Abstract
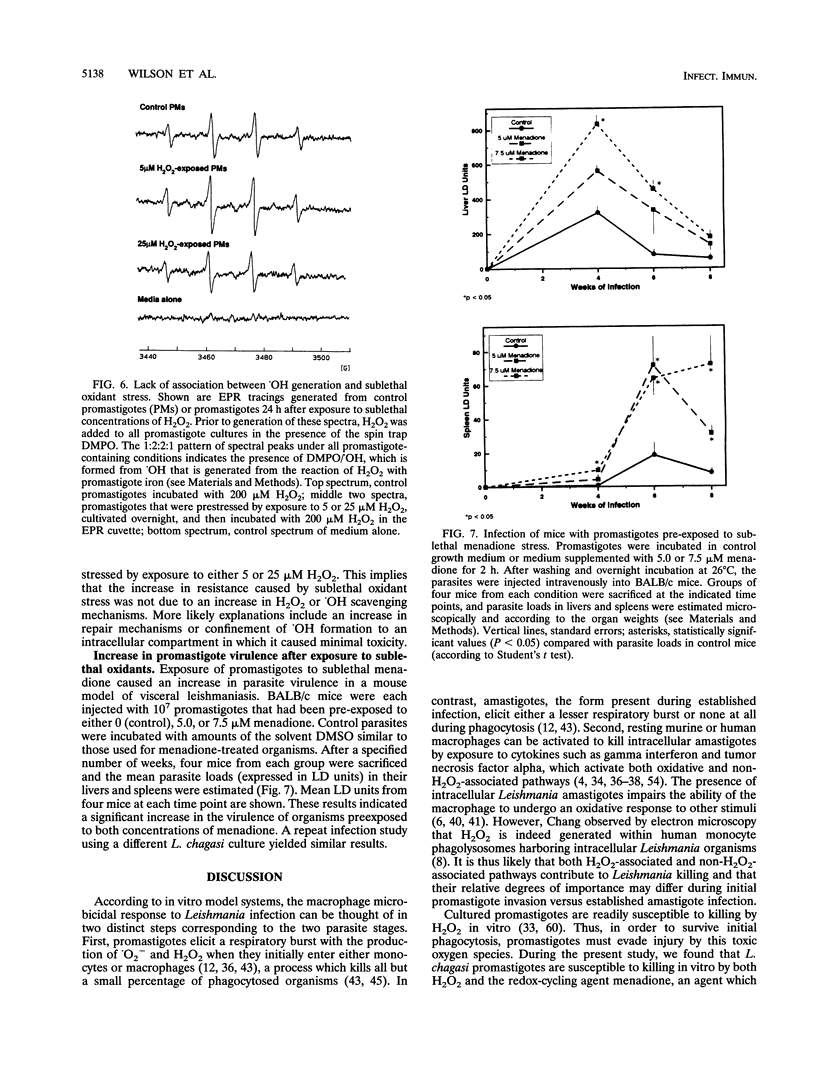
At the onset of infection, Leishmania promastigotes are phagocytized by mammalian macrophages. They must survive despite exposure to toxic oxidants such as hydrogen peroxide (H2O2) and superoxide (.O2-) generated during phagocytosis. We investigated the effects of these oxidants on Leishmania chagasi promastigotes and promastigote mechanisms for oxidant resistance. According to spin trapping and electron paramagnetic resonance spectrometry, .O2- could be generated by exposure of promastigotes to the redox-cycling compound menadione. Incubation in either menadione or H2O2 caused a concentration-dependent loss of promastigote viability. However, incubation in sublethal concentrations of H2O2 or menadione caused a stress response in promastigotes. This oxidant-induced response was associated with an increase in the amount of heat shock protein hsp70. Induction of a stress response by exposure of promastigotes either to heat shock or to sublethal oxidants (H2O2 or menadione) caused promastigotes to become more resistant to H2O2 toxicity. Sublethal menadione also caused promastigotes to become more virulent in a BALB/c mouse model of leishmaniasis. We previously correlated H2O2 cytotoxicity for promastigotes with the formation of hydroxyl radical (.OH) from H2O2. However, according to electron paramagnetic resonance spectrometry, the increase in H2O2 resistance after exposure to sublethal oxidants was not associated with diminished generation (i.e., scavenging) of .OH. These data suggest that there is a cross-protective stress response that occurs after exposure of L. chagasi promastigotes to heat shock or to sublethal H2O2 or .O2-, exposures that also occur during natural infection. This response results in increased resistance to H2O2 toxicity and increased virulence for a mammalian host.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang D., Liberek K., Skowyra D., Zylicz M., Georgopoulos C. Biological role and regulation of the universally conserved heat shock proteins. J Biol Chem. 1991 Dec 25;266(36):24233–24236. [PubMed] [Google Scholar]
- Blackwell J. M., Ezekowitz R. A., Roberts M. B., Channon J. Y., Sim R. B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985 Jul 1;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C., Moll H., Solbach W., Röllinghoff M. Tumor necrosis factor-alpha in combination with interferon-gamma, but not with interleukin 4 activates murine macrophages for elimination of Leishmania major amastigotes. Eur J Immunol. 1990 May;20(5):1131–1135. doi: 10.1002/eji.1830200528. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Cohen M. S., Rosen G. M. Detection of the production of oxygen-centered free radicals by human neutrophils using spin trapping techniques: a critical perspective. J Leukoc Biol. 1987 Apr;41(4):349–362. doi: 10.1002/jlb.41.4.349. [DOI] [PubMed] [Google Scholar]
- Buchmüller-Rouiller Y., Mauël J. Impairment of the oxidative metabolism of mouse peritoneal macrophages by intracellular Leishmania spp. Infect Immun. 1987 Mar;55(3):587–593. doi: 10.1128/iai.55.3.587-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Fujiwara T., Brennan P., McNeil M., Turco S. J., Sibille J. C., Snapper M., Aisen P., Bloom B. R. Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2453–2457. doi: 10.1073/pnas.86.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P. Cellular and molecular mechanisms of intracellular symbiosis in leishmaniasis. Int Rev Cytol Suppl. 1983;14:267–305. [PubMed] [Google Scholar]
