Abstract
Phenotype-driven screens in larval zebrafish have transformed our understanding of the molecular basis of cardiovascular development. Screens to define the genetic determinants of physiological phenotypes have been slow to materialize as a result of the limited number of validated in vivo assays with relevant dynamic range. To enable rigorous assessment of cardiovascular physiology in living zebrafish embryos, we developed a suite of software tools for the analysis of high-speed video microscopic images and validated these, using established cardiomyopathy models in zebrafish as well as modulation of the nitric oxide (NO) pathway. Quantitative analysis in wild-type fish exposed to NO or in a zebrafish model of dilated cardiomyopathy demonstrated that these tools detect significant differences in ventricular chamber size, ventricular performance, and aortic flow velocity in zebrafish embryos across a large dynamic range. These methods also were able to establish the effects of the classic pharmacological agents isoproterenol, ouabain, and verapamil on cardiovascular physiology in zebrafish embryos. Sequence conservation between zebrafish and mammals of key amino acids in the pharmacological targets of these agents correlated with the functional orthology of the physiological response. These data provide evidence that the quantitative evaluation of subtle physiological differences in zebrafish can be accomplished at a resolution and with a dynamic range comparable to those achieved in mammals and provides a mechanism for genetic and small-molecule dissection of functional pathways in this model organism.
Keywords: heart, inotropy
phenotype-driven developmental screens, largely focused on organogenesis, have established the zebrafish (Danio rerio) as a powerful system to dissect evolutionarily conserved pathways and explore the unique attributes of vertebrate embryogenesis. One of the most important advancements in the evolution of vertebrates is the transformation of the simple peristaltic heart found in invertebrate phyla. Early zebrafish genetic screens revealed that the key pathways and the basic structural and functional modules required to fashion the heart are shared among vertebrates (33). The emergence of distinct atrial and ventricular chambers with sequential contraction, an effective atrioventricular return valve, and the rapid coordinated ventricular contraction required to generate higher pressure are all key innovations (11). In larval zebrafish, a fully functioning cardiovascular system with all these attributes develops by 48 hours post fertilization (hpf). While the original screens were not designed to identify functional phenotypes, they nevertheless detected some mutants with obvious and discrete functional abnormalities such as a slow heartbeat (slo mo) (4) or isolated contraction defects (silent heart and weak atrium) (6, 30). These mutants strongly suggest that integrated physiology also might be tractable through screening strategies, if the appropriate phenotyping technologies were available.
Several physiological assays have been employed successfully in the larval fish to study cardiovascular function, for example, electrocardiography (25) and invasive hemodynamics (37) using pulled glass micropuncture needles and servo-null systems. Doppler techniques have been used to measure intracardiac flow (15). Several techniques to which the zebrafish is particularly amenable have also been employed, including laser confocal scanning microscopy, which has shed light on the sophisticated interaction between form and function in the developing heart (16, 17). These tools require special instrumentation and are not scalable for high-throughput screening.
Quantitative phenotyping of large numbers of embryos must be feasible with methodologies capable of resolving the subtle physiological differences in cardiovascular performance seen in varied disease states. The larval zebrafish at 72 hpf is ∼1 mm in length, and as such many embryos can live for several days in a single well of a 96- or even 384-well plate. Capitalizing on the optical lucency of the embryo, image processing techniques such as digital subtraction analysis have been used to measure circulatory flow and cardiac function and defined normal values through development (3, 12, 29). Similarly, simple determination of fractional shortening (FS) has been used to characterize cardiovascular defects seen in mutant fish with grossly abnormal hearts and circulation. However, the ability of methods based on the relatively low frame rate of standard video [∼30 frames per second (fps)] to discriminate subtle changes across a broad physiological range is unproved.
Several studies have found remarkable conservation of drug responses between human and zebrafish, including a broad range of compounds with effects on cardiac electrophysiology (26). Genome sequencing has enabled identification and direct comparison of orthologous genes shared between zebrafish and humans, and in many cases a high degree of sequence conservation may underlie the shared biological response to pharmacological agents. Because of a genome duplication event at the teleost radiation more than 400 million years ago (35), in silico approaches frequently identify two or more paralogous genes for a single mammalian counterpart (31). As a result, biological experimentation is required to assess the function of each gene and the consequences of genetic or small-molecule perturbations that target specific genes or pathways. The most common cardiovascular drug targets have been identified in zebrafish [β-adrenergic receptors, calcium channels, nitric oxide (NO), and Na+-K+-ATPase], and roles for these genes have been identified during early cardiac development (28, 32, 38). However, the absence of validated tools for in vivo measurement of subtle perturbations in myocardial contractility has limited the ability to explore the functional contractile orthologies that exist between humans and the zebrafish.
To enable phenotype-driven screens of myocardial function and contractility, we have developed a set of analytic tools to quantitate cardiovascular physiology, applying established algorithms to digital images obtained with readily available technology. We have validated these methods, demonstrating their ability to detect the subtle functional effects of genetic or pharmacological perturbation of conserved biological targets. Adaptation of these assays makes accessible high-throughput screens of basic cardiovascular physiology using simple light microscopy, enabling the study of small-molecule responses in the intact zebrafish.
MATERIALS AND METHODS
Aquaculture.
Experiments were performed on zebrafish (D. rerio) from wild-type (WT) or mutant breeding colonies in accordance with Institutional Animal Care and Use Committee (IACUC)-approved protocols. Care and breeding of zebrafish were performed as described previously (34) at 28.5°C with standard media (E3). Zebrafish embryos were maintained in the absence of methylene blue. All measurements were made in larvae at 72 hpf. The valentine (vtn) mutants have been described previously (22).
Pharmacological manipulations.
To assess the effects of NO on the physiology of developing zebrafish embryos, the NO donor S-nitroso-l-glutathione (GSNO; Alexis, San Diego, CA) was dissolved in E3 to a final concentration of 1 mM from concentrated stock solutions. Embryos were pipetted into this solution in a minimal volume, swirled briefly, and then imaged. As a control for physiological effects for the degradation products of GSNO on zebrafish embryos, GSNO was allowed to decompose for 24 h before use. To block NO-induced signal transduction, 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ; Sigma), a specific inhibitor of soluble guanylate cyclase (sGC), was dissolved in egg water to a final concentration of 5 μmol/l stock solution in dimethyl sulfoxide (DMSO). Zebrafish embryos at 60 hpf were added for incubation and imaged subsequently as described below.
For other drug exposures, final concentrations were achieved by preparing concentrated stock solutions of isoproterenol (Iso), ouabain (Oua), verapamil (Ver), epinephrine (Epi), and dobutamine (Dob) (Sigma) in DMSO and adding stock solutions to E3 to achieve final working concentrations (Iso, 1.9 μg/ml; Oua, 16 μg/ml; Ver, 1 μg/ml; Epi, 5 μg/ml; Dob, 5 μg/ml) with a DMSO concentration of <1 %. Stock solutions of drugs were added to E3 12 h before measurements.
Image acquisition.
Individual embryos were checked for lateral positioning and allowed to acclimate to microscope illumination for ∼1 min. At this stage in development, the embryos are still transparent, with excellent visibility of internal structures including the heart and circulation. Video microscopy was performed on an Axioplan (Zeiss) upright microscope with either the ×5 or the ×10 objective lens, with integrated incandescent illumination. A FastCam-PCI high-speed digital camera (Photron USA) with a 512 × 480 pixel gray scale image sensor was mounted on the microscope. The data acquisition system consisted of the FastCam-PCI image capture board mounted in a personal computer (Optiplex 260, Dell Computers). Sequential images of the heart and dorsal aorta were obtained with the embryo positioned on its side from the lateral position (Fig. 1A) at 250 fps with a shutter speed of 0.002 or 0.004 s; 1,088 frames (4.35 s encompassing ∼8 cardiac cycles) were acquired of the heart and dorsal aorta for multiple fish per condition. To ensure that a comparable plane of focus was examined for ventricular analysis, zebrafish hearts were imaged in a standardized lateral position, with the ventricle clearly visible in the plane of focus throughout the cardiac cycle (Fig. 1, B and C). The atrium usually lay outside the plane of focus.
