Abstract
P19 cells, a pluripotent cell line derived from a teratocarcinoma induced in C3H/HeHa mice, have been widely used as a model system to study cardiac differentiation. We have used these cells to evaluate the extent to which exposure to DMSO and/or cardiogenol C for 4 days in suspension culture enhanced their differentiation into cardiomyocytes. Cardiac differentiation was assessed by observing beating clusters and further confirmed using immunocytochemical, biochemical, and pharmacological approaches. The presence of functional gap junctions in differentiated P19 cells was identified through calcium wave analyses. Proliferation rate and cell death were analyzed by BrdU incorporation and activated caspase-3 immunodetection, respectively. Beating clusters of differentiated P19 cells were only found in cultures treated with DMSO. In addition, groups treated with DMSO up-regulated cardiac troponin-T expression. However, when DMSO was used together with cardiogenol C the up-regulation was less than that with DMSO alone, ∼1.5 times. Moreover, P19 cells cultured in DMSO or DMSO plus 0.25 μM cardiogenol C had lower proliferation rates and higher numbers of activated caspase-3-positive cells. In summary, using several methodological approaches we have demonstrated that DMSO can induce cardiac differentiation of P19 cells but that cardiogenol C does not.
Introduction
Teratocarcinomas are highly malignant tumors containing a disorganized array of many somatic and extraembryonic cells, together with a niche of embryonal carcinoma (EC) cells [1,2]. These cells can be found in malignant tumors arising spontaneously in mice and human testicles from defective germ cells and they can be induced artificially by transplantation of early murine embryos to extra-uterine sites [3,4].
Distinct from embryonic stem (ES) cells, frequently EC cells have limited ability for differentiation [5], but some EC cells have morphological, biochemical, and phenotypic properties in common with pluripotent embryonic cells [6–8]. P19 cells were derived from a teratocarcinoma artificially induced in C3H/HeHa mice [9] and represent one of the most widely studied pluripotent EC cell lines. These cells have been used as an in vitro model system to study embryonic development and differentiation [10]. Due to their ability to maintain an undifferentiated state without a feeder-cell layer and their high susceptibility to exogenous gene incorporation and expression, EC cells provide some important advantages over ES cells [11–13]. P19 cells are capable of differentiating into a variety of cell types representative of all 3 germ layers when induced by chemical agents [14]. Moreover, these cells are an excellent cell differentiation model that mimics the events of early cardioembryogenesis [15].
The formation of embryoid bodies (EB) in response to exposure to dimethyl sulfoxide (DMSO) is the main protocol that has been used to induce the differentiation of P19 cells into cardiomyocytes [16–20]. This protocol also induces cardiac differentiation in ES cells [21]. In addition, other factors have been found to induce cardiac differentiation in P19 cells, including 5-azacytidine [13], oxytocin [15,22], and retinoic acid [23,24]. Recently, it was reported that cardiogenol C (a diaminopyrimidine) induces cardiac differentiation in P19 and in P19Cl6 cells [25], the latter cells being a P19 cell subline with greater ability for cardiac differentiation [26,27]. In addition, the authors showed that this compound induced substantial cardiac differentiation in R1 mouse ES cells [25].
In this study, we determined whether 2 compounds already described as cardiogenic induced more substantial cardiac differentiation in P19 cells when used in association rather than individually. Surprisingly, we observed that when DMSO was used without cardiogenol C cardiac differentiation was higher than when it was used associated with cardiogenol C. In addition, treatment of P19 cells with cardiogenol C alone did not induce efficient cardiac differentiation.
Materials and Methods
P19 cell culture and differentiation
P19 cells were obtained from the American Type Culture Collection (ATCC, CRL 1825) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen Inc., Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 2 mM l-glutamine, 50 U/mL penicillin (Sigma-Aldrich Co., St. Louis, MO), and 50 μg/mL streptomycin (Sigma-Aldrich) in a 5% CO2 atmosphere at 37°C. In the present study, we used cultures of P19 cells with little variation at passage numbers in the different experiments.
To induce cardiac differentiation, 106 cells were cultured in suspension in 100 mm bacteriological Petri dishes in control medium (CTRL) or supplemented with: 1% DMSO (Sigma-Aldrich) (DS); 1% DMSO plus 0.25 μM cardiogenol C (Sigma-Aldrich) (DS+C25); or 0.25, 0.5, or 3.75 μM cardiogenol C (C25, C50, or C375). After 4 days in suspension, the EBs were transferred to adherent culture dishes with control medium. The medium was renewed every 2 days and the differentiation rate was analyzed 6–12 days after the formation of the EBs.
Quantitative analysis of EB area and number
The area and number of live EBs were analyzed after 4 days in suspension. EBs exposed to different treatments were transferred to 35 mm Petri dishes and 5 images (each covering a 2,200 mm2 area) from random fields of each treatment were obtained on a microscope with a 5× objective (NA = 0.15) for quantification. EBs with areas <2,000 μm2 were not included in the quantification.
Immunocytochemistry
For immunofluorescence, cells or EBs were grown on glass coverslips coated with 0.2% gelatin and fixed for 20 min in 4% paraformaldehyde. The cells or EBs were washed 3 times with phosphate-buffered saline (PBS) with 0.1% Triton X-100, incubated with 5% normal goat serum (NGS; Sigma) in PBS for 30 min, and then incubated with the primary antibody overnight at 4°C. The cells and EBs were then incubated with the secondary antibody and mounted with VectaShield (Vector, Burlingame, CA).
