Abstract
Purpose
In persons with infantile nystagmus (IN), visual acuity correlates with the duration of the foveation period of the nystagmus waveform, i.e., when the retinal image is on or near the fovea and moves with low velocity. In this study, we asked how acuity is affected by the non-foveating phases of the nystagmus waveform, when the velocity of retinal image motion is substantially higher.
Methods
Visual acuity was measured in three normal observers for high-contrast, 4-orientation Ts, presented during image motion that simulated either the whole jerk-IN waveform (whole-waveform) or only the foveation periods of the IN waveform (foveation-only). Simulated foveation durations ranged from 20 to 120 ms. For both motion waveforms, we displayed the acuity target for different number of cycles to examine if acuity benefits from multiple presentations of the stimulus.
Results
As expected, visual acuity improves with longer simulated foveation durations in both the whole-waveform and foveation-only conditions. Acuity is consistently better (by approximately 0.1 logMAR) in the foveation-only than the whole-waveform condition, indicating that the high-velocity image motion during the simulated IN waveform has a detrimental effect. This difference in acuity between the two waveform conditions increases with the number of cycles, apparently because summation occurs across cycles in the foveation-only condition but not in the whole-waveform condition.
Conclusions
In normal observers, visual acuity in the presence of a simulated nystagmus waveform is limited not only by the duration of the foveation periods, but also by the non-foveating phases of the waveform. However, because persons with IN report little or no motion smear in association with their nystagmus, it remains unclear whether the rapid retinal image motion during the non-foveating phases of the nystagmus waveform generates a similar degradation of visual acuity in IN.
Keywords: image motion, visual acuity, temporal summation, probability summation, infantile nystagmus, foveation period
Under optimal conditions, the normal human visual system can resolve fine details as small as 1 arc min of visual angle. This ability to resolve fine details (visual acuity) is compromised when the image of the object of regard moves across the retina at a speed faster than approximately 2°/s.1–3 The incessant to-and-fro eye movements that characterize infantile nystagmus (IN) have peak slow-phase velocities that are typically between 20 and 180°/s.4 Although these retinal image velocities would be expected to reduce visual acuity to between 20/80 and 20/320 (logMAR 0.6 to 1.22),2 visual acuity for people with IN is generally within the range of 20/32 to 20/63 (logMAR 0.2 to 0.5).5
Relatively good acuity in spite of the high retinal image velocities in IN is attributed to the presence of foveation periods in the nystagmus waveforms, i.e., brief intervals of relatively low eye-velocity when the target is imaged on or near the fovea.4,6,7 Presumably, reliable and useful information about the details of a target can be gathered and integrated during the foveation periods while the retinal image motion is slow. Evidence that the foveation periods contribute to good visual acuity is provided by the observations that visual acuity correlates with the average duration of the foveation periods in people with IN,4,8–11 and that acuity improves systematically with the duration of the foveation period when observers with normal vision view targets that move according to a simulated IN waveform.12–15 On the other hand, visual acuity has been reported not to improve significantly in observers with IN during near viewing, despite an attenuation of the intensity of the nystagmus and a prolongation of the foveation duration during convergence.16,17
A consequence of fast image motion is the production of motion smear that can blur the retinal image along the direction of motion. Bedell and Bollenbacher18 asked 10 observers with normal vision to estimate the length and brightness of perceived smear for small disk targets with suprathreshold luminances in the presence of retinal image motion to simulate that in jerk nystagmus. All observers reported a substantial amount of smear that increased with the luminance of the disk targets. In contrast, observers with IN reported substantially less motion smear than the normal observers, for disk targets that were set to equal multiples of the luminance detection threshold (see also Bedell & Tong19).
