Abstract
The activation of nuclear factor kappa B (NF-κB) p50/RelA is a key event in ischemic neuronal injury, as well as in brain ischemic tolerance. We tested whether epigenetic mechanisms affecting the acetylation state of RelA might discriminate between neuroprotective and neurotoxic activation of NF-κB during ischemia. NF-κB activation and RelA acetylation were investigated in cortices of mice subjected to preconditioning brain ischemia or lethal middle cerebral artery occlusion (MCAO) and primary cortical neurons exposed to preconditioning or lethal oxygen-glucose deprivation (OGD). In mice subjected to MCAO and in cortical neurons exposed to lethal OGD, activated RelA displayed a high level of Lys310 acetylation in spite of reduced total acetylation. Also, acetylated RelA on Lys310 interacted strongly with the CREB-binding protein (CBP). Conversely, RelA activated during preconditioning ischemia appeared deacetylated on Lys310. Overexpressing RelA increased Bim promoter activity and neuronal cell death both induced by lethal OGD, whereas overexpressing the acetylation-resistant RelA-K310R, carrying a mutation from Lys310 to arginine, prevented both responses. Pharmacological manipulation of Lys310 acetylation by the sirtuin 1 activator resveratrol repressed the activity of the Bim promoter and reduced the neuronal cell loss. We conclude that the acetylation of RelA in Lys310 dictates NF-κB-dependent pro-apoptotic responses and represents a suitable target to dissect pathological from neuroprotective NF-κB activation in brain ischemia.
Keywords: RelA acetylation, MCAO, OGD, ischemic preconditioning
Brain ischemia is a leading cause of mortality and neurological disability. During brain ischemia, nuclear factor kappa B (NF-κB) rapidly activates in neurons and glial cells, where it regulates inflammatory and apoptotic events characterizing the pathophysiology of post-ischemic injury.1, 2, 3, 4 NF-κB activation in neurons, rather than in glial cells, has a prominent role in post-ischemic cell loss.4, 5 NF-κB is a dimeric transcription factor created by the association of different subunits: p50, p65/RelA, p52, RelB and c-Rel. The most prevalent activated dimer observed after occlusion of the middle cerebral artery (MCAO) is p50/RelA.6, 7 Accordingly, the MCAO-induced infarct size is reduced in p50 knockout mice,2 as well as in mice carrying a brain-conditional deletion of RelA.7 We showed that activation of the p50/RelA dimer is associated with inhibition of c-Rel-containing dimers in ischemic brain tissues and in primary cortical neurons exposed to oxygen-glucose deprivation (OGD).8 Selective targeting of c-Rel and RelA revealed that activation of c-Rel-containing dimers increases cell resistance to OGD, whereas activation of p50/RelA contributes to the cell-death program. The effect of c-Rel dimers relies on promoter activation of the anti-apoptotic Bcl-xL gene8 and mediates neuroprotection induced by leptin9 or agonists at metabotropic glutamate receptor type 5.10 The deleterious effect of p50/RelA in brain ischemia depends on RelA-induced expression of pro-apoptotic Bim and Noxa genes.7, 8 However, p50/RelA is also involved in the regulation of a variety of physiological processes. Its constitutive activity is required for brain neuron survival and neurite elongation during brain development.11, 12 In mature neurons, p50/RelA selectively localizes at the synaptic level, from which point it moves to the nucleus to transmute synaptic signals into altered gene expression, regulation of synaptic plasticity and memory formation.13, 14, 15 Furthermore, the activation of p50/RelA has a role in brain tolerance, the adaptive response induced by a sublethal insult, which preserves brain health against subsequent lethal injury.16 Thus, the opposing effects elicited by NF-κB activation in cell survival remain to be elucidated. Recent studies in tumor and peripheral cells have revealed the post-translational regulation of RelA, including reversible phosphorylation and acetylation,17 which modulate p50/RelA transcriptional activity on target genes.18 RelA is acetylated after cell activation by tumor necrosis factor-α, phorbol myristate acetate or other stimuli. RelA acetylation may occur at multiple sites, including lysines (Lys) 122, 123, 218, 221 and 310.17 The epigenetic regulators of histone proteins, acetyltransferases p300/CREB-binding protein (CBP) and p/CAF, appear to have a major role in the in vivo acetylation of RelA.19 Site-specific acetylation of RelA regulates discrete biological actions of the NF-κB complex. For example, acetylation of Lys218 and 221 increases the DNA binding affinity of RelA for the κB enhancer and impairs RelA assembly with newly synthesized IκBα,19 whereas acetylation of RelA at Lys122 and 123 inhibits its transcriptional activity.17 Acetylation of Lys310 does not modulate DNA binding or IκBα assembly, but markedly enhances the NF-κB transactivation of pro-inflammatory genes. Acetylation of Lys310 is required for the full transcriptional activity of RelA. Abolishing Lys310 acetylation by mutating Lys310 to arginine significantly inhibits the transactivation of NF-κB and the expression of inflammatory cytokines,19, 20 possibly by stabilizing Set-9 factor, which leads to the methylation of Lys314-315 and proteasomal degradation of RelA.21 Selective deacetylation of Lys310 by sirtuin 1 (SIRT1), a class III histone deacetylase, inhibits the transcriptional activity of RelA22 and prevents the β-amyloid-induced release of neurotoxic factors from microglial cells.23 However, the role of RelA acetylation in NF-κB-mediated neuronal injury during brain ischemia remains elusive.
We investigated changes in c-Rel activation and RelA acetylation in response to preconditioning or lethal ischemia and the role of these changes in ischemia-mediated gene transcription and neuronal cell death.
