Abstract
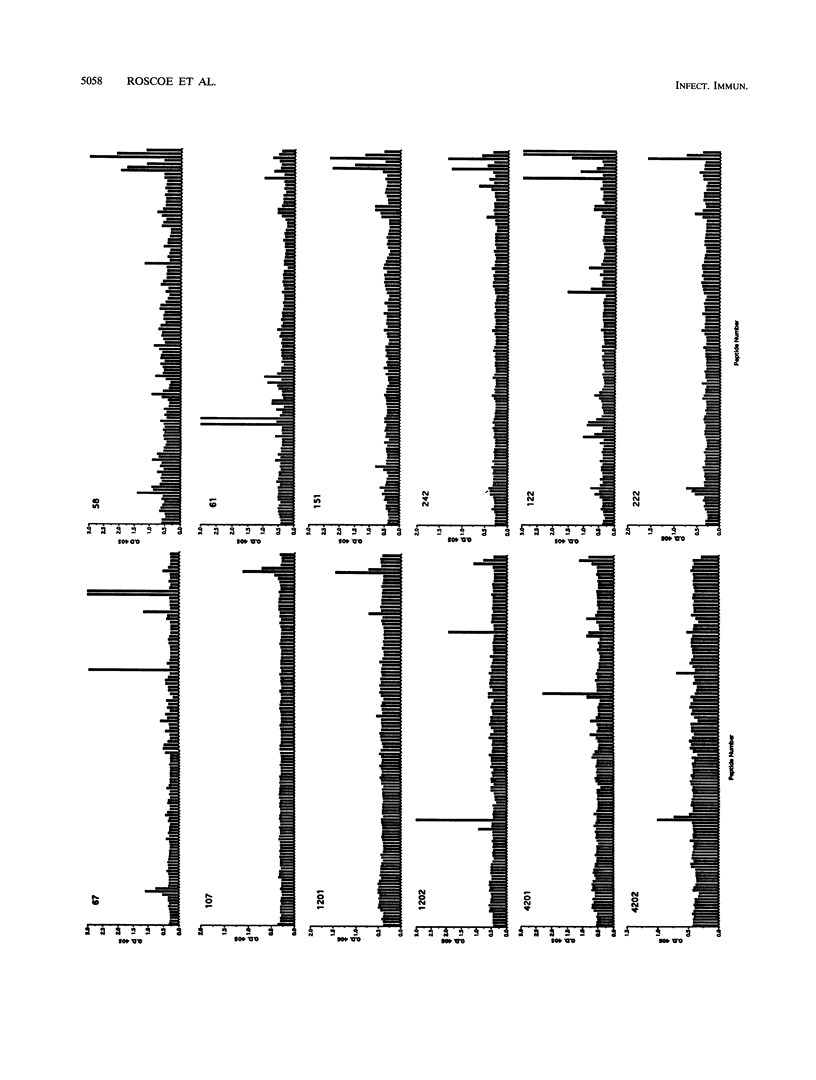
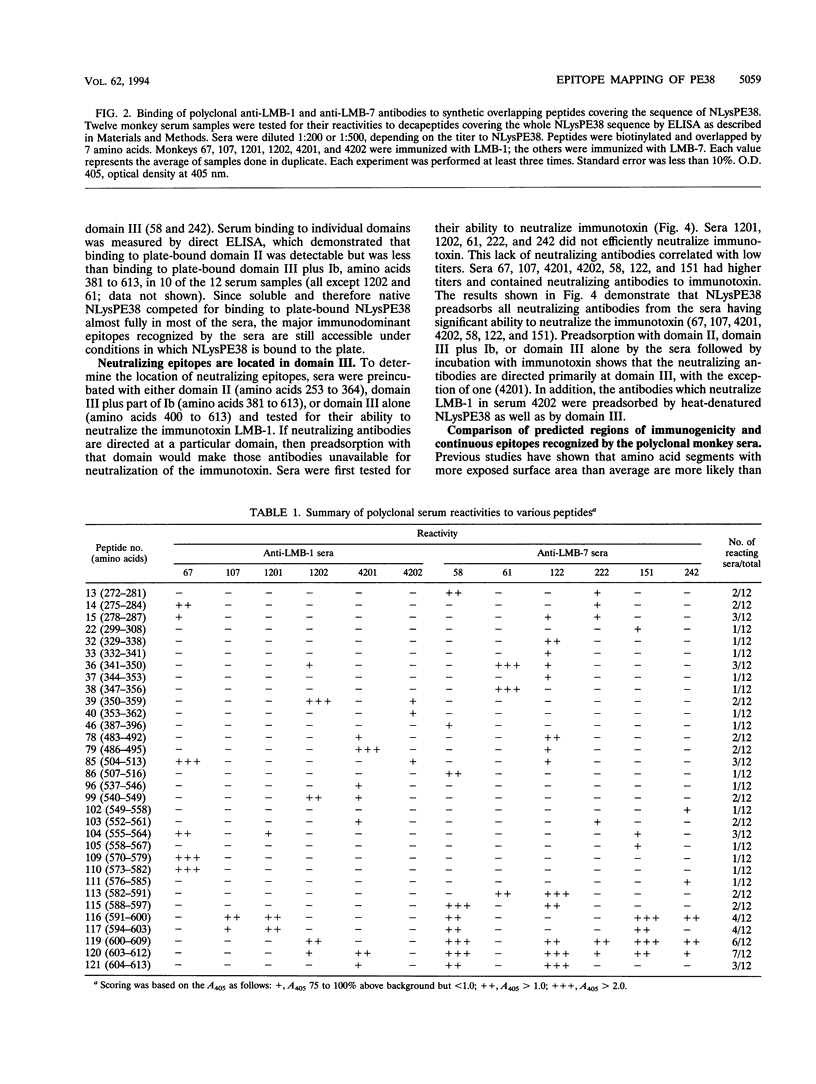
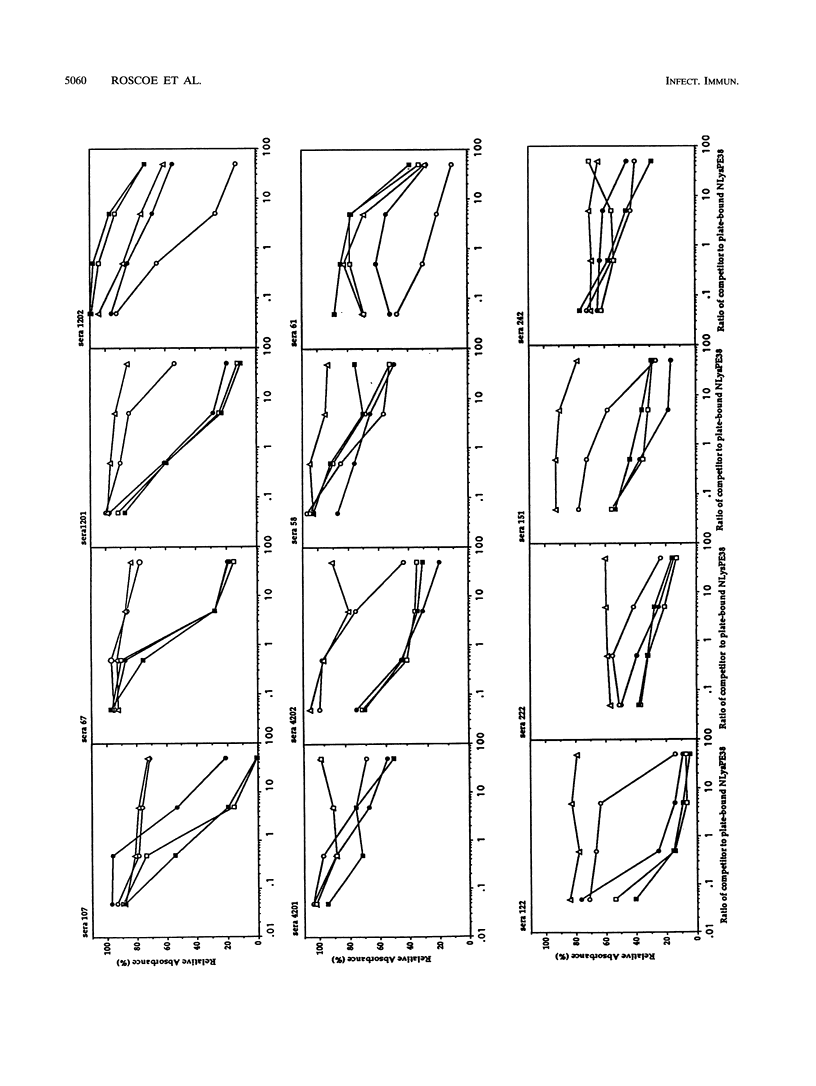
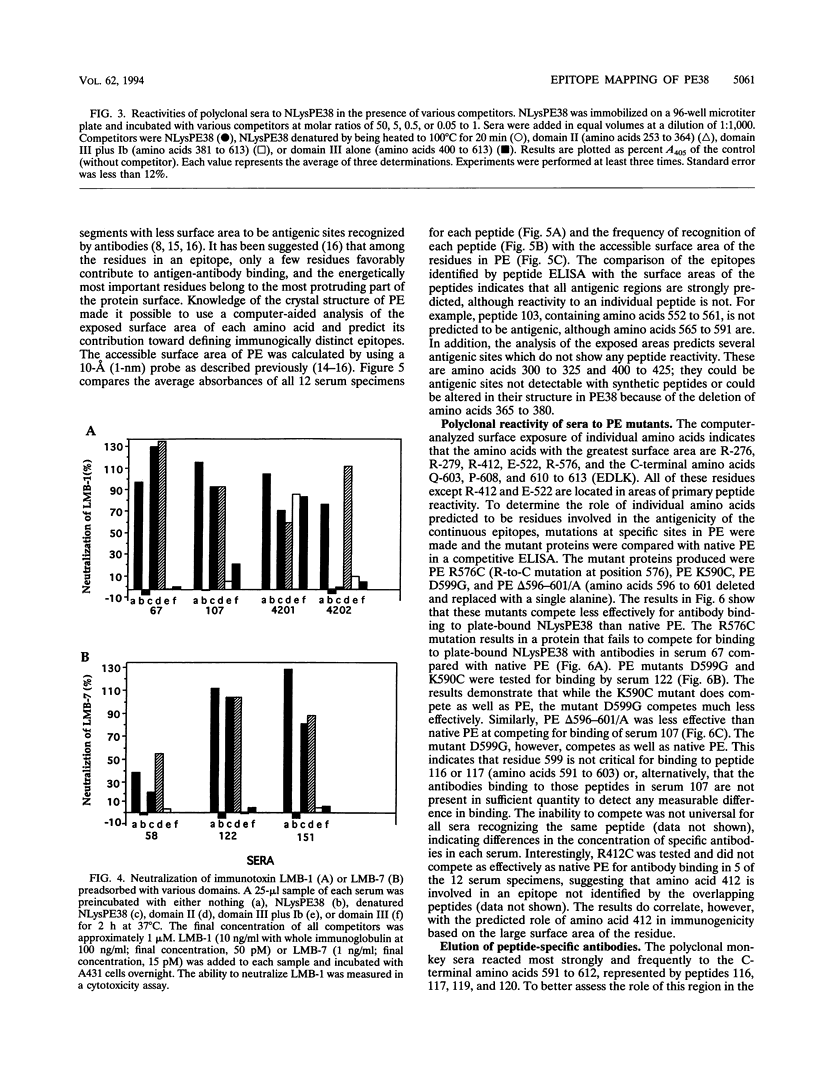
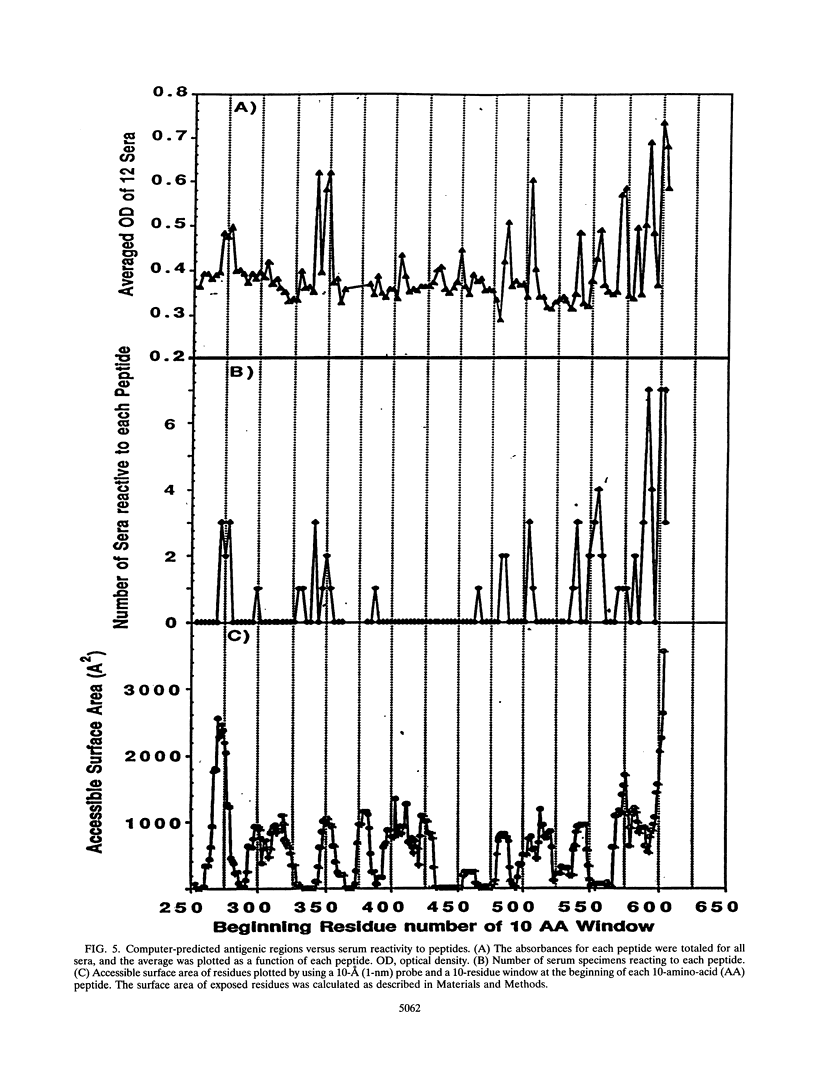
NLysPE38 is a 38-kDa derivative of Pseudomonas exotoxin (PE) in which domain Ia (amino acids 1 to 252) and part of domain Ib (365 to 380) are deleted and an 11-amino-acid N-terminal peptide is added. LMB-1 is an immunotoxin in which the monoclonal antibody B3 is coupled to NLysPE38 near its N terminus. LMB-7 is a single-chain immunotoxin in which the Fv fragment of B3 is fused to PE38. To identify the antigenic regions of PE38, 12 polyclonal serum samples from monkeys immunized with the immunotoxins LMB-1 (six monkeys) and LMB-7 (six monkeys) were tested for their reactivity to a panel of 120 synthetic, overlapping peptides representing the amino acid sequence of NLysPE38. The antibody responses to peptides were similar among the 12 serum specimens, identifying several major immunodominant B-cell epitopes. Predominant reactivity was seen in six locations: amino acids 272 to 287, 341 to 359, 504 to 516, 540 to 564, and 573 to 591 and the C-terminal amino acids 591 to 613. The sera did not react with approximately 75% of the peptides. Furthermore, a computer-aided analysis was done to predict the immunologically relevant areas and revealed the same antigenic regions defined by serum