Abstract
The hypothalamus integrates peripheral signals to regulate food intake, energy metabolism, and ultimately growth rate and body composition in vertebrates. Deviations in hypothalamic regulatory controls can lead to accumulation of excess body fat. Many regulatory genes involved in this process remain unidentified, and comparative studies may be helpful to unravel evolutionarily conserved mechanisms controlling body weight and food intake. In the present study, divergently selected fat (FL) and lean (LL) lines of chickens were used to characterize differences in hypothalamic gene expression in these unique genetic lines that develop differences in adiposity without differences in food intake or body weight. Hypothalamic transcriptional profiles were defined with cDNA microarrays before and during divergence of adiposity between the two lines. Six differentially expressed genes identified in chickens are related to genes associated with control of body fat in transgenic or knockout mice, supporting the importance of these genes across species. We identified differences in expression of nine genes involved in glucose metabolism, suggesting that alterations in hypothalamic glycolysis might contribute to differences in levels of body fat between genotypes. Expression of the sweet taste receptor (TAS1R1), which in mammals is involved in glucose sensing and energy uptake, was also higher in FL chickens, suggesting that early differences in glucose sensing might alter the set point for subsequent body composition. Differences in expression of genes associated with tumor necrosis factor (TNF) signaling were also noted. In summary, we identified alterations in transcriptional and metabolic processes within the hypothalamus that could contribute to excessive accumulation of body fat in FL chickens in the absence of differences in food intake, thereby contributing to the genetic basis for obesity in this avian model.
Keywords: microarray, obesity, glycolysis, glucose sensor, genetic susceptibility, tumor necrosis factor-α
in humans and other vertebrates, differences in body composition and development of an obese phenotype are controlled by genetics as well as environmental factors (11, 37). The hypothalamus maintains whole body energy homeostasis by regulating energy expenditure, integrating metabolic processes, and influencing food intake (2, 20). In most experimental models, it is difficult to dissociate genes within the hypothalamus that regulate the development of adiposity from genes that also regulate food intake, because these alterations often occur simultaneously. Divergently selected fat (FL) and lean (LL) lines of chicken provide a unique experimental model in which excessive adiposity has been dissociated from alterations in food intake. These lines have been selected for extremes in fatness or leanness at similar body weights and without altered food intake (17, 25, 50). It has been hypothesized that these two phenotypes are the results of polygenic changes (24) leading to discrete changes in metabolism (reviewed in Ref. 43). We reported previously (7) that important genes known to regulate metabolism in mammals are differentially expressed in the hypothalamus of FL and LL chickens, supporting the usefulness of this unique genetic model for studies aimed at identifying the underlying genetic basis for differences in the propensity to accumulate body fat among individuals.
After completion of genomic sequencing and development of high-throughput genomic tools for functional exploration, the chicken is now one of the premier National Institutes of Health-sponsored model organisms for biomedical research (http://www.nih.gov/science/models/gallus/). These genetic tools and unique genetic lines have not been used for identification of genes that function within the hypothalamus to control metabolic rate and adiposity. Currently, there are more than 600 genes identified to be associated with obesity (mostly in humans and mice), and new candidate genes are still emerging (35, 37). Here, we utilized a high-throughput transcriptional screen to identify novel candidate genes and biological pathways associated with differences in adiposity. In this study, gene expression patterns within the hypothalamus of FL and LL chickens were determined with cDNA microarrays. Our goal was to identify genes that were differentially expressed before and during the divergence of adiposity. The gene expression data were then clustered by using self-organizing maps (SOMs) to identify 1) biologically relevant patterns in the data set, 2) phenotypic marker genes distinguishing fat and lean animals, and 3) neighborhood marker genes centered around specific genes of interest that have been identified in transgenic or knockout mice. Gene expression results were then subjected to pathway analysis to identify putative genetic interactions predicted by the data set. Our results indicate that two novel pathways in the hypothalamus are divergently expressed between the lines before observed differences in adiposity. These pathways contain genes involved in glucose sensing and metabolism and genes associated with tumor necrosis factor (TNF) signaling.
MATERIALS AND METHODS
Animals and tissue preparation.
Divergently selected fat (FL) and lean (LL) chicken lines developed at the Institut National de la Recherche Agronomique (INRA, UR83 Recherches Avicoles) were used in this study (25). These chickens have been selected for either high or low abdominal fat at similar body weight at 9 wk of age for 7 generations. The samples used in the present experiment were described previously (7). Briefly, male FL and LL chickens were reared in a floor pen (4.4 × 3.9 m) together to eliminate environmental differences. They were given ad libitum access to feed and water throughout the study with a conventional pelleted starter diet (0–3 wk, 3,125 kcal/kg metabolizable energy and 20.9% protein) or a grower pellet diet (3–11 wk, 3,025 kcal/kg metabolizable energy and 17.9% protein). The light-dark cycle was 24 h of light for the first 2 days after hatch and then 14 h of light and 10 h of dark thereafter. Animals were killed at 1, 3, 5, and 7 wk of age (n = 4 for each age and group), with the hypothalamus being immediately dissected, snap frozen in liquid nitrogen, and stored at −80°C until further processing. Abdominal fat, which is the predominant site for storage of fat and highly correlated to total body fat in the chicken (8, 21, 34), was dissected and weighed as an index of fattening. All procedures were conducted according to protocols approved by the Institutional Animal Care and Use Committees at INRA, the University of Delaware, and the University of Maryland.
Microarray processing and data analysis.
Individual tissue samples (n = 4 per genotype and age group) were homogenized and total RNA extracted with RNeasy Midi kits (Qiagen, Valencia, CA) according to the manufacturer's protocol, as described previously (7). Extracted RNA was quantified with UV absorbance (260/280), and integrity was verified with a Bioanalyzer (Agilent Technologies, Palo Alto, CA). Total RNA samples were analyzed with the Del-Mar 14K Chicken Integrated Systems microarrays, using a reference RNA hybridization design as described previously by our group (16, 36). A description of these arrays along with the annotations for each cDNA has been published (10, 16) and is available in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO Platform accession no. GPL1731). An equal amount of RNA from all animals was pooled for use as an internal reference, with experimental samples (25 μg) labeled with Cy3 and the pooled reference RNA (25 μg) labeled with Cy5 during first-strand cDNA synthesis. Thirty-two microarrays were used in a reference RNA design for hybridization of four biological replicates of FL and LL chickens at four ages (1, 3, 5, and 7 wk of age). Labeling of the cDNA samples with either Cy3 or Cy5 and hybridization of the microarrays was performed by the University of Maryland Biotechnology Institute's Microarray Core Facility.
Data were analyzed as described previously (16) with the TM4 microarray suite from The Institute for Genome Research (TIGR). Images were processed with Spotfinder, and data were LOWESS normalized without background correction and regularized for standard deviation with MIDAS. Background expression was determined from the mean pixel intensities of eight spots of salmon testis DNA printed on each microarray. Spots having mean pixel intensities less than this background were deleted from the analysis. Data for genes were also deleted if more than half the data points were not present. Statistical analysis was then performed on 6,041 spots, and significant differences in gene expression by line or for the line by age interaction were identified for 919 genes (P < 0.05). The data were further trimmed to ensure that there were at least two data points per group, resulting in a total of 760 significant genes identified (P < 0.05) by analysis of variance using the GLM (general linear model) procedure in the Statistical Analysis System (SAS v.8.02; SAS Institute, Cary, NC). Results were further trimmed to remove redundant cDNAs representing the same gene and analyzed to identify genes with more than a 1.5-fold difference in mRNA levels. The significant genes were then preprocessed in GeneCluster version 2.1.7 (Broad Institute, Cambridge, MA) by normalizing expression levels to have mean = 0 and variance = 1 and then clustered into a 3 × 6 SOM to identify 18 different patterns of expression for the two genetic lines. Lists of differentially expressed clone names were submitted to our own database (GeneBase) for annotation with human protein IDs, HUGO gene names, UniProt gene description and ID, and highest BlastX hits (http://cogburn.dbi.udel.edu/file_upload.html). The data set was analyzed further to identify Gene Ontology (GO) terms (http://www.geneontology.org/) and gene networks (Pathway Miner) with BioRag (Bioresource for array genes) at www.biorag.org. This was possible for 108 genes with homologous human genes using the human protein IDs, which were not available for all genes, and for which the fold difference between the FL and LL was at least 150% at any age. It should be noted that the results for the GO analysis are as described by the Pathway Miner website, including the order and hierarchy, and that not all genes were analyzed because of the limited parameters required. A more detailed gene network analysis was then conducted with Ingenuity Pathway Analysis (IPA7.3) software (Ingenuity Systems, Redwood City, CA) using GenBank human protein IDs.