Immunostaining with anti-SSEA-1 (1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and anti-Oct-4 (1:500; Abcam Inc., Cambridge, MA) was used as positive control for undifferentiated P19 cells. To verify cardiac differentiation, the following antibodies were used: polyclonal anti-troponin-I (1:150; Santa Cruz); monoclonal anti-cardiac troponin-T (1:100; Abcam); monoclonal anti-myosin heavy chain (1:50; Santa Cruz); monoclonal anti-connexin43 (1:200; Chemicon International, Temecula, CA), monoclonal anti-sarcomeric tropomyosin (1:200; Sigma-Aldrich); and monoclonal MF20 (1:20; Developmental Studies Hybridoma bank, Iowa City, IA).
The secondary antibodies used in this study were: Alexa 488-conjugated goat-anti-rabbit IgG (1:400; Invitrogen Inc, Carlsbad, CA); Cy3-conjugated goat-anti-mouse IgG (1:1,000; Jackson ImmunoResearch Inc., West Grove, PA); and Cy3-conjugated goat-anti-mouse IgM μ-chain specific (1:1,000; Jackson ImmunoResearch Inc.). The cell nuclei were counterstained with 0.1% 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich).
Expression of Oct-3/4 and cardiac troponin-T in differentiated P19 cells
P19 cells cultured in 35 mm culture dishes were lysed in lysis buffer (1 mM NaHCO3, 2 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 5 mM EDTA) supplemented with protease inhibitor cocktail (Roche Laboratories, Basel, Switzerland) and protein concentration was determined by BCA protein assay kit (Pierce, Rockford, IL). The protein extracted was electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Bio-Rad, Hercules, CA) and proteins transferred onto nitrocellulose membranes (Whatman Int., Dassel, Germany). After 30-min incubation with blocking buffer (2% nonfat dry milk in tris buffer saline [TBS]) containing 0.05% Tween-20 (Sigma-Aldrich), the membranes were incubated overnight with monoclonal anti-Oct-3/4 (1:500; Santa Cruz) or monoclonal anti-cardiac troponin-T (1:1,000; Abcam) or rabbit polyclonal anti-BIP/GRP78 antibody (1:10,000; Affinity Bioreagent Inc., Golden, CO) at 4°C. Following three washes in TBS-Tween-20, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies goat anti-mouse or anti-rabbit IgG (1:10,000; Santa Cruz) for 1 h at room temperature. Detection of bands was performed on x-ray film (Kodak, Rochester, NY) after incubation of the membranes with the enhanced chemiluminescence reagents (Amersham Pharmacia Biotechnology, Piscataway, NJ).
Densitometric analysis of the western blots was performed using Scion Image for Windows (alfa 4.0.3.2, Scion Corporation; Frederick, MD).
Spontaneous calcium oscillations
To perform calcium wave analyses, the EBs plated on glass bottom MatTek dishes (MatTek Co., Ashland, MA) were loaded for 30 min at 37°C with Fluo-3-AM (5 μM; Invitrogen) and then bathed in Tyrode’s salt solution (pH 7.4). Intercellular Ca2+ wave images were acquired using a Nikon Eclipse TE2000E inverted microscope equipped with a CoolSNAP-HQ2 CCD camera (Photometrics) and a 20× Plan Fluor objective (NA 0.45). Changes in Fluo-3 (Invitrogen) fluorescence intensity emitted at 488 nm excitation (Lambda DG-4: Sutter Instruments, CA) were acquired at a rate of 20 Hz using Metafluor software (Molecular Devices, CA).
The gap junction blockers heptanol (Sigma-Aldrich) or carbenoxolone (Sigma-Aldrich) were used at 1 mM and 100 μM concentration, respectively. Fluo-3 fluorescence intensity obtained from regions of interest (F) was normalized by basal fluorescence (F0) and expressed as relative changes in fluorescence intensity (F/F0).
Analysis of proliferation and cell death rate
To measure proliferation rate for the different treatments, we incubated EBs with 10 μM of 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich) 2 h before fixation. In these experiments, 106 cells were cultivated in suspension on 100 mm bacteriological Petri dishes with or without the chemical inducers.
For fixation, EBs were incubated with 4% paraformaldehyde for 20 min, washed with PBS, and incubated with 20% sucrose for at least 30 min for cryopreservation. Thereafter, EBs were incubated in optimal cutting temperature resin (Sakura Finetek USA, Inc., Torrance, CA) and 12 μm frozen sections were collected on gelatin-coated slides.
Proliferation and cell death rates of EBs were analyzed on the second and fourth days after suspension culture for each experimental group. After fixation, EBs previously incubated with BrdU were exposed to anti-BrdU primary antibody (1:2; GE Healthcare, Piscataway, NJ), and these same EBs were used for immunoreactions for activated caspase-3 (1:500; Abcam).
Image acquisition
The photomicrographs shown in this study were obtained using an Axiovert 200M microscope (Zeiss, GmbH, Germany) equipped with ApoTome system or a LSM 510 Meta NLO confocal microscope (Zeiss) or a LSM 510 Meta Duo confocal microscope (Zeiss). Quantifications were performed using AxioVision 4.3 software (Zeiss).
Statistical analysis
Statistical significance was evaluated using one-way ANOVA for comparison among multiple groups. At least 3 independent experiments were performed for each analysis. All calculations were done using GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA).
Results
Characteristics of undifferentiated and differentiated P19 cells
After treatment, differentiated P19 cells were readily distinguishable from undifferentiated cells under phase contrast microscopy. Undifferentiated P19 cells were relatively small with a high nucleus to cytoplasm ratio, a common characteristic of pluripotent cells. In addition, rhythmically contracting cardiac muscle is easily distinguished by visual inspection of live cultures [16]. Undifferentiated P19 cells used in our experiments expressed pluripotency makers such as Oct-4 and SSEA-1 (Fig. 1A–1A″) and when transferred to bacteriological Petri dishes, these cells spontaneously formed EBs.
