Abstract
Last year’s Advances in Pediatric Asthma concluded with the following statement “If we can close these [remaining] gaps through better communication, improvements in the health care system and new insights into treatment, we will move closer to better methods to intervene early in the course of the disease and induce clinical remission as quickly as possible in most children”. This year’s summary will focus on recent advances in pediatric asthma that take steps moving forward as reported in Journal of Allergy and Clinical Immunology publications in 2010.
Some of those recent reports show us how to improve asthma management through steps to better understand the natural history of asthma, individualize asthma care, reduce asthma exacerbations, manage inner city asthma, and some potential new ways to use available medications to improve asthma control. It is clear that we have made many significant gains in managing asthma in children but we have a ways to go to prevent asthma exacerbations, alter the natural history of the disease, and to reduce health disparities in asthma care.
Perhaps new directions in personalized medicine and improved health care access and communication will help maintain steady progress in alleviating the burden of this disease in children, especially young children.
Keywords: asthma, asthma control, asthma impairment, asthma risk, asthma severity, early intervention in asthma, biomarkers, genetics, inhaled corticosteroids, leukotriene receptor antagonists, long-acting β-adrenergic agonists, omalizumab, personalized medicine, therapeutics
Introduction
Last year’s summary in the Advances in Pediatric Asthma series included a discussion of elements that would be necessary to implement the asthma guidelines, such as focusing on asthma control in adjusting therapy, applying techniques from managed asthma care to understand populations at risk for poor control, identifying early indicators of developing asthma, anticipating asthma exacerbations, and monitoring progression (1). That summary ended with some thoughts regarding early intervention and the potential to induce clinical remission in most children. The review by Andrea Apter on adult asthma diagnosis and treatment focused on standardizing methodology and reporting for translational research, as well as the influence of the environment, therapeutics, and management of asthma (2).
This review will highlight 2010 JACI publications that provide new information that will assist us in better understanding the natural history of asthma that could facilitate the development of strategies for early intervention and a personalized approach to asthma management. In addition, new studies related to asthma exacerbations, inner city asthma, and opportunities to improve asthma care will be summarized. Several key studies published in other journals will also be included to highlight these advances.
Natural history of asthma
The August 2010 issue was devoted to the natural history of asthma. Hans Bisgaard and Klaus Bonnelykke (3) indicated that segmentation of children with asthma and other wheezy disorders remains the main research challenge today. They proposed a translational research approach based on long-term clinical studies of birth cohorts with comprehensive and objective assessments of intermediate phenotypes and environmental exposures combined with interdisciplinary basic research and a systems biology approach (3). Until the time that one study pulls all of this information together, we must carefully evaluate reports of individual studies.
Birth history
There is continued interest in prenatal factors that might predispose an infant to the development of asthma. In regards to the environment, Pfefferle et al (4) as part of the PASTURE study reported that maternal exposure to farming activities and farm dairy products modulated cytokine production patterns of offspring at birth. Maternal contact with different farm animal species and barns and consumption of farm-produced butter during pregnancy enhanced the production of pro-inflammatory cord blood cytokines, whereas maternal consumption of farm-produced yogurt resulted in significantly lower levels of interferon-gamma and interferon-alpha in umbilical blood. Lange et al (5) examined whether overall maternal dietary pattern during pregnancy was associated with recurrent wheeze in children. They failed to find that any specific dietary pattern was associated with the primary outcome of recurrent wheeze.
Maternal distress has also been associated with the development of childhood asthma. Dreger et al (6) investigated the association between children’s cortisol levels and the combined predictors of exposure to maternal distress and childhood asthma. Among children exposed to recurrent maternal stress, they found an elevation in cortisol levels occurs in response to an acute stressor when there is no diagnosis of asthma, however, children with asthma tend to exhibit lower cortisol levels. Wright et al (7), evaluating an inner city population, reported that prenatal stress was associated with altered innate and adaptive immune responses in cord blood mononuclear cells. They suggested that stress-induced perinatal immunomodulation may impact the expression of allergic disease in children.
In regards to managing maternal asthma, concern has been raised regarding adverse effects of inhaled corticosteroids (ICS). Breton et al (8) evaluated this question from a study of three administrative databases and reported that the risk of perinatal mortality was not significantly associated with ICS use during pregnancy, however, their conclusions regarding high-dose ICS was guarded due to lack of statistical power and a possibility of residual confounding by asthma severity and control.
Genetics
Holloway et al (9) provided on overview of current knowledge related to genetics in predicting the natural history of asthma. They concluded that the application of genetic testing to clinical practice is not ready for predicting the natural history of individual patients. However, the field is moving quickly to identify genes associated with susceptibility to atopy, altered lung development, and susceptibility to more severe disease.
Several new insights related to genetics and asthma were reported. DeWan et al (10) reported an association of polymorphisms within PDE11A, a member of the phosphodiesterase superfamily of genes, for allergic asthma and worth further investigation. Sun et al (11) provided information related to S1PR1 (sphingosine-1-phosphate) gene variants in asthma susceptibility and severity. The S1P receptor is a participant in regulating lung vascular integrity and responses to lung inflammation. Li et al (12) reported on a genome-wide association study (GWAS) on a population of severe or difficult-to-treat asthma participants. They identified single polymorphonuclear polymorphisms (SNPs) in the RAD50-IL13 region on chromosome 5q31.1 and HLA-DR/DQ on chromosome 6p21.3 associated with asthma. They indicated additional investigation of these 2 regions to delineate their biologic function in the development of asthma.
Munthe-Kaas et al (13) investigated associations between CD14 SNPs and sCD14 levels at different points in childhood. CD14 is a pattern-recognition receptor for environmental lipopolysaccharide. They reported that CD14 methylation increased significantly from age 2 to 10 years, and the level of methylation was inversely correlated with sCD14 levels at 10 years of age. They suggest that this age-related change could play a role in differential influence of gene polymorphisms on gene transcription and subsequent disease. Thomsen et al (14) studied variation in age at onset of asthma in relation to genetic and environmental factors in a twin study. They concluded that host-related differences in genetic makeup cause different individuals to develop asthma at different ages. They reported that genetic factors explained 34% of the variation in the age at onset of asthma, and environmental factors accounted for 66%.
Another aspect to evaluate the influence of genetics is consideration for variation in race and ethnicity. Mathias et al (15) performed a GWAS study on African-ancestry populations with asthma and controls. They highlighted key genes and regions that might be distinct from genes important in non-African populations. Further identification of such genes might lead to a better understanding of the disparities in this minority group. In addition, Wu et al (16) examined previously implicated asthma candidate genes with childhood asthma in a Mexico City population. They concluded that SNPs in multiple genes, including TGFB1, IL1RL1, IL18R1, and DPP10, might contribute to childhood asthma susceptibility in a Mexican population.
In relation to concomitant host factors, Melen et al (17) sought to evaluate genetic variants associated with both asthma and obesity through GWAS. They did not find convincing evidence of known candidate genes that asthma and obesity share genetic determinants, however they did identify other genes that deserve further study for a role in influencing both conditions.
Infection
Another proposed factor related to onset of asthma is infection. Most studies have focused on viral infections. Rosenthal et al (18) in a rostrum article, identify important knowledge gaps and future research questions, as well as new investigational tools, including respiratory tract virus detection techniques. That will permit current and future researchers to define relationships of host, virus, development and environmental mechanisms that regulate these factors. This could lead to more effective primary prevention of asthma measures. Sly et al (19), in a Current Perspectives review, indicate the link between viral infection is not clear. However, recent studies have raised questions regarding the role of rhinovirus and the role of allergy in enhancing viral-induced inflammation. They suggest that perhaps interventions that inhibit atopy-related effectors mechanisms from participating in the host response to respiratory viral infections could break the connection between viral respiratory infections and asthma.
Horvat et al (20) using an animal model to study early-life origins of allergic airways disease, concluded that chlamydial infections also modulate immune responses, alter lung function and structure and enhance the severity of allergic airways disease in later life.
Prediction
The Asthma Predictive Index (API), developed by the Tucson Respiratory Center, has been a useful checklist to identify young children at risk for developing persistent asthma. Dr. Castro-Rodriguez provided a Current Perspective review on the API and emphasized that its most impressive aspect is its ability to rule out the likelihood of asthma by school age in young children with wheezing (21). Future refinements of such an index could occur but they must be easy to apply, validated in different populations, and shown to improve patient outcome.
Holt et al (22) sought to identify clinical and laboratory biomarkers in 2-year-olds that are predictive of the risk for persistent atopy and wheeze at age 5 years. Attaining mite-specific IgE titers of greater than 0.20 kU/L by age 2 years was associated with a 12.7% risk of persistent wheeze, increasing progressively to an 87.2% risk with increasing numbers of severe lower respiratory tract illnesses experienced. The recommended follow-up studies in larger unselected populations in order to refine this approach. Simpson et al (23) in a study of patterns of sensitization in a birth cohort study reported that IgE antibody response do not reflect a single phenotype of atopy, but rather multiple different atopic vulnerabilities which differ in their relation with asthma presence and severity. Only one of these classes (multiple early atopic vulnerability) predicted asthma. Yao et al (24) reported that milk sensitization, egg sensitization, or both were associated with heightened airway reactivity before wheezing and after the onset of wheezing; however, these factors were not associated with an increased risk of wheezing. They suggested that airway reactivity, atopy, cytokine production by stimulated PBMCs, and the number of dendritic cells contribute to the risk of recurrent wheezing and airway hyperreactivity in early life.
In regards to associated allergic disorders, Chawes et al (25) characterized asthma and intermediary asthma endpoints in young children with allergic and non-allergic rhinitis. They reported that asthma is similarly associated with allergic and non-allergic rhinitis, suggesting a link between upper and lower airways beyond allergy-associated inflammation. Only children with allergic rhinitis had increased bronchial responsiveness and elevated FeNO, suggesting different endotypes of asthma symptoms in young children with allergic and non-allergic rhinitis. Rochat et al (26) investigated whether rhinitis in childhood is an independent predictor for developing wheezing between the ages of 5 and 13 years in a birth cohort study. They concluded that allergic rhinitis in preschool children is a predictor for subsequent wheezing onset. They found that association is not attributable to the type of sensitization, the severity of sensitization or atopic dermatitis in early life.