Results
Neuronal activation of NF-κB p50/RelA in preconditioning and lethal ischemia
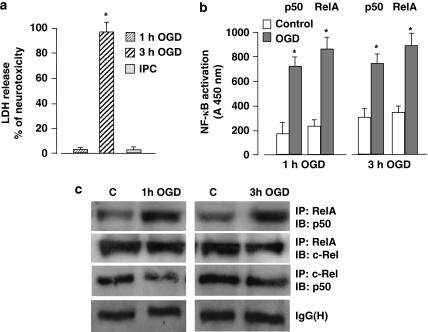
It has been shown that as an early response to ischemic brain injury, NF-κB p50 and RelA rapidly activate and drive post-ischemic neuronal apoptosis. 4, 5, 7, 8 We previously showed that in primary mouse cortical neurons exposed to OGD apoptosis precedes necrosis, as indicated by the early TUNEL-positivity displayed by the cells within 6 h after the OGD and the parallel release of cytochrome c in the cytosol in absence of lactate dehydrogenase (LDH) release. Subsequent necrosis causes progressive elevation of extracellular LDH level that becomes clearly detectable in the medium 24 h after the OGD exposure.8 Thus, we here measured delayed LDH level as a final marker of cell death. Either in primary cortical neurons or in mouse ischemic cortices, enhanced activation of p50/RelA correlates with the diminished activation of RelA/c-Rel, an event that contributes to neuronal vulnerability. In order to analyze and compare the NF-κB dimers induced by preconditioning or lethal OGD, we set up an ischemic preconditioning (IPC) model by exposing the cortical neurons to 1 h OGD and, the next day, to 3 h OGD. The 1 h OGD condition did not affect cell viability per se and completely abolished the cell death induced by longer OGD applied 24 h later (Figure 1a). Enzyme-linked immunosorbent assay (ELISA) analysis of NF-κB showed similar increases in RelA and p50 DNA-binding activity in nuclear extracts of cells exposed to 1 and 3 h OGD (Figure 1b). The co-immunoprecipitation analysis of heterodimers composed of p50, RelA and c-Rel revealed superimposable increases in levels of p50/RelA under both conditions. No increase was evident in the levels of p50/c-Rel and RelA/c-Rel complexes (Figure 1c). These results indicate that p50/RelA, but not c-Rel dimers contribute to NF-κB activation in cells exposed to preconditioning, as well as lethal OGD.
Figure 1.
(a) Primary cortical neurons were exposed to 1 h OGD and then 24 h later to 3 h OGD. The next day, cell viability was measured by LDH assay. Sub-lethal ischemic injury totally prevented the 3 h OGD-mediated neurotoxicity. Bars are means±S.E.M. of three separate experiments run in triplicate; *P<0.01 versus the corresponding control value. (b) Activation of p50 and RelA was evaluated by ELISA analysis in nuclear extracts from cortical cells exposed to 1 or 3 h OGD. Bars are means±S.E.M. of three separate experiments; *P<0.05 versus the corresponding control value. (c) Representative picture of co-immunoprecipitation analysis of p50, RelA and c-Rel dimers in nuclear extracts from primary cortical neurons showed a high level of p50/RelA complex activation after 1 or 3 h OGD, whereas the activation of c-Rel dimers decreased slightly
RelA acetylation and interaction with CBP differentiate p50/RelA activation in neuronal cells during lethal as opposed to preconditioning ischemia
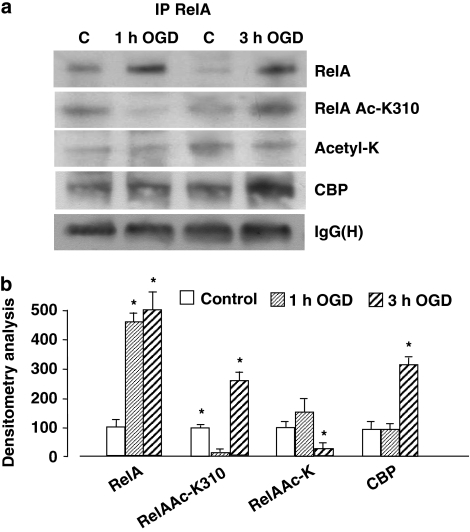
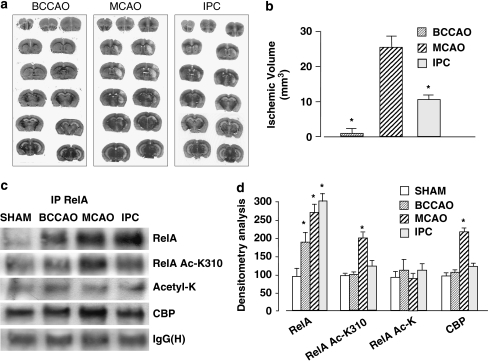
We checked the possibility of diverse RelA acetylation in two ischemic conditions, 1 and 3 h OGD. RelA was immunoprecipitated from nuclear extracts and its acetylation was checked using a specific anti-Acetyl-RelA Lys310 antibody (RelA Ac-K310) in comparison with an anti-Acetyl-Lys antibody recognizing general RelA acetylation. We observed that acetylation at Lys310 was reduced after 1 h OGD but increased significantly after 3 h OGD, in spite of reduced levels of overall RelA acetylation (Figure 2a and b). The diverse acetylation states of RelA correlated with diverse levels of interaction with the histone acetyl transferase CBP. RelA showed higher association with CBP after lethal OGD, but not after preconditioning ODG (Figure 2a and b). Similar results were obtained in an in vivo mouse model of brain ischemia and IPC.24 Mice were exposed to diverse ischemic insults: (i) 5 min bilateral common carotid arteries were occluded (BCCAO) (preconditioning ischemia); (ii) 20 min MCAO; and (iii) IPC, that is, BCCAO followed by MCAO the next day. Ischemic areas (Figure 3a) and infarct volumes (Figure 3b) were evaluated 3 days later. BCCAO induced no brain damage, but it significantly reduced the ischemic volume in mice subjected to MCAO (Figure 3b). We tested RelA acetylation in cortical nuclear extracts prepared 4 h after mice were exposed to the various conditions (Figure 3c). Higher RelA activation was evident in BCCAO, MCAO and IPC extracts, but acetylation at Lys310 increased only in mice exposed to MCAO. Concomitantly with Lys310 acetylation, RelA also displayed higher interaction with CBP in the MCAO extracts (Figure 3c and d). These results show that RelA acetylation on Lys310 discriminates between RelA activation after neurotoxic ischemia and that induced by preconditioning ischemia.
