Abstract
Fetal alcohol syndrome (FAS) is caused by maternal alcohol consumption during pregnancy. The reason why specific embryonic tissues are sensitive toward ethanol is not understood. We found that in neural crest-derived cell (NCC) cultures from the first branchial arch of E10 mouse embryos, incubation with ethanol increases the number of apoptotic cells by fivefold. Apoptotic cells stain intensely for ceramide, suggesting that ceramide-induced apoptosis mediates ethanol damage to NCCs. Apoptosis is reduced by incubation with CDP-choline (citicoline), a precursor for the conversion of ceramide to sphingomyelin. Consistent with NCC cultures, ethanol intubation of pregnant mice results in ceramide elevation and increased apoptosis of NCCs in vivo. Ethanol also increases the protein level of prostate apoptosis response 4 (PAR-4), a sensitizer to ceramide-induced apoptosis. Prenatal ethanol exposure is concurrent with malformation of parietal bones in 20% of embryos at day E18. Meninges, a tissue complex derived from NCCs, is disrupted and generates reduced levels of TGF-β1, a growth factor critical for bone and brain development. Ethanol-induced apoptosis of NCCs leading to defects in the meninges may explain the simultaneous presence of cranial bone malformation and cognitive retardation in FAS. In addition, our data suggest that treatment with CDP-choline may alleviate the tissue damage caused by alcohol.
Keywords: apoptosis, fetal alcohol syndrome, ethanol, ceramide, neural crest
Fetal alcohol syndrome (FAS) or fetal alcohol spectrum disorder (FASD) is caused by alcohol (ethanol) consumption during pregnancy. It is one of the leading causes of birth defects in the United States.1, 2 Fetal alcohol syndrome involves malformation of craniofacial bone and cognitive impairment of the child, leading to the need for treatment up to adulthood.3, 4, 5, 6 Despite widespread campaigns explaining the risk of drinking alcohol during pregnancy, 1 among 750 children is born with FAS. The high incidence of FAS is enigmatic because most women who admitted to having consumed alcohol during pregnancy did so in amounts of 4–5 standard drinks every 2 h, which translates into a blood alcohol concentration (BAC) of 0.12–0.15%. A most recent epidemiological study reported that drinking in the first trimester to the level of this BAC increases the risk of the baby being born with oral clefts (cleft lip and/or palate) by twofold.7 In mice, this BAC has also been found to cause cranial malformations.8 In most in vitro studies with cell cultures or cultivated mouse embryos, however, ethanol is toxic or teratogenic at a concentration above 0.5%, which is a lethal dose for humans. These results suggest that, in vivo, the embryo or specific embryonic tissue shows enhanced sensitivity toward ethanol.
One typical feature of FAS is malformation of the craniofacial bone, which is characterized by a smaller head (microcephaly), a flat nasal bridge, and a thin upper lip with deficient philtrum.6 As babies born with FAS are likely to develop sensory and cognitive defects, the appearance of craniofacial features is the first sign that specific treatment of FAS may be required. The craniofacial bone is derived from the neural crest (NC), a tissue complex that develops from neuroepithelial cells at the end of the first month of pregnancy in humans or at gestational day E9 in mice.9, 10, 11 A delicate balance between proliferation and apoptosis regulates cell number and morphogenesis of embryonic tissues; we hypothesized that ethanol may shift this balance toward harmful apoptosis in NC-derived cells (NCCs).
Recently, our laboratory has found that, in early neuroepithelial cells, ceramide induces apoptosis when the atypical PKCζ/λ (aPKC) inhibitor prostate apoptosis response 4 (PAR-4) is expressed. In the absence of PAR-4, ceramide directly interacts with aPKC and activates the enzyme.12 When expressed at a higher level, PAR-4 binds to ceramide-associated aPKC and inhibits the enzyme. This results in a switch from ceramide/aPKC-induced activation of NF-κB and Akt to their inhibition, which compromises at least two critical antiapoptotic pathways in neural cells.12, 13, 14
We isolated NCCs from branchial arch explant cultures and tested for the levels of PAR-4 and ceramide using immunocytochemistry and high-performance thin-layer chromatography (HPTLC). In contrast to neural progenitor cells, NCCs express PAR-4, which renders these cells vulnerable toward apoptotic stimuli such as ceramide elevation. Therefore, we hypothesized that, in NCCs, ethanol induced ceramide elevation and, in consequence, induction of apoptosis. This study provides experimental evidence in vitro and in vivo that NCCs are specifically sensitive to ethanol, which is because of co-elevation of ceramide and PAR-4. Furthermore, because NCCs promote growth and differentiation of tissues originating in other germ layers, impairment of these cells may also disturb the development of other tissues, such as the forebrain and nonfacial bones of the skull. A prominent example is the meninges, which is NC derived and generates TGF-β1, a growth factor essential for skull and brain development.15, 16, 17, 18, 19 Therefore, we also tested whether prenatal ethanol exposure may impair the development of meninges and tissues affected by meningeal growth factors.
