Abstract
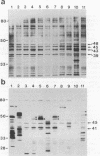
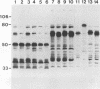
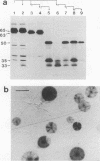
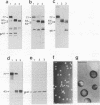
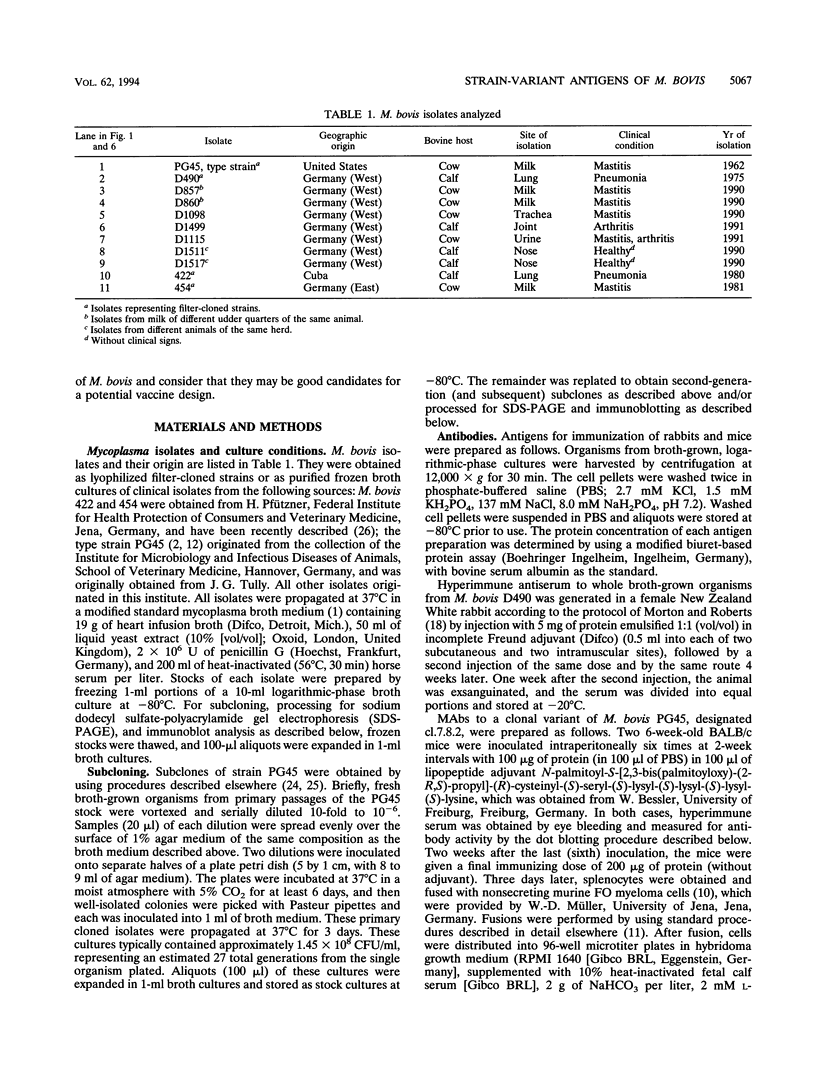
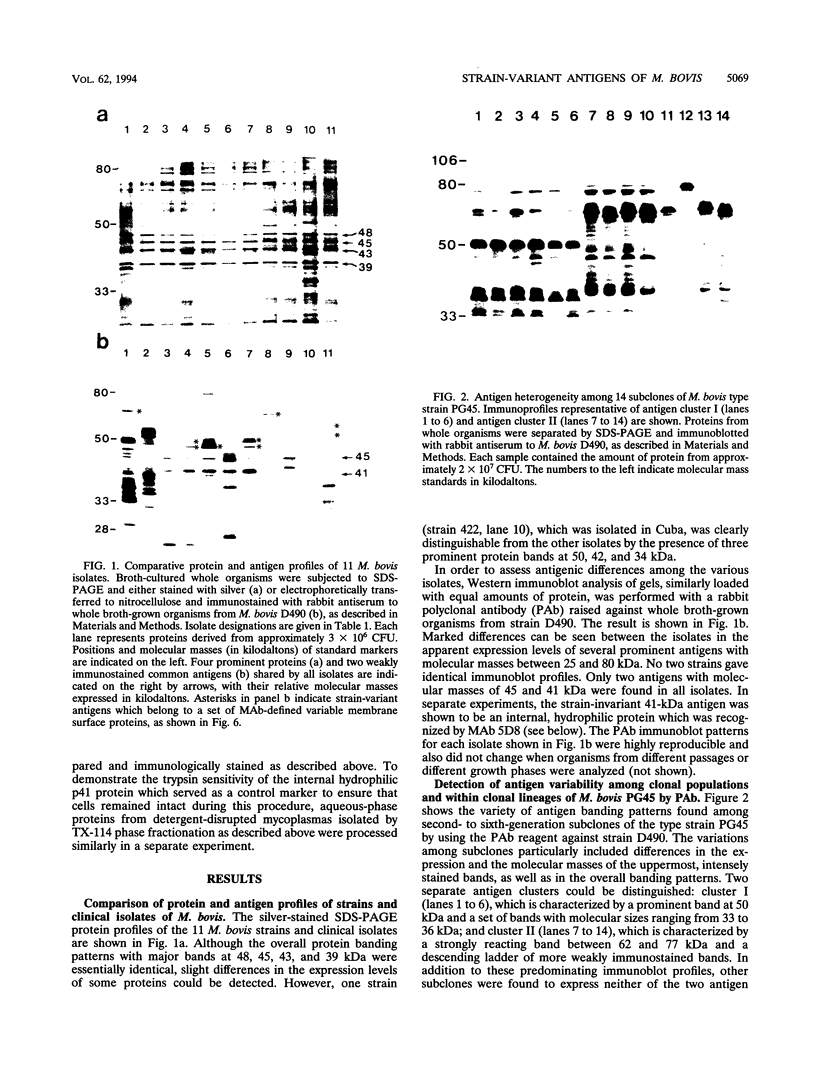
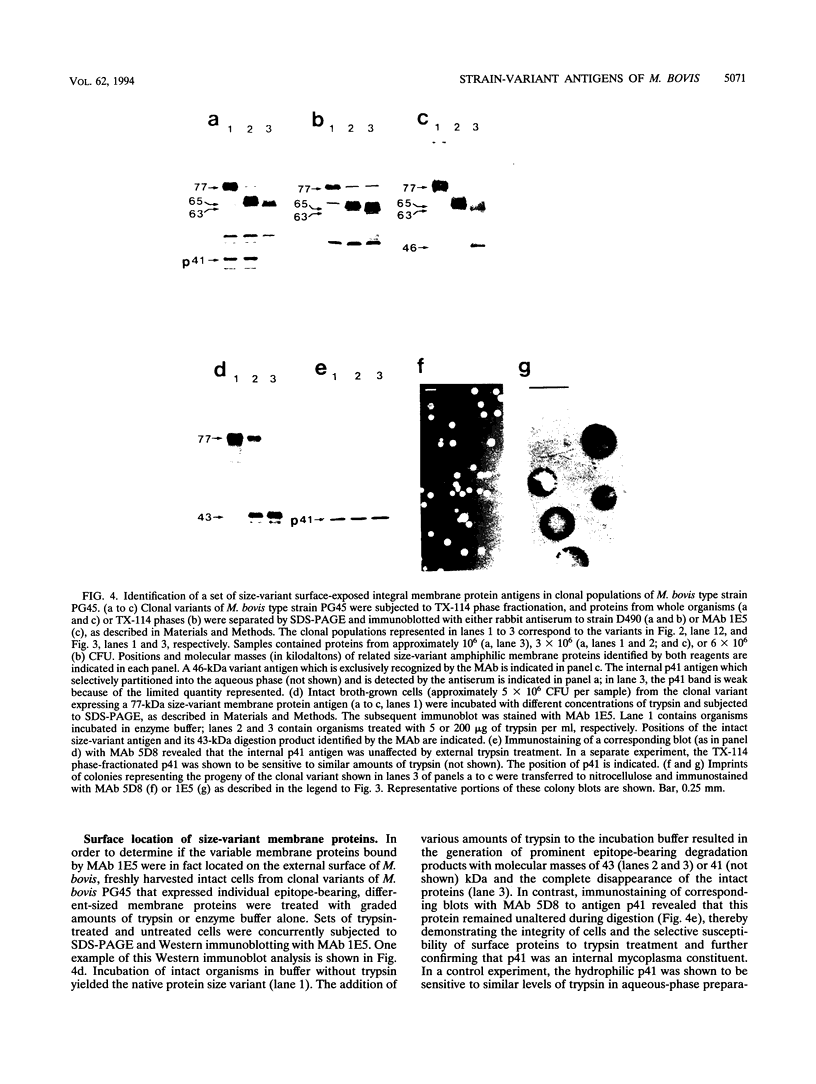
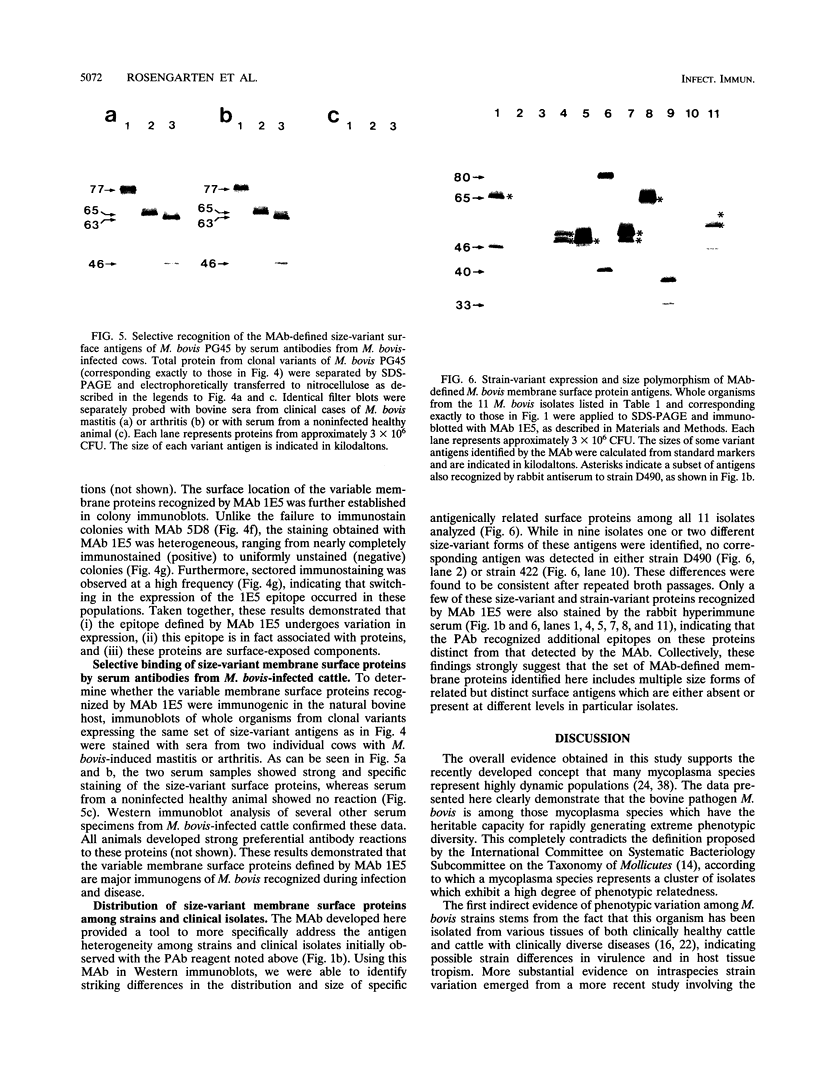
The protein and antigen profiles of 11 isolates of Mycoplasma bovis were compared by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis of whole organisms. The isolates examined included the type strain PG45 and 10 other filter-cloned strains or purified isolates both from animals without clinical signs and from clinical cases of bovine mastitis, arthritis, or pneumonia. While the overall protein patterns visualized by silver staining were very similar, marked differences in the antigen banding profiles were detected by rabbit antiserum prepared against whole organisms from one of the strains analyzed. This antigenic heterogeneity was shown to be independent of the geographical origin, the type of clinical disease, and the site of isolation and was also observed among serial isolates from a single animal. Antigen profiles were further monitored throughout sequentially subcloned populations of the PG45 strain. This clonal analysis revealed a high-frequency variation in the expression levels of several prominent antigens. All of these variable antigens were defined by detergent-phase fractionation with Triton X-114 as amphiphilic integral membrane proteins. A subset of different-sized membrane proteins was identified by a monoclonal antibody raised against a PG45 subclone expressing a 63- and a 46-kDa variant antigen within that set. The selective susceptibility of these proteins to trypsin treatment of intact organisms and their ability to bind the monoclonal antibody in colony immunoblots demonstrated