FIG. 1.
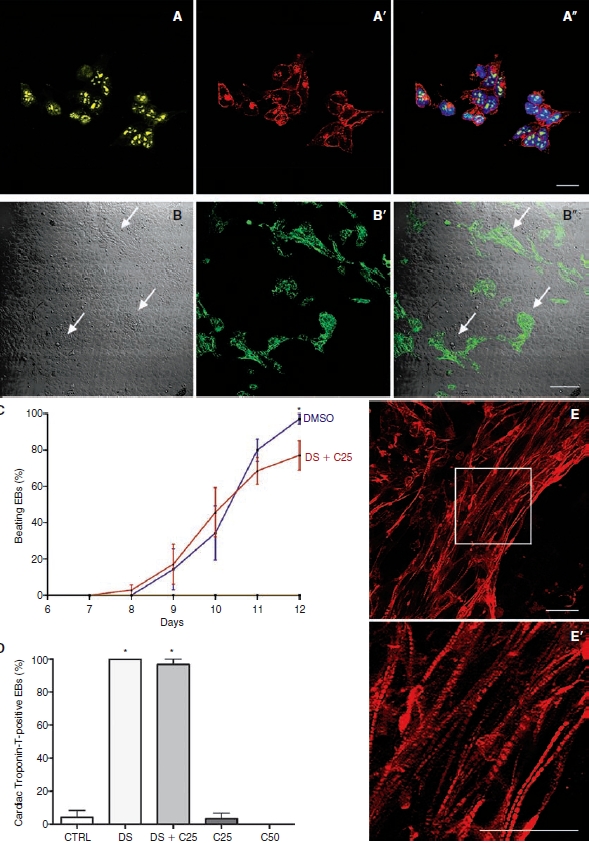
Characterization of undifferentiated and differentiated P19 cells. (A–A″) Pluripotency marker expression in undifferentiated P19 cells. (A) Oct-4. (A′) SSEA-1. (A″) Nuclear counterstaining with DAPI (blue) in the merged image. (B–B″) Beating clusters stained for troponin-I. (B) Phase contrast microscopy of beating areas (arrows). (B′) Immunofluorescence for troponin-I. (B″) Merged image (arrows showing beating cells stained with troponin-I). (C) Time course of beating embryoid body (EB) appearance after DMSO and DS+C25 treatment in cultures of P19 cells. CTRL, C25, C50, and C375 groups did not show beating clusters (N = 7). (D) Quantification of cardiac troponin-T expression in EBs at 12 days of differentiation (N = 6). (E–E′) Confocal Z-sections projected images showing cardiac troponin-T in differentiated P19 cells from the DS group. (E) Cardiac troponin-T expression. (E′) Higher magnification confocal microscopy image of the area indicated by the inset in (E) illustrating morphology and cardiac troponin-T expression pattern. Scale bars: A and E = 20 μm; B = 100 μm. Error bars represent SEM. *P < 0.05.
To confirm cardiac differentiation, beating cell clusters were fixed and stained for troponin-I after 12 days of differentiation (Fig. 1B–1B″). To determine cardiac differentiation rate among the different experimental groups, we quantified the number of EBs that showed beating clusters and the percentage of cells expressing cardiac troponin-T during the 12 days of culture. It was already possible to observe beating areas 7 days after start of aggregation (Fig. 1C). However, only EBs of the DS and DS+C25 groups showed beating clusters. On the 12th day of differentiation, we found a greater number of beating EBs from the DS group than the DS+C25 group. Beating EBs were not observed in the CTRL, C25, C50, and C375 groups. We found cells expressing cardiac troponin-T in all EBs from the DS group and in 96% from DS+C25 group (Fig. 1D–1E″). The other groups showed little or no expression of this protein.
To analyze in more detail the differentiation rate of P19 cells into cardiomyocytes, we quantified Oct-3/4 and cardiac troponin-T expression in these cells by western blotting (Fig. 2A). We observed Oct-3/4 expression in all groups after 12 days of differentiation. However, in the groups treated with DMSO this expression was lower than in the groups treated with cardiogenol C (Fig. 2B). In addition, we observed that DMSO presence in the culture medium greatly induced the up-regulation of cardiac troponin-T expression after 12 days of differentiation (Fig. 2C). Moreover, cardiac troponin-T expression was 1.52 ± 0.17 (SEM; P < 0.01)-fold higher in the DS than in the DS+C25 group. The CTRL, C25, and C50 groups did not detectably express this cardiac protein.
FIG. 2.
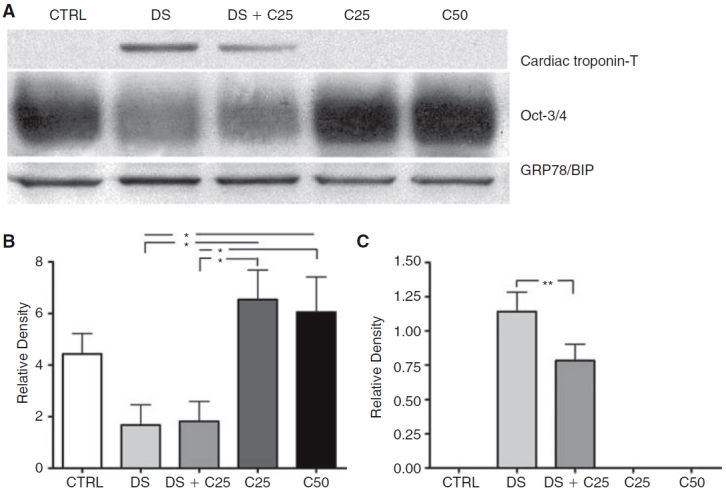
Quantification of differentiation rate in P19 embryoid bodies (EBs) at 12 days of differentiation. (A) Representative western blot showing cardiac troponin-T and Oct-3/4 expression; GRP78/BIP was used as a loading control. (B) Quantification of Oct-3/4 expression showing that expression of this protein decreases in the groups containing DMSO (N = 5). (C) Measure of the cardiac troponin-T expression. Only the DS and DS+C25 groups showed this protein. When DMSO was used alone this expression was 1.52-fold greater than when DMSO was used with cardiogenol C (N = 6). Error bars represent SEM. *P < 0.05 and **P < 0.01.