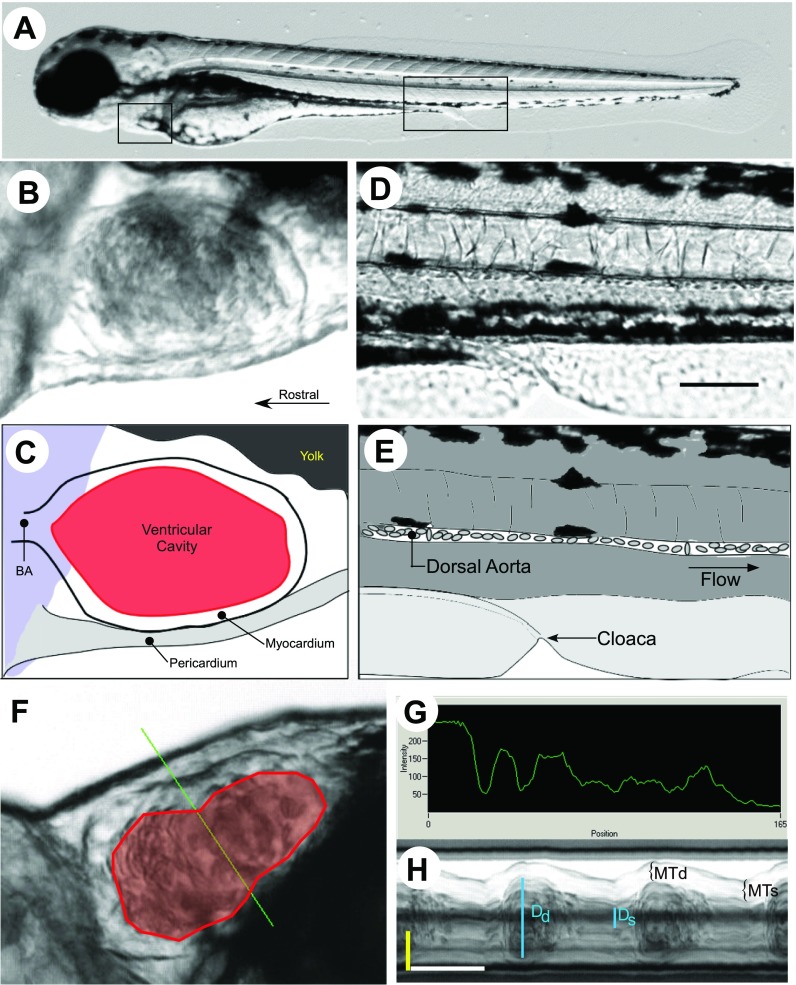
Fig. 1.
Measurement of cardiovascular function in zebrafish. A: developing zebrafish embryo at 3 days post fertilization (dpf). The zebrafish is a vertebrate model organism that is transparent during early development. The zebrafish heart and circulation are easily visible with light microscopy. The heart consists of a single atrium and ventricle that lie anteriorly on the ventral surface of the fish (A, left box) To identify a reproducible location for analysis of circulation, the aorta adjacent to the cloaca (A, right box) was chosen as a defined imaging landmark. The heart and area adjacent to the cloaca are shown in higher magnification in B and D. A brightfield image (B) and illustration (C) show the important anatomy of the zebrafish ventricle. The ventricle is oriented so that it empties rostrally with each contraction through the bulbus arteriosus (BA). At the ventral side of the fish the pericardium lies adjacent to the myocardium on the outer curvature of the heart, with the yolk lying adjacent to the inner curvature of the ventricle. The ventricular cavity (red, C) is readily identified by the blood within it. The cloaca identified a consistent landmark for microscopy to examine flow in the dorsal aorta (D and E, oriented with the rostrum to the left). Individual erythrocytes could be visualized as they transited the artery. Image analysis tools allowed the delineation of the endomyocardial border (red polygon, F) either at end systole or diastole to define the ventricular area or to place manually a scan line across the midventricular short axis (green line). The image intensity across the pixels of the scan line was then measured (G), stored in an array (materials and methods), and converted into an M-mode image (H). Yellow bar, 50 μm; white bar, 250 ms; blue bars, short-axis end-diastolic (Dd) and end-systolic (Ds) dimensions. Myocardial thickness at end diastole (MTd) and end systole (MTs) were also measured (brackets). Bar in D = 100 μm.
For imaging of tail circulation, the cloaca was chosen as a uniform anatomic landmark for dorsal aortic images (Fig. 1, A, D, and E). Acquired movies were stored as serial still images on fixed optical media for subsequent off-line analysis. Image calibration was performed by using an internal standard photographed at each magnification.
Quantitative image analysis.
To quantitate physiological parameters of cardiovascular performance in the zebrafish, we authored a set of image analysis applications, using the Measurement Studio and IMAQ Vision software packages (National Instruments) with Visual Studio 6.0 (Microsoft). The application is available for academic use and can be obtained by e-mail request to the authors.
For two-dimensional analysis of ventricular performance, sequential still frames were analyzed to identify frames capturing ventricular end systole (ES, defined as the frame after the last forward flow from the outflow tract) and end diastole (ED, the frame after the last flow into the ventricle from the atrium). The endocardial boundary was traced, and the area of the region defined by this trace was recorded (Fig. 1, C and F). At a minimum, five sequential cardiac cycles (pairs of systolic and diastolic frames) were recorded. To compare contractile function in embryonic zebrafish, fractional area change (FAC), an established parameter of ventricular performance (13), was calculated [FAC = 100 × (EDA − ESA)/EDA, where EDA and ESA are ED and ES areas].
Linear motion analysis.
To graphically depict continuous changes in myocardial wall position and to quantitate wall position throughout the cardiac cycle, a separate application was authored that permitted a linear region of interest to be drawn on either the short axis (SAx) or the long axis (LAx) of the heart (Fig. 1F). The pixel intensity of each point along this line (lp) could be measured (Fig. 1G) and those values recorded in a two-dimensional array (I). In this array, the first coordinate (t) represents the ordinal image number in the series of high-speed images and the second coordinate (m) represents the position of the pixel along the fixed line (eq. 2). Thus any particular value lt,m in the array represents the intensity (an integer value between 0 and 255) in frame t, at the m position along the inscribed line.
| (1) |
The array generated by serial analysis of images acquired from an individual heart is converted into a gray scale image (Fig. 1H). In images generated in this fashion, the x-axis corresponds to elapsed time and the y-axis to position along the ventricular axis of the developing zebrafish heart, analogous to M-mode visualization of chamber dimensions in echocardiography. With the acquisition frame rate and the calibration data above, the images are readily calibrated to time (s) and distance (μm). Measurements were made on exported gray scale images with ImageJ image analysis software (NIH). Short-axis measurements of ED diameter (Dd) and ES diameter (DS) were defined as the largest and narrowest diameters during the cardiac cycle and were made from endocardial margin to endocardial margin as defined by the blood/tissue interface (Fig. 1H, long and short blue lines, respectively). Additionally, myocardial thickness was also measured at ED (MTd) and ES (MTs) on both the greater and lesser curvature of the heart. Fractional shortening (FS) was calculated from the formula FS = (Dd − Ds)/Dd.