that they were exposed on the cell surface. In addition, their preferential recognition by serum antibodies from individual cattle with naturally induced M. bovis mastitis or arthritis confirmed that they were major immunogens of this organism. These studies establish that the apparent antigenic heterogeneity among M. bovis isolates reported here does not represent stable phenotypic strain differences generated from accumulated mutational events but reflects distinct expression patterns of diverse, highly variable membrane surface proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avakian A. P., Kleven S. H., Ley D. H. Comparison of Mycoplasma gallisepticum strains and identification of immunogenic integral membrane proteins with Triton X-114 by immunoblotting. Vet Microbiol. 1991 Nov;29(3-4):319–328. doi: 10.1016/0378-1135(91)90139-7. [DOI] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Behrens A., Heller M., Kirchhoff H., Yogev D., Rosengarten R. A family of phase- and size-variant membrane surface lipoprotein antigens (Vsps) of Mycoplasma bovis. Infect Immun. 1994 Nov;62(11):5075–5084. doi: 10.1128/iai.62.11.5075-5084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer M. J., Wise K. S. Lipid-modified surface protein antigens expressing size variation within the species Mycoplasma hyorhinis. Infect Immun. 1989 Jan;57(1):245–254. doi: 10.1128/iai.57.1.245-254.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker T. M., Boyer M. J., Keith J., Watson-McKown R., Wise K. S. Association of lipids with integral membrane surface proteins of Mycoplasma hyorhinis. Infect Immun. 1988 Feb;56(2):295–301. doi: 10.1128/iai.56.2.295-301.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- HALE H. H., HELMBOLDT C. F., PLASTRIDGE W. N., STULA E. F. Bovine mastitis caused by a Mycoplasma species. Cornell Vet. 1962 Oct;52:582–591. [PubMed] [Google Scholar]