In relation to dietary influences, Kull et al (27) investigated the relation between breast-feeding and asthma and/or sensitization during the first 8 years of life. They found that exclusive breast-feeding for 4 months or more seemed to reduce the risk of asthma up to 8 years. At this age, a reduced risk was observed particularly for asthma combined with sensitization. They also indicated that breast-feeding seems to have a beneficial effect on lung function. Zhang et al (28) examined weight status from birth to age 5 years in relation to the occurrence of asthma at age 6 and 8 years in the Childhood Origin of Asthma (COAST) project. Longitudinal analyses revealed complex relationships between overweight and asthma. In children genetically at high risk of developing asthma, overweight at age 1 year was associated with a decreased risk of asthma and better lung function at age 6 and 8 years. However, being overweight beyond infancy did not have any protective effect and even could confer a higher risk for asthma.
The course of asthma varies among individuals who present during early childhood. Risk factors for persistent or remitting asthma are of interest to counsel children and parents. Covar et al (29) determined the rate and predictors for remitting, periodic, and persistent asthma in adolescence from participants in the NHLBI Childhood Asthma Management Program (CAMP). Asthma was identified as remitting in 6%, periodic in 39%, and persistent in 55% of the 909 participants, with no effect noted from earlier anti-inflammatory treatment during the CAMP trial. Therefore, remission of asthma in adolescence was infrequent and not affected by 4 years of anti-inflammatory controller therapy. Factors such as sensitization and exposure, low lung function, and airway greater hyper-responsiveness decreased the likelihood of remitting asthma. Toelle et al (30) reported on eight-year outcomes from the Childhood Asthma Prevention study. They showed that in children with a family history of asthma, neither house dust mite avoidance nor dietary fatty acid modification, from birth to 5 years, reduced the prevalence of asthma, atopy, or other atopic disorders at age 8 years. However, the house dust mite avoidance intervention lead to a significant reduction in the prevalence of poorly controlled asthma among atopic children, but not among non-atopic children, at this age.
Vink et al (31) assessed associations of pubertal stages and transition through puberty with (1) the prevalence, incidence, and remission of asthma in male and female subjects; (2) total IgE levels; and (3) peak expiratory flow (PEF) fall during a shuttle run test in the TRacking Adolescents’ Individual Lives Survey study. They observed that a shift in the prevalence of asthma occurs between 11.1 and 16.3 years, which is due to both an increased incidence and decreased remission of asthma in female compared with male subjects. They indicated that pubertal stages could not explain the gender shift in asthma prevalence.
Management
Theresa Guilbert (32) provided a comprehensive overview of studies related to the identification and management of the infant and toddler at risk for developing asthma. She concluded that, in children with recurrent wheeze, the beneficial effects of asthma therapies need to be balanced with regular assessments of asthma risk and symptom control and consideration of reduction, substitution, or discontinuation of medication in order to capitalize on the benefit and lessen the risk of treatment. Brasholt et al (33) studied the association between physical activity in preschool children and objectively assessed intermediary asthma phenotypes. They concluded that physical activity in preschool children was reduced with increasing bronchial responsiveness. The reduced physical activity was subclinical and not realized by parents or doctors despite daily diary cards and close clinical follow-up since birth. They recommended that awareness of even very mild asthma symptoms is important.
Controversy has raged over the role of acetaminophen on either causing or exacerbating asthma in children. Several reports addressed this issue. Tapiainen et al (34) commented that confounding factors are difficult to control in observational studies related to acetaminophen because the allocation to treatment groups does not take place at random. They conducted an evaluation of a data base to evaluate the association of febrile illness and antipyretic medication in early childhood with the later development of asthma. They concluded that the greater number of febrile days in early childhood observed in adolescents with asthma in their prospective study was explained by increased morbidity from respiratory infections because no difference was observed in febrile episodes caused by gastroenteritis. Schnabel et al (35) also used an available database to study the relationship of acetaminophen during infancy with asthma development and concluded that the increased number of respiratory tract infections in children vulnerable to asthma and not the acetaminophen medication per se are associated with the development of asthma. Therefore, they also concluded that the previously reported effects of acetaminophen on asthma are confounded by increased respiratory tract infection morbidity in asthma susceptible children.
Shaheen et al (36) explored potential interactions between prenatal and infant acetaminophen exposure and antioxidant genotypes on childhood asthma. They found evidence to suggest interactions between prenatal acetaminophen exposure and maternal antioxidant genotypes on childhood asthma risk. They indicated that this observation increases the likelihood that the acetaminophen-asthma link is causal. However to confirm this hypothesis, randomized controlled trials must be conducted. Therefore, until such studies are available, it will be difficult to make specific recommendations regarding the use of acetaminophen either prior to or after birth of the infant potentially at risk for developing asthma. Furthermore, studies are needed to assess the appropriate antipyretic therapy for managing fever in children who have established asthma.
Environment
Information continues to evolve regarding the role of the environment in the development of asthma. Bottema et al (37) evaluated the main effects and gene-gene interactions of haplotype tagging single nucleotide polymorphisms of genes involved in regulatory T–cell function - IL6, IL6R, IL10, heme-oxygenase 1 (HMOX1), IL2, Toll-like receptor 2 (TLR2), TGFB1, TGF-β receptor (TGFBR)–1, TGFBR2, IL2RA, and forkhead box protein 3 (FOXP3) - in relation to atopy and asthma. They concluded that genes involved in the development and function of regulatory T cells, specifically IL2RA, TLR2, TGFBR2, and FOXP3, associate with atopy and asthma by gene-gene interaction. Therefore, modeling of multiple gene-gene interactions is important to unravel further the genetic susceptibility to atopy and asthma.
Juhn et al (38) examined the impact of neighborhood environment on asthma incidence by applying the propensity score method to match children who lived in census tracts facing or not facing intersections with major highways or railroads. They found that children who lived in census tracts facing intersections with major highways or railroads had a higher risk of asthma (hazard ratios, 1.385–1.669 depending on the matching methods) compared with the matched counterparts who lived in census tracts not facing intersections with major highways or railroads.
Another type of environmental exposure is in utero smoke (IUS) exposure. Cohen et al (39) explored whether IUS exposure is associated with increased airway responsiveness among children with asthma and whether IUS modifies the response to treatment with inhaled corticosteroids (ICSs). IUS exposure reduced age-related improvements in airway responsiveness among children with asthma. They also found that IUS exposure appeared to blunt the beneficial effects of ICS use on airways responsiveness. These results emphasize the importance of preventing this exposure with pregnant women.
Personalized Medicine
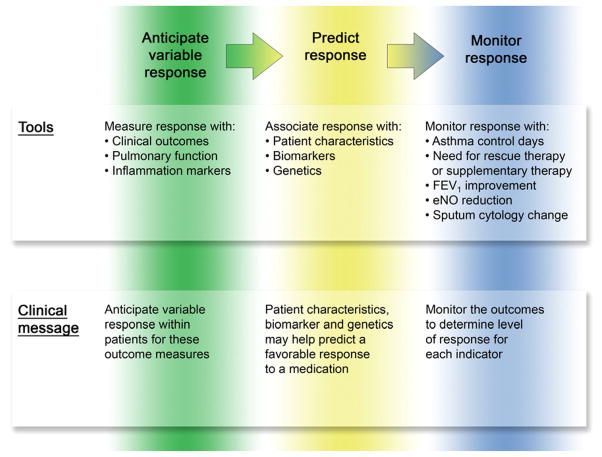
Dr. Margaret Hamburg, Commissioner of the Food and Drug Administration and Dr. Frances Collins, Director of the National Institutes of Health, leaders in the development and application of personalized medicine indicate that they have been focusing on the best ways to develop new therapies and optimize prescribing by steering patients to the right drug at the right dose at the right time. Moving from concept to clinical use requires basic, translational, and regulatory science (40). Our February 2010 theme issue focused on personalized medicine. Szefler and Martin (41) reviewed key studies conducted in the NHLBI Asthma Clinical Research Network (ACRN) and the Childhood Asthma Research and Education (CARE) Network. These two NIH asthma networks identified variable response to several long-term controller therapies, especially inhaled corticosteroids (ICS) and leukotriene receptor antagonists (LTRA). These networks also identified potential methods to use patients’ characteristics, such as age and allergic status, and biomarkers, such as bronchodilator response, exhaled nitric oxide, and urinary leukotrienes, to help predict response to ICS and LTRA and to determine which of the 2 treatments might be more effective in individual patients. This information now assists the clinician in personalizing asthma treatment at the time of initiating long-term control therapy. Physicians should now be aware that response to is variable, that certain patient features including biomarkers and genetics can be used to predict response, and treatment response should be monitored for continued effect (Figure 1).
Application of clinical trial information to clinical care.
Based on available studies, the clinician should anticipate that variability to response will occur with each treatment, that this variability in response can be associated with patient characteristics, biomarkers and genetics, and that response to treatment should be monitored for the various outcomes measures, especially symptom control and pulmonary function to assure rapid and sustained achievement of asthma control. Reproduced with permission from Szefler SJ and Martin RJ. Lessons learned from variation in response to therapy in clinical trials. J Allergy Clin Immunol 2010;125:285–92.
Kazani et al (42) in this same theme issue summarize available information related to genetic markers associated with variable response to treatments that include β-adrenergic agonists, short and long-acting, as well as ICS and LTRA. They present information related to the early pharmacogenomic studies and also discuss caveats in interpreting such information. This section will summarize JACI articles that build on the concepts of personalized medicine.
Patient characteristics associated with clinical presentation and drug response
Sonnappa et al (43) investigated whether multiple-trigger wheezers were more likely to have abnormal pulmonary function and increased fraction of exhaled nitric oxide (FeNO) than episodic (viral) wheezers and whether multiple-breath wash-out was more sensitive at detecting abnormal pulmonary function than specific airways resistance (sRaw) in preschool wheezers. They concluded that multiple-trigger wheeze is associated with pulmonary function abnormalities independent of atopic and current wheeze status. Also, conductive airways inhomogeneity is the most sensitive indicator of abnormal pulmonary function in preschool wheezers. Garcia-Marcos and Martinez (44) commented on these findings as a breakthrough study in mechanistic evaluation, since it may be that multiple-trigger wheezers have intrinsic airway abnormalities, perhaps due to altered airway responsiveness, which predisposes them to more frequent wheezing. This could set the stage for evaluation of genetic markers and therapeutic interventions associated with different forms of wheezing.
Gould et al (45) sought to determine the degree to which African American patients respond to ICS medication and whether the level of response is influenced by other factors including genetic ancestry. They reported that the degree of baseline bronchodilator reversibility was the only factor consistently associated with ICS responsiveness. The proportion of African ancestry was not significantly associated with ICS responsiveness.