Cells positive for cardiac troponin-T also expressed troponin-I after DMSO induction (Fig. 3A–3A″). We confirmed the cardiac phenotype through staining for MHC (Fig. 3B–3B″), MF20 (Fig. 3C–3C″), and tropomyosin (sarcomeric) (Fig. 3D–3D″). Additionally, we observed the expression of the gap junction protein connexin43 in cells positive for troponin-I (Fig. 3E–3E″).
FIG. 3.
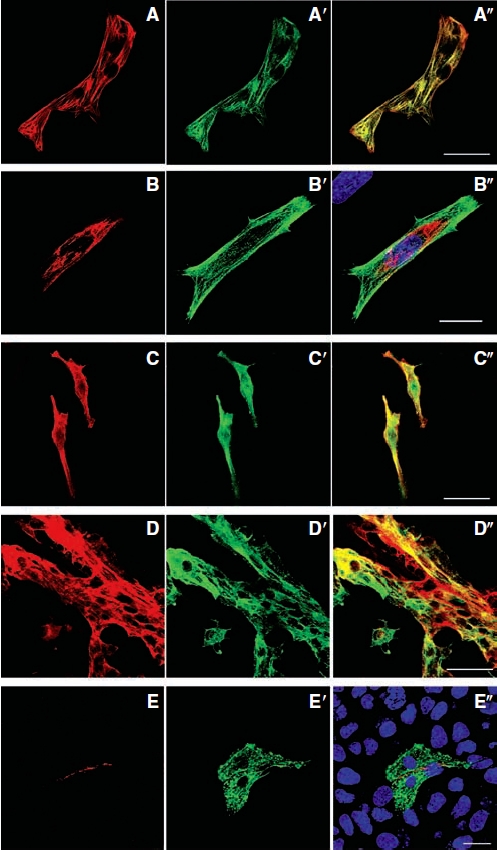
Detection of typical cardiac proteins in differentiated P19 cells by immunocytochemistry after 12 days of DMSO induction. (A–A″) P19 cell-derived cardiomyocytes that expressed cardiac troponin-T also expressed troponin-I. (A) Cardiac troponin-T. (A′) Troponin-I. (A″) Merged image. (B–B″) Representative images showing differentiated P19 cells expressing myosin heavy chain. (B) Myosin heavy chain. (B′) Troponin-I. (B″) Nuclear counterstaining with DAPI (blue) in the merged image. (C–C″) These cells also expressed MF20. (C) MF20. (C′) Troponin-I. (C″) Merged image. (D–D″) Tropomyosin (sarcomeric) expression in these same cells. (D) Tropomyosin (sarcomeric). (D′) Troponin-I. (D″) Merged image. (E–E″) Connexin43 was found among P19 cell-derived cardiomyocytes. (E) Connexin43. (E′) Troponin-I. (E″) Nuclear counterstaining with DAPI (blue) in the merged image. Scale bars: A, C, and D = 40 μm and B and E = 20 μm.
Spontaneous calcium oscillations
We observed that spontaneously beating cell clusters showed great synchrony of contraction and calcium oscillation (Fig. 4A), suggesting high intercellular coupling. To confirm this, we incubated the cells with heptanol or carbenoxolone, two commonly used gap junction channel blockers [28–30]. The beating clusters incubated with 1 mM heptanol very quickly lost the synchronization and several cells stopped beating (Fig. 4A″). This effect was rapidly reversed when we washed the culture, removing the alcohol (Fig. 4A″). Similar results were observed when we incubated the cells with 100 μM carbenoxolone (data not shown).
FIG. 4.