- Heller M., Berthold E., Pfützner H., Leirer R., Sachse K. Antigen capture ELISA using a monoclonal antibody for the detection of Mycoplasma bovis in milk. Vet Microbiol. 1993 Oct;37(1-2):127–133. doi: 10.1016/0378-1135(93)90187-c. [DOI] [PubMed] [Google Scholar]
- Jasper D. E. Bovine mycoplasmal mastitis. Adv Vet Sci Comp Med. 1981;25:121–157. [PubMed] [Google Scholar]
- Jasper D. E. The role of Mycoplasma in bovine mastitis. J Am Vet Med Assoc. 1982 Jul 15;181(2):158–162. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morton H. E., Roberts R. J. Production of anti-Mycoplasma (PPLO) antibodies in rabbits. Proc Soc Exp Biol Med. 1967 Jun;125(2):538–543. doi: 10.3181/00379727-125-32140. [DOI] [PubMed] [Google Scholar]
- Olson L. D., Renshaw C. A., Shane S. W., Barile M. F. Successive synovial Mycoplasma hominis isolates exhibit apparent antigenic variation. Infect Immun. 1991 Sep;59(9):3327–3329. doi: 10.1128/iai.59.9.3327-3329.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L. D., Shane S. W., Karpas A. A., Cunningham T. M., Probst P. S., Barile M. F. Monoclonal antibodies to surface antigens of a pathogenic Mycoplasma hominis strain. Infect Immun. 1991 May;59(5):1683–1689. doi: 10.1128/iai.59.5.1683-1689.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panangala V. S., Morsy M. A., Gresham M. M., Toivio-Kinnucan M. Antigenic variation of Mycoplasma gallisepticum, as detected by use of monoclonal antibodies. Am J Vet Res. 1992 Jul;53(7):1139–1144. [PubMed] [Google Scholar]
- Riethman H. C., Boyer M. J., Wise K. S. Triton X-114 phase fractionation of an integral membrane surface protein mediating monoclonal antibody killing of Mycoplasma hyorhinis. Infect Immun. 1987 May;55(5):1094–1100. doi: 10.1128/iai.55.5.1094-1100.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengarten R., Wise K. S. Phenotypic switching in mycoplasmas: phase variation of diverse surface lipoproteins. Science. 1990 Jan 19;247(4940):315–318. doi: 10.1126/science.1688663. [DOI] [PubMed] [Google Scholar]