- Chang K. P., Chaudhuri G., Fong D. Molecular determinants of Leishmania virulence. Annu Rev Microbiol. 1990;44:499–529. doi: 10.1146/annurev.mi.44.100190.002435. [DOI] [PubMed] [Google Scholar]
- Channon J. Y., Blackwell J. M. A study of the sensitivity of Leishmania donovani promastigotes and amastigotes to hydrogen peroxide. I. Differences in sensitivity correlate with parasite-mediated removal of hydrogen peroxide. Parasitology. 1985 Oct;91(Pt 2):197–206. doi: 10.1017/s0031182000057309. [DOI] [PubMed] [Google Scholar]
- Channon J. Y., Blackwell J. M. A study of the sensitivity of Leishmania donovani promastigotes and amastigotes to hydrogen peroxide. II. Possible mechanisms involved in protective H2O2 scavenging. Parasitology. 1985 Oct;91(Pt 2):207–217. doi: 10.1017/s0031182000057310. [DOI] [PubMed] [Google Scholar]
- Channon J. Y., Roberts M. B., Blackwell J. M. A study of the differential respiratory burst activity elicited by promastigotes and amastigotes of Leishmania donovani in murine resident peritoneal macrophages. Immunology. 1984 Oct;53(2):345–355. [PMC free article] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Davies K. J. Intracellular proteolytic systems may function as secondary antioxidant defenses: an hypothesis. J Free Radic Biol Med. 1986;2(3):155–173. doi: 10.1016/s0748-5514(86)80066-6. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Gebicki J., Gieseg S., Grant A. J., Simpson J. A. Hypothesis: a damaging role in aging for reactive protein oxidation products? Mutat Res. 1992 Sep;275(3-6):387–393. doi: 10.1016/0921-8734(92)90041-m. [DOI] [PubMed] [Google Scholar]
- Descoteaux A., Matlashewski G., Turco S. J. Inhibition of macrophage protein kinase C-mediated protein phosphorylation by Leishmania donovani lipophosphoglycan. J Immunol. 1992 Nov 1;149(9):3008–3015. [PubMed] [Google Scholar]
- Descoteaux A., Turco S. J., Sacks D. L., Matlashewski G. Leishmania donovani lipophosphoglycan selectively inhibits signal transduction in macrophages. J Immunol. 1991 Apr 15;146(8):2747–2753. [PubMed] [Google Scholar]
- Fairlamb A. H., Cerami A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu Rev Microbiol. 1992;46:695–729. doi: 10.1146/annurev.mi.46.100192.003403. [DOI] [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. A global response induced in Escherichia coli by redox-cycling agents overlaps with that induced by peroxide stress. J Bacteriol. 1989 Jul;171(7):3933–3939. doi: 10.1128/jb.171.7.3933-3939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudi T., Gupta C. M. hsp 70-like protein in rhesus erythrocyte cytosol and its interactions with membrane skeleton under heat and pathologic stress. J Biol Chem. 1993 Oct 5;268(28):21344–21350. [PubMed] [Google Scholar]
- Haas A., Goebel W. Microbial strategies to prevent oxygen-dependent killing by phagocytes. Free Radic Res Commun. 1992;16(3):137–157. doi: 10.3109/10715769209049167. [DOI] [PubMed] [Google Scholar]
- Hightower L. E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991 Jul 26;66(2):191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Ismail S. O., Skeiky Y. A., Bhatia A., Omara-Opyene L. A., Gedamu L. Molecular cloning, characterization, and expression in Escherichia coli of iron superoxide dismutase cDNA from Leishmania donovani chagasi. Infect Immun. 1994 Feb;62(2):657–664. doi: 10.1128/iai.62.2.657-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jättelä M. Overexpression of major heat shock protein hsp70 inhibits tumor necrosis factor-induced activation of phospholipase A2. J Immunol. 1993 Oct 15;151(8):4286–4294. [PubMed] [Google Scholar]