Calculation of ventricular volume.
For measuring ventricular volume, we assumed that the ventricle is a prolate spheroid and employed the following standard formula to calculate the volume:
| (2) |
Where l is the long-axis and s the short-axis radius.
Quantitative flow velocity.
For analysis of flow velocity (FV) in the dorsal aorta, a third application was authored that allows the user to track the absolute coordinates of individual blood cells in sequential still images by highlighting individual erythrocytes as they move between frames (Fig. 1, D and E). To ensure that an entire cardiac cycle was analyzed in each case, analysis began at least 10 frames before the increase in flow velocity associated with the onset of systole and continued at least 10 frames into the second systolic acceleration.
In addition to tracking the coordinates of the blood cell in each recorded image, the program also calculates the displacement (di) between frames with the formula:
| (3) |
where (xi, yi) and (xi−1, yi−1) are the coordinates of the blood cell in sequential frames. The instantaneous flow velocity (fvi) of an erythrocyte in frame i is then estimated from the formula
| (4) |
where Δt is the interval between frames, which for our studies was 4 × 10−3 s. The relevant calibration factor, obtained from the internal standard, is used to convert between pixels and length. A smoothing algorithm was used in which the normalized FV was the arithmetic average of the two nearest values in each direction. The peak flow velocity for each averaged curve was recorded, and statistics were performed on the peak FV. For graphical representation, FV curves were normalized to the onset of systole, which was defined as the first time point at which the instantaneous FV increased by >15 % over baseline.
Bioinformatics.
Protein sequences for Na+-K+-ATPase, β1- and β2-adrenergic receptors, and the L-type calcium channel (LTCC) were obtained from the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov). When available, sequence names are given by SWISSPROT primary accession number and entry name. The predicted cDNA translation was used to generate the protein sequences when the protein sequence was not available. Amino acid sequences were aligned with MAFFT (18, 19) and visualized with JalView (9). Residues conserved above an identity threshold of 70 % are highlighted in the multiple sequence alignment. Previously known drug binding sites were identified in the protein data bank annotation (for LTCC) or from literature review [β1-adrenergic receptor (1, 5, 21, 36); Na+-K+-ATPase (10, 20)]. Amino acids identified as important in mediating ligand binding or the pharmacological responses are identified by a gray background and/or notation in the upper margin above the residue(s).
Statistics.
Data are expressed as means ± SE of the measure. Values were compared with Student's t-test or ANOVA with Bonferroni/Dunn post hoc testing for equal sample sizes and Scheffé's for disparate sample sizes (Statview, SAS Institute); an overall value of P ≤ 0.05 was chosen for statistical significance.
RESULTS
Quantitative measurements effectively identify contractile defects in vtn−/− embryos.
To establish that our tools discriminate defects in cardiac structure and contractile function, we first examined the structural and physiological changes associated with one of the cardiac mutants identified in the original developmental genetic screens. Zebrafish embryos homozygous for a point mutation in the ccm2 gene (vtn) develop enlarged and poorly contractile hearts (22) (Fig. 2, A and B), paralleling human dilated cardiomyopathy.
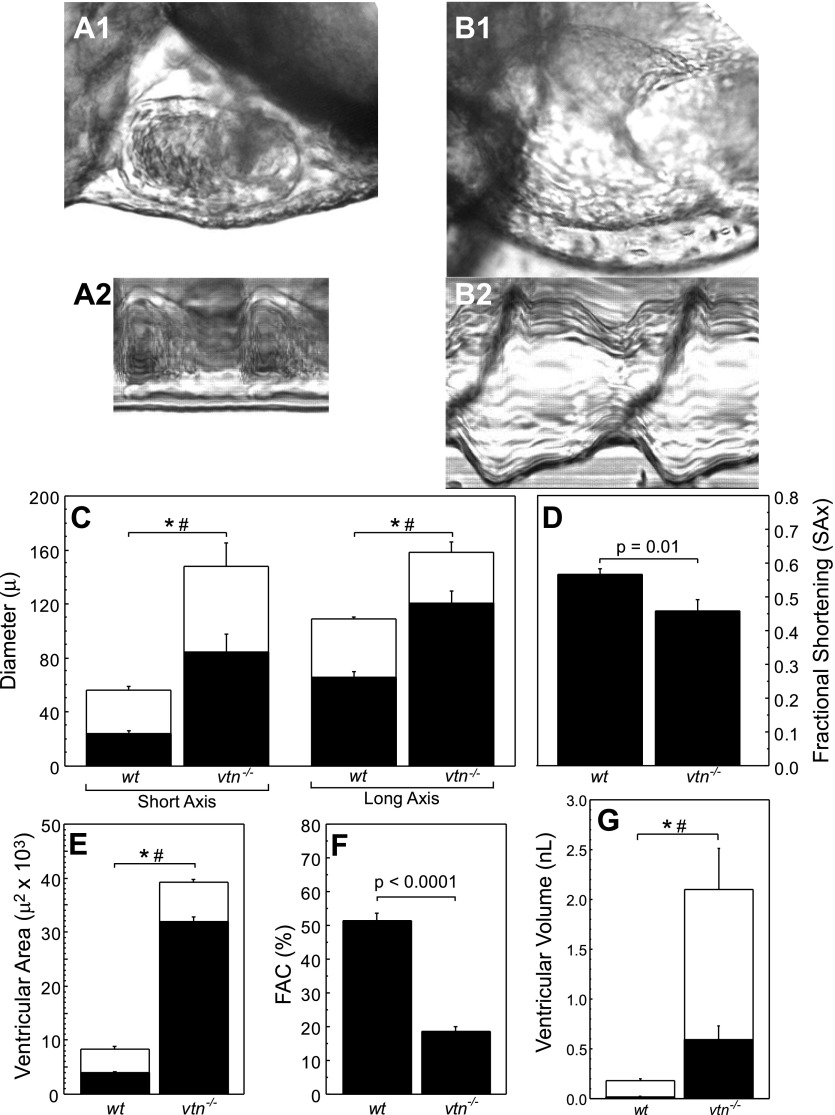
Fig. 2.
Measurement of ventricular size and function in vtn−/− mutants. Brightfield image of zebrafish ventricles from wild-type (wt; A1) and vtn−/− (B1) hearts in lateral projection and M mode of wt (A2) and vtn−/− (B2) ventricles. C: short- and long-axis ventricular diameter in wt and vtn−/− hearts. D: fractional shortening (FS) measured in wt and vtn−/− hearts. SAx, short axis. E–G: ventricular area, ventricular performance index [fractional area change (FAC)], and ventricular volume compared between wt and vtn−/− ventricles. All data are expressed as means ± SE for n = 9 measurements per condition. Differences in which P < 0.05 are denoted in diastole (*) and systole (#) or with the exact P value.
Single-dimension measurements showed that the vtn−/− embryos had larger ventricles compared with WT siblings. For WT zebrafish ventricles, linear motion analysis at 72 hpf measured 56.2 ± 2.6 and 109 ± 1.7 μm (SAx and LAx, respectively) at ED and 24.5 ± 1.7 and 66.3 ± 3.7 μm at ES. In vtn−/− mutants, the ventricular SAx diameter at ED was 2.4-fold larger (148 ± 16.8 μm) and at ES measured 3.5-fold larger (84.9 ± 13.0 μm). Similarly, LAx diameters were 158 ± 7.7 μm at ED and 121 ± 9.0 μm at ES, which were 1.4 and 1.8 times larger than control, respectively (Fig. 2C). In each case, measured LAx and SAx ventricular chamber dimensions in vtn−/− mutants were significantly larger at both ES and ED compared with their WT siblings (P < 0.001 for each comparison).