Biomarkers for diagnosis and medication selection
Measurable biomarkers could be useful in asthma management. Extensive information has become available on the use of exhaled nitric oxide, an easily measured biomarker that could be easily applied in the clinical setting based on easy access and standardization of equipment and measurement technique. It can thus serve as template for translating the application of a biologic measure to clinical care. Steps have been taken to measure FeNO in infants. Debley et al (46) reported that single-breath eNO might predict changes in lung function and risk of future wheezing and holds promise as a biomarker to predict asthma in wheezy infants and toddlers. Gerald Teague (47) commented on the mechanistic source of nitric oxide and suggested that further study of these pathways may improve the application of FeNO levels to the care of children with asthma. In a related study, Chawes et al (48) reported an elevated FeNO in asymptomatic neonates born to mothers with asthma preceded the development of transient early wheezing, but not persistent wheezing during preschool age, and was unrelated to atopy.
Dweik et al (49) evaluated FeNO in severe asthma in the NHLBI Severe Asthma Research Program. They reported that high FeNO identified those patients with severe asthma characterized by the greatest airflow obstruction and hyperinflation and most frequent use of emergency care, the most reactive and worrisome asthma phenotype. Of interest, Nair et al (50) evaluated the relationship between FeNO and sputum eosinophils in patients with prednisone-dependent asthma and demonstrated the dissociation of these two measures and, therefore, limitation of using FeNO to evaluate and monitor therapy of eosinophilic bronchitis in this patient population. Van Muylem et al (51) studied changes in alveolar nitric oxide concentration (CANO) and FeNO after administration of ICS and reported that ICS have a positive effect on the preacinar and acinar airways and at the alveolar level. They also noted that a low alveolar concentration of NO does not preclude peripheral inflammation. To date, it has been difficult to identify biomarkers that are associated with a more favorable response to LTRA as compared to ICS. Rabinovitch et al (52) addressed this question based on data from several NHLBI CARE network studies and concluded that urinary LTE4/FeNO ratios predict a better response to montelukast than fluticasone propionate in children with mild-to-moderate asthma.
There has been ongoing interest in measuring biomarkers in exhaled breath condensates, an easily accessible, noninvasive source, even in young children. Van de Kant (53) et al, after measuring cytokines and chemokines, in exhaled breath condensates (EBC) in preschool children reported elevated markers suggesting increased airway inflammation in preschool children with recurrent wheeze. Further studies will determine whether these markers could distinguish various wheezing phenotypes in this age group. In addition, Sachs-Olsen et al (54) investigated whether metabolites of the 5- and 15-lipoxygenase (LO) pathway in EBC are associated with childhood asthma, asthma severity and clinical parameters. Their results pointed to increased activity of the 15-LO inflammatory pathway in childhood asthma. Mass spectrometry analyses of EBC demonstrated that elevated eoxins not only accompany the increased 5-LO product LTC4, but are also associated with airway hyperresponsiveness (AHR). These markers may represent a new therapeutic target for asthma treatment.
Genetics
The September 2010 theme issue was devoted to genetics in allergic disease. Deborah Meyers (55) presented an update on genetic approaches, including pharmacogenetics, to understand the susceptibility and severity of common diseases, such as asthma and allergy, and to assess the potential usefulness of DNA in personalized medicine. She emphasized the importance of a systematic approach for studying these disorders and the importance of well-characterized cohorts with in-depth phenotyping, such as lung imaging, along with assessment of gene expression in a systems biology approach. Baye et al (56) provided an in-depth review of genomic approaches to identify genes and biologic networks involved in allergic diseases including genetic and phenotypic variation, statistical approaches for gene discovery, public databases, functional genomics, and clinical implications of this information.
Another consideration in understanding asthma is the interplay between genetic and environmental factors now known to be mediated by epigenetics. Shuk-Mei Ho (57) reviewed this area and indicated that discovery and validation of epigenetic biomarkers linked to exposure, asthma, or both might lead to better epigenotyping of risk, prognosis, treatment prediction including steroid resistance, and development of novel therapies.
In a study of multiethnic cohorts among various age groups, Jin et al (58) reported that variants in the dual-specificity phosphatase 1 (DUSP1) gene were associated with clinical response to ICS and might be useful in the future to identify asthmatic patients more likely to respond to this controller treatment. Tcheurekdjian et al (59) sought to identify modulating effects of arachidonate 5- lipoxygenase-activating protein (ALOX5AP) and leukotriene A4 hydrolase (LTA4H) gene polymorphisms on the drug-drug interaction between leukotriene modifiers and albuterol in Mexicans and Puerto Ricans. They concluded that LTA4H and ALOX5AP gene polymorphisms modify the augmentation of bronchodilator responsiveness by leukotriene modifiers in Puerto Ricans but not Mexicans with asthma.
Severe asthma in children
Techniques of personalized medicine could be of greatest use in improving management of severe asthma in children. This will necessitate a better understanding of the biologic pathways relevant in severe asthma and the best predictors of treatment response. Bush and Saglani (60) summarized principles around the management of severe asthma in children pointing out that they are a heterogeneous group, with substantial morbidity. In many children with severe asthma, the diagnosis is wrong or adherence to treatment is poor. Alternative treatment, such as immunomodulator therapy, for example cytokine-specific monoclonal antibodies, and thermoplasty, have not been studied in children. Perhaps, improved diagnostic approaches will determine those likely to respond to such approaches.
In addition, Fitzpatrick and Teague (61) provided insights on severe asthma in children from the NHLBI Severe Asthma Research Program (SARP). They commented that severe asthma in children, unlike adults with severe asthma, are more likely to fall in a more narrow cluster that is characterized by marked atopy and reversible airflow obstruction. However, considerable gaps remain for which additional studies are needed. Fitzpatrick et al (62) reported on the molecular phenotype of severe asthma by applying linear discriminant analysis, a supervised method of high-dimensional data reduction, to cytokines and chemokines measured in the bronchoalveolar lavage fluid and alveolar macrophage lysate. They concluded that severe asthma in children is characterized by a distinct airway molecular phenotype that does not have a clear TH1 or TH2 pattern. They feel that improved classification of children with severe asthma may assist with the development of targeted therapeutics for this group of children who are difficult to treat. Gamble et al (63), studying NHLBI SARP participants sought to assess the extent to which racial disparities in severe asthma between Blacks and Whites are attributable to physiologic, immunoinflammatory, and sociodemographic variables. They reported that biologic factors were distinctly associated with severe asthma only in Blacks. They suggested that studies which incorporate comprehensive evaluation of biologic factors associated with asthma may lead to the development of therapies which target biologic abnormalities in Blacks.
In looking forward, Bousquet et al (64) reported on a World Health Meeting convened to propose a uniform definition of severe asthma. They stated that severe asthma includes three groups, each carrying different public health messages and challenges: 1) Untreated severe asthma, 2) Difficult-to-treat severe asthma and 3) Treatment resistant severe asthma. The latter group includes asthma for which control is not achieved despite the highest level of recommended treatment and asthma for which control can be maintained only with the highest level of recommended treatment. If these new terms for severe asthma become useful in the research world, the next logical step would be to incorporate them into asthma guidelines and practice, including electronic medical records (65). That will help assure communication across medical systems to facilitate the identification of patients with emerging, inadequately controlled, and severe asthma in health care systems. From a public health perspective, that effort would prompt the identification of problem areas, such as poor access to care, poor adherence, poor technique for inhaled medications, refractoriness to treatment, excessive exposure to environmental factors, and the need for a higher level of intervention (65).
Treatment decisions meriting an individualized approach
ICS are considered the preferred long-term controller therapy for the management of persistent asthma including young children. Amirav et al (66) pointed out that infants (0–1 years of age) and young children (1–3 years of age) are a unique subpopulation with regard to inhaled therapies. There are various anatomic, physiological, and emotional factors peculiar to this age group that present significant difficulties and challenges for aerosol delivery. They concluded that the benefit of ICSs in this age group requires further evaluation to determine whether better therapeutic outcomes might be achieved with smaller particles. In regards to continuing safety concerns regarding prolonged use of ICS therapy, Raissy et al (67) provided reassuring information from the NHLBI Childhood Asthma Management Program that the long-term use of ICS in the recommended range in combination with occasional bursts of prednisone during childhood was not associated with an increased risk of cataracts. Thus regular monitoring for cataracts does not appear to be warranted in children, adolescents, and young adults with asthma being treated with low/medium-dose ICS without other significant risk factors.
A recent study from Lemanske et al (68) from the CARE Network attempted to evaluate Step 3 therapy in school-age children with asthma uncontrolled on low-dose ICS. Nearly all children demonstrated differential responses, with best response more than one-and-a-half times as likely with LABA step-up. However, many children demonstrated best response to ICS or LTRA step-up, highlighting the importance of continually re-evaluating an individual child’s response to therapy. Unfortunately, the biomarkers and genetics evaluated in this study were not helpful in selecting the participants most likely to respond to one of the three treatment choices.
Exacerbations
The June 2010 theme issue was focused on virus infections in asthma. David Jackson and Sebastian Johnston (69) reviewed the role of viral respiratory infections, the most common cause of an acute asthma exacerbation, in both children and adults representing a significant global health burden. These infections cause a greater degree of morbidity in asthmatic subjects than in the healthy population, emphasizing a discrepancy in the antiviral response of asthmatics. They indicated that studies of the molecular pathways underlying virus-induced inflammation are required to identify new targets for development of novel therapies. Patrick Holt and Deborah Strickland (70) reviewed the interactions between innate and adaptive immunity in asthma pathogenesis, specifically in relation to acute exacerbations. They indicated that the primary risk factor for atopic asthma is sensitization to perennial aeroallergens resulting from a failure to generate protective immunologic tolerance. Perturbation of the balance between the interlinked innate and adaptive immune pathways is increasingly believed to be the basis for disease expression, and in the specific case of atopic asthma, the prototypic example of this is acute exacerbations triggered by viral infections.
Human rhinovirus (HRV) infection is the cause of about one half of asthma and chronic obstructive pulmonary disease exacerbations. Palmenberg et al (71) reviewed the information related to the analysis of the complete genome sequences of HRV. Techniques have now been developed using next-generation sequencing to generate complete genomes from patient isolates with high throughput, deep coverage, and low costs. Therefore, relationships can now be evaluated between obstructive lung phenotypes and variation in HRV genomes in infected patients and potential novel therapeutic strategies developed based on HRV sequence. Olenec et al (72) sought to evaluate rhinovirus infections during peak seasons in children with asthma and to analyze relationships between viral infection and illness severity. Viruses were detected in 36% to 50% of the specimens, and 72% to 99% of the viruses were rhinoviruses. They concluded that RV infections are nearly universal in children with asthma during common cold seasons, likely because of a plethora of new strains appearing each season. Illnesses associated with viruses have greater duration and severity and atopic asthmatic children experienced more frequent and severe virus-induced illnesses.