- Lawrence F., Robert-Gero M. Induction of heat shock and stress proteins in promastigotes of three Leishmania species. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4414–4417. doi: 10.1073/pnas.82.13.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Atkinson B. L., Giannini S. H., Van der Ploeg L. H. Structure and expression of the hsp 70 gene family of Leishmania major. Nucleic Acids Res. 1988 Oct 25;16(20):9567–9585. doi: 10.1093/nar/16.20.9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Millott S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990 Dec 15;145(12):4306–4310. [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- McNeely T. B., Turco S. J. Inhibition of protein kinase C activity by the Leishmania donovani lipophosphoglycan. Biochem Biophys Res Commun. 1987 Oct 29;148(2):653–657. doi: 10.1016/0006-291x(87)90926-0. [DOI] [PubMed] [Google Scholar]
- Meshnick S. R., Eaton J. W. Leishmanial superoxide dismutase: a possible target for chemotherapy. Biochem Biophys Res Commun. 1981 Oct 15;102(3):970–976. doi: 10.1016/0006-291x(81)91633-8. [DOI] [PubMed] [Google Scholar]
- Morgan R. W., Christman M. F., Jacobson F. S., Storz G., Ames B. N. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cartelli D. M. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest. 1983 Jul;72(1):32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Cell-mediated immune response in experimental visceral leishmaniasis. II. Oxygen-dependent killing of intracellular Leishmania donovani amastigotes. J Immunol. 1982 Jul;129(1):351–357. [PubMed] [Google Scholar]
- Murray H. W. Cellular resistance to protozoal infection. Annu Rev Med. 1986;37:61–69. doi: 10.1146/annurev.me.37.020186.000425. [DOI] [PubMed] [Google Scholar]
- Murray H. W. Interaction of Leishmania with a macrophage cell line. Correlation between intracellular killing and the generation of oxygen intermediates. J Exp Med. 1981 Jun 1;153(6):1690–1695. doi: 10.1084/jem.153.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Murray H. W. Susceptibility of Leishmania to oxygen intermediates and killing by normal macrophages. J Exp Med. 1981 May 1;153(5):1302–1315. doi: 10.1084/jem.153.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Teitelbaum R. F. L-arginine-dependent reactive nitrogen intermediates and the antimicrobial effect of activated human mononuclear phagocytes. J Infect Dis. 1992 Mar;165(3):513–517. doi: 10.1093/infdis/165.3.513. [DOI] [PubMed] [Google Scholar]
- Olivier M., Baimbridge K. G., Reiner N. E. Stimulus-response coupling in monocytes infected with Leishmania. Attenuation of calcium transients is related to defective agonist-induced accumulation of inositol phosphates. J Immunol. 1992 Feb 15;148(4):1188–1196. [PubMed] [Google Scholar]
- Olivier M., Brownsey R. W., Reiner N. E. Defective stimulus-response coupling in human monocytes infected with Leishmania donovani is associated with altered activation and translocation of protein kinase C. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7481–7485. doi: 10.1073/pnas.89.16.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Harcus J. L., Roberts D., Donowitz G. R. Differential survival of Leishmania donovani amastigotes in human monocytes. J Immunol. 1983 Oct;131(4):1994–1999. [PubMed] [Google Scholar]