Figure 2.
(a) Immunoprecipitation analysis of RelA acetylation and association with CBP in nuclear extracts of primary cortical neurons exposed to 1 or 3 h OGD. RelA Lys310 acetylation decreased after 1 h OGD and increased after 3 h OGD. Total RelA acetylation was not altered by 1 h OGD but was reduced by 3 h OGD. RelA association with CBP increased in nuclear extracts of cells subjected to 3 h OGD. The signal given by IgG(H) was used as a control for the quality of the immunoprecipitation. Similar results were obtained in at least four separate experiments. (b) Values from densitometric analysis of immunoblot bands are expressed as a percentage of the corresponding control value. Error bars depict means±S.E.M.; *P<0.05 versus control
Figure 3.
(a) Representative images of infarct areas in brain coronal sections of mice exposed to BCCAO for 5 min or to MCAO for 20 min. In preconditioning experiments (IPC), mice were subjected to BCCAO and the next day to MCAO. Ischemic lesions were evaluated 3 days later. (b) Infarct volume in mice subjected to BCCAO, MCAO or IPC. Data are reported as means±S.E.M. (n=9 or 10 animals per group); *P<0.05 versus MCAO value. (c) Representative picture of co-immunoprecipitation analysis of RelA acetylation in nuclear extracts of mice exposed to BCCAO, MCAO or IPC (n=3 per group). Nuclear extracts were prepared 4 h after the end of each experimental condition. Acetylation on the Lys310 residue, as well as levels of the RelA/CBP complex, increased in mice exposed to MCAO. (d) Densitometric analysis of immunoblot bands. Values are expressed as a percentage of the control (Sham) value. Error bars depict means±S.E.M.; *P<0.05 versus control
Acetylation of RelA on Lys 310 is required for toxic effects elicited by NF-κB activation
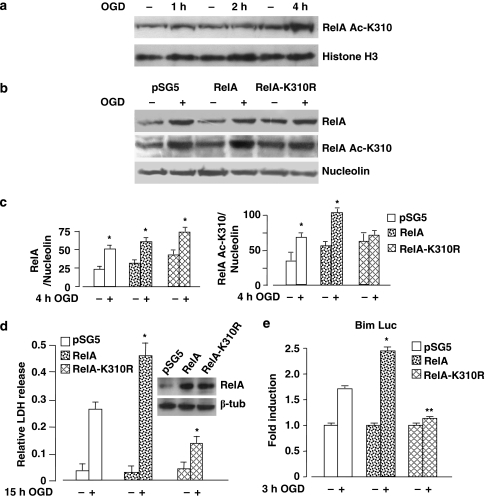
The relevance of RelA acetylation on Lys310 in the NF-κB-mediated neurotoxicity after acute ischemic injury was initially investigated in neuronally differentiated SK-N-SH cells. Under exposure to retinoic acid (RA), SK-N-SH neuroblastoma cells switch from a non-neuronal to a neuronal-like phenotype25 and express vulnerability to OGD by NF-κB RelA activation.8 Neuronal cells were exposed to OGD for 1, 2 and 4 h. Western blot analysis of nuclear extracts confirmed increased acetylation of RelA on Lys310 after 4 h ODG, as observed in primary cortical neurons (Figure 4a). The neuronal cultures were transiently transfected with control empty vector (pSG5) or with expression plasmids coding for human wild-type RelA or RelA carrying a mutation from Lys310 to arginine (RelA-K310R). As arginine has the same polar side chain and charge as lysine but cannot be acetylated, this mutated form of RelA was used as a negative control to test the biological effects of specific Lys310 acetylation.19 Around 24 h after transfection, the nuclear translocation of RelA and its acetylation were verified by immunoblot analysis in nuclear extracts of cells exposed to 4 h OGD. We found that OGD promoted a marked upregulation of nuclear RelA in cells transfected with empty vector, as well as in cells overexpressing wild-type RelA or RelA-K310R (Figure 4b and c). As expected, the concomitant increase in acetylation on Lys310 after OGD was evident in control and RelA-overexpressing cells, but negligible in the RelA-K310R-transfected cells (Figure 4b and c). In order to test the possibility that this unique modification was specifically responsible for the regulation of neuronal injury, transfected cells were exposed to OGD for 15 h and cell viability was measured by LDH release in the medium 24 h later. Cells transfected with the empty vector were vulnerable to OGD and the overexpression of wild-type RelA significantly increased cell death (Figure 4d), in line with previous findings.8 Instead, the RelA-induced enhancement of OGD-mediated neurotoxicity was completely abolished in cells overexpressing the RelA-K310R mutant construct. Similar experimental conditions were used to analyze the NF-κB-dependent activation of mouse Bim promoter-luciferase plasmid in cortical neurons during 3 h OGD followed by 4 h recovery, a time during which neurons express Bim and undergo apoptosis.7, 8 We previously showed that OGD induces the activity of the Bim promoter but not the activity of BimΔκB plasmid carrying a mutation at the κB site, demonstrating that Bim transcription during OGD is NF-κB specific.8 The OGD-induced Bim promoter was significantly enhanced by RelA overexpression, but fell to basal levels in cells overexpressing RelA-K310R (Figure 4e). These results show that NF-κB-mediated Bim transcription is completely dependent on Lys310 acetylation of RelA. This mechanism is pivotal in driving the deleterious effects of NF-κB activation during lethal ischemia.