Simoes et al (73) evaluated the causal relationship of respiratory syncytial virus (RSV) lower respiratory tract infections (LRTIs) in early life to determine the association of an atopic diathesis. They observed that RSV prophylaxis in non-atopic children decreased by 80% the relative risk of recurrent wheezing but did not have any effect in infants with an atopic family history. They felt this observation suggests that RSV predisposes to recurrent wheezing in an atopy-independent mechanism. Despite campaigns by public health officials to promote seasonal influenza vaccination, there remains significant public reluctance among persons in high-risk groups. Leo et al (74) studied 2009 seasonal and H1N1 influenza vaccination compliance in asthmatic children and adults. They found that despite significant vaccination rates in asthmatic adults and children compared with their non-asthmatic counterparts, there still remain large proportions of adults and children who are unlikely to receive seasonal, H1N1, or both influenza vaccinations. Information derived from such studies may help to improve future vaccination programs.
Ambient fine particles (particular matter <2.5 mm diameter [PM2.5]) and ozone exacerbate respiratory conditions including asthma. Silverman et al (75) investigated the relationship between severe asthma morbidity and PM2.5 and ozone in the warm season, and to determine whether there is an age-related susceptibility to pollution. They reported that warm weather patterns of ozone and PM2.5 disproportionately affect children with asthma and appear responsible for severe attacks that could have been avoided. Similarly, Strickland et al (76) investigated short-term associations between ambient air pollutant concentrations and emergency department visits for pediatric asthma and reported that even at relatively low ambient concentrations, ozone and primary pollutants from traffic sources independently contributed to the burden of emergency department visits for pediatric asthma.
Roy et al (77) evaluated demographic risk factors for asthma hospitalizations in urban versus rural areas of Mississippi. They found that asthma hospitalization rates were significantly higher among all demographic groups in the rural Delta region compared with the urban Jackson Metropolitan Statistical Area (P < .001). They also noted that Blacks with asthma are more likely to have multiple asthma hospitalizations in Mississippi. Higher odds of multiple asthma discharges for Delta residents were not explained by race, sex, age, or income, indicating that other contributing factors (eg., environmental, social, and access to care factors) need further investigation.
Asthma guidelines emphasize both maintaining current control and reducing future risk, but the relationship between these 2 targets is not well understood. Bateman et al (78) assessed the relationship between an asthma control questionnaire and Global Initiative for Asthma-defined clinical asthma control and future risk of instability and exacerbations. They reported that current control predicts future risk of instability and exacerbations. They also noted that budesonide/formoterol maintenance and reliever therapy reduces exacerbations versus comparators and achieves at least similar control. Kenneth Chapman (79) in an associated editorial pointed out that the data from this study utilizing as needed combination therapy indicated that although severe exacerbations occurred less frequently, they occurred often enough to be significant and an ongoing concern. He felt that despite the availability of newer medications and formulations, there is no evidence that life-saving strategies of symptom prevention should be abandoned for a program of symptom-driven care. Concern has been raised regarding the safety of long-acting β-adrenergic agonists (LABA). Nelson et al (80) assessed the risks of formoterol-containing versus non- LABA treatment by using a large asthma database. They provided some reassurance that there was no evidence of increased risk of asthma-related hospitalization, no asthma-related deaths, and a low incidence of all-cause death and asthma-related intubation seen with formoterol-containing versus non-LABA treatment.
Inner City Asthma
The March 2010 theme issue was focused on inner city asthma in children. Asthma in inner-city children is prevalent, has increased severity, and for many patients poses difficulty in achieving control. Although some of the limitations to asthma control in inner-city children include socioeconomic burdens, other factors, such as environmental allergens, pollutants, infections, and stress, contribute significantly to the disease burden found in these children. NIAID focuses its asthma and allergy programs on understanding the interaction of the immune system with allergens and infectious agents and identifying genetic and epigenetic elements that influence the immune system. Togias et al (81) indicated that a key goal in this field is to define mechanisms of immune system deviation and immune tolerance and apply this knowledge to generate improvements in asthma care and allergen immunotherapy. A related goal is to further understand the environmental, social, and immunological elements that impact on the development of inner-city asthma through in-depth characterization and longitudinal follow-up of inner-city children from the time of birth.
Dr. William Busse, Principal Investigator for the NIAID Inner City Asthma Consortium (ICAC), reviewed the Inner-City Asthma Network Program that began in 1991 and has, over the ensuing years, funded 3 distinct networks and showed how newly proposed efforts under ICAC 2 propose to improve our understanding and eventual control of asthma in inner-city children (82). Szefler et al (83) highlighted features of the NIAID ICAC Asthma Control Evaluation (ACE) study that enhanced our knowledge regarding the application of the asthma guidelines and the lessons learned from that important study. Essentially, they were impressed that a systematic guidelines-based approach improved asthma control significantly over the course of the 1-year treatment period in almost all patients. The future challenges for improving management of asthma in inner city children include further studies on defining the cause of asthma, assessing the role of specific allergens, such as cockroach allergen, in predisposing to persistent asthma and asthma exacerbations, and the interaction of viral infections with the atopic phenotype. In addition, it will be important to determine if asthma management could be enhanced by refinements in electronic medical records, community and school support programs, and public health collaboration.
The NIAID Urban Environment and Childhood Asthma (URECA) birth cohort study was initiated in 2004 to test the hypothesis that exposure in early life to adverse environmental and lifestyle factors associated with disadvantaged urban environments modifies immune development to increase the risk for allergic diseases and asthma. Dr. James Gern summarized the study design, methods, and early findings of the URECA study (84). The major study outcomes are recurrent wheeze at 3 years of age and asthma at age 7 years.
Pongracic et al (85) examined relationships between fungal sensitization, exposure, and asthma morbidity in 5 to 11 years old inner city children enrolled in the Inner-City Asthma Study. They reported that outdoor fungal exposure is primarily associated with increased asthma symptoms and increased risk of exacerbations in this population. Kattan et al (86) sought to understand the relationships among adiposity, sex, and asthma control in 368 adolescents with moderate-to-severe asthma (ages 12–20 years) living in 10 urban areas for 1 year. They reported that adiposity is associated with poorer asthma control in female subjects. Adiponectin is associated with improved asthma control in male subjects.
Sheehan et al (87) provided a Work Group Report of the American Academy of Allergy, Asthma & Immunology Indoor Allergy/Air Pollution Committee on the importance of pest allergen exposure in inner-city asthma. They discussed how exposure is associated with allergen sensitization and asthma morbidity. They also reviewed different methods of intervention and the effectiveness of these tactics. Future studies will continue to evaluate the effectiveness of inner city allergen reduction in improving health outcomes of asthma.
Studies in urban and rural children could be important in understanding variability in asthma presentation. Calvert et al (88) investigated the association between infection with the parasite Ascaris allergic sensitization, and exercise-induced bronchospasm (EIB) in a cross-sectional prevalence survey conducted in urban and rural South African children. They observed that in areas of high parasite endemicity, Ascaris might induce an inflammatory response in the lungs independent of its effect on IgE production. This could explain some of the contradictory findings seen in studies examining the association between geohelminth infection, atopy, and asthma. Platts-Mills and Cooper (89) indicated that despite this interesting information, there are limitations to these observations. We still need to understand the factors that leave some rural villages with no significant asthma compared with the increased prevalence of wheezing that has appeared associated with urbanization and the severity that are common in western countries including minority populations in the United States.
Much has been done to promote population-based childhood asthma screening; however, concerns remain regarding its cost-effectiveness. Gerald et al (90) conducted a cost-effectiveness analysis of school based asthma screening strategies. They suggest that the most efficient approach is to screen for previously diagnosed but poorly controlled asthma. Linking screening with better treatment, and long-term adherence strategies might yield future cost-effective approaches. McElligott and Polsky (91) commenting on this study suggest that once issues related to adherence, treatment, and follow-up screening are addressed, it may be possible to implement a cost-effective childhood asthma screening program.
Opportunities to improve control
Over the last 20 years we have seen the reduction in asthma mortality, the introduction of new medications, and less steroid-requiring and resistant asthma, especially in children. Now we must look at new targets to improve overall asthma control, for example, reducing exacerbations, especially in young children, addressing issues of health disparities, continuing to reduce school absence due to asthma, and early intervention to alter the course of the disease. Fitzgerald and Shahidi (92) provided a Current Perspectives review to address asthma control in patients with moderate disease. They are usually treated initially with low-dose inhale corticosteroids (ICSs) supplemented with a short-acting bronchodilator as a rescue medication. For patients in whom adherence, inhaler technique, environmental control, and co-morbidities have been addressed but who still have uncontrolled symptoms, the addition of a long-acting β-adrenergic agonist should be considered. Some patients might require a higher dose of ICS. Leukotriene receptor antagonists might be considered as alternate initial therapy or as an add-on to maintenance therapy with an ICS. Williams et al (93) assessed the effect of supplying patient adherence information to primary care providers. They found that providing adherence information to clinicians did not improve ICS use among patients with asthma. However, they noted that physician use of this information was a barrier.
In relation to asthma control measures, Liu et al (94) sought to determine a second cut point on the Childhood Asthma Control Test (C-ACT) to identify children, ages 4 to 11 years, with “very poorly controlled” asthma. They determined that a second cut point of 12 or less on the C-ACT identifies children with the lowest level of control, who are at risk for poorer outcomes, and is conceptually consistent with the classification of “very poorly controlled” asthma adopted by asthma management guidelines. Simon et al (95) sought to determine whether the measurement of FEF25–75 percent predicted offers advantages over FEV1 percent predicted and FEV1/forced vital capacity (FVC) percent predicted for the evaluation of childhood asthma based on data evaluation from several NIH CARE Network studies. They noted that FEF25–75 percent predicted was well correlated with bronchodilator responsiveness in asthmatic children with normal FEV1. They suggested that FEF25–75 percent predicted should be evaluated in clinical studies of asthma in children and might be of use in predicting the presence of clinically relevant reversible airflow obstruction. Regis McFadden (96), in an accompanying editorial, provided several interesting comments on this report including the possibility that FEF25–75 could be explored to assess the natural history of asthma and its management. However, long-term studies would be needed to reach definitive conclusions about the ultimate clinical utility of FEF25–75.
In regards to co-morbidites related to asthma, Brozek et al (97) developed clinical recommendations for systematically treating allergic rhinitis on the basis of current best evidence. The guideline panel developed evidence profiles for each recommendation and considered health benefits and harms, burden, patient preferences, and resource use, when appropriate, to formulate recommendations for patients, clinicians, and other health care professionals.