At least for observers with normal vision, the presence of perceived motion smear might interfere with the perception of acuity targets and thus degrade the measured acuity. However, to date it remains unclear if motion-induced smear affects visual acuity when the acuity target remains stationary and clear for part of the viewing period, and if acuity is affected, to what extent. The primary goal of this study was to examine if the motion smear that occurs during nystagmus-like image motion affects visual acuity. To do so, we measured visual acuity in observers with normal vision while the acuity target underwent image motion simulating a typical jerk-nystagmus waveform. This simulated waveform comprised three components: (1) a zero-velocity simulated foveation period, followed by (2) an accelerating ramp to simulate the nystagmus slow phase, and (3) a fast-return phase (the “beat”). By comparing visual acuity measured during only the simulated foveation period (i.e. when a stationary acuity target was briefly visible) with that during the entire waveform, we could assess whether or not the motion-induced smear produced by the slow and fast-return phases of the nystagmus waveform has an impact on visual acuity. Because nystagmus-like image motion produces a substantial amount of perceived motion smear in observers with normal vision,18 we predicted that the spatio-temporal interference between moving and stationary images of the target would degrade observers’ visual acuity.
In addition to the temporal integration of information across relatively brief intervals of time, the visual system also can integrate information that is presented in discrete packages that are separated in time. The presumed explanation for this effect is probability summation.20 Previously, Baron and Westheimer21 investigated how visual acuity varied with the time between a pair of briefly presented stationary targets and found no systematic effect. When observers with IN view an acuity target during successive foveation periods, probability summation predicts that acuity should improve with the number of times that the stationary target is presented. On the other hand, if motion smear results in a degradation of acuity, then this degradation may be exaggerated if a moving acuity target is presented multiple times during the non-foveating phases of the IN waveform. An auxiliary goal of this study was to evaluate the effect of repeated stimulus presentations on visual acuity for targets presented during only the foveation periods of the simulated nystagmus waveform, and for targets presented continuously during multiple cycles of the entire simulated IN waveform, when fast retinal image motion accompanied the stationary images during the simulated foveation periods.
METHODS
Stimuli and Apparatus
Visual acuity was measured psychometrically using four-orientation single T-stimuli constructed according to the Sloan configuration, i.e., with a stroke-width equal to one-fifth the height or the width of the letter. The T-stimuli were rendered as black letters (1.4 cd/m2) on a bright background of 58 cd/m2, at 98% Weber’s contrast. Stimuli were generated using a VSG2/3 graphics board (Cambridge Research Ltd., U.K.) and presented on a monochrome monitor (Image Systems, Hopkins, MN) at a frame rate of 239 Hz. This monitor was equipped with an ultra-fast decay phosphor (DP-104). Retinal image motion simulating that in IN was generated by having observers view the T-stimuli presented on the monitor after reflection from a galvanometer-mounted first-surface mirror located close to the right eye. The left eye was covered. The galvanometer-mounted mirror oscillated around a vertical axis to produce horizontal image motion simulating that in individuals with jerk IN (see below for details of the simulated nystagmus waveforms). During testing, we instructed observers to fixate one end of the excursion of the target, and not to attempt to track the moving stimuli. After each trial, observers used a joystick to indicate the orientation of the T-stimulus — up (⊤), down (⊥), right (⊣) or left (⊢). Feedback was not provided.