- Rosengarten R., Wise K. S. The Vlp system of Mycoplasma hyorhinis: combinatorial expression of distinct size variant lipoproteins generating high-frequency surface antigenic variation. J Bacteriol. 1991 Aug;173(15):4782–4793. doi: 10.1128/jb.173.15.4782-4793.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse K., Grajetzki C., Pfützner H., Hass R. Comparison of Mycoplasma bovis strains based on SDS-PAGE and immunoblot protein patterns. Zentralbl Veterinarmed B. 1992 Jun;39(4):246–252. doi: 10.1111/j.1439-0450.1992.tb01165.x. [DOI] [PubMed] [Google Scholar]
- Talkington D. F., Fallon M. T., Watson H. L., Thorp R. K., Cassell G. H. Mycoplasma pulmonis V-1 surface protein variation: occurrence in vivo and association with lung lesions. Microb Pathog. 1989 Dec;7(6):429–436. doi: 10.1016/0882-4010(89)90023-5. [DOI] [PubMed] [Google Scholar]
- Thirkell D., Spooner R. K., Jones G. E., Russell W. C. Polypeptide and antigenic variability among strains of Mycoplasma ovipneumoniae demonstrated by SDS-PAGE and immunoblotting. Vet Microbiol. 1990 Jan;21(3):241–254. doi: 10.1016/0378-1135(90)90035-t. [DOI] [PubMed] [Google Scholar]
- Thomas C. B., Jasper D. E., Willeberg P. Clinical bovine mycoplasmal mastitis. An epidemiologic study of factors associated with problem herds. Acta Vet Scand. 1982;23(1):53–64. doi: 10.1186/BF03546822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn L. R., Hirsch S. Comparison of four Mycoplasma arthritidis strains by enzyme immunoassay, metabolism inhibition, one- and two-dimensional electrophoresis, and immunoblotting. J Clin Microbiol. 1990 Sep;28(9):1974–1981. doi: 10.1128/jcm.28.9.1974-1981.