Lv X., Zhan C., Zeng S., Chen W. R., and Luo Q., “Construction of multiphoton laser scanning microscope based on dual-axis acousto-optic deflector,” Rev. Sci. Instrum. 77(4), 046101 (2006). 10.1063/1.2190047 [DOI] [Google Scholar]
- Zeng S., Lv X., Bi K., Zhan C., Li D., Chen W. R., Xiong W., Jacques S. L., and Luo Q., “Analysis of the dispersion compensation of acousto-optic deflectors used for multiphoton imaging,” J. Biomed. Opt. 12, 24015 (2007). 10.1117/1.2714061 [DOI] [PubMed] [Google Scholar]
- Grewe B. F., Langer D., Kasper H., Kampa B. M. and Helmchen F., “High-speed in vivo calcium imaging reveals neuronal network activity with near-millisecond precision,” Nature Meth. 7(5), 399–405 (2010). 10.1038/nmeth.1453 [DOI] [PubMed] [Google Scholar]
- Grace A. A. and Bunney B. S., “The control of firing pattern in nigral dopamine neurons: burst firing,” J. Neurosci. 4(11), 2877–2890 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., “Bursts as a unit of neural information: making unreliable synapses reliable,” Trends Neurosci. 20(1), 38–43 (1997). 10.1016/S0166-2236(96)10070-9 [DOI] [PubMed] [Google Scholar]
- Harris K. D., Hirase H., Leinekugel X., Henze D. A., and Buzsáki G., “Temporal interaction between single spikes and complex spike bursts in hippocampal pyramidal cells,” Neuron 32(1), 141–149 (2001). [DOI] [PubMed] [Google Scholar]
- Iyer V., Hoogland T. M., and Saggau P., “Fast functional imaging of single neurons using random-access multiphoton (RAMP) microscopy,” J. Neurophysiol. 95(1), 535–545 (2006). 10.1152/jn.00865.2005 [DOI] [PubMed] [Google Scholar]
- Wu Y., Liu X., Zhou W., Lv X., and Zeng S., “Observing neuronal activities with random access two-photon microscope,” J. Innov. Opt. Health Sci. 12(1), 67–71 (2009). 10.1142/S179354580900036X [DOI] [Google Scholar]
- Bel W. G. and Helmchen F., “In vivo calcium imaging of neural network function,” Physiology (Bethesda) 22, 358–365 (2007). [DOI] [PubMed] [Google Scholar]
- Grewe B. F. and Helmchen F., “Optical probing of neuronal ensemble activity,” Curr. Opin. Neurobiol. 19(5), 520–529 (2009). 10.1016/j.conb.2009.09.003 [DOI] [PubMed] [Google Scholar]
- Neher E. and Augustine G. J., “Calcium gradients and buffers in bovine chromaffin cells,” J. Physiol. 450(1), 273–301 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D. and Bekkers J. M., “Detection of spontaneous synaptic events with an optimally scaled template,” Biophys. J. 73(1), 220–229 (1997). 10.1016/S0006-3495(97)78062-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J., Greenberg D., and Helmchen F., “Imaging input and output of neocortical networks in vivo,” Proc. Natl. Acad. Sci. U.S.A. 102(39), 14063–14068 (2005). 10.1073/pnas.0506029102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smetters D., Majewska A., and Yuste R., “Detecting action potentials in neuronal populations with calcium imaging,” Methods 18(2), 215–221 (1999). 10.1006/meth.1999.0774 [DOI] [PubMed] [Google Scholar]
- Moreaux L. and Laurent G., “Estimating firing rates from calcium signals in locust projection neurons in vivo,” Front. Neural Circ. 1, 2 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel E. A., Nimmerjahn A., and Schnitzer M. J., “Automated analysis of cellular signals from large-scale calcium imaging data,” Neuron 63(6), 747–760 (2009). 10.1016/j.neuron.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Gray N., Mainen Z., and Svoboda K., “The functional microarchitecture of the mouse barrel cortex,” Plos Biol. 5(7), 1440–1452 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Takahashi N., Matsuki N., and Ikegaya Y., “Fast and accurate detection of action potentials from somatic calcium fluctuations,” J. Neurophysiol. 100(3), 1668–1676 (2008). 10.1152/jn.00084.2008 [DOI] [PubMed] [Google Scholar]
- Vogelstein J. T., Watson B. O., Packer A. M., Yuste R., Jedynak B., and Paninski L., “Spike inference from calcium imaging using sequential monte carlo methods,” Biophys. J. 97(2), 636–655 (2009). 10.1016/j.bpj.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishchencko Y., Vogelstein J. T., and Paninski L., “A Bayesian approach for inferring neuronal connectivity from calcium fluorescence imaging data,” Ann. Appl. Stat. (in press). [Google Scholar]
- Watanabe W., Matsunaga S., Fukui K., and Itoh K., “Intracellular manipulation by femtosecond lasers: review,” J. Innov. Opt. Health Sci. 2(1), 1–8 (2009). 10.1142/S1793545809000310 [DOI] [Google Scholar]
- Marhl M., Schuster S., and Brumen M., “Mitochondria as an important factor in the maintenance of constant amplitudes of cytosolic calcium oscillations,” Biophys. Chem. 71(2–3), 125–132 (1998). 10.1016/S0301-4622(97)00139-7 [DOI] [PubMed] [Google Scholar]
- Wallace D. J., Zum Alten Borgloh S. M., Astori S., Yang Y., Bausen M., Kügler S., Palmer A. E., Tsien R. Y., Sprengel R., and Kerr J., “Single-spike detection in vitro and in vivo with a genetic Ca2+ sensor,” Nature Meth. 5(9), 797–804 (2008). [DOI] [PubMed] [Google Scholar]
- Perreault P. and Avoli M., “4-Aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus,” J. Neurosci. 12(1), 104–115 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar P., Tapia R., and Rogawski M. A., “Effects of neurosteroids on epileptiform activity induced by picrotoxin and 4-aminopyridine in the rat hippocampal slice,” Epilepsy Res. 55(1–2), 71–82 (2003). 10.1016/S0920-1211(03)00112-8 [DOI] [PubMed] [Google Scholar]
- Usachev Y. M. and Thayer S. A., “All-or-none Ca2+ release from intracellular stores triggered by Ca2+ influx through voltage-gated Ca2+ channels in rat sensory neurons,” J. Neurosci. 17(19), 7404–7414 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]