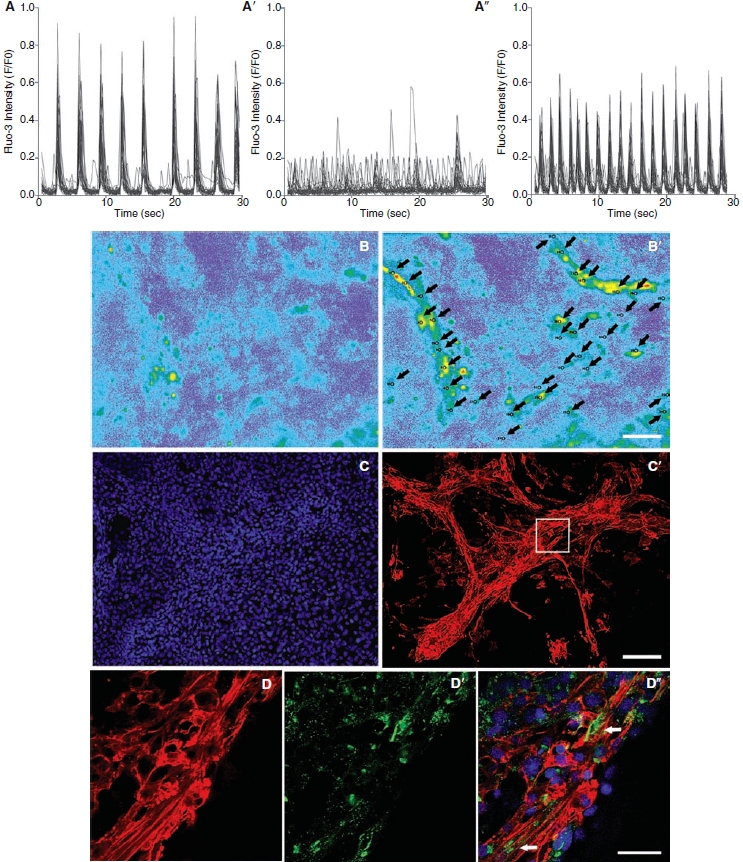
Representative images demonstrating spontaneous calcium (Fluo-3) oscillations in P19 cell-derived cardiomyocytes after 12 days of culture. (A–A″) Heptanol effect on the coordinated contractions of differentiated P19 cells (N = 7). (A) Spontaneous beating areas showing rhythmic intracellular calcium fluctuations. (A′) This rhythmic contraction was lost immediately after exposure to heptanol. (A″) After washing, rhythmic intracellular calcium fluctuations quickly returned. (B–B′) Frame scan of typical spontaneous beating areas with regions of interest marked (arrows). Proximally 30 regions of interest were marked for each experiment. (B) Before contraction. (B′) During contraction. (C–C′) The same cells used previously to analyze calcium fluctuations expressed cardiac troponin-T. (C) Nuclei stained with DAPI (blue). (C′) Cardiac troponin-T. (D–D″) Higher magnification confocal images of the area indicated by the inset in (C′) illustrating connexin43 expression among cardiac troponin-T-positive cells. (D) Cardiac troponin-T. (D′) Connexin43. (D″) Nuclear counterstaining with DAPI (blue) in the merged image. Arrows indicate regions with greater quantity of connexin43 among P19 cell-derived cardiomyocytes. Scale bars: B = 50 μm; C =100 μm; and D = 20 μm.
By immunocytochemistry we observed that the same beating clusters used to analyze calcium oscillations (Fig. 4B–4B″) also expressed cardiac troponin-T (Fig. 4C–4C″) and connexin 43 (Fig. 4D–4D″), confirming efficient differentiation into cardiomyocytes.
Quantitative analysis of EB area and number
We analyzed EB area and number on the fourth day in suspension culture among the different groups. The CTRL, DS, and DS+C25 groups showed lower EB area when compared with C50 group (Fig. 5A). Furthermore, the DS+C25 group showed lower EB area than the C25 group. However, we did not find differences in EB number among treatments (Fig. 5B–5G).
FIG. 5.
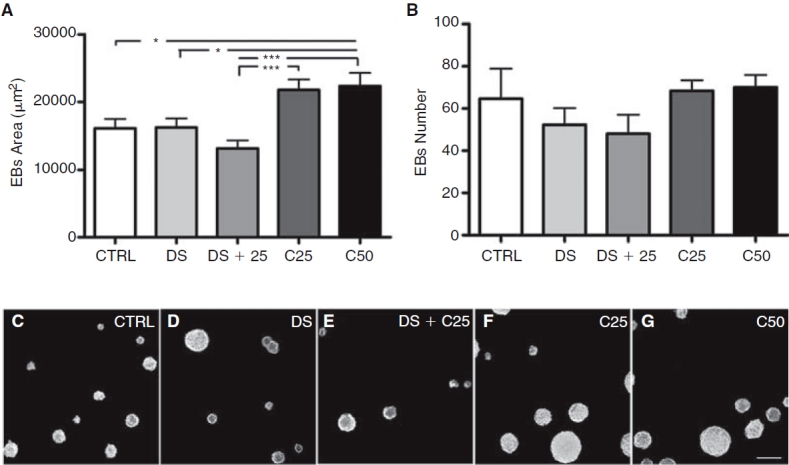
Quantitative analysis of embryoid body (EB) numbers and areas after 4 days of treatment in non-adherent dishes. (A) Measure of EB areas (N = 116). (B) Measure of EB numbers (N = 3). (C–G) Representative images showing EB numbers and areas for the different groups. (C) Control. (D) 1% DMSO. (E) 1% DMSO plus 0.25 μM cardiogenol C. (F) 0.25 μM cardiogenol C. (G) 0.50 μM cardiogenol C, scale bar = 200 μm. Error bars represent SEM, *P < 0.05 and ***P < 0.001.
Proliferation and cell death rate of EBs
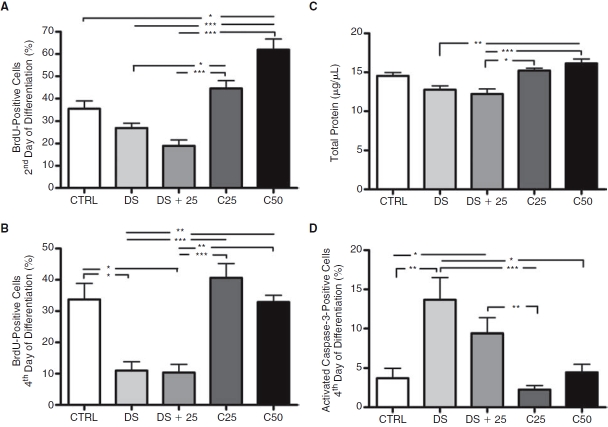
To analyze proliferation rate, BrdU was added to the culture on the second and fourth days of treatment before fixation. On the second day of treatment, the DS and DS+C25 groups showed lower numbers of proliferating cells in EBs when compared to C25 and C50 groups. The CTRL group also showed lower number of proliferating cells than the C50 group (Fig. 6A). On the fourth day of treatment, the DS and DS+C25 groups still showed lower number of proliferating cells in EBs when compared with other groups (Fig. 6B).
FIG. 6.