Results
Ethanol induces apoptosis of NCCs
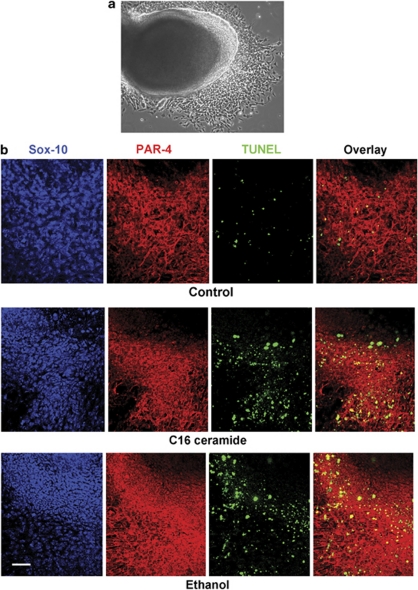
NC-derived cells, rapidly migrating out of explant cultures obtained by cultivation of the first branchial arch (Figure 1a), were tested for the effect of ethanol. Immunocytochemistry was performed for the expression of the NCC marker Sox-10 and apoptosis was determined by TUNEL staining (Figure 1b). Figure 1b and Table 1 show that incubation with 0.3 and 0.5% ethanol or 2 μM C16 ceramide induced apoptosis (TUNEL staining) in Sox10(+) cells migrating out of the branchial arch explant. The TUNEL(+) cells expressed PAR-4, a sensitizer protein toward ceramide-induced apoptosis. Sox10(+)/PAR-4(−) cells in the center of the branchial arch were TUNEL(−), suggesting that the expression of PAR-4 was enhanced in migrating NCCs, which rendered these cells susceptible to ceramide- or ethanol-induced apoptosis.
Figure 1.
Branchial arch NCCs express PAR-4 and undergo apoptosis when exposed to ceramide or ethanol. (a) Branchial arch explant culture generates emigrating NCCs. (b) Immunocytochemistry for Sox-10 (blue) and PAR-4 (red) shows that emigrating NCCs are sensitive toward C16 ceramide (2 μM overnight) and ethanol (0.5% overnight) as determined by TUNEL assay (green). The overlay (TUNEL and PAR-4) shows that PAR-4-expressing cells undergo C16 ceramide or ethanol-induced apoptosis. This experiment was performed five times with at least 20 images for each count (for quantitation, see Table 1). Bar=50 μm
Table 1. Counts of PAR-4(+)/TUNEL(+) NCCs exposed to C16 ceramide or ethanol as determined from Figure 1.
| %PAR-4(+)/TUNEL(+) cells | |
|---|---|
| Control | 3±1% |
| 0.3% ethanol | 9±2% |
| 0.5% ethanol | 15±3% |
| 2 μM C16-ceramide | 13±3% |
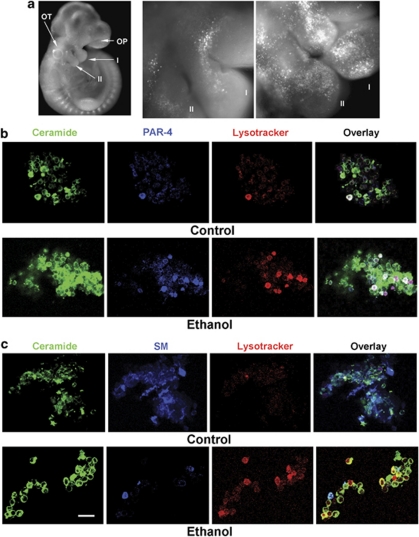
To test whether the ethanol effect was also observed in vivo, pregnant mice were intubated at day E8.5 and 9.5 with ethanol (0.5 ml of 20% ethanol in distilled water) using an oral gavage. This dose has been reported to lead to a BAC of 0.1–15% ethanol (45–90 min after administration) and to induce craniofacial malformation.8 Pregnant control mice were fed with the same volume of an isocaloric solution. At day E10.5, embryos were extracted and apoptotic cells stained with lysotracker, a fluorescent dye known to accumulate in lysosomes, indicating apoptosis in embryonic tissue.20 Figure 2a shows that many apoptotic cells were found at the first branchial arch and at the cranial neural tube. The branchial arch tissue was dissociated and immunocytochemistry was performed using antibodies against ceramide and PAR-4. Consistent with the results from NCC cultures (Figure 1b), the number of apoptotic cells (lysotracker) in the first branchial arch was significantly increased after ethanol intubation (Figure 2b and Table 2a). In summary, the observation that apoptosis was specifically induced in PAR-4(+) cells of branchial arch explant cultures and that these cells were also sensitive toward C16 ceramide suggested that, in vivo, ethanol induces apoptosis by elevation of ceramide in PAR-4(+) cells.
Figure 2.