Figure 4.
(a) Immunoblot analysis of nuclear extracts from differentiated SK-N-SH cells exposed to 1–4 h OGD. RelA Lys310 acetylation significantly increased after 4 h OGD. (b) Neuronal SK-N-SH cells were transfected with wild-type RelA and RelA-K310R plasmids or with a pSG5 empty vector for 24 h and then exposed to OGD for 4 h. The OGD-induced RelA acetylation on Lys310 was significantly reduced in RelA-K310R expressing cells. (c) Data from densitometric analysis of RelA and RelA Ac-K310 immunoblots are expressed as ratios to relative nucleolin levels. Bars are the means±S.E.M. of three separate experiments; *P<0.05 versus corresponding control value. (d) Cell survival was measured in SK-N-SH cells exposed to 15 h OGD. RelA overexpression significantly enhanced OGD toxicity, whereas RelA Ac-K310R overexpression prevented cell loss. Bars are the means±S.E.M. of three separate experiments; *P<0.01 versus OGD in pSG5-expressing cells. (e) Primary cortical neurons were transfected with Bim luciferase reporter plasmid together with wild-type RelA or RelA-K310R plasmids or pSG5 empty vector for 24 h and then exposed to 3 h OGD. Luciferase activity was measured after 4 h. RelA overexpression enhanced OGD-induced Bim promoter activity, whereas RelA-K310R overexpression totally inhibited such activity. Bars are the means±S.E.M. of three experiments run in triplicate; *P<0.01, **P<0.05 versus OGD value in pSG5-expressing neurons
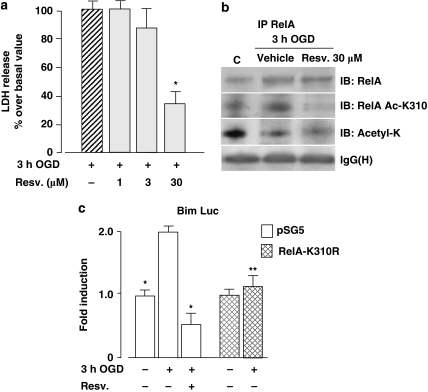
Resveratrol-mediated neuroprotection is associated with inhibition of RelA acetylation at the Lys310 residue as well as with the inhibition of Bim promoter activity
Deacetylation of Lys310 by the class III histone deacetylase SIRT1 inhibits the transcriptional activity of RelA.22 To determine whether pharmacological manipulation of RelA acetylation could repress NF-κB-mediated pro-apoptotic transcription during OGD, we tested the SIRT1-activating molecule resveratrol. Cortical neurons were exposed to OGD for 3 h and then treated for 24 h with resveratrol (1, 3 and 30 μM). Even when added during the post-ischemic period, resveratrol showed significant neuroprotective activity at a concentration of 30 μM (Figure 5a). Immunoprecipitation analysis of RelA in nuclear extracts confirmed that after OGD, the acetylation of Lys310 increased and total acetylation decreased. Treatment with 30 μM resveratrol for 2 h after OGD only partially reduced RelA activation, weakly affected general RelA acetylation and completely deacetylated the Lys310 residue (Figure 5b). In line with this drastic effect on Lys310 acetylation, resveratrol repressed the Bim promoter activity to levels below the baseline (Figure 5c). These results represent further evidence that during ischemia, the neurotoxic activation of NF-κB is associated with RelA acetylation at Lys310. Drugs that deacetylate RelA at Lys310, similar to the actions of resveratrol, can block post-ischemic transcription and neurodegeneration.
Figure 5.
(a) Cell death was evaluated in cortical neurons exposed to 3 h OGD and then treated with 1, 3, 30 μM resveratrol for 24 h. Resveratrol produced a significant neuroprotective effect at the 30 μM concentration. Bars are the means±S.E.M. of three separate experiments run in quadruplicate; *P<0.05 versus the corresponding control value. (b) Representative picture of co-immunoprecipitation analysis of RelA in nuclear extracts of primary cortical neurons exposed to 3 h OGD and to 30 μM resveratrol for 2 h during the post-OGD period. (c) Luciferase activity in cortical neurons co-transfected with Bim luciferase reporter plasmid and control vector (pSG5) or RelA-K310R expression plasmid. OGD stimulated luciferase activity in pSG5 but not in RelA-K310R-expressing cells. Resveratrol (30 μM), added after the OGD and maintained for 4 h, repressed luciferase activity in pSG5-cells. Bars are the means±S.E.M. of three separate experiments; *P<0.01, **P<0.05 versus OGD value in pSG5-expressing neurons
Discussion
Here, we report that although activation of NF-κB c-Rel dimers promotes neuronal resistance to environmental noxae,8, 9, 10 NF-κB activation following preconditioning OGD does not involve c-Rel-containing dimers. Moreover, the p50/RelA complex is activated in preconditioning, as well as in lethal OGD. The aberrant activation of p50/RelA dimer in neuronal cells, responsible for pro-apoptotic events in the post-ischemic period,6, 7, 8 is characterized by RelA acetylation at the Lys310 residue. It is unknown whether the acetylation of other lysine residues is also involved, though the increase of Lys310 acetylation occurred in spite of decreased total RelA acetylation. Moreover, acetylation of RelA on Lys310 differentiated the p50/RelA activation during lethal ischemia from that induced by preconditioning ischemia. The level of acetylation in Lys310 during OGD paralleled the increased interaction of RelA with CBP, in line with evidence that RelA acetylation on Lys310 is strictly dependent on the histone acetyltransferase activity of CBP/p300.19 Similar results were obtained in a model of in vivo brain ischemia and IPC. RelA is highly activated in cortices of mice exposed to either BCCAO or MCAO, as previously shown.16 Notably, the nuclear RelA level was high also in mice exposed to 1-day IPC, that is, BCCAO followed by MCAO on the next day. This result is in contrast to what was observed in models of 3-day IPC, in which severe ischemia induced 3 days after sublethal ischemia elicited weaker NF-κB activation as a consequence of enhanced IκBα expression during the 3-day interval.16 Our data support the idea that brain tolerance does not impair NF-κB translocation, but does affect RelA acetylation and transactivation. Thus, compared with BCCAO or IPC mice, the MCAO mice displayed a higher level of Lys310 acetylation that correlated with increased interaction of RelA with CBP.