We also used ventricular diameter measurements to calculate the dimensionless parameter of FS. WT zebrafish embryos exhibited a SAx FS of 0.57 ± 0.02 compared with 0.46 ± 0.03 in vtn−/− mutant embryos (P = 0.01, Fig. 2D). When long-axis FS was calculated, WT embryos had an FS of 0.39 ± 0.03 compared with mutant FS of 0.24 ± 0.02 (P = 0.0003; data not shown).
Two-dimensional measurements of ventricular structure and function in vtn−/− mutant embryos were also determined. The WT ventricular area was 8.36 × 103 ± 4.91 × 102 μm2 at ED and 3.97 × 103 ± 2.49 × 103 μm2 at ES. In vtn−/− embryos, ventricular area increased between 5 and 8 times control, to 3.92 × 104 ± 5.98 × 102 μm2 and 3.20 × 104 ± 8.15 × 102 μm2 at ED and ES, respectively (P < 0.0001 for each compared with WT; Fig. 2E). To compare ventricular emptying and contraction between mutant and WT groups, we calculated the FAC based on the difference between end-systolic and diastolic ventricular areas. WT FAC was 51.4 ± 2.4 % and decreased to 18.5 ± 1.4 % in vtn−/− embryos.
We also estimated the ventricular volume at 72 hpf. Because the zebrafish ventricle at 3 days of development approximates a prolate spheroid in shape (cf. Fig. 1, B and C), we employed the standard formula for volume. The calculated ventricular volume in WT embryos was 0.18 ± 0.02 nl at ED and 0.02 ± 0.003 nl at ES. In vtn−/− mutants, the ED volume increased >10-fold to 2.10 ± 0.41 nl (P = 0.0003 compared with WT) and 0.59 ± 0.14 nl (P = 0.0008) at end diastole and end systole, respectively (Fig. 2G). The calculated stroke volume increased from 0.16 ± 0.01 nl for WT embryos to 1.51 ± 0.28 nl for vtn−/− embryos (P = 0.0002).
We conclude that differences in ventricular size and function between mutant and WT zebrafish embryos are successfully discriminated by 1) direct linear, 2) area, and 3) calculated volume metrics. Because the directly measured (1 and 2 dimensional) parameters of cardiac size made from high-speed video images are sufficient to discriminate changes in ventricular size and performance in living zebrafish embryos with major cardiovascular defects, we subsequently relied on these metrics rather than on volume, a calculated measure. Furthermore, FS and FAC are routinely used in human and mammalian imaging studies and afford the opportunity to translate results across species.
NO exposure causes increased flow velocity and aortic diameter.
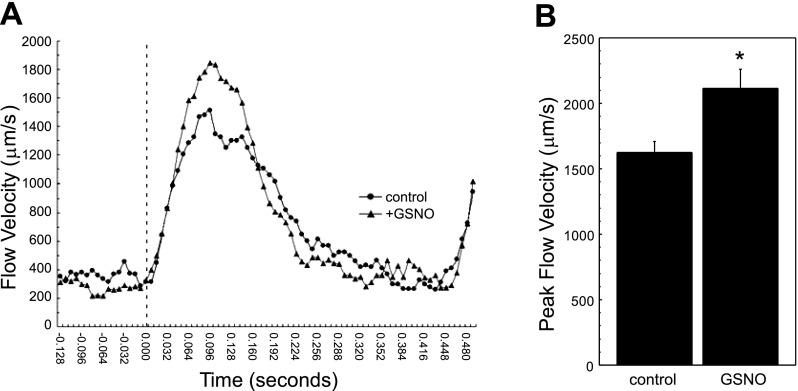
Exposure of larval zebrafish to NO donors has previously been shown to increase cardiac output and the aortic diameter in developing zebrafish embryos (12). To validate the ability of our acquisition and analysis to measure subtle physiological changes in zebrafish cardiovascular function, we exposed larvae to the NO donor GSNO and measured blood FV in high-speed video series in control and GSNO-exposed larvae. Differential displacement calculated from individual cell tracking was used to calculate the instantaneous flow velocity of erythrocytes tracked throughout an entire cardiac cycle (representative curves shown in Fig. 3A). The diastolic FV was similar between groups, but peak systolic FVs increased significantly in GSNO-exposed groups compared with control. Peak FV (Fig. 3B) demonstrates a 28 % increase following NO exposure compared with controls (1,644 ± 82 vs. 2,112 ± 148 μm/s; P = 0.0195). Measurement of aortic dimensions at the level of the cloaca during diastole reveals an 8.3 % increase in aortic diameter following GSNO exposure: the average control diameter measured 16.9 ± 0.12 μm, with an increase to 18.3 ± 0.30 μm (P < 0.001) following GSNO treatment. These data are consistent with previously observed changes in cardiovascular physiology seen after exposure of developing zebrafish embryos to NO and validate the use of our high-speed video microscopic methods for the detection of subtle physiological changes caused by small molecules.
Fig. 3.
Flow velocity (FV) is increased after exposure to a nitric oxide donor. A: representative instantaneous FV profiles for single erythrocytes in the dorsal aorta of control and S-nitroso-l-glutathione (GSNO)-treated zebrafish larvae. Index time 0 (dotted line) denotes the onset of systole. B: average maximum FV in control and GSNO-treated zebrafish larvae. Data are expressed as means ± SE for n = 9 measurements per group. *P = 0.0195.
Measurement of nitric oxide effects on ventricular morphology and physiology.
While no changes in myocardial function were grossly visible, we sought to determine whether alterations in myocardial performance explained the increased flow velocity and cardiac output caused by NO exposure, using high-speed video microscopy to measure ventricular size and function in GSNO-treated embryos.
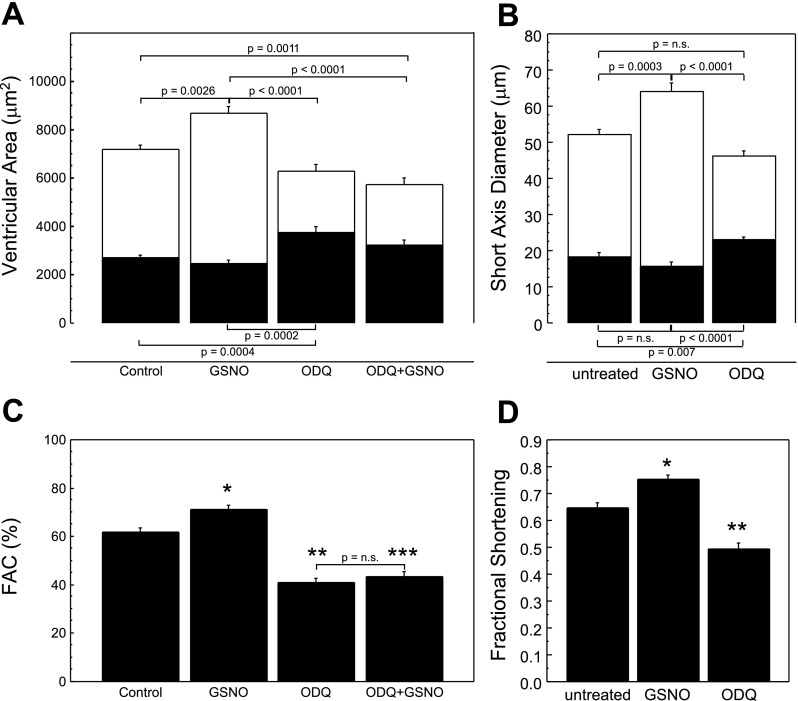
Exposure of zebrafish embryos to NO specifically increases ventricular chamber size at ED without effecting end-systolic measurements. EDA in 72 hpf zebrafish embryos increased from 7,095 ± 191 μm2 to 8,680 ± 289 μm2 (P = 0.0002) after exposure to 1 mM GSNO (Fig. 4A). GSNO exposure did not change ES ventricular area (control: 2,901 ± 113 μm2, GSNO: 2,474 ± 144 μm2; P = 0.16). The changes in ED ventricular size observed after GSNO treatment were specific to NO and did not reflect nonspecific effects of GSNO or its decomposition products. Preincubation of embryos with ODQ, a small-molecule inhibitor of sGC, blocked the increase in EDA caused by NO exposure (ODQ+GSNO: 5,741 ± 275 μm2; P < 0.0001 compared with GSNO exposure; Fig. 4A). In embryos treated with decomposed GSNO, no difference in ventricular size at either systole or diastole was measured (data not shown).