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn L. R., Hirsch S., Voelker L. L. Mechanisms of attachment of Mycoplasma arthritidis to host cells in vitro. Infect Immun. 1993 Jun;61(6):2670–2680. doi: 10.1128/iai.61.6.2670-2680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson H. L., Blalock D. K., Cassell G. H. Variable antigens of Ureaplasma urealyticum containing both serovar-specific and serovar-cross-reactive epitopes. Infect Immun. 1990 Nov;58(11):3679–3688. doi: 10.1128/iai.58.11.3679-3688.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson H. L., McDaniel L. S., Blalock D. K., Fallon M. T., Cassell G. H. Heterogeneity among strains and a high rate of variation within strains of a major surface antigen of Mycoplasma pulmonis. Infect Immun. 1988 May;56(5):1358–1363. doi: 10.1128/iai.56.5.1358-1363.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S. Adaptive surface variation in mycoplasmas. Trends Microbiol. 1993 May;1(2):59–63. doi: 10.1016/0966-842X(93)90034-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Kim M. F. Major membrane surface proteins of Mycoplasma hyopneumoniae selectively modified by covalently bound lipid. J Bacteriol. 1987 Dec;169(12):5546–5555. doi: 10.1128/jb.169.12.5546-5555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Kim M. F., Theiss P. M., Lo S. C. A family of strain-variant surface lipoproteins of Mycoplasma fermentans. Infect Immun. 1993 Aug;61(8):3327–3333. doi: 10.1128/iai.61.8.3327-3333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev D., Rosengarten R., Watson-McKown R., Wise K. S. Molecular basis of Mycoplasma surface antigenic variation: a novel set of divergent genes undergo spontaneous mutation of periodic coding regions and 5' regulatory sequences. EMBO J. 1991 Dec;10(13):4069–4079. doi: 10.1002/j.1460-2075.1991.tb04983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]