Simulated Nystagmus Waveforms
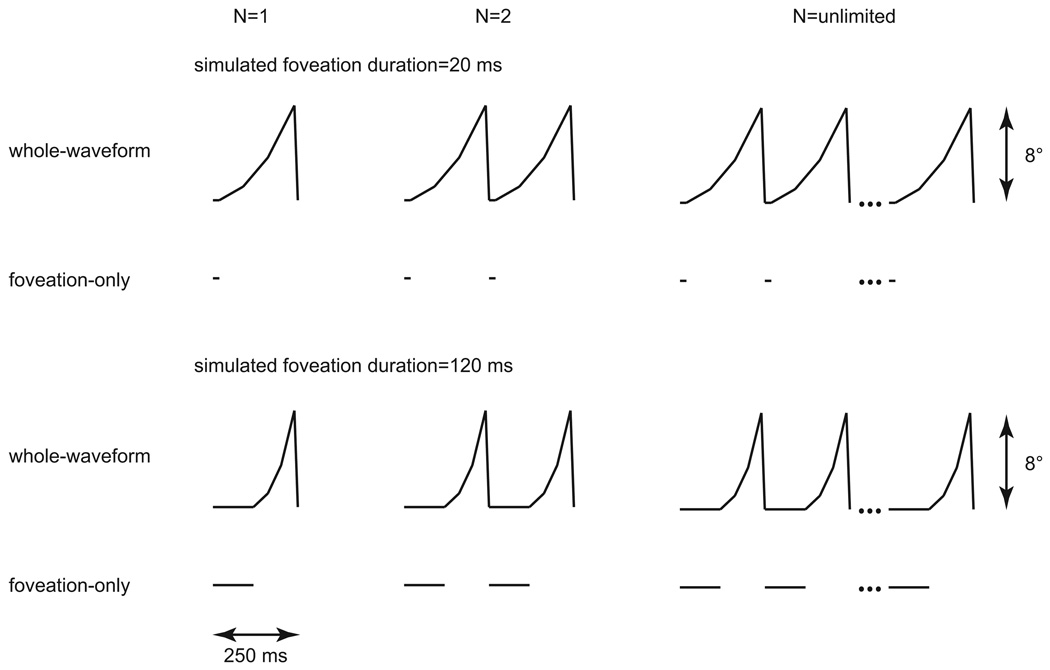
An accelerating ramp waveform drove the galvanometer-mounted mirror so that the resulting image motion simulated that in individuals with jerk nystagmus.4,12–15,22 The waveform comprised a zero-velocity component, designated as the “simulated foveation period”, followed by three ramp components of increasing velocity, in the ratio of 2:4:7, and then a rapid-velocity segment in the opposite direction to simulate the fast-return phase of jerk IN (see Fig. 1). The three ramp components were of the same duration, which was constrained by the duration of the simulated foveation period. The amplitude of the waveform was 8° and the frequency of the waveform was 4 Hz, which is typical of the frequency of idiopathic IN and more rapid than pursuit tracking can follow.12,23 Five simulated foveation durations were tested: 20, 40, 60, 80 and 120 ms.
Figure 1.
Idealized position traces showing the accelerating ramp waveforms used to simulate the retinal image motion in jerk nystagmus for the whole-waveform condition with simulated foveation durations of 20 and 120 ms. Each waveform has a frequency of 4 Hz and an amplitude of 8°. For comparison, traces for the foveation-only condition are shown underneath. Three different numbers of cycles of stimulus presentation are illustrated: one (N=1), two (N=2) and unlimited.
Testing Parameters
To evaluate whether the image motion that occurs outside of the zero-velocity simulated foveation periods has an impact on visual acuity, we compared the acuity measured for the “whole-waveform” condition of the simulated IN image motion (described above) with the acuity measured when the T-stimulus was presented only during the zero-velocity simulated foveation duration, in the “foveation-only” condition. Because there was no image motion during the simulated foveation duration, the foveation-only condition essentially measured static acuity for different exposure durations.
We also examined whether or not visual acuity benefited from summation across cycles of the nystagmus waveform. To do so, in different blocks of trials, we measured visual acuity when the T-stimulus was exposed for 1, 2, 3, 5 or 8 complete cycles of the waveform (see Fig. 1 for examples). In the case of the foveation-only condition, the T-stimulus was exposed only during the simulated foveation durations. For comparison, we also measured visual acuity when the T-stimulus was exposed for an unlimited number of waveform cycles, until the observer responded.
Experimental Design and Procedures
Testing conditions were blocked by the two primary parameters of interest — (1) whether or not the entire waveform was used and (2) the number of cycles of stimulus presentation. In each block of trials, we used the Method of Constant Stimuli to present the T-stimuli at five letter sizes such that observers’ performance accuracy of identifying the orientation of the Ts spanned a range between chance (25% correct) and perfect performance. For each set of data relating the percent-correct identification of the orientation of the T-stimuli with letter size, we used probit analysis to determine the letter size that corresponded to an identification accuracy of 62.5% on the psychometric function, which we took to represent the acuity threshold. Each datum point reported in this paper represents the averaged acuity measurement of the three observers (see below), with at least three repeated measurements of the same condition for each observer. The error bar associated with each datum point takes into account both within- and between-observer variability.