Proliferation and cell death rate in P19 cell culture. (A and B) Percentage of BrdU-positive cells at early times after culture of P19 cells in non-adherent dishes. (A) BrdU expression on the second day of embryoid body (EB) formation (N = 13). (B) BrdU expression on the fourth day of EB formation (N = 11). (C) Total protein quantification in differentiated P19 cells after 12 days of culture (N = 6). (D) Percentage of cells immunopositive for activated caspase-3 on the fourth day of EB formation (N = 14). Error bars represent SEM, *P < 0.05, **P < 0.01, and ***P < 0.001.
After 12 days of differentiation, we observed lower total protein in the DS and DS+C25 groups when compared with C50 group (Fig. 6C). In addition, the DS+C25 group showed lower total protein quantity than the C25 group.
To analyze cell death rate, the EBs were stained with an antibody against activated caspase-3 after 2 or 4 days in suspension. Due to low numbers of cells expressing activated caspase-3 on the second day of treatment, it was not possible to make any significant comparisons among the different groups. The cells expressing activated caspase-3 increased on the fourth day of differentiation and we observed that the DS and DS+C25 groups showed greater cell death (Fig. 6D). The DS group showed significant difference in relation to CTRL, C25, and C50 groups and the DS+C25 group showed significant difference when compared to CTRL and C25 group.
Discussion
In this study, we tested different protocols with compounds previously described as cardiogenic in P19 cells. DMSO has been widely used in studies of cardiac differentiation [31,32]; cardiogenol C was discovered recently, and there is only one published study showing its capacity for inducing cardiac differentiation [25].
Cardiac differentiation of P19 cells
The results obtained in the present study show that treatment with DMSO plus cardiogenol C was able to induce cardiac differentiation. However, we observed lower differentiation rates in this group when compared with the group that received only DMSO treatment. The cardiogenic properties of DMSO observed in this study corroborates several studies that showed this agent to be able to induce cardiac differentiation in P19 and ES cells [16,20,21]. In addition, the CTRL group and the group treated with cardiogenol C alone showed few or no cells expressing cardiac makers. Additionally, beating areas of the cultures were not observed in these groups. We obtained similar results in a study testing the cardiac differentiation of P19 cells in monolayers, without EB formation. In these experiments the DS group also showed higher cardiac troponin-T expression than the DS+C25 group (data not shown).
The results reported here are in disagreement with those reported by Wu et al. (2004). These authors reported that 0.25 μM cardiogenol C increased cardiac differentiation of P19 and R1 mouse ES cells. In their study, cardiac differentiation was measured by increased expression of atrial natriuretic factor (ANF) in P19 cells. In R1 mouse ES cells, they showed cardiac differentiation after 3 days of exposure to cardiogenol C, without EB formation. After 7 days in culture, they observed that >50% of the cells were positive for MHC and >90% of the cells were positive for GATA-4 [25].
Although in our experiments the C25 group showed some cells positive for cardiac troponin-T, this differentiation was similar to that observed in the CTRL group. It is known that P19 cells have a low frequency of spontaneous cardiac differentiation. Only 1%–2% of the P19 cells show spontaneous contractions when the differentiation occurs in the absence of inducing factors [20]. In addition, we used cardiogenol C in a concentration 15 times higher than Wu et al. (2004) [25], and we still did not observe beating clusters after 12 days of differentiation. Moreover, the results obtained in western blots of Oct-3/4 show that cardiogenol C alone maintains the expression of this protein at a level similar to that seen in undifferentiated cells (control group). Therefore, we can suggest that cardiogenol C does not induce cardiac differentiation or any other differentiated phenotype in P19.
Functional gap junctions in differentiated P19 cells
We observed connexin43 expression among cells positive for cardiac troponin-T. Connexin43 can also be found in undifferentiated human [33] and mouse ES cells [34] and P19 cells [35]. It is known that connexin43 expression is reduced during early cardiac differentiation in ES cells and often this protein is not detected in premature cardiomyocytes [35]. However, with cardiac maturation connexin43 levels increase again, similar to what occurs during heart formation [36].
The presence of connexin43 in the spontaneous beating clusters that lost rhythmic contractions when incubated with heptanol or cabenoxolone suggests the formation of functional gap junction channels and that these intercellular channels play an important role in the propagation and coordination of contractions that occurred spontaneously in the groups treated with DMSO.
The ability of ES cell-derived cardiomyocytes to improve structure and function of lesioned hearts [37,38, for review see 39] encourages the study and characterization of chemical induction of these cells and their subsequent usage in animal models of myocardial diseases that closely match human diseases. In this respect, it is critical that the transplanted cells retain the capacity to be functionally integrated into the host tissue. In this report, we present strong evidence that DMSO-induced P19 cells differentiate into functionally mature cardiomyocytes that present the ability to propagate coordinated calcium waves, a crucial phenotype that must be expressed by putative transplanted cells into lesioned hearts. These observations bring some light in the perspective of cell therapy with chemically induced pluripotent cells in animal models of heart failure.
Proliferation and cell death rate of EBs
In this study, we showed that the treatment with DMSO or DMSO plus cardiogenol C decreased the proliferation rate of EBs. We observed similar results in P19 cells differentiated in monolayers (data not shown). These results corroborate the principle that the more committed a cell is with a specific phenotype, the lower is its proliferation rate [12].
In addition, on the second day of differentiation the CTRL group showed a lower proliferation rate when compared with the C50 group, suggesting that cardiogenol C induces proliferation in P19 cells.
In spite of the DS and DS+C25 groups showing a greater apoptosis rate when compared with the other groups, we propose that the lower proliferation rate in these groups was not due to increased cell death. In fact, on the second day of EB treatment we already observed different proliferation rates among groups, although there was practically no apoptosis detected in any of the groups.