reactivity to peptides. Competition enzyme-linked immunosorbent assays and neutralization assays were performed with domain II, III, or III plus Ib of PE38 and confirmed the immunodominance of domain III. To analyze the role of specific amino acids in antibody binding, individual amino acids of PE38 with large accessible surface areas were altered by site-directed mutagenesis. These results also show that the predicted areas of immunogenicity agree with the reactivity of the anti-PE38 antibodies to peptides and to the mutants of PE.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allured V. S., Collier R. J., Carroll S. F., McKay D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhar I., Wang Q. C., FitzGerald D., Pastan I. Pseudomonas exotoxin A mutants. Replacement of surface-exposed residues in domain III with cysteine residues that can be modified with polyethylene glycol in a site-specific manner. J Biol Chem. 1994 May 6;269(18):13398–13404. [PubMed] [Google Scholar]
- Brinkmann U., Pai L. H., FitzGerald D. J., Willingham M., Pastan I. B3(Fv)-PE38KDEL, a single-chain immunotoxin that causes complete regression of a human carcinoma in mice. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8616–8620. doi: 10.1073/pnas.88.19.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. F., Collier R. J. Active site of Pseudomonas aeruginosa exotoxin A. Glutamic acid 553 is photolabeled by NAD and shows functional homology with glutamic acid 148 of diphtheria toxin. J Biol Chem. 1987 Jun 25;262(18):8707–8711. [PubMed] [Google Scholar]