New techniques, such as a challenge with mannitol, have recently been developed to measure AHR. Andregnette-Roscigno et al (98) reported that bronchoprovocation with a directly acting stimulus such as methacholine is extremely sensitive, demonstrating bronchial hyper-responsiveness as a result of the presence of current asthma symptoms more reliably than mannitol in their population of children with asthma. They felt the mannitol test might be a clinically relevant marker for assessing the clinical course of asthma, perhaps indicating that the two tests are complementary but not interchangeable for clinical application. Sverrild et al (99) compared AHR to mannitol and methacholine and exhaled nitric oxide (eNO) in a non-selected population sample of young adults, ages 14 to 24 years. They noted that AHR to mannitol was less sensitive but more specific than methacholine in diagnosing asthma. Furthermore, AHR to mannitol was more closely associated with ongoing airway inflammation in terms of increased eNO. Both studies seemed to suggest that the two tests provide different pieces of information in assessing asthma.
Old therapies – potential new applications
It is unlikely that a new medication for the treatment of asthma will be introduced in the next several years. Therefore, it is important to look at other available techniques or medications that could add to asthma control. Beebe et al (100) tested an art therapy intervention, consisting of 60-minute art therapy sessions once a week for 7 weeks, in a randomized controlled trial in children with asthma. They found that children with asthma received benefit from art therapy that included decreased anxiety and increased quality of life. Even at 6 months, the active group maintained some positive changes relative to the control group.
Several reports evaluated the effects of medications and allergen immunotherapy. Zielen et al (101) investigated the efficacy of an allergen-specific immunotherapy with a high-dose hypoallergenic mite preparation (allergoid) as steroid-sparing agent in 65 children with asthma (Global Initiative for Asthma treatment levels II and III; 6–17 years old), after reaching asthma control with inhaled steroids. They concluded that adding subcutaneous mite allergoid immunotherapy to pharmacologic treatment is an effective and safe strategy to reduce corticosteroid doses while maintaining disease control in children with mite-induced allergic asthma. Franklin Adkinson commented that this study adds substantial credibility to this controversial claim in the list of potential benefits from aeroallergen immunotherapy (102). He indicated that more clinically significant than the changes in mean fluticasone requirements is the observation that almost half of the children receiving immunotherapy were able to reduce inhaled steroids by 2 or more dosage steps over the two years, compared with only 19% of the control group. He indicated that factors such as asthma severity, breadth of allergic sensitivities, ongoing allergen exposure, age and asthma duration may influence the steroid-sparing effect attributable to allergen immunotherapy.
Majak et al (103) assessed the effect of montelukast treatment on early clinical and immunologic effects of allergen-specific immunotherapy (SIT) in children with asthma. They concluded that their study failed to show a beneficial effect of montelukast on SIT. In fact, compared with placebo, montelukast intervention led to less effectiveness of SIT. Of interest, from an animal study, Han et al (104) determined the effects of montelukast in primary and secondary RSV-infected newborn and adult mice. The found that montelukast attenuated the initial responses to primary RSV infection in both age groups and altered the consequences of RSV reinfection in mice initially infected as neonates. They felt that their data supported an important role for cysteinyl leukotrienes in RSV-induced AHR.
Concern lingers regarding the safety of LABA therapy in asthma management. Lemanske and Busse (105), responding to an earlier FDA communication with new recommendations regarding LABA labeling changes, indicated that current data are not sufficient or convincing to mitigate against the long-term use of LABA as adjunctive therapy. Restrictions could set back effective asthma care and its sustained control in the United States. They suggested that an open discussion with transparent examination of data will be critical to an appropriate conclusion and will provide the safest and most effective asthma care. Furthermore, re-examination of treatment use can also ensure that combination therapy is prescribed to those who need it and will most benefit from this approach.
For those who have lingering concerns regarding LABA use, alternative strategies were studied in children and discussed in the previously mentioned report by Lemanske et al (68) from the CARE Network step-up study. In addition, Peters et al (106) studied tiotropium bromide in a three-way, double-blind, triple-dummy crossover trial involving 210 adult patients with asthma in the ACRN. They concluded that tiotropium bromide when added to an inhaled glucocorticoid, improved symptoms and lung function in patients with inadequately controlled asthma. Its effects appeared to be equivalent to those with the addition of salmeterol. However, further studies in adults and children are needed to establish its position in the treatment of asthma.
Recent attention has been directed to dietary insufficiency, including vitamin D, and its potential relationship to childhood asthma. Jartti et al (107) hypothesized that serum 25-hydroxyvitamin D (25[OH]D) levels would be inversely associated with the risk of rhinovirus (the main viral trigger of wheezing) and/or >1 concurrent viral infections, i.e., viral co-infections. Among 284 hospitalized wheezing children in Finland, low serum 25(OH)D levels were associated with increased risk for rhinovirus, respiratory syncytial virus and virus co-infections. In 31% of children, 25(OH)D was <50 nmol/L despite national recommendations for supplementation. Brehm et al (108) sought to assess the relationship between serum vitamin D levels and subsequent severe asthma exacerbations from sera collected from 1,024 children with mild-to-moderate persistent asthma at the time of enrollment in a multicenter clinical trial, the NHLBI Childhood Asthma Management Program. They also reported that vitamin D insufficiency is common in this population of North American children with mild-to-moderate persistent asthma and is associated with higher odds of severe exacerbation over a 4-year period. In addition, having sufficient vitamin D levels conferred protection against severe exacerbations that was in addition to the effect of inhaled corticosteroids. Searing et al (109) investigated disease variables associated with vitamin D insufficiency in patients with childhood asthma and interaction of vitamin D with corticosteroid-mediated anti-inflammatory responses. They observed that corticosteroid use and worsening airflow limitation were associated with lower vitamin D serum levels in asthmatic patients. They also indicated that vitamin D enhanced glucocorticoid action in PBMCs from asthmatic patients and enhanced the immunosuppressive function of dexamethasone. Therefore, they felt that vitamin D supplementation might potentiate anti-inflammatory function of corticosteroids in asthmatic patients and thus improve asthma control. Ginde and Sutherland (110) in an editorial relating to the Brehm and Searing reports (108, 109) along with other recent studies indicated that the emerging story relating vitamin D and asthma remains compelling and in need of further development through prospective studies along with carefully developed mechanistic components. Indeed, this is a target area for the newly developed NHLBI AsthmaNet that will conduct clinical trials in children and adults.
In another approach to dietary supplementation, Covar et al (111) evaluated the efficacy, compliance, and safety of NNF in asthmatic children of a novel nutritional formula (NNF) enriched in eicosapentaenoic (EPA) and gamma-linolenic fatty acids and antioxidants. They reported that both NNF and control groups demonstrated improvement in asthma-free days. The NNF-treated group had reduced biomarkers of disease activity. Rapid PBMC fatty acid composition changes reflected an anti-inflammatory profile. Dietary supplementation with NNF was safe and well tolerated. Therefore, further studies are needed to follow up on this well-designed pilot, feasibility study to evaluate the role of this nutritional formula in reducing airway inflammation and improving asthma control in a wider patient population.
In regards to other potential new treatment directions, an interesting report was provided by Razi et al (112) related to OM-85 BV (Broncho-Vaxom; OM Pharma, Meyrin/Geneva, Switzerland), an immunostimulant extracted from 8 bacterial pathogens of the upper respiratory tract, to assess prevention of acute respiratory tract illnesses (ARTI) in preschool children. They conducted a randomized, double-blind, placebo-controlled, parallel group study. Participants were given either OM-85 BV or a placebo (one capsule per day, for 10 days each month, for 3 consecutive months) at the start of the trial and followed for 12 months. Subjects given OM-85 had a lower rate of wheezing attacks. The cumulative difference in wheezing attacks between the 2 groups was 2.18 wheezing attacks per patient in 12 months— there was a 37.9% reduction in the group given OM-85 BV, compared with the group given placebo (P<0.001). They therefore concluded that OM-85 significantly reduced the rate and duration of wheezing attacks in preschool children with ARTIs. Miles Weinberger in an associated editorial (113) commented that the absence of currently available alternatives warrants particular interest in OM-85 BV but questions remain to be answered regarding the potential mechanism of action, dose and duration of treatment, and whether this product could ever be evaluated in the United States despite its availability in many other countries for the past 20 years.
Another step is to identify new therapeutic targets for asthma management. Mukhopadhyay et al (114) sought to explore the hypothesis that matrix metalloproteinase (MMP)-12 represents a novel therapeutic target in asthma. The role of MMP-12–specific inhibition was tested in vitro, as well as in animal models of allergic airway inflammation. Their studies on human participants with asthma and COPD showed that the risk of a MMP-12 gene variant was associated with disease severity. In allergen-sensitized sheep pharmacologic inhibition of MMP-12 down-regulated both early and late airway responses in response to allergic stimuli. Therefore, specific inhibition of MMP-12 driven airway inflammation and remodeling might represent a new therapeutic target for asthma and COPD.
Systems management to improve asthma control
With all of the fascination around personalized medicine and the potential insights to new therapeutic targets, we must not lose sight of barriers that will impair achieving optimal asthma control in all children. One area to address is access to care. Kogan et al (115) recently reported that the number of underinsured children exceeded the number of children without insurance for all or part of the year studied. Access to health care and the quality of health care are suboptimal for uninsured and underinsured children. Whether or not the Affordable Care Act will answer those questions remains a concern for all health practitioners and patients (116). It is also clear that tobacco regulation must continue to advance in order to reduce the significant risk of addiction in older children and young adults, as well as reducing tobacco-associated disabilities (117). Other issues like improved record keeping and communication via electronic medical records hold promise to advance health care (118). While some of these may be encouraged by forms of regulation, hopefully roadmaps such as the Healthy Vision 2020 will prompt a closer look to advance the quantity and quality of life, including healthy places and environments, health equity, and disease prevention (119).
Conclusion
In summary, based on recent advances, including the studies summarized in this review, we can feel some pride in accomplishments in managing childhood asthma over the past 20 years with some indication of reduced mortality and morbidity. However, there is still much more to do before we can say that we conquered this disease. Some experts feel that we have entered an era of complacency around asthma, perhaps due to the absence of new drugs that promise to replace our current therapy. A careful look at asthma hospitalizations in children, racial differences in exacerbations, and the impact that asthma has on life choices for children should quickly sober the complacent individual into taking the next steps to improve asthma care for children. Better use of our current medications will be sufficient for some but not all patients.
Table I.
Key Advances in Pediatric Asthma, 2010
|
Acknowledgments
Supported in part by Public Health Services Research Grants HR-16048, HL64288, HL 51834, AI-25496, HL081335, HL075416, and the Colorado Cancer, Cardiovascular and Pulmonary Disease Program. Supported in part by Colorado CTSA grant 1 UL1 RR025780 from the National Institutes of Health (NIH) and National Center for Research Resources (NCRR).