Branchial arch cells from ethanol-exposed embryos show high levels of PAR-4 and ceramide, concurrent with apoptosis. (a) Ethanol intubation at E8.5 and E9.5 (0.5 ml 20% ethanol; 3 g/kg) induces apoptosis in migrating NCCs of the branchial arches as shown by lysotracker staining of embryos extracted at day E10.5. This result was found in 36 embryos matched by somite stage from eight pregnant mice (four isocaloric controls, four ethanol-intubated mice). (b, c) Cells from embryo heads were dissociated, fixed, and immunocytochemistry for PAR-4 (blue in b) and ceramide (green), or for SM (blue in c), was performed. Note that the number of apoptotic (red), ceramide(+) (green), PAR-4(+) (blue) cells is increased in ethanol-exposed embryos (these cells appear white in overlay). Also note that SM(+) cells are not apoptotic. This experiment was performed five times with at least 20 images for each count (for quantitation see Tables 2 and 3). Bar=20 μm
Table 2a. Counts of dissociated cells from branchial arches with positive staining for the markers indicated in the Table. (see also Figure 2).
| Ceramide (+) | Lysotracker(+) | Ceramide (+)/ lysotracker (+) | Ceramide(+)/PAR-4 (+)/ lysotracker (+) | |
|---|---|---|---|---|
| Control | 28±4% | 2±1% | 2±1% | 2±1% |
| Ethanol | 48±6% | 10±2% | 8±2% | 7±2% |
Ethanol stimulates hydrolysis of sphingomyelin and elevates PAR-4 level
To determine ethanol-induced ceramide elevation in embryonic tissues, we performed lipid analysis using HPTLC and immunocytochemistry using an antibody against ceramide. Figures 2c and 3a and Table 2a show that the level of ceramide and the number of ceramide(+) cells were increased when embryos were extracted from a pregnant mouse that was intubated with 0.5 ml 20% ethanol. To identify the cells that showed increased ceramide levels, embryos were stained with lysotracker, the first branchial arches dissociated, and immunocytochemistry for ceramide and sphingomyelin (SM) was performed on individual cells. Sphingomyelin is a plasma membrane-bound sphingolipid precursor that is hydrolyzed to ceramide by the activation of acid or neutral sphingomyelinase (aSMase or nSMase). Figure 2c and Table 2b show that lysotracker staining was concurrent with a strong signal for ceramide, but not with SM. Ethanol reduced the number of SM(+) cells by more than 70%, concurrent with a fourfold elevation of apoptotic ceramide(+) cells (Table 2a and b). These results suggested that ceramide elevation from ethanol-stimulated SM hydrolysis induced apoptosis in the embryo.
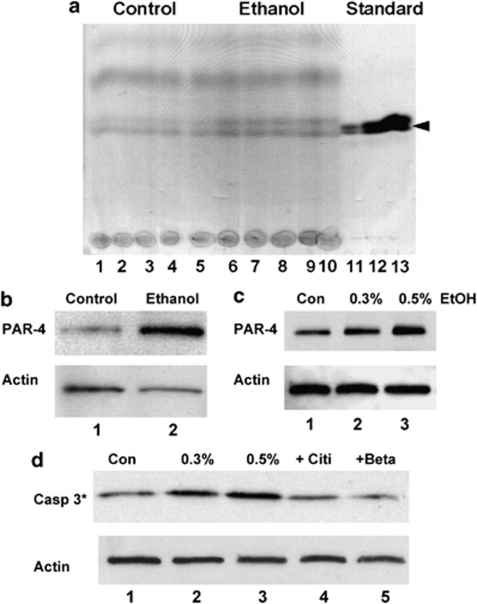
Figure 3.
Ethanol-induced elevation of ceramide and PAR-4 activates caspase 3 in NCCs, which is prevented by betaine and citicoline. (a) Pregnant mice (three isocaloric controls, three treated) were intubated with 3 g/kg ethanol at days E8.5 and 9.5. Lipids were extracted from embryos at day E10.5 and analyzed by HPTLC. Lanes 1–5, vehicle-treated controls; lanes 6–10, ethanol-treated mice; lanes 11–13, ceramide standard (0.1, 0.2, 0.5 μg) (b) Immunoblot showing elevation of PAR-4 in embryos at day E10.5 after prenatal ethanol exposure at day E8.5 and E9.5. Lane 1, vehicle control; lane 2, embryo from ethanol-treated mouse. (c) Immunoblot showing elevation of PAR-4 in NCCs from explant culture exposed to ethanol (overnight). Lane 1, no ethanol; lane 2, 0.3% ethanol; lane 3, 0.5% ethanol. (d) Ethanol incubation (overnight) of NCCs leads to the activation of caspase 3, which is prevented by simultaneous incubation with citicoline (CDP-choline, 100 μM) or betaine (1 mM). For further quantitation by counting apoptotic cells, see Table 3. Lane 1, no ethanol; lane 2, 0.3% ethanol; lane 3, 0.5% ethanol; lane 4, 0.5% ethanol+citicoline; lane 5, 0.5% ethanol+betaine
Table 2b. Counts as in Table 2a with positive staining for the markers as indicated (see also Figure 2).
| SM (+) | SM (+)/ lysotracker (+) | Ceramide(+)/ lysotracker (+) | |
|---|---|---|---|
| Control | 31±4% | <1±1% | 2±1% |
| Ethanol | 8±2% | 2±1% | 8±2% |
To test whether ceramide-induced apoptosis in vivo was specific for PAR-4-expressing cells, we performed immunocytochemistry on dissociated first branchial arch cells from ethanol-exposed embryos. Figure 2b indicates that apoptosis (lysotracker staining) was specific for PAR-4-expressing cells. We also determined whether apoptosis was specific for cells that co-stain for PAR-4 and ceramide. Table 2a and b shows that the number of ceramide(+) cells was increased by 1.7-fold, whereas the number of apoptotic, ceramide(+), and ceramide(+)/PAR-4(+) cells was increased by 3.5- to fourfold. This result suggested that ceramide induced apoptosis specifically in PAR-4(+) cells. However, the data also suggested that the increase in the number of apoptotic cells was higher than that of ceramide(+) cells, which was potentially because of an increase in the number of PAR-4(+) cells. Therefore, we hypothesized that ethanol induced apoptosis by both the elevation of ceramide and PAR-4.