To elucidate the relevance of Lys310 acetylation to RelA-mediated effects during ischemia, we transfected the neuronally differentiated SK-N-SH cells that were displaying RelA-dependent vulnerability to OGD8 with the RelA-K310R mutant construct. We found that OGD greatly enhanced Lys310 acetylation in control cells, as well as in cells transfected with RelA, whereas acetylation did not increase in cells overexpressing RelA-K310R. This result confirmed that the mutation of lysine to arginine in the RelA sequence impairs acetylation of the RelA 310 residue.19 Thus, OGD-mediated cell death was enhanced in RelA-overexpressing cells,8 but was completely impaired in RelA-K310R-transfected cultures. The occurrence of cell death at levels even lower than those observed in cells expressing empty vector suggests that deacetylated RelA-K310R can compete with native Acetyl-RelA Lys310 to maximally reduce neuronal vulnerability, as observed in the preconditioned cells.
NF-κB fine-tunes cell survival by regulating transcription of Bcl2 family genes endowed with either anti-apoptotic activity, as the Bcl-xL, or pro-apoptotic activity, as the BH3-only members Bim and Noxa.26 It has been shown that Bim and Noxa genes, being under the transcriptional control of RelA, are significantly induced 6 h after MCAO and their upregulation is abolished in mice carrying a brain-conditional deletion of RelA.7 Evidence that cortical neurons exposed to lethal OGD display NF-κB-dependent induction of Bim, but not Bcl-xL promoter, highlights the transcriptional specificity of p50/RelA activation during anoxic injury.8 Bim, similar to other BH3-only proteins, contributes to increase the mitochondrial permeability and activation of caspase cascade by directly activating pro-apoptotic Bax and Bak27 or by releasing Bax and Bak from their complexes with pro-survival Bcl-2 homologs.28, 29 Bim represents a converging point of diverse pro-apoptotic pathways during brain ischemia. In addition to NF-κB, Bim can be transcriptionally induced by Forkhead transcription factors (FOXO)3a as downstream target of the PTEN–Akt–FOXO3a pathway activated in ischemic brains.30 The interaction of Bim with the c-Jun N-terminal protein kinase (JNK), enhances Bim affinity to Bax and increases both proteins translocation to the mitochondria.31, 32 Intriguingly, JNK and FOXO3a signaling can be negatively modulated by the crosstalk with NF-κB in pro-survival pathways33, 34 raising additional queries about the mode of Bim regulation by NF-κB in brain ischemia. Here, we show that Bim transcription during OGD strictly depends on Lys310 acetylation of RelA. Bim transcription increased during OGD, it was enhanced in cortical neurons overexpressing RelA, but was completely inhibited in RelA-K310R-expressing cells. This result suggests that RelA acetylation on Lys310, through the recruitment of the coactivator CBP/p300, is a mechanism evolved to regulate inducible pro-apoptotic genes during ischemia in neuronal cells. Notably, the transactivation potential of c-Rel was reported not to be influenced by the CBP/p300 interaction.35 This difference may also account for diverse regulation of NF-κB target genes by c-Rel and RelA during ischemia.8 The specific RelA acetylation on Lys310 represents a molecular target to modify gene transcription and neuronal resilience to ischemic injury.
Yeung and colleagues22 originally demonstrated that the histone deacetylase SIRT1 can interact with RelA to inhibit gene transcription by deacetylating RelA at Lys310, without modifying the other lysine residue. These results suggested that acetylated Lys310 might form a platform for the binding of a bromodomain-containing protein that is required for full transcriptional activity of RelA.36 Interestingly, SIRT1 protects against neurodegeneration, and SIRT1 levels rise rapidly in various neurotoxic and neurodegenerative conditions; these results suggest SIRT1 may represent a stress sensor molecule that is important for the neuroprotective adaptation response.37 We checked the effects induced in cortical neurons by resveratrol, a pharmacological activator of SIRT1.22 In line with previous evidence,37, 38 resveratrol protected neuronal cells in a concentration-dependent manner, even if added after exposure to OGD. This effect correlated with the capability of the compound to abolish Lys310 acetylation, in spite of a modest inhibition of RelA nuclear translocation and a minor reduction in total RelA acetylation. As a consequence of specific RelA deacetylation, resveratrol repressed the acetyl-RelA Lys310-dependent transcription of Bim promoter during OGD. We cannot rule out the fact that additional targets of SIRT1 deacetylase activity, including FOXO3, p53 and peroxisome proliferator, activated receptor gamma co-activator 1α (PGC-1α) transcription factors,37, 39 as well as mechanism other than SIRT1 activation38 may contribute to the neuroprotection by resveratrol. Nonetheless, the present data are consistent with the hypothesis that the use of pharmacological agents modulating SIRT1 activity affects the acetylation status of RelA protein at Lys310, as well as its pro-apoptotic transactivation potential.
The issue of RelA acetylation has been thought to explain the pro-inflammatory activity of p50/RelA in immune cells,40, 41 reactive astrocytes42 and microglial cells.23 Here, we demonstrate that in neuronal cells does the acetylation of RelA at Lys310 function as an intranuclear molecular switch that discriminates between the neurotoxic and the neuroprotective NF-κB pathway in brain ischemia. The acetylation of RelA at Lys310 may represent a new drugable target to reproduce the preconditioning-induced activation of NF-κB.