Preparation of cDNA and quantitative reverse transcription-PCR analysis.
All primers were designed with Primer Express (v.2.0, Applied Biosystems) to a homologous region of the chicken genes when aligned with the mouse sequences. Primers were 18–30 nucleotides in length with a melting temperature between 58 and 64°C or 69 and 72°C, and the amplicons were between 100 and 150 base pairs in length (Supplemental Table S1).1 One microgram of total RNA and an oligo(dT) primer were used for cDNA synthesis with the manufacturer's protocol for SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA). A negative control was included to measure genomic DNA contamination by performing the reverse transcription reaction with no reverse transcriptase added. Transcript levels were quantified by using 2 μl of diluted cDNA (1:200) in a 20-μl PCR reaction using SYBR Green real-time quantitative PCR master mix [2× PCR buffer, 0.12 U/μl Taq polymerase, 400 nM dNTPs, 40 nM fluorescein (Invitrogen), and SYBR Green I Nucleic Acid Gel Stain (Invitrogen) diluted 1:10,000] and analyzed with the iCycler iQ Real-Time PCR Detection System (Bio-Rad, Hercules, CA). A three-step PCR cycle was used with an initial denaturation at 95°C for 3 min followed by 40 cycles of 94°C for 45 s, 60°C for 45 s, and 72°C for 45 s, which was followed by a melting curve analysis. PCR products were verified for the appropriate size by gel electrophoresis.
The quantitative reverse transcription-PCR (qRT-PCR) data were normalized with geNorm software and methods (51). Briefly, the data were first transformed to a ΔCt value by subtracting the sample threshold cycle (Ct) value from the sample with the highest expression level in order to control for amplification efficiency. The ΔΔCt value was then calculated by normalizing gene expression to two housekeeping genes, β-actin (ACTB) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The formula used for the normalization is the same as previously described (51). The same 32 RNA samples used for microarray analysis were used for qRT-PCR to confirm expression patterns of 13 selected genes. Differences in expression of individual genes were tested with a two-way (line by week) analysis of variance (ANOVA) with a PDIFF post hoc analysis to identify differences between the groups at different ages (SAS v.8.02, SAS Institute). Values reported are means ± SE, and P < 0.05 was required to reach statistical significance. All data for both qRT-PCR and microarray analyses are presented with the mean data point equal to 1, to place both data sets on the same scale for graphic presentation. Although the microarray and qRT-PCR data were placed on the same relative scale, absolute quantitative comparisons should not be made between the qRT-PCR and microarray data sets since the fold changes were determined with different normalization parameters and different techniques.
RESULTS
General features of phenotypes and hypothalamic gene expression in FL and LL chickens.
Body weight, abdominal fat weight, and percent abdominal fat are presented in Table 1. Abdominal fat pad weight and percentage were similar at 1 wk but diverged thereafter, being 2.5 times greater in FL than in LL at 3 wk of age. This difference continued through 11 wk.
Table 1.
Phenotypic data for FL and LL chickens
| 1 wk | 3 wk | 5 wk | 7 wk | 9 wk | 11 wk | |
|---|---|---|---|---|---|---|
| Body weight, g | ||||||
| FL | 115 ± 4.0 | 544 ± 21 | 1,297 ± 37* | 1,983 ± 36 | 2,693 ± 91 | 3,222 ± 154 |
| LL | 123 ± 3.0 | 551 ± 16 | 1,204 ± 21* | 1,964 ± 40 | 2,787 ± 53 | 3,281 ± 302 |
| Abdominal fat, g | ||||||
| FL | 0.5 ± 0.1 | 13 ± 1.0* | 38 ± 2.0* | 88 ± 3.0* | 124 ± 8.0* | 150 ± 20* |
| LL | 0.4 ± 0.1 | 5 ± 0.5* | 15 ± 2.0* | 31 ± 2.0* | 54 ± 8.0* | 59 ± 10* |
| Abdominal fat:body weight, % | ||||||
| FL | 0.4 ± 0.1 | 2.3 ± 0.1* | 2.9 ± 0.2* | 4.4 ± 0.2* | 4.6 ± 0.3* | 4.6 ± 0.5* |
| LL | 0.3 ± 0.1 | 1.0 ± 0.1* | 1.2 ± 0.1* | 1.6 ± 0.1* | 1.9 ± 0.3* | 1.8 ± 0.3* |
Values are means ± SE for n = 8 chickens at ages of 1–11 wk.
P < 0.05 in a 1-way ANOVA comparing fat (FL) and lean (LL) lines of the same age.
Of the 14,053 genes represented on the microarrays, expression of 715 genes was significantly different by line or for the line-by-age interaction. Of these, 420 genes were differentially expressed by line (without any line-by-age interaction) and 331 genes were significant for line-by-age interactions by a two-way ANOVA (P < 0.05). The MIAME-compliant microarray data were deposited in the NCBI Gene Expression Omnibus as Series GSE10052. Means, P values, cDNA clone names, highest BlastX hits, corresponding human protein identification numbers, and HUGO gene symbols are provided for each differentially expressed transcript in Supplemental Table S2.