Fig. 4.
Measurement of ventricular size and function with modulation of nitric oxide signaling. A: ventricular area in control, GSNO-treated, and 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ)-treated embryos. B: short-axis ventricular diameter measured in control and treated embryos. Statistically significant differences are denoted with actual P value when a significant difference was measured. P values above the bars refer to differences in end-diastolic measurements, while those below reference end-systolic data. n.s., Not significant. C and D: measurement of ventricular contractility by 2-dimensional FAC (C) and FS (D). Data are expressed as means ± SE for n ≥ 9 measurements per group. In A and B, end-diastolic measurements are shown by open bars and end-systolic measurements are shown by filled bars; significant differences are denoted with exact P value. In C, statistically significant differences are denoted by * (P = 0.0074 compared with control), ** (P < 0.0001 compared with control and GSNO), or n.s. (no significant difference was measured). In D, * denotes P = 0.0022 compared with control and ** denotes P < 0.0001 compared with both control and GSNO-exposed embryos.
To complement measurements of ventricular size made by direct measurement of ventricular area, we also directly measured SAx diameter with linear motion mode. Exposure to GSNO increased ED SAx (control 52.2 ± 1.4 μm vs. GSNO 64.1 ± 2.4 μm; P = 0.0003), with no change in ES SAx (control 18.4 ± 1.2 vs. GSNO 15.7 ± 1.1 μm, P = 0.17; Fig. 4B). ODQ did not impact ED ventricular size but did cause an increase in SAx diameter at ES. These data show that the measurements of ventricular size using a single-dimensional technique parallel measurements made with a two-dimensional area-based method.
Changes in cardiac contractile performance with manipulation of NO signaling were assessed with both 1) FAC calculated from area measurements and 2) FS calculated from single-dimension measurements. We found that that GSNO exposure enhanced FAC compared with control measurements (71.2 ± 1.8 % and 61.9 ± 1.6 %, respectively, P = 0.007; Fig. 4B). No difference was observed with the addition of decomposed GSNO (data not shown). Pretreatment with ODQ before exposure to GSNO (FAC = 43.4 ± 2.0 %) not only blocks the increase in FAC caused by NO (P < 0.001), but also, in and of itself, causes a significant decrease in ventricular performance compared with baseline (P < 0.001 vs. control). Augmented ventricular performance with GSNO treatment is corroborated with FS measurements (control 0.65 ± 0.02 vs. GSNO 0.75 ± 0.01, P = 0.0022; Fig. 4D). Again, ODQ treatment before exposure to GSNO resulted in a significant decrease in FS, suggesting a decrease in contractility.
These data confirm that enhanced ventricular performance is associated with the increase in peak FV measured after GSNO exposure. Our observations suggest that the increased performance results from an increase in ventricular ED size caused specifically by NO, with no measurable impact on ES size. These differences are evident with both one- and two-dimensional measurements, and we conclude that the two methods for quantifying ventricular performance are equivalent. In view of the relative simplicity and scalability of the linear motion mode (M mode) analysis, we subsequently used the single SAx dimensions and FS for measurement of ventricular size and function.
Sequence conservation in functionally important domains.
Previous studies have demonstrated the conservation of some small-molecule responsiveness within the cardiovascular system of the developing zebrafish embryo and suggested that several prototypic drug-responsive pathways are intact in zebrafish embryos even at an early developmental stage. The important role of the Na+-K+-ATPase α1β1-subunit in cardiac development has been demonstrated through mutations that cause the heart and mind and small heart defects (32, 38). Loss of the α1β1-subunit is associated with small heart, diminished cardiac contraction, and absence of circulation (32). This subunit is extensively conserved at the amino acid level across all vertebrates. The human gene product is 1,023 aa while the zebrafish form is 1,025 aa in length. There is extensive similarity in these proteins, with 88 % identity. Of nine residues implicated in binding to the prototypic cardiac glycoside Oua (27), seven are absolutely conserved across seven species spanning over 400 million years of evolution (Supplemental Fig. S1).1 Early Oua has been shown to affect developing zebrafish by phenocopying the loss of function mutations.
Similarly, mutations in the voltage-gated LTCC result in impaired circulation and contractility and morphologically in a small ventricle and atrial arrhythmia (island beat) (28). The LTCC is also one of the targets of the calcium channel blocker Ver. Alignment of the polypeptide sequences for the human and zebrafish orthologs of the α-subunit of the LTCC reveals 76 % identity over 2,251 aa. Similarly, domains implicated in drug binding are highly conserved, with complete identity across five aligned species in the first three domains linked to binding and extensive identity (78 %) across species for the remaining domain (Supplemental Fig. S2). Ver has been shown to decrease heart rate in embryonic zebrafish hearts (24).
Finally, the β-adrenergic receptors mediate catecholamine signaling in the cardiovascular system modulating inotropy, chronotropy, and, as a result, cardiac output. While no mutants have been reported in zebrafish β-adrenergic receptors, the agonist Iso has been shown to increase zebrafish heart rate, demonstrating an intact signaling pathway (24). β1- and β2-adrenergic receptors are conserved at a level of 56 % and 31 %, respectively, between humans and zebrafish. The three amino acids shown to mediate catechol binding are absolutely conserved across multiple species (Supplemental Fig. S3).
Thus, for drugs known to impact cardiovascular physiology in zebrafish, evolutionary conservation of drug binding residues and regions is important in conserving drug responsiveness. Specifically, Oua, Ver, and Iso have defined myocardial effects in mammals, but little has been published regarding inotropy-modulating effects in developing zebrafish embryos. Because these drugs have well-defined contractility-modulating effects in higher vertebrates, we explored whether orthologous physiological effects could be measured in embryonic zebrafish.
Pharmacological manipulation of zebrafish cardiac performance reveals functional orthology.
We next tested the functional conservation of responses to several prototypic drugs associated with the structural conservation of their cognate targets. We assessed cardiac performance in zebrafish embryos exposed to agents modulating cardiac performance acting on three separate pathways: 1) Iso, a prototypic β-adrenergic agonist, 2) Oua, an inhibitor of Na+-K+-ATPase, and 3) Ver, a voltage-gated calcium channel blocking agent. Iso and Oua act as positive inotropes in mammals, while Ver would increase ventricular ED and ES size and may negatively impact contractility (7, 14). We hypothesized, because of sequence conservation at drug binding residues, that these agents would act similarly in embryonic fish.