- Chia J. K., Pollack M., Avigan D., Steinbach S. Functionally distinct monoclonal antibodies reactive with enzymatically active and binding domains of Pseudomonas aeruginosa toxin A. Infect Immun. 1986 Jun;52(3):756–762. doi: 10.1128/iai.52.3.756-762.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Tainer J. A., Rodda S. J., Mason T. J., Alexander H., Getzoff E. D., Lerner R. A. Chemistry of antibody binding to a protein. Science. 1987 Mar 6;235(4793):1184–1190. doi: 10.1126/science.3823878. [DOI] [PubMed] [Google Scholar]
- Helzlsouer K. J., Gordon G. B., Alberg A. J., Bush T. L., Comstock G. W. Relationship of prediagnostic serum levels of dehydroepiandrosterone and dehydroepiandrosterone sulfate to the risk of developing premenopausal breast cancer. Cancer Res. 1992 Jan 1;52(1):1–4. [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe L. K. Humanized antibodies: enhancing therapeutic utility through antibody engineering. Int Rev Immunol. 1993;10(2-3):241–250. doi: 10.3109/08830189309061699. [DOI] [PubMed] [Google Scholar]
- Kondo T., FitzGerald D., Chaudhary V. K., Adhya S., Pastan I. Activity of immunotoxins constructed with modified Pseudomonas exotoxin A lacking the cell recognition domain. J Biol Chem. 1988 Jul 5;263(19):9470–9475. [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Smith-Gill S. J. Epitopes on protein antigens: misconceptions and realities. Cell. 1990 May 18;61(4):553–556. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Novotny J., Bruccoleri R. E., Saul F. A. On the attribution of binding energy in antigen-antibody complexes McPC 603, D1.3, and HyHEL-5. Biochemistry. 1989 May 30;28(11):4735–4749. doi: 10.1021/bi00437a034. [DOI] [PubMed] [Google Scholar]
- Novotny J. Protein antigenicity: a thermodynamic approach. Mol Immunol. 1991 Mar;28(3):201–207. doi: 10.1016/0161-5890(91)90062-o. [DOI] [PubMed] [Google Scholar]
- Ogata M., Pastan I., FitzGerald D. Analysis of Pseudomonas exotoxin activation and conformational changes by using monoclonal antibodies as probes. Infect Immun. 1991 Jan;59(1):407–414. doi: 10.1128/iai.59.1.407-414.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. C., Hamood A. N., Vincent T. S., Beachey E. H., Iglewski B. H. Identification of functional epitopes of Pseudomonas aeruginosa exotoxin A using synthetic peptides and subclone products. Mol Immunol. 1990 Oct;27(10):981–993. doi: 10.1016/0161-5890(90)90121-f. [DOI] [PubMed] [Google Scholar]
- Oppenheimer N. J., Bodley J. W. Diphtheria toxin. Site and configuration of ADP-ribosylation of diphthamide in elongation factor 2. J Biol Chem. 1981 Aug 25;256(16):8579–8581. [PubMed] [Google Scholar]