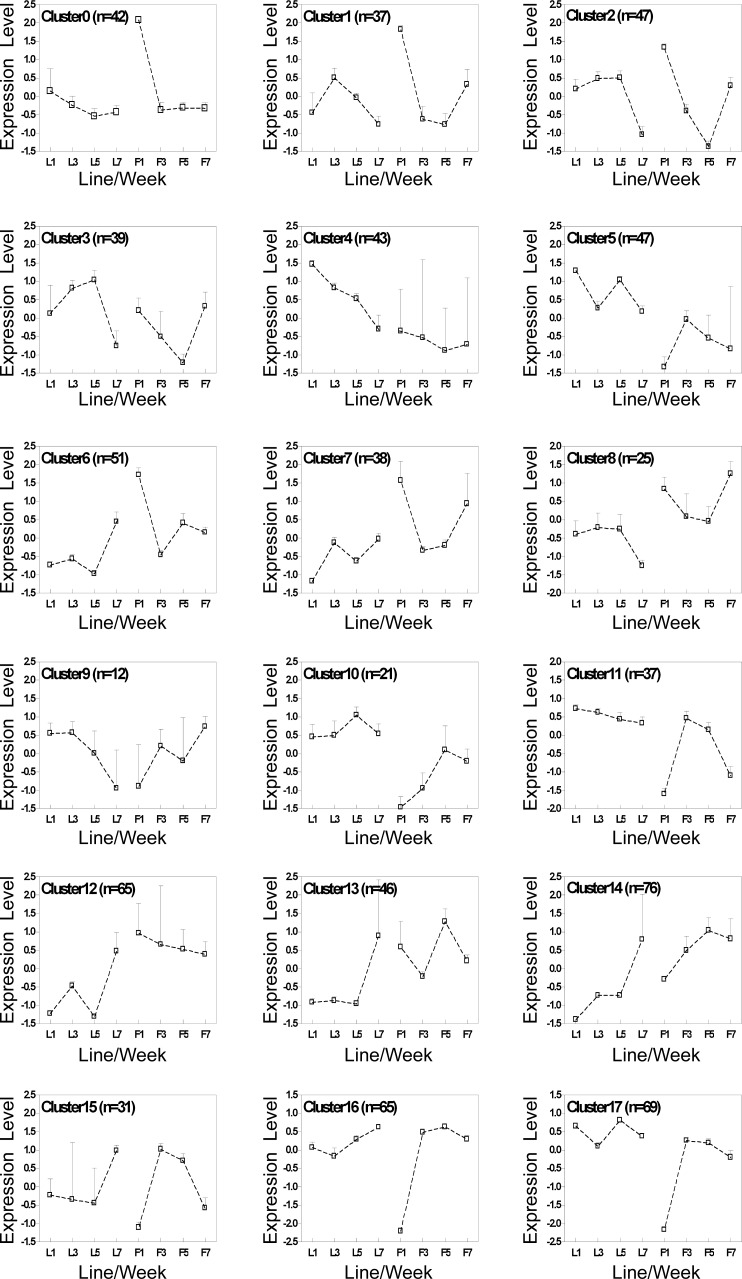
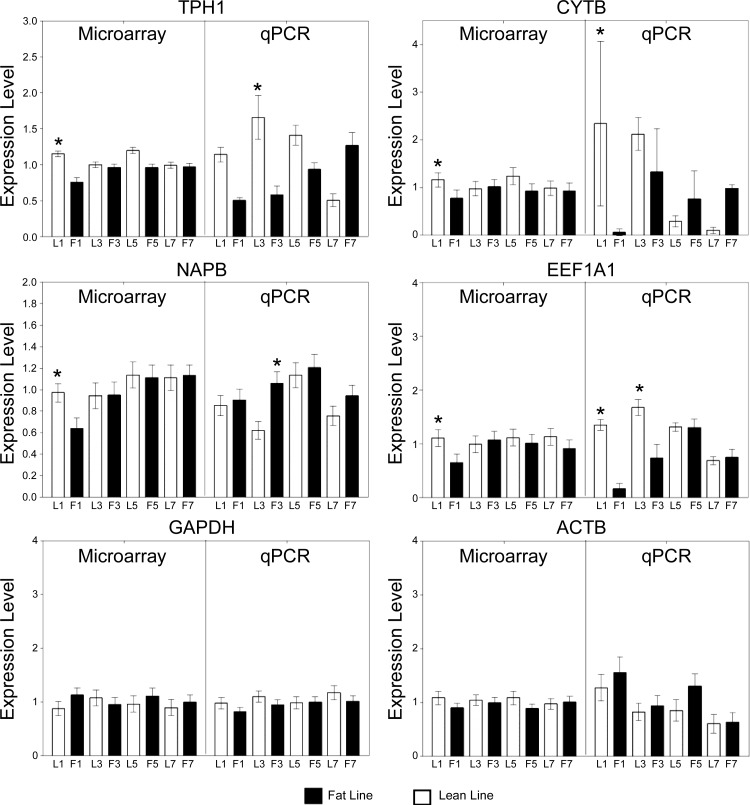
Differentially expressed genes were analyzed with GeneCluster to identify sets of genes that discriminate between FL and LL. This analysis indicated that many genes were consistently downregulated or upregulated in FL versus LL during development (Supplemental Fig. S1). Genes differentially expressed between FL and LL were clustered with SOMs to identify clusters related to the divergence of the fat and lean phenotypes (Fig. 1). Each graph represents the mean level of genes within the cluster, with expression levels normalized to have mean = 0 and SD = 1 across time points (48). The data are presented in a 3 × 6 SOM, which was the smallest number of clusters without substantial redundancy of profiles. Every cluster but two (clusters 4 and 14) exhibited striking differences in expression profiles during development between FL and LL. The expression patterns for 11 genes (belonging to 6 clusters), plus GAPDH and ACTB, were confirmed by qRT-PCR analysis (Table 2). Results for six of these are presented in Fig. 2. The remaining seven are presented below. In general, results from the microarray and qRT-PCR were in agreement. However, results from the two analyses were more similar to one another at week 1 than at later ages. The basis for the differences observed between the microarray results and the qRT-PCR results is not clear. One possibility is that the two different normalization strategies employed for the two techniques, LOWESS and block normalization for the microarray data and geNorm for the qRT-PCR data, contributed to the apparent differences between the two approaches. Overall, we consider qRT-PCR more robust and less prone to systematic errors.
Fig. 1.
Self-organizing map (SOM) analysis reveals biologically relevant clusters and expression patterns between the fat (FL) and lean (LL) lines. The number of genes included in each cluster is provided at top left; values reported are mean ± SE expression levels for FL (F) and LL (L) by week.
Table 2.
Verification of expression profiles by qRT-PCR was performed on specified genes from different SOM clusters
| Clone ID | GenBank Accession No. | Gene Name | Cluster |
|---|---|---|---|
| pgp1n.pk005.c24 | BI391670 | Cytochrome b (CYTB) | 5 |
| pgp1n.pk006.h4 | BI392043 | Elongation factor 1α (EEF1A) | 17 |
| pgp1n.pk004.l24 | BI394654 | Phosphoglycerate mutase (PGAM1) | 17 |
| pgp1n.pk013.i12 | BI395062 | N-ethylmaleimide-sensitive factor attachment protein β (NAPB) | 16 |
| pgf2n.pk005.o4 | BM427174 | 5-Tryptophan hydroxylase (TPH1) | 6 |
| pgp1n.pk012.j21 | BI393839 | Neural enolase (ENO2) | 17 |
| pgr1n.pk006.l9 | CD218118 | CCAAT/enhancer-binding protein ζ (CEBPZ) | 12 |
| pgp1c.pk001.j21 | BI390429 | Dihydropyrimidinase like protein 2 (DPYSL2) | 16 |
| pgf2n.pk006.k5 | BM427410 | Transmembrane protein induced by tumor necrosis factor-α (TMEM120A) | 14 |
| pgp2n.pk004.a23 | BM490642 | Growth hormone-releasing hormone receptor (GHRHR) | 6 |
| pgf1n.pk010.k1 | BI067093 | Pitrilysin metalloprotease 1 (PITRM1) | 6 |
Clone ID is the identification number of the cDNA clone used in the production of the microarray. GenBank accession numbers allow access to partial cDNA sequences. HUGO gene names are provided in parentheses. Cluster number relates to the results presented in Fig. 1. qRT-PCR, quantitative reverse transcription-PCR; SOM, self-organizing map.
Fig. 2.
Quantitative reverse transcription-PCR (qRT-PCR) verification of expression profiles. Microarray data are shown on left and qRT-PCR data are presented on right of each graph. Microarray data have been converted from a log value, compressing the data points to be centered around the value of 1. Values reported are means ± SE. *Differential expression between the lines at the same age (P < 0.05). Gene symbols are defined in the text.
Expression profiles and cell type specificity of genes during divergence of adiposity.