The effect of ethanol on the expression of PAR-4 in the embryo was tested directly using immunoblotting. Figure 3b shows that in embryos from ethanol-intubated mice, PAR-4 was elevated by more than twofold. Ethanol-induced elevation of PAR-4 was also tested in vitro with NCCs and found to be dose dependent (Figure 3c). These results suggested that the combined elevation of ceramide and PAR-4 inactivates critical cell survival pathways downstream of aPKC, the mutual target of ceramide and PAR-4. Previously, we have found that ceramide/PAR-4-mediated inhibition of aPKC induces activation of caspase 3. Therefore, we tested whether caspase 3 is also activated in ethanol-treated NCCs. Figure 3d shows that there is clearly a dose-dependent activation of caspase 3 by ethanol in first branchial arch-derived NCC cultures. Caspase 3 activation and apoptosis of NCCs were prevented by treatment with the SM precursor CDP choline (citicoline) and by the methyl group donor betaine, respectively (Figure 3d and Table 3). These data suggest that providing substrates for SM biosynthesis from ceramide may reverse ethanol-induced apoptosis in NCCs.
Table 3. Counts as in Table 1 to test effect of citicoline or betaine on ethanol-induced apoptosis of NCCs.
| %PAR-4(+)/TUNEL(+) cells | |
|---|---|
| Control | 3±1% |
| 0.5% ethanol | 15±3% |
| 0.5% ethanol plus 100 μM citicoline | 5±1% |
| 0.5% ethanol plus 1 mM betaine | 6±2% |
Cranial malformation and TGF-β1 deficiency induced by ethanol in vivo
Previously, it has been reported that ethanol intubation leads to cranial malformation and neuronal cell death in mice.8, 21 Our results suggested that this may be the result of aberrant development of NCC-derived tissues. We focused on meninges, as this tissue complex is derived from NCCs.22 Furthermore, specific meninges-derived growth factors such as TGF-β1 regulate bone and cortical development.15 Transforming growth factor-β1 has also been identified as a candidate gene for the pathology observed with FAS.23
Control and ethanol-exposed embryos were extracted at day E17.5 and bone structure was visualized using staining with Alizarin Red. Consistent with a recent study using a similar intubation protocol and ethanol dose, we observed aberrant craniofacial morphogenesis in 15–20% of ethanol-exposed embryos (Figure 4a).8 This malformation was characterized by delayed facial and parietal bone formation and incomplete closure of the suture between the parietal bones.
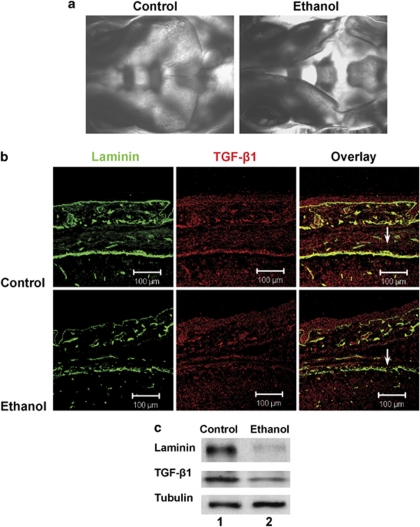
Figure 4.
Prenatal ethanol exposure leads to defects in parietal bone development and loss of laminin and TGF-β1 expression in the meninges. (a) Pregnant mice were intubated with 3 g/kg ethanol at days E8.5 and E9.5. At day E17.5, embryos were extracted and subjected to Alizarin Red staining (a) or coronal cryosectioning and immunocytochemistry for laminin (green) and TGF-β1 (red, b). Note ethanol-induced malformation of the parietal bone. Also note disrupted laminin staining and loss of TGF-β1 in ethanol-exposed embryos (arrows). (c) Immunoblot showing reduced levels of laminin and TGF-β1 in 20% of embryos, concurrent with defects in head development. This experiment was performed three times with a total of 25 embryos from isocaloric control and ethanol-intubated pregnant mice. Lane 1, embryo from control mouse, lane 2, embryo from ethanol-treated mouse
In contrast to facial bones, parietal bones are not derived from neuroectodermal NCCs, but from mesodermal precursor cells. However, parietal bone development and suture closure are regulated by the meninges. We tested whether ethanol intubation at day E8.5 and 9.5 impairs meninges development and the generation of meninges-derived growth factors of mouse embryos at day E17.5. Immunocytochemistry showed that the TGF-β1 signal was almost completely diminished (Figure 4b), concurrent with deficient suture closure and interrupted formation of the basal menigeal cell layer, as determined by staining for laminin. The loss of laminin and TGF-β1 in the meninges of ethanol-exposed embryos was confirmed by immunoblotting (Figure 4c). Therefore, it is likely that prenatal ethanol exposure at the time of NCC proliferation and migration will severely impair the development of embryonic tissues that are regulated by NCC derivatives.