In summary, our results clearly show that cardiogenol C does not induce cardiac commitment in P19 cells. Conversely, cardiogenol C-induced P19 cells proliferate more than non- or DMSO-induced P19 cells and express high amounts of Oct-3/4, a marker for undifferentiated cells. Moreover, our results contribute to an improved understanding of the cardiac differentiation process in P19 cells, in particular by showing the generation of cardiomyocytes with functional intercellular communication.
Acknowledgments
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), and the National Institutes of Health. We gratefully acknowledge Dr Sylvia O. Suadicani, Mrs. Marcia Urban-Maldonado, and Mrs. Andreza Martins Bastos for technical assistance and thoughtful comments during the elaboration of this work.
Contributor Information
Jasmin, Instituto de Biofísica Carlos Chagas Filho, Programa de Terapia Celular and Instituto Nacional de Ciência e Tecnologia de Biologia Estrutural e Bioimagem, UFRJ, Rio de Janeiro, Brazil..
David C. Spray, Dominick P. Purpura Department of Neuroscience, Albert Einstein College of Medicine, Bronx, New York.
Antonio Carlos Campos de Carvalho, Instituto de Biofísica Carlos Chagas Filho, Programa de Terapia Celular and Instituto Nacional de Ciência e Tecnologia de Biologia Estrutural e Bioimagem, UFRJ, Rio de Janeiro, Brazil..
Rosalia Mendez-Otero, Instituto de Biofísica Carlos Chagas Filho, Programa de Terapia Celular and Instituto Nacional de Ciência e Tecnologia de Biologia Estrutural e Bioimagem, UFRJ, Rio de Janeiro, Brazil..
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gudjonsson T, Magnusson MK. Stem cell biology and the cellular pathways of carcinogenesis. Apmis. (2005);113:922–929. doi: 10.1111/j.1600-0463.2005.apm_371.x. [DOI] [PubMed] [Google Scholar]
- 2.Rajaraman R, Guernsey DL, Rajaraman MM, Rajaraman SR. Stem cells, senescence, neosis and self-renewal in cancer. Cancer Cell Int. (2006);6:25. doi: 10.1186/1475-2867-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers I, Smith A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene. (2004);23:7150–7160. doi: 10.1038/sj.onc.1207930. [DOI] [PubMed] [Google Scholar]
- 4.Solter D. From teratocarcinomas to embryonic stem cells and beyond: a history of embryonic stem cell research. Nat Rev Genet. (2006);7:319–327. doi: 10.1038/nrg1827. [DOI] [PubMed] [Google Scholar]
- 5.Andrews PW, Matin MM, Bahrami AR, Damjanov I, Gokhale P, Draper JS. Embryonic stem (ES) cells and embryonal carcinoma (EC) cells: opposite sides of the same coin. Biochem Soc Trans. (2005);33:1526–1530. doi: 10.1042/BST0331526. [DOI] [PubMed] [Google Scholar]
- 6.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. (1981);78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews PW. From teratocarcinomas to embryonic stem cells. Philos Trans R Soc Lond B Biol Sci. (2002);357:405–417. doi: 10.1098/rstb.2002.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desbaillets I, Ziegler U, Groscurth P, Gassmann M. Embryoid bodies: an in vitro model of mouse embryogenesis. Exp Physiol. (2000);85:645–651. [PubMed] [Google Scholar]
- 9.McBurney MW, Rogers BJ. Isolation of male embryonal carcinoma cells and their chromosome replication patterns. Dev Biol. (1982);89:503–508. doi: 10.1016/0012-1606(82)90338-4. [DOI] [PubMed] [Google Scholar]
- 10.Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol. (2004);51:1–28. doi: 10.1016/j.critrevonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. (1998);282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 12.van der Heyden MA, Defize LH. Twenty one years of P19 cells: what an embryonal carcinoma cell line taught us about cardiomyocyte differentiation. Cardiovasc Res. (2003);58:292–302. doi: 10.1016/s0008-6363(02)00771-x. [DOI] [PubMed] [Google Scholar]
- 13.Choi SC, Yoon J, Shim WJ, Ro YM, Lim DS. 5-Azacytidine induces cardiac differentiation of P19 embryonic stem cells. Exp Mol Med. (2004);36:515–523. doi: 10.1038/emm.2004.66. [DOI] [PubMed] [Google Scholar]
- 14.McBurney MW. P19 embryonal carcinoma cells. Int J Dev Biol. (1993);37:135–140. [PubMed] [Google Scholar]
- 15.Paquin J, Danalache BA, Jankowski M, McCann SM, Gutkowska J. Oxytocin induces differentiation of P19 embryonic stem cells to cardiomyocytes. Proc Natl Acad Sci USA. (2002);99:9550–9555. doi: 10.1073/pnas.152302499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards MK, Harris JF, McBurney MW. Induced muscle differentiation in an embryonal carcinoma cell line. Mol Cell Biol. (1983);3:2280–2286. doi: 10.1128/mcb.3.12.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arreola J, Spires S, Begenisich T. Na+ channels in cardiac and neuronal cells derived from a mouse embryonal carcinoma cell line. J Physiol. (1993);472:289–303. doi: 10.1113/jphysiol.1993.sp019947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skerjanc IS, Slack RS, McBurney MW. Cellular aggregation enhances MyoD-directed skeletal myogenesis in embryonal carcinoma cells. Mol Cell Biol. (1994);14:8451–8459. doi: 10.1128/mcb.14.12.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abilez O, Benharash P, Miyamoto E, Gale A, Xu C, Zarins CK. P19 progenitor cells progress to organized contracting myocytes after chemical and electrical stimulation: implications for vascular tissue engineering. J Endovasc Ther. (2006);13:377–388. doi: 10.1583/06-1844.1. [DOI] [PubMed] [Google Scholar]