As previously observed (46), differences in adiposity began to develop by 3 wk of age in FL relative to LL (Table 1). Our goal was to identify genes that were differentially expressed before and during the divergence of adiposity. Our rationale was that these genes could contribute to the difference in abdominal fat accumulation between FL and LL birds. Criteria for upregulated (FL > LL) or downregulated (FL < LL) genes were as follows: 1) gene expression was significantly different by line or line by age (P < 0.05, 760 genes total) and 2) at the age of interest there must be no overlapping error bars between the data points, as presented in the SOM cluster data (Fig. 1). More genes were differentially expressed between the lines at week 1 than at any other age. Nine clusters representing 427 genes were upregulated in FL at 1 wk (Fig. 1; clusters 0, 1, 2, 6, 7, 8, 12, 13, and 14). Eight clusters representing 325 genes were downregulated in FL at 1 wk (Fig. 1; clusters 4, 5, 9, 10, 11, 15, 16, and 17), while cluster 3 had no difference between the lines at this age. With the onset of adiposity differences at week 3, fewer genes exhibited upregulated (Fig. 1; clusters 12, 13, 14, 16, and 17; n = 321 genes) or downregulated (Fig. 1; clusters 1, 2, 3, and 10; n = 144 genes) expression patterns in FL relative to LL (Table 3). By week 7, only 160 genes were upregulated in FL, while 223 genes were downregulated. A more detailed analysis by biological process is provided in Supplemental Table S3. In short, the greatest number of genes differentially expressed in the hypothalamus was found at week 1, before divergence of abdominal fat weight.
Table 3.
Numbers of differentially expressed genes in FL relative to LL by age
| FL vs. LL | Week 1 | Week 3 | Week 5 | Week 7 |
|---|---|---|---|---|
| Upregulated | 427 (54%) | 321 (40%) | 372 (47%) | 160 (20%) |
| Downregulated | 325 (41%) | 144 (18%) | 266 (34%) | 223 (28%) |
Numbers in parentheses are percentage of all significantly differentially expressed genes.
The hypothalamus is comprised of neurons and glial subtypes, such as astrocytes and oligodendrocytes. Thus differences in gene expression observed could be attributable to glial rather than neuronal gene expression. To address this issue, we utilized a database for genes specifically expressed in neurons, astrocytes, or oligodendrocytes in mice (9). Genes that were differentially expressed between FL and LL were compared with genes identified for having specific expression in just one cell type. Of the 56 genes found in both data sets, 20 were expressed in neurons, 24 in astrocytes, and 12 in oligodendrocytes (Supplemental Table S4). These findings suggest that neurons, although smaller in number, account for a significant portion of genes that are differentially expressed between FL and LL.
Gene Ontology analysis.
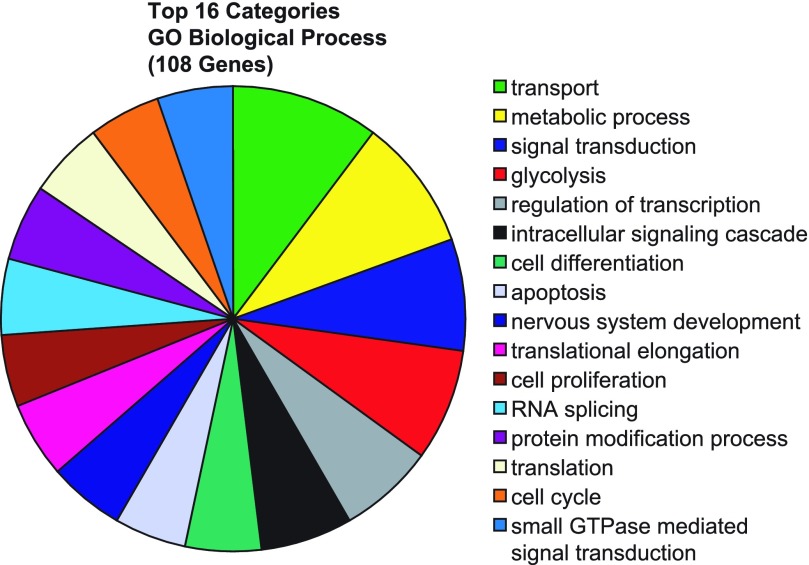
GO analysis was used to identify GO biological processes that could contribute to the fat or lean phenotype. Regulation of transcription and signal transduction were GO biological processes that encompassed the largest number of genes (112 genes) among all 760 genes differentially expressed between the two lines. However, when only the 108 genes that exhibited >1.5-fold differences in expression levels between the lines were considered, transport, metabolic process, signal transduction, and glycolysis (27 genes total) were the most prevalent biological processes represented (Fig. 3). Interestingly, glycolysis was represented by a small number of all significant genes (0.06%) but comprised a larger percentage of genes with >1.5-fold differences between FL and LL (4.6%). Although these genes are involved in gluconeogenesis as well, we suggest that in the hypothalamus carbohydrate metabolism is primarily oriented toward glycolysis, since gluconeogenesis has not been demonstrated in the central nervous system. Next, we chose to annotate SOM cluster 17 with GO terms for biological processes, because this cluster contains two of the genes involved in glycolysis, ENO2 and PGAM1. The 69 genes in cluster 17 were all linked to a GO term. Presented in Supplemental Table S5 are biological processes having at least two genes in a category. Carbohydrate metabolism, inherently related to the glycolytic pathway, contained one additional gene. Interestingly, the genes in cluster 17 were primarily involved with synthesis and metabolism of proteins.
Fig. 3.
Gene Ontology (GO) and pathway analysis of significant genes. Genes significantly different (P < 0.05) and expressed at 1.5-fold or greater levels between FL and LL animals (108 genes total) were analyzed for GO terms for biological process. Shown are the results for GO terms represented by ≥4 genes.
Gene network analysis.
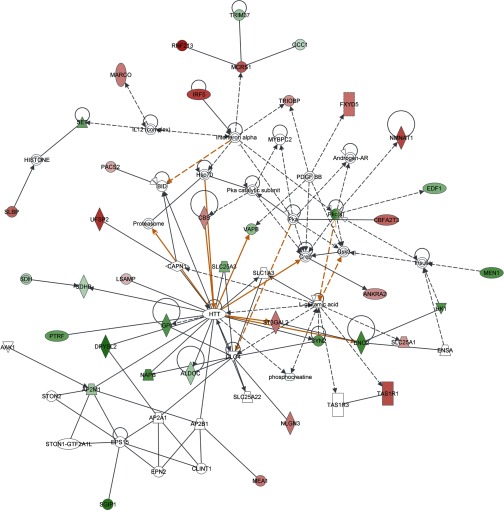
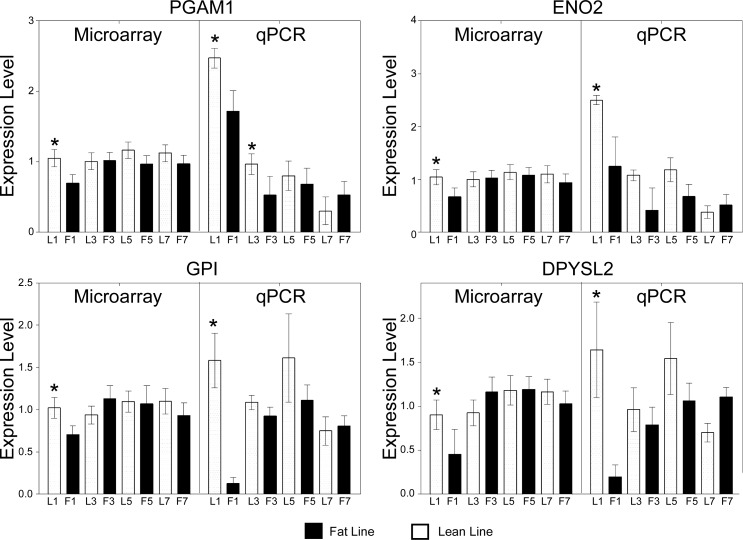
The list of differentially expressed genes was initially submitted to Pathway Miner to identify known cellular and metabolic pathways that were differentially expressed between FL and LL (Supplemental Table S6). The pathway with the greatest number of elements represented by differentially expressed genes was again glycolysis, with five genes represented in the pathway. This suggested that genes involved in glycolysis could play a large role within the hypothalamus in driving the difference between the two phenotypes. Results from week 1 were then analyzed by Ingenuity Pathway Analysis (IPA). A total of 528 genes were IPA network eligible from the 715 differentially expressed genes (Supplemental Table S2). Again, a predicted network of genes involved in glucose metabolism was identified (Fig. 4). This network shows an equal number of genes that were either upregulated or downregulated in the hypothalamus of FL compared with LL at 1 wk of age. Interestingly, seven genes involved in glycolysis were downregulated (ALDOC, ENO2, GAPDH, GPI, HK1, PGAM1, and PGK1) whereas two genes were upregulated (GALM and PTGR1) in FL compared with LL (Fig. 4; Supplemental Table S7). Three of these [phosphoglucose isomerase (GPI), phosphoglycerate mutase (PGAM1), and neural enolase (ENO2)] were confirmed by qRT-PCR to be downregulated in FL relative to LL at week 1 (Fig. 5). These genes are involved in catalyzing different steps of glycolysis, which suggests that before the divergence of adiposity FL could have a lower level of glucose metabolism in the hypothalamus relative to LL, as indicated by lower plasma glucose in FL (26). After adiposity differences began to appear at 3 wk of age, this trend reversed. The same genes that were downregulated at week 1 were upregulated at 3 wk in FL relative to LL. This pattern continued at 5 wk, with two genes regulating glycolysis still being upregulated. By 7 wk, when the divergence in adiposity had started to stabilize between the two lines (Table 1), the expression patterns of the glycolytic genes were similar to the patterns observed at 1 wk. qRT-PCR analysis of three glycolytic genes (GPI, PGAM1, and ENO2) confirmed lower expression at 1 wk in FL chickens (Fig. 5). Hypothalamic expression of the gene encoding acid α-glucosidase (GAA) was slightly higher in FL chickens. This enzyme is responsible for breakdown of glycogen to glucose in lysosomes, and mutations in GAA cause Pompe disease (or glycogen storage disease II) (47). Together, these results suggest that genes regulating glycolysis could promote divergence of the fat or lean phenotype.