Discussion
Fetal alcohol syndrome is one of the most prominent birth defects, although its etiology is still unclear. Although it is obvious that prenatal consumption of ethanol is the primary cause of the defect, it is still unclear regarding which metabolic pathway is critically impaired by alcohol and which embryonic or fetal tissues are specifically sensitive to alcohol. The phenotypic similarity of genetic defects in neural crest development and FAS has prompted several groups to study the effect of ethanol on NCCs and tissues derived from or regulated by NCCs. Our group has focused on the effect of ethanol on sphingolipid metabolism in NCCs, because dysregulation of this metabolism has been shown to evoke teratogenic phenotypes similar to genetic defects in NCCs.24, 25, 26
The consumption of maize contaminated with the fungus Fusarium leads to neural tube and craniofacial defects in humans.25, 26 The fungal toxin fumonisin B1 causing these defects is a potent inhibitor of sphingolipid biosynthesis. It has been suggested that this inhibition leads to the reduction of SM-dependent lipid rafts in neural tube-associated cells, which induces malfunction of folate-binding protein 1 (Folbp1), and in turn folate deficiency.25 Folate deficiency is a well-known cause of birth defects with a phenotype similar to that of genetic defects in neural crest and tube development. In agreement with the previous hypothesis that SM deficiency can lead to neural tube and craniofacial phenotypes, we proposed that SM deficiency is also caused by prenatal ethanol exposure and evokes the respective phenotypes in FAS. However, we suggest the new hypothesis that SM deficiency is concurrent with ceramide elevation in NCCs, which will lead to ceramide-induced apoptosis and aberrant development of tissues derived from NCCs. Further, we hypothesize that ceramide elevation and SM deficiency emanate from ethanol-evoked hydrolysis of SM to ceramide or impaired conversion of ceramide to SM. This hypothesis implies that stimulating SM biosynthesis from ceramide may alleviate FAS or other birth defects with a similar phenotype.
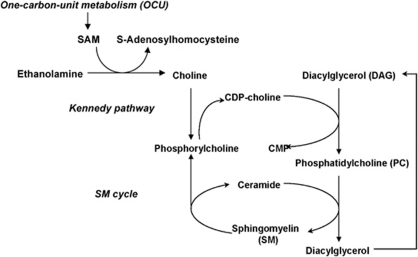
Central to our hypothesis is the intimate connection between SM biosynthesis and one unit carbon (OCU) metabolism, which we have proposed previously.13 Figure 5 shows a link among OCU metabolism providing methyl group donors such as S-adenosyl methionine (SAM) to generate choline from ethanolamine, the Kennedy pathway for phosphatidylcholine (PC) biosynthesis using CDP-choline as a substrate, and the SM cycle generating SM from PC and ceramide. The Kennedy pathway contributes to more than 60% of de novo PC biosynthesis. Alternatively, PC can also be generated by OCU-dependent methylation of phosphatidylethanolamine, which contributes to 20–40% of de novo PC biosynthesis.27, 28 In the Kennedy pathway, CDP-choline is regenerated from phosphoryl choline, a co-product of SM hydrolysis to ceramide in the SM cycle. Regeneration of CDP-choline can also happen from choline provided by OCU-dependent methylation of ethanolamine. It is well known that OCU metabolism essentially relies on folate and the B vitamin complex, cofactors sensitive to alcohol consumption. In line with these observations, prenatal alcohol consumption reduces the levels of SAM, PC, and SM, suggesting that OCU metabolism and de novo biosynthesis of PC and SM are impaired by ethanol. Reduction in SAM, PC, and SM levels is also observed in folate-deficient embryos, suggesting that disturbance of OCU metabolism, the Kennedy pathway, or the SM cycle leads to similar biochemical deficiencies and birth defect phenotypes.25
Figure 5.
Interconnection of OCU metabolism, Kennedy pathway, and SM cycle. CDP-choline is required for de novo biosynthesis of phosphatidylcholine (PC) in the Kennedy pathway. PC is used to convert ceramide to SM in the SM cycle. Diacylglycerol (DAG) and phosphorylcholine, two products of SM biosynthesis and degradation, can be used to regenerate CDP-choline and PC. Ethanol-induced shortage of CDP-choline or PC, or hydrolysis of SM, will result in reduced SM and increased ceramide levels. CDP-choline can either be directly administered (citicoline) or synthesized from choline. Choline can be regenerated from betaine through OCU metabolism
A new finding in our study is the observation that these deficiencies may result in ceramide-induced apoptosis in NCCs and, therefore, to alcohol-induced defects in embryonic tissues derived from NCCs. Although we do not know yet which of these metabolic pathways is critically affected in FAS, providing methyl group donors such as betaine to drive OCU-dependent methylation or CDP choline (citicoline) to drive the Kennedy pathway may enhance de novo PC biosynthesis and foster regeneration of SM from ceramide. This assumption is consistent with previous studies showing that betaine and citicoline can restore SM levels in embryos or adult tissues with disturbed OCU metabolism.29, 30 More importantly, it is supported by our results showing that betaine or CDP-choline alleviates ethanol-induced apoptosis in NCCs.