Dr. Szefler would like to thank Gretchen Hugen for assistance with this manuscript preparation.
Abbreviations
- ACRN
NHLBI Asthma Clinical Research Network
- AHR
airway hyper-responsiveness
- ALOX5AP
arachidonate 5- lipoxygenase-activating protein
- ARTI
acute respiratory tract infections
- BAL
bronchoalveolar lavage
- BHR
bronchial hyper-responsiveness
- C-ACT
Childhood Asthma Control Test
- CARE
NHLBI Childhood Asthma Research and Education Network
- DPP10
Dipeptidyl-peptidase 10 gene
- DUSP1
Dual-specificity phosphatase 1
- gene EIB
exercise-induced bronchoconstriction
- ED
Emergency department
- eNO
exhaled nitric oxide
- EPA
eicosapentaenoic acid
- FEF25-75
forced expiratory flow between 25% and 75% of vital capacity
- FeNO
fraction of exhaled nitric oxide (ppb)
- FEV1
forced expiratory volume in one second
- FOXP3
forkhead box protein 3
- GWAS
genome-wide association study
- HMOX1
heme-oxygenase 1
- HRV
Human rhinovirus
- ICAC
NIAID Inner City Asthma Consortium
- ICS
inhaled corticosteroid
- IL1R1
IL1 receptor-like gene
- IL2RA
interleukin 2 receptor A
- IL6
interleukin 6
- IL6R
interkeukin 6 receptor
- IL10
interleukin 10
- IL18R1
IL18 receptor 1 gene
- LABA
long acting β-adrenergic agonists
- LO
lipoxygenase
- LRTIs
lower respiratory tract infections
- LTA4H
leukotriene A4 hydrolase
- LTC4
leukotriene C4
- LTE4
leukotriene E4
- LTRA
leukotriene receptor antagonist
- MMP
matrix metalloproteinase
- NIAID
National Institute of Allergy and Infectious Diseases
- NHLBI
National Heart, Lung and Blood Institute
- NNF
novel nutritional formula
- NO
nitric oxide
- PBMCs
peripheral blood mononuclear cells
- RSV
respiratory syncytial virus
- RV
rhinovirus
- SARP
NHLBI Severe Asthma Research Program
- sCD14
soluble CD14
- SIP
specific immunotherapy
- SNPs
single polymorphonuclear polymorphisms
- TGFB1
TGF-β1 gene
- TGFBR
TGF-β receptor
- TLR2
Toll-like receptor 2
- 25[OH]D
25-hydroxyvitamin D
- URECA
Urban Environment and Childhood Asthma birth cohort study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Szefler SJ. Advances in pediatric asthma in 2009: Gaining control of childhood asthma. J Allergy Clin Immunol. 2010;125:69–78. doi: 10.1016/j.jaci.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Apter AJ. Advances in adult asthma diagnosis and treatment in 2009. J Allergy Clin Immunol. 2010;125:79–84. doi: 10.1016/j.jaci.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisgaard H, Bennelykke K. Long-term studies of the natural history of asthma in childhood. J Allergy Clin Immunol. 2010;126:187–97. doi: 10.1016/j.jaci.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Pfeferle PI, Buchele G, Blumer N, Roponen M, Ege MJ, Krauss-Etschmann S, et al. Cord blood cytokines are modulated by maternal farming activities and consumption of farm dairy products during pregnancy: The PASTURE Study. J Allergy Clin Immunol. 2010;125:108–15. doi: 10.1016/j.jaci.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Lange NE, Rifas-Shiman SL, Camargo CA, Gold DR, Gillman MW, Litonjua AA. Maternal dietary pattern during pregnancy is not associated with recurrent wheeze in children. J Allergy Clin Immunol. 2010;126:250–5. doi: 10.1016/j.jaci.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreger LC, Kozyrskyj AL, Hay Glass KT, Becker AB, MacNeil BJ. Lower cortisol levels in children with asthma exposed to recurrent maternal distress from birth. J Allergy Clin Immunol. 2010;125:116–22. doi: 10.1016/j.jaci.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 7.Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, et al. Prenatal maternal stress and cord blood innate and adaptive cytokines responses in an inner-city cohort. Am J Respir Crit Care Med. 2010;182:25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breton MC, Beauchesne MF, Lemiere C, Rey E, Forget A, Blaise L. Risk of perinatal mortality associated with inhaled corticosteroid use for the treatment of asthma during pregnancy. J Allergy Clin Immunol. 2010;126:772–7. doi: 10.1016/j.jaci.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Holloway JW, Arshad SH, Holgate ST. Using genetics to predict the natural history of asthma? J Allergy Clin Immunol. 2010;126:200–9. doi: 10.1016/j.jaci.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 10.DeWan AT, Triche EW, Xu X, Hsu LI, Zhao C, Belanger K, et al. PDE11A associations with asthma: results of a genome-wide association scan. J Allergy Clin Immunol. 2010;126:871–3. doi: 10.1016/j.jaci.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Ma SF, Wade MS, Flores C, Pino-Yanes M, Moitra J, et al. Functional variants of the sphingosine-1-phosphate receptor 1 gene associate with asthma susceptibility. J Allergy Clin Immunol. 2010;126:241–9. doi: 10.1016/j.jaci.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Howard TD, Zheng SL, Haselkorn T, Peters SP, Meyers DA, et al. Genome-wide association study of asthma identifies RAD50-IL13 and HLA-DR/DQ regions. J Allergy Clin Immunol. 2010;125:328–35. doi: 10.1016/j.jaci.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munthe-Kaas MC, Maehre Torjussen T, Gervin K, Ledrup Carlsen KC, Hakon Carlsen K, Granum B, et al. CD14 polymorphisms and serum CD14 levels through childhood: a role for gene methylation? J Allergy Clin Immunol. 2010;125:1361–8. doi: 10.1016/j.jaci.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Thomsen SF, Duffy DL, Ohm Kyvik K, Backer V. Genetic influence on the age at onset of asthma: a twin study. J Allergy Clin Immunol. 2010;126:626–30. doi: 10.1016/j.jaci.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Mathias RA, Grant AV, Rafaels N, Hand T, Gao L, Vergara C, et al. A genome-wide association study on African-ancestry populations for asthma. J Allergy Clin Immunol. 2010;125:336–46. doi: 10.1016/j.jaci.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu H, Romieu I, Shi M, Hancock DB, Huiling L, Sienra-Monge JJ, et al. Evaluation of candidate genes in a genome-wide association study of childhood asthma in Mexicans. J Allergy Clin Immunol. 2010;125:321–7. doi: 10.1016/j.jaci.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melen E, Himes BE, Brehm JM, Boutaoui N, Klanderman BJ, Sylvia JS, et al. Analysis of shared genetic factors between asthma and obesity in children. J Allergy Clin Immunol. 2010;126:631–7. doi: 10.1016/j.jaci.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenthal LA, Avila PC, Heymann PW, Martin RJ, Miller EK, Papadopoulos NG, et al. Viral respiratory tract infections and asthma: The course ahead. J Allergy Clin Immunol. 2010;125:1212–7. doi: 10.1016/j.jaci.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sly PD, Kusel M, Holt PG. Do early-life viral infections cause asthma? J Allergy Clin Immunol. 2010;125:1202–5. doi: 10.1016/j.jaci.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Horvat JC, Starkey MR, Kim RY, Phipps S, Gibson PG, Beagley KW, et al. Early-life chlamydial lung infection enhances allergic airways disease through age-dependent differences in immunopathology. J Allergy Clin Immunol. 2010;125:617–25. doi: 10.1016/j.jaci.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Castro-Rodriguez JA. The Asthma Predictive Index: a very useful tool for predicting asthma in young children. J Allergy Clin Immunol. 2010;126:212–6. doi: 10.1016/j.jaci.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Holt PG, Rowe J, Kusel M, Parsons F, Hollams EM, Bosco A, et al. Toward improved prediction of risk for atopy and asthma among preschoolers: A prospective cohort study. J Allergy Clin Immunol. 2010;125:653–9. doi: 10.1016/j.jaci.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Simpson A, Tan VYP, Winn J, Svensen M, Bishop CM, Heckerman DE, et al. Beyond atopy. Multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181:1200–1206. doi: 10.1164/rccm.200907-1101OC. [DOI] [PubMed] [Google Scholar]
- 24.Yao W, Barbe-Tuana FM, Llapur CJ, Jones MH, Tiller C, Kimmel R, et al. Evaluation of airway reactivity and immune characteristics as risk factors for wheezing early in life. J Allergy Clin Immunol. 2010;126:483–8. doi: 10.1016/j.jaci.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chawes BLK, Bennelykke K, Kreiner-Moller E, Bisgaard H. Children with allergic and nonallergic rhinitis have a similar risk of asthma. J Allergy Clin Immunol. 2010;126:567–73. doi: 10.1016/j.jaci.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Rochat MK, Illi S, Ege MJ, Lau S, Keil T, Wahn U, et al. Allergic rhinitis as predictor for school age wheezing. J Allergy Clin Immunol. 2010 December;126:■■■–■■■. doi: 10.1016/j.jaci.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Kull I, Melen E, Alm J, Hallberg J, Sartengren M, van Hage M, et al. Breast-feeding in relation to asthma, lung function and sensitization in young schoolchildren. J Allergy Clin Immunol. 2010;125:1013–9. doi: 10.1016/j.jaci.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, La HCJ, Roberg KA, Gangnon RE, Evans MD, Anderson EL, et al. Early childhood weight status in relation to asthma development in high-risk children. J Allergy Clin Immunol. 2010 December;126:■■■–■■■. doi: 10.1016/j.jaci.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Covar RA, Strunk R, Zeiger RS, Wilson LA, Liu AH, Weiss S, et al. for the Childhood Asthma Management Program Research Group. Predictors of remitting, periodic, and persistent childhood Asthma. J Allergy Clin Immunol. 2010;125:359–66. doi: 10.1016/j.jaci.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toelle BG, Ng KKW, Crisafulli D, Belousova EG, Almqvist C, Webb K, et al. Eight-year outcomes of the Childhood Asthma Prevention Study. J Allergy Clin Immunol. 2010;126:388–9. doi: 10.1016/j.jaci.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 31.Vink NM, Postma DS, Schouten JP, Rosmalen JGM, Boezen HM. Gender differences in asthma development and remission during transition through puberty: the Tracking Adolescents’ Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol. 2010;126:498–504. doi: 10.1016/j.jaci.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Guilbert T. Identifying and managing the infant and toddler at risk for asthma. J Allergy Clin Immunol. 2010;126:417–22. doi: 10.1016/j.jaci.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 33.Brasholt M, Baty F, Bisgaard H. Physical activity in young children is reduced with increasing bronchial responsiveness. J Allergy Clin Immunol. 2010;125:1007–12. doi: 10.1016/j.jaci.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Tapiainen T, Dunder T, Mottonen M, Pokka T, Uhari M. Adolescents with asthma or atopic eczema have more febrile days in early childhood: A possible explanation for the connection between paracetamol and asthma? J Allergy Clin Immunol. 