Fig. 4.
Gene interaction network in hypothalamus of FL and LL chickens at 1 wk of age. Results for differentially expressed genes were analyzed with Ingenuity Pathway Analysis. The network shown included a number of genes involved in glucose metabolism. Red gene symbols indicate upregulated and green symbols represent downregulated genes in FL relative to LL.
Fig. 5.
Expression pattern verification for genes involved in glycolysis. Data expression patterns were similar for microarray (left) and qRT-PCR (right). The log scale microarray data have been converted, which compresses the data points around the value of 1. Values reported are means ± SE. *Differential expression between the lines at the same age (P < 0.05). Gene symbols are defined in the text.
Marker analysis in FL and LL chickens.
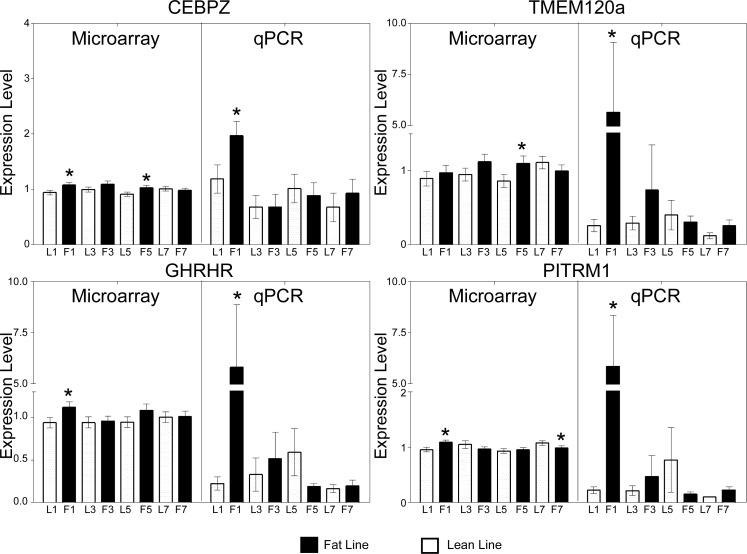
More than 600 genes have been associated with body weight and adiposity in other species, of which 166 have been linked directly to body composition or metabolism by knockout and transgenic technologies in mice (35). We screened whether any of the genes that were differentially expressed between FL and LL were among the 166 candidate genes validated in mice. From this comparison, we identified 24 genes that were either homologous to or directly related to the 166 genes validated in mice. We then selected four of these [CAAT enhancer binding protein ζ (CEBPZ), a gene induced by TNF-α (TMEM120A), growth hormone-releasing hormone receptor (GHRHR), and pitrilysin metallopeptidase 1 (PITRM1)] to confirm by qRT-PCR (Supplemental Table S8; Fig. 6). In each case, gene expression was greater in FL than in LL at 1 wk of age, in agreement with the microarray results. At subsequent ages, levels of each mRNA were similar between lines. To generate a hypothetical model for gene interactions within the hypothalamus, we next performed marker-specific analysis with GeneCluster software. The genes presented in Figs. 5 and 6 were used, one at a time, as a marker for analyzing the microarray data to identify genes whose expression levels are most closely correlated with one another (Supplemental Table S9). Two gene interaction models were developed from correlations among the gene expression patterns (Fig. 7).
Fig. 6.
Expression pattern verification for genes identified by comparison of significant genes. Expression patterns for FL relative to LL at 1 wk of age were increased for genes presented in our model. The log scale microarray data have been converted, which compresses the data points around the value of 1. Values reported are means ± SE. *Differential expression between the lines at the same age (P < 0.05). Gene symbols are defined in the text.
Fig. 7.
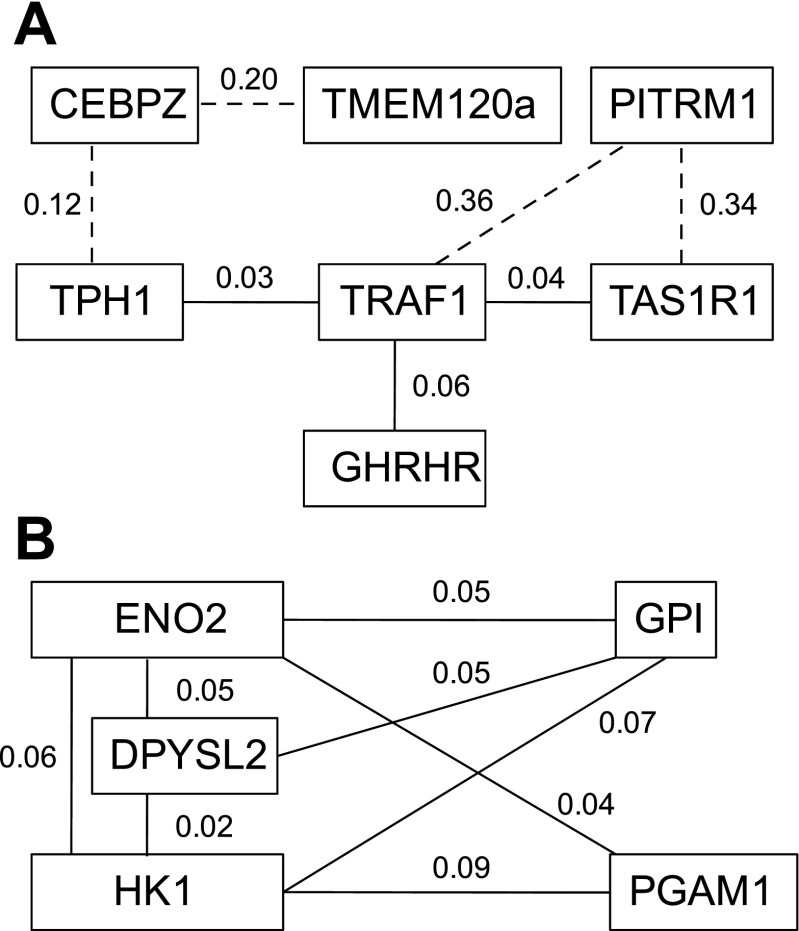
Potential genetic interactions identified by GeneCluster software gene marker analysis. A: genes known to modulate body composition were identified as being linked to the tumor necrosis factor (TNF)-α signaling processes. B: genes known to modulate glycolysis are linked to dihydropyrimidinase like-2 (DPYSL2). Numbers indicate the significance of the correlation. Dashed lines indicate that the genes connected have a correlation significance >0.10 and are less likely to be biologically relevant. Gene symbols are defined in the text.