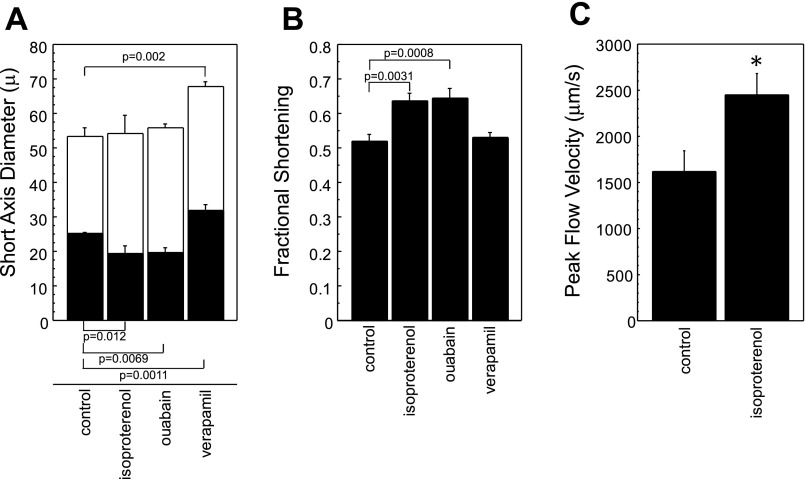
Iso treatment of zebrafish embryos resulted in increased cardiac performance at 72 hpf (Fig. 5). Specifically, FS increased by 0.12 to 0.64 ± 0.02 versus the control value of 0.52 ± 0.02 (P = 0.0031). Increased contractility resulted entirely from a smaller ES size following Iso exposure (SAx dimension = 19.5 ± 2.2 μm) compared with controls (25.2 ± 0.5 μm; P = 0.0117). ED size was similar between treated (54.1 ± 5.3 μm) and control (53.3 ± 2.7 μm) embryos.
Fig. 5.
Drug treatment modulates cardiovascular function in developing zebrafish. A: SAx diameter at both end systole (ES; filled bars) and end diastole (ED; open bars) after drug treatment. Significant differences at ES are noted below the bars, while significant ED differences are identified above the open bars. B: FS following drug treatment; significant differences are as noted. C: dorsal aortic peak FV following isoproterenol treatment. Statistically significant differences are presented with exact P values in A and B; in C * denotes P < 0.05.
Treatment of embryos with Oua resulted in a significant decrease in ES ventricular size (SAx diameter = 19.7 ± 1.4 μm; P = 0.0069 vs. control) but did not impact ED size (55.9 ± 1.1 μm; P = nonsignificant vs. control). The significantly smaller ES size again resulted in a net increase in contractile performance following Oua exposure: FS in treated embryos increased by 0.12 to 0.64 ± 0.03 (P = 0.0008 vs. control).
Ver-mediated inhibition of voltage-gated calcium channels caused significant increases in ventricular size at both ED and ES (Fig. 5A). The SAx diameter at ED measured 67.9 ± 1.3 μm (P = 0.0002 vs. control) and at ES measured 32.0 ± 1.5 μm (P = 0.0011 vs. control). Because of concomitant and offsetting changes in both ES and ED dimensions, ventricular performance measured by FS did not differ between control and Ver-treated embryos (0.53 ± 0.02).
In Iso-treated embryos, we measured FV in addition to FS to assess the impact of contractility-modifying drugs on cardiac output. Peak FV increased significantly from 1,622 ± 220 μm/s in control-treated embryos to 2,449 ± 231 μm/s in Iso-treated embryos (Fig. 5C). These findings confirm that the measured increase in cardiac performance is associated with augmented cardiac output in the periphery.
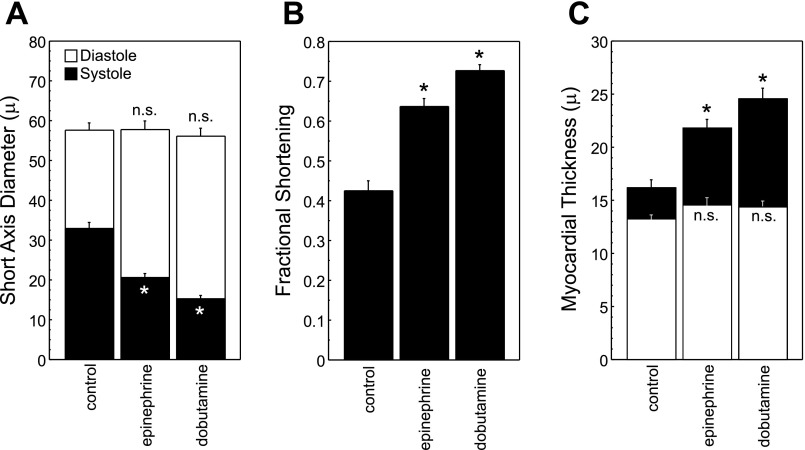
To confirm that the measured changes in cardiac physiology represent findings that can be generalized across multiple agents affecting the same pathway, we measured cardiac function in response to Epi and Dob, which also bind β-adrenergic receptors (Fig. 6). We predicted that these agents would evoke findings parallel to those measured in response to Iso treatment. Ventricular size at ES was smaller after treatment with either Epi (SAx = 20.7 ± 1.0 μm; P < 0.0001) or Dob (15.3 ± 0.9 μm; P < 0.0001) compared with control (32.9 ± 1.5 μm) but did not differ at ED (control: 57.6 ± 1.9 μm) suggesting that β-adrenergic stimulation promotes inotropy through enhanced ventricular emptying. As expected, Epi and Dob treatment also increased FS (Epi 0.64 ± 0.02, Dob 0.73 ± 0.02; P < 0.001) compared with control (0.43 ± 0.02). Finally, we measured myocardial wall thickness in response to β-adrenergic stimulation (Fig. 6C). MTd in Epi- and Dob-treated embryos (14.5 ± 0.7 and 14.4 ± 0.6 μm, respectively) did not differ significantly from control (13.2 ± 0.4 μm; P = 0.21). MTs increased after exposure to Epi (21.8 ± 0.8 μm; P = 0.0001) and Dob (24.6 ± 1.0 μm; P < 0.0001) compared with control (16.2 ± 0.8 μm). We therefore conclude that, using a variety of inotropy-modulating agents, our methods are capable of measuring significant changes in cardiovascular physiology.
Fig. 6.
Drug treatment confirms increased ventricular performance with β-adrenergic stimulation. A: SAx diameter at both ES and ED after treatment with epinephrine and dobutamine. Epinephrine and dobutamine treatment (5 μg/ml) cause a decrease in SAx diameter at ES but do not impact the ED dimension. B: calculation of FS shows augmented contractility with drug treatment. C: measurement of myocardial thickness demonstrates increased ES but not ED wall thickness with β-adrenergic agonist exposure. *P < 0.05; n.s., nonsignificant difference.
Thus these data demonstrate that small molecules known to exert contractility-modulating effects in human and mammalian hearts also cause orthologous functional responses in zebrafish cardiac physiology. In contrast to the 50–100 % differences in cardiac size measured in vtn−/− mutants, pharmacologically mediated differences in contractility are relatively small (on the order of 10 %) and would not be appreciated without the temporal and spatial resolution of these methods.
DISCUSSION
Genetic screens have established the zebrafish as a model system for studying the developmental biology of cardiovascular form. Emerging technologies are beginning to provide a foundation for the exploration of comparative physiology in several organ systems, but the measurement of subtle phenotypic differences necessary for robust screens of physiology or pharmacology remains challenging. We have developed a system using standard light microscopy with a commercial high-speed camera and custom software that is capable of efficient measurement of key aspects of cardiac physiology that is scalable to screening-level throughput.