- Pai L. H., Batra J. K., FitzGerald D. J., Willingham M. C., Pastan I. Anti-tumor activities of immunotoxins made of monoclonal antibody B3 and various forms of Pseudomonas exotoxin. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3358–3362. doi: 10.1073/pnas.88.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai L. H., Bookman M. A., Ozols R. F., Young R. C., Smith J. W., 2nd, Longo D. L., Gould B., Frankel A., McClay E. F., Howell S. Clinical evaluation of intraperitoneal Pseudomonas exotoxin immunoconjugate OVB3-PE in patients with ovarian cancer. J Clin Oncol. 1991 Dec;9(12):2095–2103. doi: 10.1200/JCO.1991.9.12.2095. [DOI] [PubMed] [Google Scholar]
- Pai L. H., Pastan I. Immunotoxin therapy for cancer. JAMA. 1993 Jan 6;269(1):78–81. [PubMed] [Google Scholar]
- Pai L. H., Pastan I. The use of immunotoxins for cancer therapy. Eur J Cancer. 1993;29A(11):1606–1609. doi: 10.1016/0959-8049(93)90306-z. [DOI] [PubMed] [Google Scholar]
- Pastan I., Chaudhary V., FitzGerald D. J. Recombinant toxins as novel therapeutic agents. Annu Rev Biochem. 1992;61:331–354. doi: 10.1146/annurev.bi.61.070192.001555. [DOI] [PubMed] [Google Scholar]
- Pastan I., Lovelace E. T., Gallo M. G., Rutherford A. V., Magnani J. L., Willingham M. C. Characterization of monoclonal antibodies B1 and B3 that react with mucinous adenocarcinomas. Cancer Res. 1991 Jul 15;51(14):3781–3787. [PubMed] [Google Scholar]