Also critical to our hypothesis is the fact that there is an embryonic tissue-specific sensitivity toward ethanol to explain the FAS complex. As NCC derivatives such as the meninges secrete growth factors that promote bone and brain development, NCC malfunction induced by ethanol will lead to craniofacial and cognitive deficiencies. This prediction is supported by our data showing that the level of meningeal TGF-β1 is significantly reduced in ethanol-exposed embryos, concurrent with delayed development of the parietal bones and defective suture closure. Transforming growth factor-β1 is known to promote bone formation and neurogenesis.31 Therefore, it is reasonable to speculate that ethanol-induced apoptosis during the formation of the neural crest and migration of NCCs is not completely compensated by enhanced proliferation, but leads to functional deficiencies in tissues derived from or regulated by NCCs at a later stage in development. Previous studies have focused on the direct effect of ethanol on NCC-derived tissues such as facial bones or on cells that show ethanol-induced damage such as neurons. The delayed or indirect effect of ethanol on tissues that are regulated by NCCs has not been analyzed yet. Our data support the idea that indirect effects of early NCC damage may be responsible for a major portion of deficiencies related to FAS. These data also suggest that providing dietary substrates for SM biosynthesis from ceramide, such as betaine or citicoline, may prevent or alleviate the ethanol-induced damage of NCCs. This strategy has been tremendously successful in preventing neural tube defects by dietary supplementation with folate. In future studies, we will test whether dietary supplementation with SM precursors can alleviate FAS-related deficiencies in a similar way as folate has achieved for neural tube-related birth defects.
Materials and Methods
Materials
Mice (C57BL/6) were bred in house. Fetal bovine serum was purchased from Atlanta Biologicals (Louisville, GA, USA). Anti-Sox-10 antibody was purchased from Cemines (Evergreen, CO, USA), and anti-laminin and anti-TGF-β1 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-ceramide rabbit IgG was generated as described previously.32 Anti-ceramide mouse IgM MAS00020 was purchased from Glycobiotech (Kuekels, Germany). Lysenin was purchased from Peptide International (Louisville, KY, USA) and anti-lysenin rabbit IgG and Alizarin Red were obtained from Sigma-Aldrich (St. Louis, MO, USA). Fluorophore-conjugated secondary antibodies were obtained from Jackson ImmunoResearch Laboratory (Bar Harbor, MN, USA). High-performance thin-layer chromatography plates were purchased from Merck (Darmstadt, Germany). All reagents and solvents were of analytical grade or better.
Methods
Explant culture of NCCs
Embryos were extracted from gestational day E9.5–10.5 mice (35±3 somite stage) and deciduas were removed. Using microscissors and scalpels, the first branchial arch was extracted and tissue was cultivated on collagen-coated dishes in F12/DMEM (1:1, vol/vol) medium supplemented with 10% fetal bovine serum. Within 24 h, NCCs emigrated from the explants. At this stage, ethanol and other reagents were added as desired. The neural crest cells were incubated for another 48 h and then fixed with 4% paraformaldehyde in PBS for immunocytochemistry or collected for RT-PCR.
Ethanol intubation of pregnant mice and processing of extracted embryos
At day E8.5 and 9.5, pregnant mice were fed with 0.5 ml of 20% ethanol in distilled and sterilized water using an oral gavage. Controls were fed with the same volume of isocaloric solution. At day E10.5, embryos were extracted and incubated for 60 min with 1 μM lysotracker green or red in NCC cultivation medium. Embryos were either fixed for cryostat sectioning or NCC-derived tissues were dissected as described in the previous section. The head and branchial arches were dissociated by trituration in 0.25% trypsin/EDTA/PBS. The dissociated cells were washed and then fixed with 4% paraformaldehyde in PBS. The cell suspension was used for immunocytochemistry.
To visualize bone from ethanol-treated or control mice, embryos were extracted at day E17.5. After skin maceration in 4% NaCl solution overnight at 4°C, fetuses were skinned and eviscerated, and cervical and dorsal muscles gently removed. Skinned specimens were immediately placed in acid staining solution (0.002% Alizarin Red, 12.5% glacial acetic acid, and 70% ethanol, pH 2.8) for at least 24 h at room temperature. Embryos were dehydrated in 96% ethanol for at least 6 h and maceration of soft tissues was performed by placing specimens in the basic solution (0.002% Alizarin Red, 0.7% KOH) for 30 h at room temperature. The basic solution was renewed at least three times. Specimens were then cleared in clearing solution (40% glycerin, 20% benzyl alcohol, and 30% ethanol) for at least 8 h and stored in storing solution (1:1 mixture of 70% ethanol and glycerin).
For cryostat sectioning, day E17.5-fixed embryos were embedded in OCT solution and flash frozen. Coronal sections of the embryo head were used for immunocytochemistry.