To validate these tools and methods, we first showed the ability to measure gross changes in cardiac size and function caused by a single point mutation (vtn−/−) that mimics dilated cardiomyopathy in mammals. We used the vtn−/− mutants for our preliminary validation because the cardiac defects observed in mutant embryos are grossly visible and robust metrics should be able to detect these differences easily. Directly measured parameters such as chamber diameter and ventricular area at end systole and diastole, as well as calculated volumes, differed significantly between groups and discriminated functional differences between WT and mutant embryos. Importantly, the directly measured and calculated values for WT embryos were concordant with published values derived in other studies. Baggato and Burggren (3) examined normal cardiovascular development and function over the first 6 days in normal zebrafish embryos. Their values for ventricular volume and erythrocyte velocity are similar to our measurements. Specifically, they estimated ED ventricular volumes ranging between 0.2 and 0.8 nl, which are similar to those we measured at 3 dpf in WT larvae. The values we found for WT ES volume (0.02 nl) differs somewhat from the ES volume calculated by Baggato and Burggren at 72 hpf, but our overall stroke volume (0.16 nl) both compares closely to the value in their report and is similar to the value calculated by Malone and colleagues (23) by a separate technique, dividing cardiac output by heart rate.
The higher frame rate of the images gathered with our video system enabled the calculation of an instantaneous flow velocity for erythrocytes in the dorsal aorta at high temporal resolution. While others have reported mean FV between 291 and 766 μm/s (3), we measured peak systolic and diastolic FVs of 1,644 and 206 μm/s, respectively. Calculating mean FV as of peak and of diastolic FV, our mean value of 685 μm/s falls within a range that is consistent with reported values.
The cardiac defects seen in vtn−/− embryos are striking, both grossly and quantitatively, with 2-fold increases in linear dimension and up to 10-fold changes in volume. In contrast, the physiological effects caused by drugs are more subtle and impossible to appreciate by the unassisted observer. To date, there have been no reports identifying a change in ventricular contractility in response to a pharmacological treatment. Fritsche and colleagues (12) showed that NO and epinephrine modulated aortic diameter, erythrocyte flow, and cardiac output, but detected no impact on cardiac contractility. In contrast, our high-resolution techniques were able to identify changes in ventricular function caused by NO and to document concordant increases in vascular diameter and peak flow velocity following exposure to GSNO. The effects of NO on myocardial contractility and performance in developing zebrafish were driven by significant increases in ED ventricular dimensions. In the absence of measured pressures, it is not possible to determine whether the increase in ED ventricular dimensions results from increased preload or from a direct myocardial effect of NO. However, ODQ, an inhibitor of the cytoplasmic NO receptor sGC, not only blocks the cardiac response to NO but by itself causes a significant decrease in ES dimensions as well as in ventricular performance. These data suggest that endogenous NO itself may have a tonic role in cardiovascular function in the developing heart. Furthermore, because zebrafish vascular smooth muscle is not thought to emerge until later in development, the increase in vascular diameter following exposure to GSNO may not reflect a direct effect on vascular tone but a passive response to increased cardiac output. Interestingly, when Fritsche and colleagues examined the temporal changes in vascular diameter after nitroprusside exposure, they found that the vascular changes were relatively late, peaking 10 min after exposure. Because the impact of NO on vasculature tone should be prompt, the late onset of the measured response might support an indirect mechanism of NO on vascular diameter.
While the target receptors for the agents we have tested vary in their level of peptide sequence conservation (31–88 % amino acid identity), key residues implicated in drug binding are conserved to a greater extent (78–100 %), providing a genomic basis for the functional conservation. Measurement of the contractile changes in ventricular physiology confirms functionally orthologous effects of three prototypic cardioactive agents.
In the absence of robust tools to measure cardiac performance in larval zebrafish, it has been difficult to confirm the effects of classic agents modulating cardiac contractility. We have demonstrated the detection of subtle changes in cardiovascular function inaccessible to the unassisted observer in living zebrafish with clinically relevant drugs. Because of the spatial and temporal resolution of these methods, we have been able to document orthologous functional responses in cardiac contractility between zebrafish larvae and higher vertebrates. Oua, a prototypic inhibitor of Na+-K+-ATPase, and Iso, a classic small-molecule agonist of β-adrenergic signaling, both augment ventricular inotropy, paralleling the mammalian effects of these drugs. Although enhanced inotropy following Oua treatment differs from mutants (heart and mind and small heart) that lack functional Na+-K+-ATPase α1β1 and their Oua-induced phenocopy, we exposed embryos after myocardial specification and cardiogenesis were complete and after a circulation had been established. The enhancement of contractility that we measured in Oua-treated embryos is in fact the one that is orthologous to that of higher-order vertebrates. Similarly the β-adrenergic agonist Iso augments ventricular contractility. We have also demonstrated that the effects on myocardial contractility can be generalized to other β-adrenergic agonists by showing that both Epi and Dob also elicit increases in contractility. In addition to orthologous changes in ventricular performance, small-molecular treatments are also associated with increased peak erythrocyte flow velocity (a surrogate for cardiac output) and enhanced myocardial thickening. These changes parallel measurable changes in normal human hearts treated with positive inotropes (8).
While we did not measure a negative inoptropic effect of Ver on ventricular performance in zebrafish embryos, we did find a larger ventricle, suggesting that myocardial LTCC blockade may instead impact on ventricular lusitropy and stiffness. Additionally, Ver treatment decreased zebrafish heart rate (data not shown), which may serve as the basis for increased diastolic filling and ventricular diastolic size. In normal human hearts, Arrighi and colleagues (2) measured no impact of Ver on ejection fraction, raising the possibility that the pharmacological effects of Ver on indexes of ventricular performance are particularly load dependent and may explain why we did not observe a meaningful change in FS, despite measuring significant changes in ventricular chamber size at both ED and ES.
The methods we describe above measure significant differences in ventricular performance with linear, area, and volumetric parameters. These studies not only demonstrate a functional orthology in inotropic drug response but also highlight that traditional indexes such as FS and FAC used in mammalian models and clinical practice are equally relevant in measuring cardiac function and physiology in zebrafish. This finding should enable the assessment across phyla of analogous perturbations impacting inotropy.
The suite of tools we have described not only will facilitate exploration of the role of function in early cardiac and vascular morphogenesis (6, 17) but also will open up the possibility of detailed pharmacological studies for a broad range of cardioactive drugs.(24) Adaptation of these tools on the scale necessary to conduct large-scale, genomewide analyses on automated platforms may serve as the basis for high-throughput screening of genetic determinants and pharmacological modifiers of contractile physiology and disease.
GRANTS
Support to J. T. Shin and C. A. MacRae was received from the Novartis Foundation. Support to C. A. MacRae was also received from National Institutes of Health (NIH) Grant GM-075946 and the Muscular Dystrophy Association. J. T. Shin is also supported by NIH Grant HL-085280.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Ken Bloch for helpful comments.