Microarray analysis of gene expression patterns shows that TNF receptor-associated factor 1 (TRAF1), which is associated with TNF-α signaling, is linked to other genes that modulate energy metabolism (Fig. 7A). For example, TRAF1 is potentially related to many genes that modulate body composition: 5-tryptophan hydroxylase (TPH1), GHRHR, sweet taste receptor 1 (TAS1R1), and PITRM1. At 1 wk of age, the expression of TAS1R1 in the hypothalamus of FL birds was 1.8-fold higher than that of LL chickens. This model also suggests that CEBPZ is associated with TPH1 and TMEM120a gene expression. qRT-PCR analysis verified increased expression in FL relative to LL for four of these genes, CEBPZ, TMEM120a, PITRM1, and GHRHR (Fig. 6). We also developed a model for gene interactions in which four of the five glycolytic genes are closely linked (Fig. 7B). Within this model, we also identified dihydropyrimidinase-like 2 (DPYSL2) as a gene that may interact with the glycolytic pathway in the hypothalamus during the divergence of adiposity between FL and LL. qRT-PCR analysis confirmed that PGAM1, ENO2, GPI, and DPYSL2 mRNA levels were reduced in FL relative to LL (Fig. 5).
DISCUSSION
Microarray analysis revealed dynamic changes in gene expression within the hypothalamus before and after the development of adiposity in our experimental model of genetically linked “obesity.” More genes were differentially expressed between FL and LL at 1 wk of age than at later ages. This is likely attributable to developmental processes or neural circuitry being established during this age. Considering the known central role of the hypothalamus in controlling food intake and metabolism (2, 20), this finding also suggests that differences in establishment of these hypothalamic neural processes could contribute to the difference in abdominal fat and total body fat between the two genetic lines.
Glycolysis and development of adiposity.
Enzymes of glycolysis are generally not considered as rate limiting in glucose utilization, except in specific cells or organs where the first intracellular step of glucose metabolism is rate limiting and dependent upon the activity of hexokinase 1 (HK1). In the hypothalamus of mammals, HK1 is expressed in glucose-sensitive neurons, where it exerts a major role in sensing glucose, although additional and important elements have also been proposed (22, 27). Furthermore, glucokinase (GCK) has been implicated in more direct glucose sensing in the murine anterior pituitary (53). Here, we have identified differences between FL and LL chickens in hypothalamic expression of nine genes encoding enzymes involved in glucose metabolism and glycolysis. Glycolytic gene expression was downregulated in the hypothalamus of FL relative to LL at 1 wk of age, before divergence in adiposity between the lines. In a similar manner, an enhancement in hepatic glycolysis has also been associated with a reduction in obesity (52). In contrast, the glycolytic potential of muscle has been reported to be greater in FL than LL animals (42). To our knowledge, no alternative functions besides their role in glycolysis have been proposed for enzymes identified in the present microarray experiment, including ENO2. However, α-enolase is located in multiple subcellular compartments (including the cell surface) and may exert other functions (49). We have identified DPYSL2 as a coregulated gene with four of the genes involved in glycolysis and therefore as a potential upstream regulator or downstream target of glycolysis. DPYSL2 has been previously associated with Alzheimer disease (5, 14) and is known to be involved in development of the nervous system (12). This is the first report associating the function of DPYSL2 with energy metabolism and processes contributing to development of a fat or lean phenotype.
In contrast to the situation that prevails in obese mammals, FL chickens generally exhibit low plasma glucose and high or normal plasma insulin (44). Other studies performed on isolated, perfused pancreas in chickens from the same genetic lines have also hypothesized the presence of a discrete change in the glucose-insulin balance between FL and LL chickens (39). At 61 days of age, differences in glucose levels had not been observed in cerebrospinal fluid in either the fasted or fed state, despite a clear difference in plasma glucose level in the fasted state between FL and LL chickens (45). The decrease in mRNA for glycolytic enzymes in the hypothalamus of FL chickens would most likely not depend upon changes in glucose availability. However, the possibility of a change in the membrane glucose transporter step cannot be excluded. Conversely, a discrete change in glucose metabolism within the hypothalamus, as presently suggested, may generate different peripheral signals.
Our discovery of differential expression of the sweet taste receptor gene (TAS1R1) in the hypothalamus of the FL chicken could be of major physiological significance. The sweet taste receptor T1R1 has been recently proposed as a hypothalamic glucose sensor in the mouse (38), where heterodimeric T1R2/TR1R3 senses glucose and T1R1/T1R3 monitors l-amino acid levels in the brain. In mouse small intestine, the T1R taste receptors sense sugar levels and regulate active and facilitated absorption of glucose (30, 32). Two independent computational surveys, which examined either distribution of G protein-coupled receptors in the chicken genome (23) or the evolution and diversity of sweet and bitter taste receptor genes (40), have both shown that chickens lack the TAS1R2 gene, which could account for their inability to perceive sweetness. Interestingly, TAS1R1 transcripts are also differentially expressed in both liver and abdominal fat samples from FL and LL chickens (L. A. Cogburn, unpublished observations). Increased expression of TAS1R1 transcripts in multiple tissues (hypothalamus, liver, and abdominal fat) of FL chickens supports a role of the TAS1R1 gene in glucose sensing and utilization in this unique model of genetic obesity.
Homologous genes associated with obesity in knockout or transgenic mice.
The present analysis identified several genes previously known to modulate adiposity in mice (37). TNF-α-associated genes were another family of genes that had expression differences between the lines. TNF-α is a cytokine secreted from many cell types, including adipocytes, and has been shown to act on the hypothalamus to suppress appetite (3). We demonstrated upregulation of genes related to TNF-α signaling in LL relative to FL, potentially explaining the decreased adiposity in LL relative to FL.
The TRAF1 molecule has been associated with gene expression for the sweet taste receptor (TAS1R1), which is also present in the small intestine of mice and is associated with glucose uptake (29). The FL chicken has lower plasma glucose and higher plasma insulin relative to the LL bird (26), with hypothalamic TAS1R1 and TRAF1 mRNA levels being lower in FL relative to LL. This suggests that TAS1R1 expression in the hypothalamus may be associated with TRAF1 to modulate glucose uptake in the neuron, in a manner similar to that demonstrated in the intestine of mice. TRAF1 was also associated in our present study with GHRHR and TPH1, the rate-limiting enzyme for serotonin synthesis, and indirect relationships have been shown to exist between TNF-α, GHRH, and serotonin production. For example, the antitumor effects of TNF-α were attenuated by blocking serotonin receptors (31). We demonstrate here that decreased levels of TPH1 mRNA correlate with decreased levels of TRAF1 mRNA in the hypothalamus of FL relative to LL birds. Mice with deficits in GHRH signaling were not able to increase mRNA expression for GHRHR or TNFα after virus treatment, suggesting that a direct relationship between the two genes may exist (1).