2010;125:751–2. doi: 10.1016/j.jaci.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 35.Schnabel E, Heinrich J and the LISA Study Group. Respiratory tract infections and not paracetamol medication during infancy are associated with asthma development in childhood. J Allergy Clin Immunol. 2010 December;126:■■■–■■■. doi: 10.1016/j.jaci.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 36.Shaheen SO, Newson RB, Ring SM, Rose-Zerilli MJ, Holloway JW, Henderson AJ. Prenatal and infant acetaminophen exposure, antioxidant gene polymorphisms and childhood asthma. J Allergy Clin Immunol. 2010 December;126:■■■–■■■. doi: 10.1016/j.jaci.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bottema RWB, Kerkhof M, Reijmerink NE, Thijs C, Smit HA, van Schayck CP, et al. Gene-gene interaction in regulatory T-cell function in atopy and asthma development in childhood. J Allergy Clin Immunol. 2010;126:338–46. doi: 10.1016/j.jaci.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 38.Juhn YJ, Qin R, Urm S, Katusic S, Vargas-Chanes D. The influence of neighborhood environment on the incidence of childhood asthma: a propensity score approach. J Allergy Clin Immunol. 2010;125:838–43. doi: 10.1016/j.jaci.2009.12.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen RT, Raby BA, VanSteen K, Fuhlbrigge AL, Celedon JC, Rosner BA, et al. In utero smoke exposure and impaired response to inhaled corticosteroids in children with asthma. J Allergy Clin Immunol. 2010;126:491–7. doi: 10.1016/j.jaci.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363:301–04. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 41.Szefler SJ, Martin RJ. Lessons learned from variation in response to therapy in clinical trials. J Allergy Clin Immunol. 2010;125:285–92. doi: 10.1016/j.jaci.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kazani S, Wechsler ME, Israel E. The role of pharmacogenomics in improving the management of asthma. J Allergy Clin Immunol. 2010;125:295–302. doi: 10.1016/j.jaci.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonnappa S, Bastardo CM, Wade A, Saglani S, McKenzie SA, Bush A, et al. Symptom-pattern phenotype and pulmonary function in preschool wheezers. J Allergy Clin Immunol. 2010;126:519–26. doi: 10.1016/j.jaci.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Marcos L, Martinez LD. Multitrigger versus episodic wheeze in toddlers: new phenotypes or severity markers? J Allergy Clin Immunol. 2010;126:489–90. doi: 10.1016/j.jaci.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 45.Gould W, Peterson EL, Karungi G, Zoratti A, Gaggin J, Toma G, et al. Factors predicting inhaled corticosteroid responsiveness in African American patients with asthma. J Allergy Clin Immunol. 2010 December;126:■■■–■■■. doi: 10.1016/j.jaci.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Debley JS, Stamey DC, Cochrane ES, Gama KL, Redding GJ. Exhaled nitric oxide, lung function and exacerbations in wheezy infants and toddlers. J Allergy Clin Immunol. 2010;125:1228–34. doi: 10.1016/j.jaci.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teague WG. Exhaled nitric oxide in wheezy infants: a marker of inflammation determined by airways acidification and S-nitrosothiol degradation? J Allergy Clin Immunol. 2010;125:1235–6. doi: 10.1016/j.jaci.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chawes BLK, Buchvald F, Bischoff AL, Loland L, Hermansen M, Halkjaer LB, et al. Elevated exhaled nitric oxide in high-risk neonates precedes transient early but not persistent wheeze. Am J Respir Crit Care Med. 2010;182:138–42. doi: 10.1164/rccm.200909-1377OC. [DOI] [PubMed] [Google Scholar]
- 49.Dweik RA, Sorkness RL, Wenzel S, Hammel J, Curran-Everett D, Comhair SAA, et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med. 2010;181:1033–1041. doi: 10.1164/rccm.200905-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nair P, Kjarsgaard M, Armstrong S, Efthimiadis A, O’Byrne PM, Hargreave FE. Nitric oxide in exhaled breath is poorly correlated to sputum eosinophils in patients with prednisone-dependent asthma. J Allergy Clin Immunol. 2010;126:404–5. doi: 10.1016/j.jaci.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 51.Van Muylem A, Kerckx Y, Michils A. Acinar effect of inhaled steroids evidenced by exhaled nitric oxide. J Allergy Clin Immunol. 2010;126:730–5. doi: 10.1016/j.jaci.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 52.Rabinovitch N, Graber NJ, Chinchilli VM, Sorkness CA, Zeiger RS, Strunk RC, et al. Urinary leukotriene E4/exhaled nitric oxide ratio and montelukast response in childhood asthma. J Allergy Clin Immunol. 2010;126:545–51. doi: 10.1016/j.jaci.2010.07.008. [erratum: J Allergy Clin Immunol 2010;126:959–60.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van de Kant KDG, Klaasen EMM, Jobsis Q, Koers K, Rijkers GT, van der Grinten CP, et al. Wheezing in preschool children is associated with increased levels of cytokines/chemokines in exhaled breath condensate. J Allergy Clin Immunol. 2010;126:669–71. doi: 10.1016/j.jaci.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 54.Sachs-Olsen C, Sanak M, Lang AM, Gielicz A, Mowinckel P, Lodrup Carlsen KC, et al. Eoxins: a new inflammatory pathway in childhood asthma. J Allergy Clin Immunol. 2010;126:859–67. doi: 10.1016/j.jaci.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 55.Meyers DA. Genetics of asthma and allergy: what have we learned? J Allergy Clin Immunol. 2010;126:439–46. doi: 10.1016/j.jaci.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baye TM, Martin LJ, Khurana Hershey GK. Application of genetic/genomic approaches to allergic disorders. J Allergy Clin Immunol. 2010;126:425–36. doi: 10.1016/j.jaci.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho SM. Environmental epigenetics of asthma: an update. J Allergy Clin Immunol. 2010;126:453–65. doi: 10.1016/j.jaci.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin Y, Hu D, Peterson EL, Eng C, Levin AM, Wells K, et al. Dual-specificity phosphatase 1 as a pharmacogenetic modifier of inhaled steroid response among asthmatic patients. J Allergy Clin Immunol. 2010;126:618–25. doi: 10.1016/j.jaci.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tcheurekdjian H, Via M, De Giacomo A, Corvol H, Eng C, Thyne S, et al. on behalf of the Genetics of Asthma in Latino Americans Study. ALOX5AP and LTA4H polymorphisms modify augmentation of bronchodilator responsiveness by leukotriene modifiers in Latinos. J Allergy Clin Immunol. 2010;126:853–8. doi: 10.1016/j.jaci.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bush A, Saglani S. Management of severe asthma in children. Lancet. 2010;376:814–25. doi: 10.1016/S0140-6736(10)61054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fitzpatrick AM, Teague WG. Severe asthma in children: insights from the National Heart, Lung and Blood Institute’s Severe Asthma Research Program. Pediatric Allergy, Immunology and Pulmonology. 2010;23:131–38. doi: 10.1089/ped.2010.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fitzpatrick AM, Higgins M, Holguin F, Brown LAS, Teague WG for the National Institutes of Health/National Heart, Lung and Blood Institute’s Severe Asthma Research Program. The molecular phenotype of severe asthma in children. J Allergy Clin Immunol. 2010;125:851–7. doi: 10.1016/j.jaci.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gamble C, Talbott E, Youk A, Holguin F, Pitt B, Silveira L, et al. Racial differences in biologic predictors of severe asthma: Data from the Severe Asthma Research Program. J Allergy Clin Immunol. 2010 December;126:■■■–■■■. doi: 10.1016/j.jaci.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bousquet J, Mantzouranis E, Cruz AA, Ait-Khaled N, Baena-Cagnani CE, Bleecker ER, et al. Uniform definition of asthma severity, control and exacerbations. J Allergy Clin Immunol. 2010 November;126:■■■–■■■. doi: 10.1016/j.jaci.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 65.Szefler SJ. Defining asthma phenotypes: Focusing the picture. J Allergy Clin Immunol. 2010 November;126:■■■–■■■. doi: 10.1016/j.jaci.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 66.Amirav I, Newhouse MT, Minocchieri S, Castro-Rodiguez JA, Schuepp KG. Factors that affect the efficacy of inhaled corticosteroids for infants and young children. J Allergy Clin Immunol. 2010;125:1206–11. doi: 10.1016/j.jaci.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 67.Raissy HH, Sternberg AL, Williams P, Jacobs A, Kelly HW for the CAMP Research Group. Risk of cataracts in the Childhood Asthma Management Program Cohort. J Allergy Clin Immunol. 2010;126:389–92. doi: 10.1016/j.jaci.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lemanske R, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, et al. for the Childhood Asthma Research and Education Network of the National Heart, Lung and Blood Institute. Step-up therapy for children with uncontrolled asthma while receiving inhaled corticosteroids. N Engl J Med. 2010;362:975–985. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jackson DJ, Johnston SL. The role of viruses in acute exacerbations of asthma. J Allergy Clin Immunol. 2010;125:1178–87. doi: 10.1016/j.jaci.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holt PG, Strickland DH. Interactions between innate and adaptive immunity in asthma pathogenesis: new perspectives from studies on acute exacerbations. J Allergy Clin Immunol. 2010;125:963–72. doi: 10.1016/j.jaci.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 71.Palmenberg AC, Rathe JA, Liggett SB. Analysis of the complete genome sequences of human rhinovirus. J Allergy Clin Immunol. 2010;125:1190–9. doi: 10.1016/j.jaci.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olenec JP, Kyung Kim W, Lee WM, Vang F, Pappas TE, Salazar LEP, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125:1001–6. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simoes EAF, Carbonell-Estrany XC, Rieger CHL, Mitchell I, Fredrick L, Groothuis JR on behalf of the Palivizumuab Long-Term Respiratory Outcomes Study Group. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J Allergy Clin Immunol. 2010;126:256–62. doi: 10.1016/j.jaci.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leo HL, Clark SJ, Butchart AT, Singer DC, Clark NM, Davis MM. 2009 Seasonal and H1N1 influenza vaccination compliance in asthmatic children and adults. J Allergy Clin Immunol. 2010;126:166–8. doi: 10.1016/j.jaci.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 75.Silverman RA, Ito K. Age-related association of fine particles and ozone withsevere acute asthma in New York City. J Allergy Clin Immunol. 