- 20.Angello JC, Kaestner S, Welikson RE, Buskin JN, Hauschka SD. BMP induction of cardiogenesis in P19 cells requires prior cell-cell interaction(s) Dev Dyn. (2006);235:2122–2133. doi: 10.1002/dvdy.20863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lako M, Lindsay S, Lincoln J, Cairns PM, Armstrong L, Hole N. Characterisation of Wnt gene expression during the differentiation of murine embryonic stem cells in vitro: role of Wnt3 in enhancing haematopoietic differentiation. Mech Dev. (2001);103:49–59. doi: 10.1016/s0925-4773(01)00331-8. [DOI] [PubMed] [Google Scholar]
- 22.Fathi F, Murasawa S, Hasegawa S, Asahara T, Kermani AJ, Mowla SJ. Cardiac differentiation of P19CL6 cells by oxytocin. Int J Cardiol. (2009);134:75–81. doi: 10.1016/j.ijcard.2008.01.046. [DOI] [PubMed] [Google Scholar]
- 23.Edwards MK, McBurney MW. The concentration of retinoic acid determines the differentiated cell types formed by a teratocarcinoma cell line. Dev Biol. (1983);98:187–191. doi: 10.1016/0012-1606(83)90348-2. [DOI] [PubMed] [Google Scholar]
- 24.Bouchard F, Paquin J. Skeletal and cardiac myogenesis accompany adipogenesis in P19 embryonal stem cells. Stem Cells Dev. (2009) doi: 10.1089/scd.2008.0288. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Ding S, Ding Q, Gray NS, Schultz PG. Small molecules that induce cardiomyogenesis in embryonic stem cells. J Am Chem Soc. (2004);126:1590–1591. doi: 10.1021/ja038950i. [DOI] [PubMed] [Google Scholar]
- 26.Habara-Ohkubo A. Differentiation of beating cardiac muscle cells from a derivative of P19 embryonal carcinoma cells. Cell Struct Funct. (1996);21:101–110. doi: 10.1247/csf.21.101. [DOI] [PubMed] [Google Scholar]
- 27.Moore JC, Spijker R, Martens AC, de Boer T, Rook MB, van der Heyden MA, Tertoolen LG, Mummery CL. A P19Cl6 GFP reporter line to quantify cardiomyocyte differentiation of stem cells. Int J Dev Biol. (2004);48:47–55. doi: 10.1387/ijdb.15005574. [DOI] [PubMed] [Google Scholar]
- 28.Rose CR, Ransom BR. Gap junctions equalize intracellular Na+ concentration in astrocytes. Glia. (1997);20:299–307. doi: 10.1002/(sici)1098-1136(199708)20:4<299::aid-glia3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Pijnappels DA, Schalij MJ, van Tuyn J, Ypey DL, de Vries AA, van der Wall EE, van der Laarse A, Atsma DE. Progressive increase in conduction velocity across human mesenchymal stem cells is mediated by enhanced electrical coupling. Cardiovasc Res. (2006);72:282–91. doi: 10.1016/j.cardiores.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. (2006);26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skerjanc IS. Cardiac and skeletal muscle development in P19 embryonal carcinoma cells. Trends Cardiovasc Med. (1999);9:139–143. doi: 10.1016/s1050-1738(99)00017-1. [DOI] [PubMed] [Google Scholar]
- 32.van der Heyden MA, van Kempen MJ, Tsuji Y, Rook MB, Jongsma HJ, Opthof T. P19 embryonal carcinoma cells: a suitable model system for cardiac electrophysiological differentiation at the molecular and functional level. Cardiovasc Res. (2003);58:410–422. doi: 10.1016/s0008-6363(03)00247-5. [DOI] [PubMed] [Google Scholar]
- 33.Wong RC, Pebay A, Nguyen LT, Koh KL, Pera MF. Presence of functional gap junctions in human embryonic stem cells. Stem Cells. (2004);22:883–889. doi: 10.1634/stemcells.22-6-883. [DOI] [PubMed] [Google Scholar]
- 34.van Kempen M, van Ginneken A, de Grijs I, Mutsaers N, Opthof T, Jongsma H, van der Heyden M. Expression of the electrophysiological system during murine embryonic stem cell cardiac differentiation. Cell Physiol Biochem. (2003);13:263–270. doi: 10.1159/000074541. [DOI] [PubMed] [Google Scholar]
- 35.van der Heyden MA, Rook MB, Hermans MM, Rijksen G, Boonstra J, Defize LH, Destree OH. Identification of connexin43 as a functional target for Wnt signalling. J Cell Sci. (1998);111:1741–1749. doi: 10.1242/jcs.111.12.1741. [DOI] [PubMed] [Google Scholar]
- 36.van Kempen MJ, Fromaget C, Gros D, Moorman AF, Lamers WH. Spatial distribution of connexin43, the major cardiac gap junction protein, in the developing and adult rat heart. Circ Res. (1991);68:1638–1651. doi: 10.1161/01.res.68.6.1638. [DOI] [PubMed] [Google Scholar]
- 37.Singla DK, Hacker TA, Ma L, Douglas PS, Sullivan R, Lyons GE, Kamp TJ. Transplantation of embryonic stem cells into the infarcted mouse heart: formation of multiple cell types. J Mol Cell Cardiol. (2006);40:195–200. doi: 10.1016/j.yjmcc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. (2007);25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 39.van Laake LW, Hassink R, Doevendans PA, Mummery C. Heart repair and stem cells. J Physiol. (2006);577:467–478. doi: 10.1113/jphysiol.2006.115816. [DOI] [PMC free article] [PubMed] [Google Scholar]