Materials and Methods
Cell culture
Primary cultures of mouse cortical neurons
Cortical neurons were prepared from 15-day-old embryonic mice, harvested with cesarean section from anaesthetized pregnant C57Bl/6 dams (Charles River, Italy) and cultured as previously described.8 Experiments were carried out at 11 days in vitro (DIV).
SK-N-SH cell culture
The human SK-N-SH neuroblastoma cell line was purchased from American Type Culture Collection (Rockville, MD, USA). Cells were grown at 37°C in a humidified atmosphere of 5% CO2, 95% O2 in Dulbecco's modified Eagle's medium (DMEM) (Euroclone, Milan, Italy) supplemented with fetal calf serum, 4 mM glutamine and 100 U/ml penicillin/streptomicin. The addition of 50 μM RA (Sigma, St. Louis, MO, USA) for 10–12 days induced mitotic arrest and differentiation into a neuronal-like phenotype.25
Cerebral ischemia models
Transient middle cerebral artery occlusion (MCAO)
Procedures involving animals were approved by the Institutional Animal Care Committee in compliance with the Italian guidelines for animal care (DL 116/92) and the European Communities Council Directive (86/609/EEC). C57Bl/6 male mice (Harlan, Milan, Italy) were exposed to IPC and/or transient (20 min) MCAO as previously described.24 For IPC, mice (n=9) were anesthetized, and bilateral common carotid arteries were occluded (BCCAO) for 5 min with microclips. After 24 h, mice underwent 20 min MCAO as reported.24 Parallel groups were subjected to BCCAO (n=9) or MCAO (n=9) alone. Infarcts were measured 3 days later to rule out transient neuroprotection. Examination of infarct volume was performed in brains frozen in liquid nitrogen to avoid post-mortem changes. To prepare nuclear extracts, mice were killed by decapitation 4 h after BCCAO (n=3), MCAO (n=3) or BCCAO and MCAO (IPC) (n=3).
OGD
Primary cortical neurons at 11 DIV were exposed to OGD as previously described,8 for 1 or 3 h. Cells recovered for 24 h in culture medium and were typically aerated in the incubator for the evaluation of cell viability. Resveratrol (1, 3 and 30 μM) (Calbiochem, Beeston Nottingham, UK) was added in the post-ischemic period. Nuclear proteins were extracted at the end of OGD or after an additional 2 h incubation with resveratrol. SK-N-SH neuronal cells were exposed to 1 to 4 h OGD for analysis of NF-κB in nuclear extracts. Cells were exposed to 15 h OGD and replaced in fresh DMEM without serum for 24 h for analysis of cell viability. Neuronal injury was evaluated by measuring the amount of LDH (Promega, Madison, WI, USA) released relative to total releasable LDH.8
Co-immunoprecipitation and western blot analyses
Nuclear protein extracts were prepared as previously described8 from primary cortical neurons and from differentiated SK-N-SH cells, immediately after OGD exposure or after an additional time as indicated, or from the cortices of mice exposed to 5 min BCCAO followed by 4 h reperfusion, 20 min of MCAO followed by 4 h reperfusion, 5 min BCCAO and 24 h later, 20 min MCAO (IPC) and 4 h reperfusion.
Co-immunoprecipitation studies and immunoblot analyses were carried out as previously described.8 In total, 20 μg of nuclear extracts were incubated at 4°C overnight with 2 μg/ml of goat polyclonal anti-RelA antibody (sc-372G, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and co-immunoprecipitated proteins were detected by immunoblotting using the following antibodies: rabbit polyclonal anti-p50 (1 : 500, ab7971 Abcam, Cambridge, UK), rabbit polyclonal anti-c-Rel (1 : 50, sc-71X Santa Cruz Biotechnology), rabbit polyclonal anti-RelA (1 : 200, sc-372, Santa Cruz Biotechnology), rabbit polyclonal anti-Acetyl-RelA (Lys310) (1 : 500, #3045, Cell Signaling, Danvers, MA, USA), rabbit polyclonal anti-Acetyl-Lys (1 : 500, #06-933 Upstate-Millipore, Billerica, MA, USA) and rabbit polyclonal anti-CBP (1 : 500, sc-583 Santa Cruz Biotechnology). For immunoblot analyses, nuclear proteins (25 μg proteins/sample) were resolved by 10% SDS/polyacrylamide gel. Immunodetection was performed by incubating the membrane overnight at 4°C, with the following primary antibodies: rabbit polyclonal anti-Acetyl-RelA (Lys310) (1 : 500, #3045 Cell Signaling), rabbit polyclonal anti-RelA (1 : 200, sc-372 Santa Cruz Biotechnology), rabbit polyclonal anti-histone H3 (1 : 1000, #9715 Cell Signaling) and rabbit polyclonal anti-C23 nucleolin (1 : 300, sc-13057 Santa Cruz Biotechnology). Quantification of protein expression was performed by densitometry analysis of immunoblots, using Gel Pro.3 analysis software (MediaCybernetics, MD, USA).
NF-κB activation
The binding of mouse p50 and RelA to the NF-κB-binding consensus sequence was measured in nuclear extracts using the ELISA-based Mercury TransFactor kit (BD Biosciences, San Jose, CA, USA) as previously described.10 Data are expressed as the absorbance difference observed in the presence of nuclear extracts and that observed in the absence of nuclear extracts.