2010;125:367–73. doi: 10.1016/j.jaci.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 76.Strickland MJ, Darrow LA, Klein M, Flanders WD, Sarnat JA, Waller LA, et al. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med. 2010;182:307–16. doi: 10.1164/rccm.200908-1201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roy SR, McGinty EE, Carr Hayes S, Zhang L. Regional and racial disparities in asthma hospitalizations in Mississippi. J Allergy Clin Immunol. 2010;125:636–42. doi: 10.1016/j.jaci.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 78.Bateman ED, Reddel HK, Eriksson G, Peterson S, Ostlund O, Sears MR, et al. Overall asthma control: The relationship between current control and future risk. J Allergy Clin Immunol. 2010;125:600–8. doi: 10.1016/j.jaci.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 79.Chapman KR. SMART isn’t. J Allergy Clin Immunol. 2010;125:609–10. doi: 10.1016/j.jaci.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 80.Nelson H, Bonuccelli C, Radner F, Ottosson A, Carroll KJ, Andersson TLG, et al. Safety of formoterol in patients with asthma: Combined analysis of data from double-blind, randomized controlled trials. J Allergy Clin Immunol. 2010;125:390–6. doi: 10.1016/j.jaci.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 81.Togias A, Fenton MJ, Gergen PJ, Rotrosen D, Fauci AS. Asthma in the inner city: the perspective of the National Institute of Allergy and Infectious Diseases. J Allergy Clin Immunol. 2010;125:540–4. doi: 10.1016/j.jaci.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 82.Busse WW. The National Institutes of Allergy and Infectious Diseases networks on asthma in inner-city children: An approach to improved care. J Allergy Clin Immunol. 2010;125:529–37. doi: 10.1016/j.jaci.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szefler SJ, Gergen PJ, Mitchell H, Morgan W. Achieving asthma control in the inner city: do the National Institutes of Health Asthma Guidelines really work? J Allergy Clin Immunol. 2010;125:521–6. doi: 10.1016/j.jaci.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 84.Gern JE. The Urban Environment and Childhood Asthma Study. J Allergy Clin Immunol. 2010;125:545–9. doi: 10.1016/j.jaci.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pongracic JA, O’Connor GT, Mullenberg ML, Vaughn B, Gold DR, Kattan M, et al. Differential effects of outdoor versus indoor fungal spores on asthma morbidity in inner-city children. J Allergy Clin Immunol. 2010;125:593–9. doi: 10.1016/j.jaci.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125:584–92. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sheehan WJ, Rangsithienchai PA, Wood RA, Rivard D, Chinratanapisit S, Perzanowski MS, et al. Pest and allergen exposure and abatement in inner-city asthma: A work group report of the American Academy of Allergy, Asthma & Immunology indoor allergy/air pollution committee. J Allergy Clin Immunol. 2010;125:575–81. doi: 10.1016/j.jaci.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Calvert J, Burney P. Ascaris, atopy, and exercise-induced bronchoconstriction in rural and urban South African children. J Allergy Clin Immunol. 2010;125:100–5. doi: 10.1016/j.jaci.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Platts-Mills TAE, Cooper PJ. Differences in asthma between rural and urban communities in South Africa and other developing countries. J Allergy Clin Immunol. 2010;125:106–7. doi: 10.1016/j.jaci.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 90.Gerald JK, Grad R, Bailey WC, Gerald LB. Cost-effectiveness of school-based asthma screening in an urban setting. J Allergy Clin Immunol. 2010;125:643–50. doi: 10.1016/j.jaci.2009.12.984. [DOI] [PubMed] [Google Scholar]
- 91.McElligott S, Polsky D. The opportunity costs of screening children for asthma. J Allergy Clin Immunol. 2010;125:651–2. doi: 10.1016/j.jaci.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 92.FitzGerald JM, Shahidi N. Achieving asthma control in patients with moderate disease. J Allergy Clin Immunol. 2010;125:307–11. doi: 10.1016/j.jaci.2009.12.978. [DOI] [PubMed] [Google Scholar]
- 93.Williams LK, Peterson EL, Wells K, Campbell J, Wang M, Chowdhry VK, et al. A cluster-randomized trial to provide clinicians inhaled corticosteroid adherence information for their patients with asthma. J Allergy Clin Immunol. 2010;126:225–31. doi: 10.1016/j.jaci.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu AH, Zeiger RS, Sorkness CA, Ostrom NK, Chipps BE, Rosa K, et al. The Childhood Asthma Control Test: retrospective determination and clinical validation of a cut point to identify children with very poorly controlled asthma. J Allergy Clin Immunol. 2010;126:267–73. doi: 10.1016/j.jaci.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 95.Simon MR, Chinchilli VM, Phillips BR, Sorkness CA, Lemanske RF, Szefler SJ, et al. Forced expiratory flow between 25% and 75% of vital capacity and FEV1/forced vital capacity ratio in relation to clinical and physiological parameters in asthmatic children with normal FEV1 values. J Allergy Clin Immunol. 2010;126:527–34. doi: 10.1016/j.jaci.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McFadden ER. Resurrection men and the FEF25–75. J Allergy Clin Immunol. 2010;126:535–6. doi: 10.1016/j.jaci.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 97.Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 Revision. J Allergy Clin Immunol. 2010;126:466–76. doi: 10.1016/j.jaci.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 98.Andregnette-Roscigno V, Fernandez-Nieto M, Garcia del Potro M, Aguado E, Sastre J. Methacholine is more sensitive than mannitol for evaluation of bronchial hyperresponsiveness in children with asthma. J Allergy Clin Immunol. 2010;126:869–71. doi: 10.1016/j.jaci.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 99.Sverrild A, Prosbjerg C, Thomsen SF, Backer V. Airway hyperresponsiveness to mannitol and methacholine and exhaled nitric oxide. A random sample population study. J Allergy Clin Immunol. 2010 November;126:■■■–■■■. doi: 10.1016/j.jaci.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 100.Beebe A, Gelfand EW, Bender B. A randomized trial to test the effectiveness of art therapy for children with asthma. J Allergy Clin Immunol. 2010;126:263–6. doi: 10.1016/j.jaci.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 101.Zielen S, Kardos P, Madonini E. Steroid-sparing effects with allergen-specific immunotheraphy in children with asthma: a randomized controlled trial. J Allergy Clin Immunol. 2010 November;126:■■■–■■■. doi: 10.1016/j.jaci.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 102.Adkinson NF. Who (if anyone) actually gets steroid-sparing effects from aeroallergen immunotherapy? J Allergy Clin Immunol. 2010 November;126:■■■–■■■. doi: 10.1016/j.jaci.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 103.Majak P, Rychlik B, Pulaski L, Blauz A, Agnieszka B, Bobrowska-Korzenlowska M, et al. Montelukast treatment may alter the early efficacy of immunotheraphy in children with asthma. J Allergy Clin Immunol. 2010;125:1220–7. doi: 10.1016/j.jaci.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 104.Han J, Jia Y, Takeda K, Shiraishi Y, Okamoto M, Dakhama A, et al. Montelukast during primary infection prevents airway hyperresponsiveness and inflammation after reinfection with respiratory syncytal virus. Am J Respir Crit Care Med. 2010;182:455–63. doi: 10.1164/rccm.200912-1811OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lemanske RF, Busse WW. The US Food and Drug Administration and long-acting Beta2-agonists: the importance of striking the right balance between risks and benefits of therapy? J Allergy Clin Immunol. 2010;126:449–52. doi: 10.1016/j.jaci.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 106.Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes WT, et al. for the National Heart, Lung and Blood Institute’s Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715–26. doi: 10.1056/NEJMoa1008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jartti T, Ruuskanen O, Mansbach JM, Vuorinen T, Camargo CA. Low serum 25-hydroxyvitamin D levels are associated with increased risk of viral co-infections in wheezing children. J Allergy Clin Immunol. 2010 November;126:■■■–■■■. doi: 10.1016/j.jaci.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. 2010;126:52–8. doi: 10.1016/j.jaci.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DYM. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125:995–1000. doi: 10.1016/j.jaci.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ginde AA, Sutherland ER. Vitamin D in asthma: panacea or true promise? J Allergy Clin Immunol. 2010;126:59–60. doi: 10.1016/j.jaci.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 111.Covar R, Gleason M, Macomber B, Stewart L, Szefler P, Engelhardt K, et al. Impact of a novel nutritional formula on asthma control and biomarkers of allergic airway inflammation in Children. Clin Experimental Allergy. 2010;40:1163–1174. doi: 10.1111/j.1365-2222.2010.03523.x. [DOI] [PubMed] [Google Scholar]
- 112.Razi CH, Harmanci K, Abaci A, Ozdemir O, Hizli S, Renda R, et al. The immunostimulant OM-85 BV prevents wheezing attacks in preschool children. J Allergy Clin Immunol. 2010;126:763–9. doi: 10.1016/j.jaci.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 113.Weinberger M. Can we prevent exacerbations of asthma caused by common cold viruses? J Allergy Clin Immunol. 2010;126:770–1. doi: 10.1016/j.jaci.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 114.Mukhopadhyay S, Sypek J, Tavendale R, Gartner U, Winter J, Li W, et al. Matrix metalloproteinase-12 is a therapeutic target for asthma in children and young adults. J Allergy Clin Immunol. 2010;126:70–6. doi: 10.1016/j.jaci.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 115.Kogan MD, Newacheck PW, Blumberg SJ, Ghandour RM, Singh GP, Strickland BB, et al. Underinsurance among children in the United States. N Engl J Med. 2010;363:841–51. doi: 10.1056/NEJMsa0909994. [DOI] [PubMed] [Google Scholar]
- 116.Perrin JM. Treating underinsurance. N Engl J Med. 2010;363:881–83. doi: 10.1056/NEJMe1007674. [DOI] [PubMed] [Google Scholar]
- 117.Deyton L, Sharfstein J, Hamburg M. Tobacco product regulation – a public health approach. N Engl J Med. 2010;36s:1753–56. doi: 10.1056/NEJMp1004152. [DOI] [PubMed] [Google Scholar]
- 118.Blumenthal D, Tavenner M. The meaningful use regulation for electronic health records. N Engl J Med. 2010;363:501–04. doi: 10.1056/NEJMp1006114. [DOI] [PubMed] [Google Scholar]
- 119.Koh HK. A 2020 vision for healthy people. N Engl J Med. 2010;363:1653–56. doi: 10.1056/NEJMp1001601. [DOI] [PubMed] [Google Scholar]