Our analysis suggests that TMEM120a may interact with CEBPZ. CEBPα and -β have been associated with adipocyte differentiation (13), and here we show that hypothalamic CEBPZ expression may be associated with the divergence of adiposity in FL and LL, with CEBPZ mRNA levels being greater in FL relative to LL. The putative transmembrane protein (TMEM43) could be a peroxisome proliferator-activated receptor γ (PPARγ)-target gene, since response elements for this adipogenic gene have been identified in mammals (33). PITRM1 was also identified in the present study as a candidate gene associated with TRAF1. Interestingly, it has been found that tissue inhibitor of metalloprotease 1 is elevated in obese children, resulting in lower levels of PITRM1 (18). This trend corresponds with the increased levels of PITRM1 mRNA observed in the hypothalamus of FL relative to LL at 1 wk of age. It has also been shown that TNF-α elevates the levels of disintegrin and metalloprotease with thrombospondin motifs 1 (ADAMTS1) (15), which has been directly associated with modulating body composition (41). Collectively, these observations suggest that TNF-α may interact with or regulate expression of PITRM1 to modulate body composition.
Genetic influences.
The present study indicates that genotype influences development of adiposity by regulating hypothalamic expression of genes that control deposition of body fat during a critical developmental period. The altered gene expression observed at 1 wk of age, before any differences in adiposity, could modulate neuronal development to regulate the long-term body fat “set point.” Similar developmental aspects of functional neural pathways between hypothalamic nuclei have been shown in the mouse (4). Therefore, we have identified a large set of genes that are differentially regulated after divergent selection of abdominal fat, which could potentially interact to regulate homeostatic mechanisms controlling long-term accumulation of body fat. It should be noted that all of our findings were restricted to levels of mRNA within the hypothalamus. Differences in mRNA levels do not necessarily translate into differences in levels of functional proteins. Furthermore, differences in levels of a specific protein within nervous tissue do not necessarily result in altered function of the neurons. Additional research is necessary to confirm differences in hypothalamic function associated with divergence in adiposity suggested by the present study.
Animal models are useful for identifying novel candidate genes that regulate energy homeostasis. For example, one rat model was bred to be susceptible or resistant to diet-induced obesity (DIO), and this model has been very useful in elucidating interactions between environment and genotype in the development of obesity (28). Here, we utilized a unique animal model to study genotype-regulated differences during development of extremes in adiposity, which are independent of food intake and total body weight. Our findings of differences in hypothalamic gene expression before the onset of differences in adiposity suggest that this chicken model has the potential to help elucidate the underpinnings of genotype-induced human obesity. In addition, the chicken genome has a greater synteny with the human genome than does the mouse (6, 19). In conclusion, we have investigated hypothalamic gene expression patterns in a unique genetic model for obesity and identified novel candidate genes that could contribute to the development of genotype-induced obesity.
GRANTS
This work was supported by a grant from the United States Department of Agriculture Initiative for Future Agricultural and Food Systems (USDA-IFAFS) Animal Genome Program (to L. A. Cogburn, T. E. Porter, S. E. Aggrey, and J. Simon; Award No. 00-52100-9614). M. S. Byerly was supported by a grant from the Cosmos Club Foundation and graduate student training fellowships from the National Institutes of Health (F31-DK-743802 and MH-20048).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
Breeding of FL-LL chickens used in this experiment and tissue samplings required the contribution of a large number of colleagues from INRA (UR83 Recherches Avicoles and UE1295 Pôle d'Expérimentation Avicole de Tours, Nouzilly, France). Dr. Paul Rault delivered the certificate of origin of the samples for U.S. import. The contributions of these colleagues are gratefully acknowledged.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Alt JA, Bohnet S, Taishi P, Duricka D, Obal F, Jr, Traynor T, Majde JA, Krueger JM. Influenza virus-induced glucocorticoid and hypothalamic and lung cytokine mRNA responses in dwarf lit/lit mice. Brain Behav Immun 21: 60–67, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Anand BK, Brobeck JR. Nutrition classics. The Yale Journal of Biology and Medicine. Volume XXIV 1951–1952. Hypothalamic control of food intake in rats and cats. Nutr Rev 42: 354–356, 1984. [DOI] [PubMed] [Google Scholar]
- 3. Bodnar RJ, Pasternak GW, Mann PE, Paul D, Warren R, Donner DB. Mediation of anorexia by human recombinant tumor necrosis factor through a peripheral action in the rat. Cancer Res 49: 6280–6284, 1989. [PubMed] [Google Scholar]
- 4. Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci 24: 2797–2805, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyd RS, Adam PJ, Patel S, Loader JA, Berry J, Redpath NT, Poyser HR, Fletcher GC, Burgess NA, Stamps AC, Hudson L, Smith P, Griffiths M, Willis TG, Karran EL, Oscier DG, Catovsky D, Terrett JA, Dyer MJ. Proteomic analysis of the cell-surface membrane in chronic lymphocytic leukemia: identification of two novel proteins, BCNP1 and MIG2B. Leukemia 17: 1605–1612, 2003. [DOI] [PubMed] [Google Scholar]
- 6. Burt DW, Bruley C, Dunn IC, Jones CT, Ramage A, Law AS, Morrice DR, Paton IR, Smith J, Windsor D, Sazanov A, Fries R, Waddington D. The dynamics of chromosome evolution in birds and mammals. Nature 402: 411–413, 1999. [DOI] [PubMed] [Google Scholar]
- 7. Byerly MS, Simon J, Lebihan-Duval E, Duclos MJ, Cogburn LA, Porter TE. Effects of BDNF, T3, and corticosterone on expression of the hypothalamic obesity gene network in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol 296: R1180–R1189, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cahaner A, Leenstra F. Effects of high temperature on growth and efficiency of male and female broilers from lines selected for high weight gain, favorable feed conversion, and high or low fat content. Poult Sci 71: 1237–1250, 1992. [DOI] [PubMed] [Google Scholar]
- 9. Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28: 264–278, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carre W, Wang X, Porter TE, Nys Y, Tang J, Bernberg E, Morgan R, Burnside J, Aggrey SE, Simon J, Cogburn LA. Chicken genomics resource: sequencing and annotation of 35,407 ESTs from single and multiple tissue cDNA libraries and CAP3 assembly of a chicken gene index. Physiol Genomics 25: 514–524, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Chagnon YC, Perusse L, Bouchard C. Familial aggregation of obesity, candidate genes and quantitative trait loci. Curr Opin Lipidol 8: 205–211, 1997. [DOI] [PubMed] [Google Scholar]
- 12. Charrier E, Reibel S, Rogemond V, Aguera M, Thomasset N, Honnorat J. Collapsin response mediator proteins (CRMPs): involvement in nervous system development and adult neurodegenerative disorders. Mol Neurobiol 28: 51–64, 2003. [DOI] [PubMed] [Google Scholar]
- 13. Chiu CH, Lin WD, Huang SY, Lee YH. Effect of a C/EBP gene replacement on mitochondrial biogenesis in fat cells. Genes Dev 18: 1970–1975, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi J, Forster MJ, McDonald SR, Weintraub ST, Carroll CA, Gracy RW. Proteomic identification of specific oxidized proteins in ApoE-knockout mice: relevance to Alzheimer's disease. Free Radic Biol Med 36: 1155–1162, 2004. [DOI] [PubMed] [Google Scholar]