Immunocytochemistry
Immunocytochemistry was performed with NCC cultures, cryostat sectioned embryos, or with suspended cells from dissociated embryonic tissues. Nonspecific binding sites on fixed cells or tissues were first saturated by incubation with 3% ovalbumin in 2% donkey serum/PBS for 1 h. Immunocytochemistry for ceramide followed a previously published protocol showing that the two antibodies used in this studies are specific for ceramide.32 Cells or tissues were exposed to antibodies or binding proteins for ceramide (anti-ceramide monoclonal IgM MAS0020 (1:50) or anti-ceramide polyclonal rabbit IgG (1:75)) or sphingomyelin (1 μg/ml lysenin) in 0.1% ovalbumin/PBS without permeabilization, and then again fixed with 4% paraformaldehyde in PBS. If intracellular proteins were to be detected, cells and tissues were permeabilized with 0.2% Triton X-100 for 5 min at room temperature. This step was followed by incubation with first antibodies against proteins (e.g., Sox10) or with anti-lysenin antibody. Finally, cells and tissues were incubated with secondary antibodies (raised in donkey) covalently linked to fluorophores following instructions provided by the supplier of the antibodies. Confocal laser scanning microscopy was performed using a Zeiss LSM510 microscope (Chester, VA, USA). Micrographs were taken for image analysis.
Image analysis
Micrographic images were taken at the same settings for laser intensity, pinhole, and signal amplification and pixel intensity was determined using Image Pro software. Images obtained with secondary antibody only were used as negative controls, representing background intensity in a particular laser channel. Pixels with at least double the background intensity were considered significant and counted as (+) value. All others were counted as (−) value. Counting was performed with at least five fields on each section or slide and with at least 50 cells in each field. Four independent samples (n=4) were taken, with each sample yielding at least one slide or section. The statistical significance of the co-distribution of two signals was calculated using χ2 analysis following published procedures. A value of P<0.05 was considered statistically significant.
Lipid analysis
Lipid analysis was performed on the upper part of the embryo (head and branchial arches) following an isolation and HPTLC procedure as described previously.33 Ceramide and other lipids were stained with cupric acetate reagent.
Miscellaneous
The amount of protein was determined using a Bio-Rad protein assay kit. Sodium dodecyl sulfate gel electrophoresis was performed according to Laemmli.34
Acknowledgments
This study was supported by a March of Dimes grant (6-FY08-322) to EB. We thank Imaging Core Facility (under supervision of Drs Paul and Ana McNeil) for assistance with confocal microscopy. We are also grateful to Drs Somsankar Dasgupta and Kannan Krishnamurthy for their help with this project. Furthermore, we acknowledge support by the Institute of Molecular Medicine and Genetics (under supervision of Dr Lin Mei), Medical College of Georgia, Augusta, GA, USA.
Glossary
- FAS
fetalalcohol syndrome
- NCC
neural crest-derived cell
- OCU
one carbon unit
- PAR-4
prostate apoptosis response 4
- aPKC
atypical PKCz/l
- PC
phosphatidylcholine
- SM
sphingomyelin
- SAM
S-adenosyl methionine
The authors declare no conflict of interest.
References
- Wattendorf DJ, Muenke M. Fetal alcohol spectrum disorders. Am Fam Physician. 2005;72:279. [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Dunty WC, Jr, Zucker RM, Sulik KK. Hindbrain and cranial nerve dysmorphogenesis result from acute maternal ethanol administration. Dev Neurosci. 2002;24:328–342. doi: 10.1159/000066748. [DOI] [PubMed] [Google Scholar]
- Willford J, Leech S, Day N. Moderate prenatal alcohol exposure and cognitive status of children at age 10. Alcohol Clin Exp Res. 2006;30:1051–1059. doi: 10.1111/j.1530-0277.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- Dunty WC, Jr, Chen SY, Zucker RM, Dehart DB, Sulik KK. Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: implications for alcohol-related birth defects and neurodevelopmental disorder. Alcohol Clin Exp Res. 2001;25:1523–1535. [PubMed] [Google Scholar]
- Sulik KK. Genesis of alcohol-induced craniofacial dysmorphism. Exp Biol Med. 2005;230:366–375. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- DeRoo LA, Wilcox AJ, Drevon CA, Lie RT. First-trimester maternal alcohol consumption and the risk of infant oral clefts in Norway: a population-based case–control study. Am J Epidemiol. 2008;168:638–646. doi: 10.1093/aje/kwn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyedele OO, Kramer B. Acute ethanol administration causes malformations but does not affect cranial morphometry in neonatal mice. Alcohol. 2008;42:21–27. doi: 10.1016/j.alcohol.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Knight RD, Schilling TF. Cranial neural crest and development of the head skeleton. Adv Exp Med Biol. 2006;589:120–133. doi: 10.1007/978-0-387-46954-6_7. [DOI] [PubMed] [Google Scholar]