Expression plasmids and transfections
The wild-type RelA plasmid8 was used as a template to produce the Lys-to-arginine mutant construct RelA-K310R using the Quick change site-directed method (Stratagene, La Jolla, CA, USA), and Pfu DNA Polymerase. The primers sequence synthesized for the mutagenesis were the following: RelA-K310R for 5′-AGGACATATGAGACCTTCAGGAGCATCATGAAGAAGAG-3′ RelA-K310R REV 5′-CTCTTCTTCATGATGCTCCTGAAGGTCTCATATGTCCT-3′ (nucleotide substitution is in bold). The results of mutagenesis were confirmed by sequencing clones with the following internal primer, K310R 5′-GCCTGCAGGCTCCTGTGCGT-3′. Restriction map analysis was further carried out to verify construct integrity, and expression was confirmed by immunoblotting with the anti-RelA antibody in differentiated SH-N-SK cells, using the following antibodies: rabbit polyclonal anti-RelA (1 : 200, sc-372, Santa Cruz Biotechnology) and mouse monoclonal anti-β-tubulin (1 : 1500, NeoMarkers, Fremont, CA, USA).
Transfection of differentiated SK-N-SH cells was carried out according to the manufacturer's instructions with Lipofectamine 2000 Reagent (LF 2000, Invitrogen Corp., Carlsbad, CA, USA), as previously described.8 Cells were transfected with expression plasmids encoding RelA, RelA-K310R or empty expression vector pSG5 as a negative control, for 24 h, before undergoing the OGD experiments.
Reporter gene assays
In order to evaluate mouse Bim promoter activity during OGD, cortical neurons were transfected at 10 DIV using LF 2000 Reagent with 0.2 μg/well of the Bim-pGL3 plasmid and 0.8 μg/well of RelA or RelA-K310R mutant construct or empty expression vector pSG5 as negative control, as previously described.8 To normalize the transfection efficiency, 0.02 μg/well Renilla luciferase (phRLTK) control plasmid (Promega) was used. After 24 h, neurons were exposed to 3 h OGD as described above. At the end of incubation, during the 4 h recovery in Neurobasal medium with 0.4% B27 supplement (Invitrogen Corp.), the cells expressing pSG5 were treated with resveratrol (30 μM). Cells were then harvested, and firefly and Renilla luciferase were measured by using Dual Luciferase Reporter Assay (Promega).
Statistics
NF-κB ELISA data were analyzed using one-way analysis of variance (ANOVA), followed by Dunnett's post hoc analysis to determine statistical significance. P<0.05 was considered significant. Columns represent the means±S.E.M. of at least four values. Data describing cell survival were analyzed by Kruskal–Wallis non-parametric ANOVA with adjustment for multiple comparisons. Data relative to densitometry analyses and luciferase reporter activity were analyzed using Student's t-test for independent data. P<0.05 was considered as significant.
Acknowledgments
This work was supported by grants from the Italian Ministry of Education, University and Scientific Research (MIUR)—PRIN 2006 and 2008; MIUR Center of Excellence for Innovative Diagnostics and Therapeutics (IDET) of Brescia University.
Glossary
- BCCAO
bilateral common carotid arteries occluded
- CBP
p300/CREB-binding protein
- DIV
days in vitro
- FOXO3a
Forkhead transcription factors
- IPC
ischemic preconditioning
- JNK
c-Jun N-terminal protein kinase
- LDH
lactate dehydrogenase
- Lys
lysine
- MCAO
occlusion of middle cerebral artery
- NF-κB
nuclear factor kappa B
- OGD
oxygen-glucose deprivation
- RA
retinoic acid
- SIRT1
sirtuin 1
The authors declare no conflict of interest.
References
- Clemens JA, Stephenson DT, Smalstig EB, Dixon EP, Little SP. Global ischemia activates nuclear factor-kappa B in forebrain neurons of rats. Stroke. 1997;28:1073–1080. doi: 10.1161/01.str.28.5.1073. [DOI] [PubMed] [Google Scholar]
- Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- Nurmi A, Perttu J, Koistinaho M, Zhang W, Juettler E, Karjalainem-Lindsberg ML, et al. Nuclear factor-κB contributes to infarction after permanent focal ischemia. Stroke. 2004;35:987–991. doi: 10.1161/01.STR.0000120732.45951.26. [DOI] [PubMed] [Google Scholar]
- Herrmann O, Baumann B, de Lorenzi R, Muhammad S, Zhang W, Kleesiek J, et al. IKK mediates ischemia-induced neuronal death. Nat Med. 2005;11:1322–1329. doi: 10.1038/nm1323. [DOI] [PubMed] [Google Scholar]
- Zhang W, Potrovita I, Tarabin V, Herrmann O, Beer V, Weih F, et al. Neuronal activation of NF-kappaB contributes to cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:30–40. doi: 10.1038/sj.jcbfm.9600004. [DOI] [PubMed] [Google Scholar]
- Crack PJ, Taylor JM, Ali U, Mansell A, Hertzog PJ. Potential contribution of NF-kappaB in neuronal cell death in the glutathione peroxidase-1 knockout mouse in response to ischemia-reperfusion injury. Stroke. 2006;37:1533–1538. doi: 10.1161/01.STR.0000221708.17159.64. [DOI] [PubMed] [Google Scholar]
- Inta I, Paxian S, Zhang W, Pizzi M, Sarnico I, Spano P, et al. Bim and Noxa are candidates to mediate the deleterious effect of the NF-κB subunit RelA in cerebral ischemia. J Neurosci. 2006;26:12896–12903. doi: 10.1523/JNEUROSCI.3670-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnico I, Lanzillotta A, Boroni F, Benarese M, Alghisi M, Schwaninger M, et al. NF-kappaB p50/RelA and c-Rel-containing dimers: opposite regulators of neuron vulnerability to ischaemia. J Neurochem. 2009;108:475–485. doi: 10.1111/j.1471-4159.2008.05783.x. [DOI] [PubMed] [Google Scholar]
- Valerio A, Dossena M, Bertolotti P, Boroni F, Sarnico I, Faraco G, et al. Leptin is induced in the ischemic cerebral cortex and exerts neuroprotection through NF-kappaB/c-Rel-dependent transcription. Stroke. 2009;40:610–617. doi: 10.1161/STROKEAHA.108.528588. [DOI] [PubMed] [Google Scholar]