- 15. Do MS, Jeong HS, Choi BH, Hunter L, Langley S, Pazmany L, Trayhurn P. Inflammatory gene expression patterns revealed by DNA microarray analysis in TNF-alpha-treated SGBS human adipocytes. Yonsei Med J 47: 729–736, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellestad LE, Carre W, Muchow M, Jenkins SA, Wang X, Cogburn LA, Porter TE. Gene expression profiling during cellular differentiation in the embryonic pituitary gland using cDNA microarrays. Physiol Genomics 25: 414–425, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Geraert PA, MacLeod MG, Larbier M, Leclercq B. Nitrogen metabolism in genetically fat and lean chickens. Poult Sci 69: 1911–1921, 1990. [DOI] [PubMed] [Google Scholar]
- 18. Glowinska-Olszewska B, Urban M. Elevated matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 in obese children and adolescents. Metabolism 56: 799–805, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Hedges SB, Kumar S. Genomics. Vertebrate genomes compared. Science 297: 1283–1285, 2002. [DOI] [PubMed] [Google Scholar]
- 20. Hetherington A, Ranson S. Nutrition classics. The Anatomical Record, Volume 78, 1940. Hypothalamic lesions and adiposity in the rat. Nutr Rev 41: 124–127, 1983. [DOI] [PubMed] [Google Scholar]
- 21. Hood RL. The cellular basis for growth of the abdominal fat pad in broiler-type chickens. Poult Sci 61: 117–121, 1982. [DOI] [PubMed] [Google Scholar]
- 22. Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eiki J, Zhang BB, Levin BE. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 55: 412–420, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Lagerström MC, Hellström AR, Gloriam DE, Larsson TP, Schiöth HB, Fredriksson R. The G protein-coupled receptor subset of the chicken genome. PLoS Comput Biol 2: e54, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leclercq B. Observations on reciprocal F1 crosses between fat and lean lines of chickens. Arch Geflügelk 4: 129–131, 1986. [Google Scholar]
- 25. Leclercq B, Blum JC, Boyer JP. Selecting broilers for low or high abdominal fat—initial observations. Br Poult Sci 21: 107–113, 1980. [Google Scholar]
- 26. Leclercq B, Kouassi-Kouakou J, Simon J. Laying performances, egg composition, and glucose tolerance of genetically lean or fat meat-type breeders. Poult Sci 64: 1609–1616, 1985. [DOI] [PubMed] [Google Scholar]
- 27. Levin BE. Neuronal glucose sensing: still a physiological orphan? Cell Metab 6: 252–254, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725–R730, 1997. [DOI] [PubMed] [Google Scholar]
- 29. Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 582: 379–392, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mace OJ, Lister N, Morgan E, Shepherd E, Affleck J, Helliwell P, Bronk JR, Kellett GL, Meredith D, Boyd R, Pieri M, Bailey PD, Pettcrew R, Foley D. An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine. J Physiol 587: 195–210, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manda T, Nishigaki F, Mori J, Shimomura K. Important role of serotonin in the antitumor effects of recombinant human tumor necrosis factor-alpha in mice. Cancer Res 48: 4250–4255, 1988. [PubMed] [Google Scholar]
- 32. Margolskee RF, Dyer J, Kokrashvili Z, Salmon KSH, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104: 15075–15080, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Merner ND, Hodgkinson KA, Haywood AF, Connors S, French VM, Drenckhahn JD, Kupprion C, Ramadanova K, Thierfelder L, McKenna W, Gallagher B, Morris-Larkin L, Bassett AS, Parfrey PS, Young TL. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a missense mutation in the TMEM43 gene. Am J Hum Genet 82: 809–821, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nir I, Nitsan Z, Keren-Zvi S. Fat deposition in birds. In: Leanness in Domestic Birds: Genetic, Metabolic and Hormonal Aspects, edited by Leclercq B, Whitehead CC. London: Butterworths, 1988. [Google Scholar]
- 35. Perusse L, Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Snyder EE, Bouchard C. The human obesity gene map: the 2004 update. Obes Res 13: 381–490, 2005. [DOI] [PubMed] [Google Scholar]
- 36. Porter TE, Ellestad LE. Gene expression profiling in the developing neuroendocrine system of the chick. In: Functional Avian Endocrinology, edited by Dawson A, Sharp PJ. New Delhi: Narosa, 2005, p. 45–56. [Google Scholar]
- 37. Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Perusse L, Bouchard C. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 14: 529–644, 2006. [DOI] [PubMed] [Google Scholar]
- 38. Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci 3: 12, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rideau N, Simon J, Leclercq B. Further characterization of insulin secretion from the perfused duodenum-pancreas of chicken: a comparison of insulin release in chickens selected for high and low abdominal fat content. Endocrinology 119: 2635–2641, 1986. [DOI] [PubMed] [Google Scholar]
- 40. Shi P, Zhang J. Contrasting modes of evolution between vertebrate sweet/umami receptor genes and bitter receptor genes. Mol Biol Evol 23: 292–300, 2006. [DOI] [PubMed] [Google Scholar]
- 41. Shindo T, Kurihara H, Kuno K, Yokoyama H, Wada T, Kurihara Y, Imai T, Wang Y, Ogata M, Nishimatsu H, Moriyama N, Oh-hashi Y, Morita H, Ishikawa T, Nagai R, Yazaki Y, Matsushima K. ADAMTS-1: a metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J Clin Invest 105: 1345–1352, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sibut V, LeBihan-Duval E, Tesseraud S, Godet E, Bordeau T, Cailleau-Audouin E, Chartrin P, Duclos MJ, Berri C. Adenosine monophosphate-activated protein kinase involved in variations of muscle glycogen and breast meat quality between lean and fat chickens. J Anim Sci 86: 2888–2896, 2008. [DOI] [PubMed] [Google Scholar]
- 43. Simon J. Chicken as a useful species for the comprehension of insulin action. Crit Rev Poult Biol 2: 121–148, 1989. [Google Scholar]
- 44. Simon J. Insulin in birds: metabolic effects and possible implications in genetically fat and lean chickens. In: Leanness in Domestic Birds: Genetic, Metabolic and Hormonal Aspects, edited by Leclercq B, Whitehead CC. London: Butterworths, 1988. [Google Scholar]
- 45. Simon J, Leclercq B. Fat and lean chickens: prefattening period and in vivo sensitivity to insulin, atropine, and propranolol. Am J Physiol Regul Integr Comp Physiol 249: R393–R401, 1985. [DOI] [PubMed] [Google Scholar]
- 46. Simon J, Leclercq B. Longitudinal study of adiposity in chickens selected for high or low abdominal fat content: further evidence of a glucose-insulin imbalance in the fat line. J Nutr 112: 1961–1973, 1982. [DOI] [PubMed] [Google Scholar]
- 47. Sugawara K, Saito S, Sekijima M, Ohno K, Tajima Y, Kroos MA, Reuser AJ, Sakuraba H. Structural modeling of mutant alpha-glucosidases resulting in a processing/transport defect in Pompe disease. J Hum Genet 54: 324–330, 2009. [DOI] [PubMed] [Google Scholar]
- 48. Tamayo P, Slonim D, Mesirov J, Zhu Q, Kitareewan S, Dmitrovsky E, Lander ES, Golub TR. Interpreting patterns of gene expression with self-organizing maps: methods and application to hematopoietic differentiation. Proc Natl Acad Sci USA 96: 2907–2912, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Terrier B, Degand N, Guilpain P, Servettaz A, Guillevin L, Mouthon L. Alpha-enolase: a target of antibodies in infectious and autoimmune diseases. Autoimmun Rev 6: 176–182, 2007. [DOI] [PubMed] [Google Scholar]
- 50. Touchburn S, Simon J, Leclercq B. Evidence of a glucose-insulin imbalance and effect of dietary protein and energy level in chickens selected for high abdominal fat content. J Nutr 111: 325–335, 1981. [DOI] [PubMed] [Google Scholar]
- 51. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu C, Kang JE, Peng LJ, Li H, Khan SA, Hillard CJ, Okar DA, Lange AJ. Enhancing hepatic glycolysis reduces obesity: differential effects on lipogenesis depend on site of glycolytic modulation. Cell Metab 2: 131–140, 2005. [DOI] [PubMed] [Google Scholar]
- 53. Zelent D, Golson ML, Koeberlein B, Quintens R, van Lommel L, Buettger C, Weik-Collins H, Taub R, Grimsby J, Schuit F, Kaestner KH, Matschinsky FM. A glucose sensor role for glucokinase in anterior pituitary cells. Diabetes 55: 1923–1929, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.