Present address of C. A. MacRae: Cardiovascular Div., Brigham and Women's Hospital, Thorn 11, 75 Francis St., Boston, MA 02115.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Ahmed M, Ishiguro M, Nagatomo T. Molecular modeling of SWR-0342SA, a beta3-selective agonist, with beta1- and beta3-adrenoceptor. Life Sci 78: 2019–2023, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Arrighi JA, Dilsizian V, Perrone-Filardi P, Diodati JG, Bacharach SL, Bonow RO. Improvement of the age-related impairment in left ventricular diastolic filling with verapamil in the normal human heart. Circulation 90: 213–219, 1994. [DOI] [PubMed] [Google Scholar]
- 3. Bagatto B, Burggren W. A three-dimensional functional assessment of heart and vessel development in the larva of the zebrafish (Danio rerio). Physiol Biochem Zool 79: 194–201, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Baker K, Warren KS, Yellen G, Fishman MC. Defective “pacemaker” current (Ih) in a zebrafish mutant with a slow heart rate. Proc Natl Acad Sci USA 94: 4554–4559, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Behr B, Hoffmann C, Ottolina G, Klotz KN. Novel mutants of the human beta1-adrenergic receptor reveal amino acids relevant for receptor activation. J Biol Chem 281: 18120–18125, 2006. [DOI] [PubMed] [Google Scholar]
- 6. Berdougo E, Coleman H, Lee DH, Stainier DY, Yelon D. Mutation of weak atrium/atrial myosin heavy chain disrupts atrial function and influences ventricular morphogenesis in zebrafish. Development 130: 6121–6129, 2003. [DOI] [PubMed] [Google Scholar]
- 7. Bonow RO, Ostrow HG, Rosing DR, Cannon RO, 3rd, Lipson LC, Maron BJ, Kent KM, Bacharach SL, Green MV. Effects of verapamil on left ventricular systolic and diastolic function in patients with hypertrophic cardiomyopathy: pressure-volume analysis with a nonimaging scintillation probe. Circulation 68: 1062–1073, 1983. [DOI] [PubMed] [Google Scholar]
- 8. Borow KM, Neumann A, Lang RM. Milrinone versus dobutamine: contribution of altered myocardial mechanics and augmented inotropic state to improved left ventricular performance. Circulation 73: III153–III161, 1986. [PubMed] [Google Scholar]
- 9. Clamp M, Cuff J, Searle SM, Barton GJ. The Jalview Java alignment editor. Bioinformatics 20: 426–427, 2004. [DOI] [PubMed] [Google Scholar]
- 10. Croyle ML, Woo AL, Lingrel JB. Extensive random mutagenesis analysis of the Na+/K+-ATPase alpha subunit identifies known and previously unidentified amino acid residues that alter ouabain sensitivity—implications for ouabain binding. Eur J Biochem 248: 488–495, 1997. [DOI] [PubMed] [Google Scholar]
- 11. Davidson B. Ciona intestinalis as a model for cardiac development. Semin Cell Dev Biol 18: 16–26, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fritsche R, Schwerte T, Pelster B. Nitric oxide and vascular reactivity in developing zebrafish, Danio rerio. Am J Physiol Regul Integr Comp Physiol 279: R2200–R2207, 2000. [DOI] [PubMed] [Google Scholar]
- 13. Haendchen RV, Wyatt HL, Maurer G, Zwehl W, Bear M, Meerbaum S, Corday E. Quantitation of regional cardiac function by two-dimensional echocardiography. I. Patterns of contraction in the normal left ventricle. Circulation 67: 1234–1245, 1983. [DOI] [PubMed] [Google Scholar]
- 14. Hanrath P, Mathey DG, Kremer P, Sonntag F, Bleifeld W. Effect of verapamil on left ventricular isovolumic relaxation time and regional left ventricular filling in hypertrophic cardiomyopathy. Am J Cardiol 45: 1258–1264, 1980. [DOI] [PubMed] [Google Scholar]
- 15. Ho YL, Shau YW, Tsai HJ, Lin LC, Huang PJ, Hsieh FJ. Assessment of zebrafish cardiac performance using Doppler echocardiography and power angiography. Ultrasound Med Biol 28: 1137–1143, 2002. [DOI] [PubMed] [Google Scholar]
- 16. Hove JR. In vivo biofluid dynamic imaging in the developing zebrafish. Birth Defects Res C Embryo Today 72: 277–289, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421: 172–177, 2003. [DOI] [PubMed] [Google Scholar]
- 18. Katoh K, Kuma K-i, Toh H, Miyata T. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33: 511–518, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katoh K, Misawa K, Kuma Ki, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30: 3059–3066, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keenan SM, DeLisle RK, Welsh WJ, Paula S, Ball WJ., Jr Elucidation of the Na+,K+-ATPase digitalis binding site. J Mol Graph Model 23: 465–475, 2005. [DOI] [PubMed] [Google Scholar]
- 21. Kurose H, Isogaya M, Kikkawa H, Nagao T. Domains of beta1 and beta2 adrenergic receptors to bind subtype selective agonists. Life Sci 62: 1513–1517, 1998. [DOI] [PubMed] [Google Scholar]
- 22. Mably JD, Chuang LP, Serluca FC, Mohideen MA, Chen JN, Fishman MC. santa and valentine pattern concentric growth of cardiac myocardium in the zebrafish. Development 133: 3139–3146, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Malone MH, Sciaky N, Stalheim L, Hahn KM, Linney E, Johnson GL. Laser-scanning velocimetry: a confocal microscopy method for quantitative measurement of cardiovascular performance in zebrafish embryos and larvae. BMC Biotechnol 7: 40, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Milan D, Peterson TA, Ruskin JN, Peterson RT, MacRae CA. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation 107: 1355–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 25. Milan DJ, Jones IL, Ellinor PT, MacRae CA. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. Am J Physiol Heart Circ Physiol 291: H269–H273, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation 107: 1355–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 27. Palasis M, Kuntzweiler TA, Arguello JM, Lingrel JB. Ouabain interactions with the H5–H6 hairpin of the Na,K-ATPase reveal a possible inhibition mechanism via the cation binding domain. J Biol Chem 271: 14176–14182, 1996. [DOI] [PubMed] [Google Scholar]
- 28. Rottbauer W, Baker K, Wo ZG, Mohideen MA, Cantiello HF, Fishman MC. Growth and function of the embryonic heart depend upon the cardiac-specific L-type calcium channel alpha1 subunit. Dev Cell 1: 265–275, 2001. [DOI] [PubMed] [Google Scholar]
- 29. Schwerte T, Pelster B. Digital motion analysis as a tool for analysing the shape and performance of the circulatory system in transparent animals. J Exp Biol 203: 1659–1669, 2000. [DOI] [PubMed] [Google Scholar]
- 30. Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DY. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet 31: 106–110, 2002. [DOI] [PubMed] [Google Scholar]
- 31. Serluca FC, Sidow A, Mably JD, Fishman MC. Partitioning of tissue expression accompanies multiple duplications of the Na+/K+ ATPase alpha subunit gene. Genome Res 11: 1625–1631, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shu X, Cheng K, Patel N, Chen F, Joseph E, Tsai HJ, Chen JN. Na,K-ATPase is essential for embryonic heart development in the zebrafish. Development 130: 6165–6173, 2003. [DOI] [PubMed] [Google Scholar]
- 33. Stainier DY, Fouquet B, Chen JN, Warren KS, Weinstein BM, Meiler SE, Mohideen MA, Neuhauss SC, Solnica-Krezel L, Schier AF, Zwartkruis F, Stemple DL, Malicki J, Driever W, Fishman MC. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development 123: 285–292, 1996. [DOI] [PubMed] [Google Scholar]
- 34. Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). Eugene, OR: Univ. of Oregon Press, 2000. [Google Scholar]
- 35. Woods IG, Kelly PD, Chu F, Ngo-Hazelett P, Yan YL, Huang H, Postlethwait JH, Talbot WS. A comparative map of the zebrafish genome. Genome Res 10: 1903–1914, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xhaard H, Rantanen VV, Nyronen T, Johnson MS. Molecular evolution of adrenoceptors and dopamine receptors: implications for the binding of catecholamines. J Med Chem 49: 1706–1719, 2006. [DOI] [PubMed] [Google Scholar]
- 37. Xu X, Meiler SE, Zhong TP, Mohideen M, Crossley DA, Burggren WW, Fishman MC. Cardiomyopathy in zebrafish due to mutation in an alternatively spliced exon of titin. Nat Genet 30: 205–209, 2002. [DOI] [PubMed] [Google Scholar]
- 38. Yuan S, Joseph EM. The small heart mutation reveals novel roles of Na+/K+-ATPase in maintaining ventricular cardiomyocyte morphology and viability in zebrafish. Circ Res 95: 595–603, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.