- Pizzi M, Sarnico I, Boroni F, Benarese M, Steimberg N, Mazzoleni G, et al. NF-kappaB factor c-Rel mediates neuroprotection elicited by mGlu5 receptor agonists against amyloid beta-peptide toxicity. Cell Death Diff. 2005;12:761–772. doi: 10.1038/sj.cdd.4401598. [DOI] [PubMed] [Google Scholar]
- Bhakar AL, Tannis LL, Zeindler C, Russo MP, Jobin C, Park DS, et al. Constitutive nuclear factor-kappa B activity is required for central neuron survival. J Neurosci. 2002;22:8466–8475. doi: 10.1523/JNEUROSCI.22-19-08466.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez H, Hale VA, Dolcet X, Davies A. NF-kappaB signalling regulates the growth of neural processes in the developing PNS and CNS. Development. 2005;132:1713–1726. doi: 10.1242/dev.01702. [DOI] [PubMed] [Google Scholar]
- West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt B, Ndiaye D, Korte M, Pothion S, Arbibe L, Prüllage M, et al. NF-kappaB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling. Mol Cell Biol. 2006;26:2936–2946. doi: 10.1128/MCB.26.8.2936-2946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Activation of the nuclear factor-kappa B is a key event in brain tolerance. J Neurosci. 2001;21:4668–4677. doi: 10.1523/JNEUROSCI.21-13-04668.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Shaping the nuclear action of NF-κB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- Campbell KJ, Rocha S, Perkins ND. Active repression of antiapoptotic gene expression by RelA(p65) NF-kappa B. Mol Cell. 2004;13:853–865. doi: 10.1016/s1097-2765(04)00131-5. [DOI] [PubMed] [Google Scholar]
- Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-κB. EMBO J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, et al. NF-κB phosphorylation regulates RelA acetylation. Mol Cell Biol. 2005;25:7966–7975. doi: 10.1128/MCB.25.18.7966-7975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XD, Tajkorshis E, Chen LF. Functional interplay between acetylation and methylation of the RelA subunit of NF-kB. Mol Cell Biol. 2010;30:2170–2180. doi: 10.1128/MCB.01343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, et al. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- Faraco G, Blasi F, Min W, Wang ZQ, Moroni F, Chiarugi A. Brain ischemic preconditioning does not require PARP-1. Stroke. 2010;41:181–183. doi: 10.1161/STROKEAHA.109.567826. [DOI] [PubMed] [Google Scholar]
- Pizzi M, Boroni F, Bianchetti A, Moraitis C, Sarnico I, Benarese M, et al. Expression of functional NR1/NR2B-type NMDA receptors in neuronally differentiated SK-N-SH human cell line. Eur J Neurosci. 2002;16:2342–2350. doi: 10.1046/j.1460-9568.2002.02403.x. [DOI] [PubMed] [Google Scholar]
- Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp FR, et al. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J Neurosci. 2002;22:5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. H3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Strasser A.Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins Cell Death Differ 20029512Review. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xing D, Chen M. Bim(L) displacing Bcl-x(L) promotes Bax translocation during TNFalpha-induced apoptosis. Apoptosis. 2008;13:950–958. doi: 10.1007/s10495-008-0226-5. [DOI] [PubMed] [Google Scholar]
- Li D, Qu Y, Mao M, Zhang X, Li J, Ferriero D, et al. Involvement of the PTEN-AKT-FOXO3a pathway in neuronal apoptosis in developing rat brain after hypoxia-ischemia. J Cereb Blood Flow Metab. 2009;29:1903–1913. doi: 10.1038/jcbfm.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno S, Saito A, Hayashi T, Chan PH. The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. J Neurosci. 2004;24:7879–7887. doi: 10.1523/JNEUROSCI.1745-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Signore AP, Yin W, Cao G, Yin XM, Sun F, et al. Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J Cereb Blood Flow Metab. 2005;25:694–712. doi: 10.1038/sj.jcbfm.9600062. [DOI] [PubMed] [Google Scholar]
- Chariot A. The NF-kappaB-independent functions of IKK subunits in immunity and cancer. Trends Cell Biol. 2009;19:404–413. doi: 10.1016/j.tcb.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Papa S, Bubici C, Zazzeroni F, Pham CG, Kuntzen C, Knabb JR, et al. The NF-kappaB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ. 2006;13:712–729. doi: 10.1038/sj.cdd.4401865. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang X, Hussain S, Zheng Y, Sanjabi S, Ouaaz F, et al. Distinct roles of different NF-kappa B subunits in regulating inflammatory and T cell stimulatory gene expression in dendritic cells. J Immunol. 2007;178:6777–6788. doi: 10.4049/jimmunol.178.11.6777. [DOI] [PubMed] [Google Scholar]
- Huang B, Yang XD, Zhou MM, Ozato K, Chen LF. Brd4 coactivates transcriptional activation of NF-kappaB via specific binding to acetylated RelA. Mol Cell Biol. 2009;29:1375–1387. doi: 10.1128/MCB.01365-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval AP, Lin HW, Dave KR, Defazio RA, Della Morte D, Kim EJ, et al. Resveratrol and ischemic preconditioning in the brain. Curr Med Chem. 2008;15:1545–1551. doi: 10.2174/092986708784638861. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Granja AG, Sabina P, Salas ML, Fresno M, Revilla Y. Regulation of inducible nitric oxide synthase expression by viral A238L-mediated inhibition of p65/RelA acetylation and p300 transactivation. J Virol. 2006;80:10487–10496. doi: 10.1128/JVI.00862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:861–870. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Jana M, Pahan K. MAPK p38 regulates transcriptional activity of NF-kappaB in primary human astrocytes via acetylation of p65. J Immunol. 2007;179:7101–7109. doi: 10.4049/jimmunol.179.10.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]