- Tapadia MD, Cordero DR, Helms JA. It's all in your head: new insights into craniofacial development and deformation. J Anat. 2005;207:461–477. doi: 10.1111/j.1469-7580.2005.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin E, Creuzet S, Le Douarin NM. The contribution of the neural crest to the vertebrate body. Adv Exp Med Biol. 2006;589:96–119. doi: 10.1007/978-0-387-46954-6_6. [DOI] [PubMed] [Google Scholar]
- Wang G, Silva J, Krishnamurthy K, Tran E, Condie BG, Bieberich E. Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J Biol Chem. 2005;280:26415–26424. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- Bieberich E. Ceramide signaling in cancer and stem cells. Future Lipidol. 2008;3:273–300. doi: 10.2217/17460875.3.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica BA, Movsesyan VA, Lea PMt, Faden AI. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3beta, and induction of the mitochondrial-dependent intrinsic caspase pathway. Mol Cell Neurosci. 2003;22:365–382. doi: 10.1016/s1044-7431(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Mecha M, Rabadan MA, Pena-Melian A, Valencia M, Mondejar T, Blanco MJ. Expression of TGF-betas in the embryonic nervous system: analysis of interbalance between isoforms. Dev Dyn. 2008;237:1709–1717. doi: 10.1002/dvdy.21558. [DOI] [PubMed] [Google Scholar]
- Gosain AK, Recinos RF, Agresti M, Khanna AK. TGF-beta1, FGF-2, and receptor mRNA expression in suture mesenchyme and dura versus underlying brain in fusing and nonfusing mouse cranial sutures. Plast Reconstr Surg. 2004;113:1675–1684. doi: 10.1097/01.prs.0000117362.33347.43. [DOI] [PubMed] [Google Scholar]
- Krieglstein K, Strelau J, Schober A, Sullivan A, Unsicker K. TGF-beta and the regulation of neuron survival and death. J Physiol. 2002;96:25–30. doi: 10.1016/s0928-4257(01)00077-8. [DOI] [PubMed] [Google Scholar]
- Heine U, Munoz EF, Flanders KC, Ellingsworth LR, Lam HY, Thompson NL, et al. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987;105 (6 Pt 2:2861–2876. doi: 10.1083/jcb.105.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders KC, Ludecke G, Engels S, Cissel DS, Roberts AB, Kondaiah P, et al. Localization and actions of transforming growth factor-beta s in the embryonic nervous system. Development. 1991;113:183–191. doi: 10.1242/dev.113.1.183. [DOI] [PubMed] [Google Scholar]
- Zucker RM, Hunter ES, III, Rogers JM. Apoptosis and morphology in mouse embryos by confocal laser scanning microscopy. Methods. 1999;18:473–480. doi: 10.1006/meth.1999.0815. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Adams JA, Hogan EL. Maternal alcohol consumption increases sphingosine levels in the brains of progeny mice. Neurochem Res. 2007;32:2217–2224. doi: 10.1007/s11064-007-9445-3. [DOI] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- Lombard Z, Tiffin N, Hofmann O, Bajic VB, Hide W, Ramsay M. Computational selection and prioritization of candidate genes for fetal alcohol syndrome. BMC Genomics. 2007;8:389. doi: 10.1186/1471-2164-8-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Powrozek T, Goodlett CR, Li TK. Moderate alcohol exposure compromises neural tube midline development in prenatal brain. Brain Res. 2003;144:43–55. doi: 10.1016/s0165-3806(03)00158-5. [DOI] [PubMed] [Google Scholar]
- Marasas WF, Riley RT, Hendricks KA, Stevens VL, Sadler TW, Gelineau-van Waes J, et al. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: a potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J Nutr. 2004;134:711–716. doi: 10.1093/jn/134.4.711. [DOI] [PubMed] [Google Scholar]
- Gelineau-van Waes J, Starr L, Maddox J, Aleman F, Voss KA, Wilberding J, et al. Maternal fumonisin exposure and risk for neural tube defects: mechanisms in an in vivo mouse model. Birth Defects Res A Clin Mol Teratol. 2005;73:487–497. doi: 10.1002/bdra.20148. [DOI] [PubMed] [Google Scholar]
- Vance JE. Molecular and cell biology of phosphatidylserine and phosphatidylethanolamine metabolism. Prog Nucleic Acid Res Mol Biol. 2003;75:69–111. doi: 10.1016/s0079-6603(03)75003-x. [DOI] [PubMed] [Google Scholar]
- Vos JP, de Haas CG, van Golde LM, Lopes-Cardozo M. Relationships between phosphatidylcholine, phosphatidylethanolamine, and sphingomyelin metabolism in cultured oligodendrocytes. J Neurochem. 1997;68:1252–1260. doi: 10.1046/j.1471-4159.1997.68031252.x. [DOI] [PubMed] [Google Scholar]
- Fisher MC, Zeisel SH, Mar MH, Sadler TW. Perturbations in choline metabolism cause neural tube defects in mouse embryos in vitro. FASEB J. 2002;16:619–621. doi: 10.1096/fj.01-0564fje. [DOI] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF, Dempsey RJ. Citicoline: neuroprotective mechanisms in cerebral ischemia. J Neurochem. 2002;80:12–23. doi: 10.1046/j.0022-3042.2001.00697.x. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Hoffmann R, Sieber-Blum M. Mitogenic and anti-proliferative signals for neural crest cells and the neurogenic action of TGF-beta1. Dev Dyn. 1997;208:375–386. doi: 10.1002/(SICI)1097-0177(199703)208:3<375::AID-AJA8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy K, Dasgupta S, Bieberich E. Development and characterization of a novel anti-ceramide antibody. J Lipid Res. 2007;48:968–975. doi: 10.1194/jlr.D600043-JLR200. [DOI] [PubMed] [Google Scholar]
- Wang G, Krishnamurthy K, Bieberich E. Regulation of primary cilia formation by ceramide. J Lipid Res. 2009;5:2103–2110. doi: 10.1194/jlr.M900097-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]