- Rutault K., Vacheron M. J., Guinand M., Michel G. Comparative immunochemistry of two fragments from domains Ib and III of Pseudomonas aeruginosa exotoxin A. Infect Immun. 1993 Dec;61(12):5417–5420. doi: 10.1128/iai.61.12.5417-5420.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoca R., Schwab C., Bosshard H. R. Epitope mapping employing immobilized synthetic peptides. How specific is the reactivity of these peptides with antiserum raised against the parent protein? J Immunol Methods. 1991 Aug 9;141(2):245–252. doi: 10.1016/0022-1759(91)90151-5. [DOI] [PubMed] [Google Scholar]
- Schwab C., Twardek A., Lo T. P., Brayer G. D., Bosshard H. R. Mapping antibody binding sites on cytochrome c with synthetic peptides: are results representative of the antigenic structure of proteins? Protein Sci. 1993 Feb;2(2):175–182. doi: 10.1002/pro.5560020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribbick G., Triantafyllou B., Lauricella R., Rodda S. J., Mason T. J., Geysen H. M. Systematic fractionation of serum antibodies using multiple antigen homologous peptides as affinity ligands. J Immunol Methods. 1991 Jun 3;139(2):155–166. doi: 10.1016/0022-1759(91)90185-i. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Fulton R. J., May R. D., Till M., Uhr J. W. Redesigning nature's poisons to create anti-tumor reagents. Science. 1987 Nov 20;238(4830):1098–1104. doi: 10.1126/science.3317828. [DOI] [PubMed] [Google Scholar]