- Pearson R. D., Harcus J. L., Symes P. H., Romito R., Donowitz G. R. Failure of the phagocytic oxidative response to protect human monocyte-derived macrophages from infection by Leishmania donovani. J Immunol. 1982 Sep;129(3):1282–1286. [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T. Mechanism of lethal effect of human serum upon Leishmania donovani. J Immunol. 1980 Nov;125(5):2195–2201. [PubMed] [Google Scholar]
- Pinelli E., Shapira M. Temperature-induced expression of proteins in Leishmania mexicana amazonensis. A 22-kDa protein is possibly localized in the mitochondrion. Eur J Biochem. 1990 Dec 12;194(2):685–691. doi: 10.1111/j.1432-1033.1990.tb15669.x. [DOI] [PubMed] [Google Scholar]
- Rey-Ladino J. A., Reiner N. E. Expression of 65- and 67-kilodalton heat-regulated proteins and a 70-kilodalton heat shock cognate protein of Leishmania donovani in macrophages. Infect Immun. 1993 Aug;61(8):3265–3272. doi: 10.1128/iai.61.8.3265-3272.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G., Talamas-Rohana P. Leishmania and the macrophage: a marriage of inconvenience. Immunol Today. 1989 Oct;10(10):328–333. doi: 10.1016/0167-5699(89)90188-6. [DOI] [PubMed] [Google Scholar]
- Searle S., Campos A. J., Coulson R. M., Spithill T. W., Smith D. F. A family of heat shock protein 70-related genes are expressed in the promastigotes of Leishmania major. Nucleic Acids Res. 1989 Jul 11;17(13):5081–5095. doi: 10.1093/nar/17.13.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle S., McCrossan M. V., Smith D. F. Expression of a mitochondrial stress protein in the protozoan parasite Leishmania major. J Cell Sci. 1993 Apr;104(Pt 4):1091–1100. doi: 10.1242/jcs.104.4.1091. [DOI] [PubMed] [Google Scholar]
- Searle S., Smith D. F. Leishmania major: characterisation and expression of a cytoplasmic stress-related protein. Exp Parasitol. 1993 Aug;77(1):43–52. doi: 10.1006/expr.1993.1059. [DOI] [PubMed] [Google Scholar]
- Smejkal R. M., Wolff R., Olenick J. G. Leishmania braziliensis panamensis: increased infectivity resulting from heat shock. Exp Parasitol. 1988 Feb;65(1):1–9. doi: 10.1016/0014-4894(88)90101-4. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Sherry B., Cerami A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med. 1989 Dec 1;170(6):2097–2104. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toye P., Remold H. The influence of temperature and serum deprivation on the synthesis of heat-shock proteins and alpha and beta tubulin in promastigotes of Leishmania major. Mol Biochem Parasitol. 1989 Jun 1;35(1):1–10. doi: 10.1016/0166-6851(89)90136-9. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Hardin K. K., Donelson J. E. Expression of the major surface glycoprotein of Leishmania donovani chagasi in virulent and attenuated promastigotes. J Immunol. 1989 Jul 15;143(2):678–684. [PubMed] [Google Scholar]
- Wilson M. E., Pearson R. D. Evidence that Leishmania donovani utilizes a mannose receptor on human mononuclear phagocytes to establish intracellular parasitism. J Immunol. 1986 Jun 15;136(12):4681–4688. [PubMed] [Google Scholar]
- Wilson M. E., Pearson R. D. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infect Immun. 1988 Feb;56(2):363–369. doi: 10.1128/iai.56.2.363-369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarley J. H., Britigan B. E., Wilson M. E. Hydrogen peroxide-mediated toxicity for Leishmania donovani chagasi promastigotes. Role of hydroxyl radical and protection by heat shock. J Clin Invest. 1991 Nov;88(5):1511–1521. doi: 10.1172/JCI115461. [DOI] [PMC free article] [PubMed] [Google Scholar]