Observers
Three young observers (in their 20s or 30s) with corrected clinical visual acuities of 20/16 in each eye and normal ocular motility participated in the study. Two of the observers were emmetropic and one was a low myope who wore her correction of −0.50 DS during testing. Written consent was obtained from each observer after the experimental procedures were explained and before the collection of data.
RESULTS
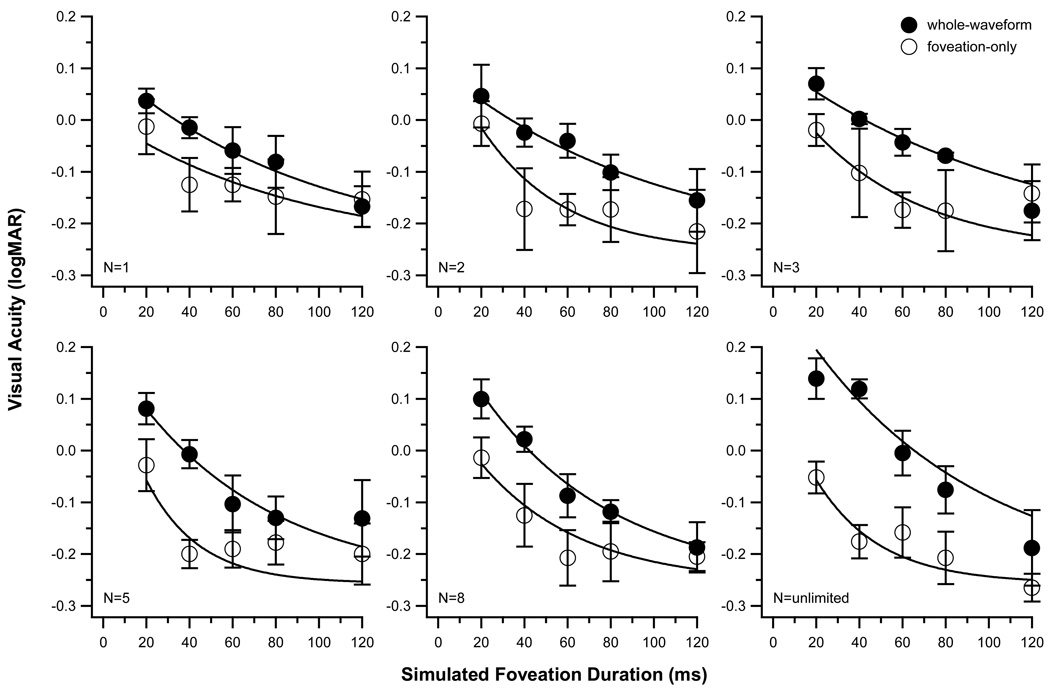
Visual acuity (in logMAR) is plotted as a function of simulated foveation duration (in ms) for different number of cycles of presentation (N) of the acuity target, for the whole-waveform (filled circles) and the foveation-only (unfilled circles) conditions in Fig. 2. Each datum point represents the averaged acuity of the three observers. To describe the change in visual acuity with the simulated foveation duration, we fit each set of data with an exponential function of the following form:
where y0 is the asymptotic acuity when the simulated foveation duration is not a limiting factor, A is the maximum degradation in acuity due to a shortening of the simulated foveation duration and τ is the time constant. The smooth line drawn through each set of data represents the exponential function fit. As in our previous studies,14,15 the asymptote (y0) was constrained to the average acuity of the observers when the stationary acuity target was presented for an unlimited duration. In the current study, the average static acuity of the three observers was −0.256 logMAR.
Figure 2.
Visual acuity (logMAR) is plotted as a function of simulated foveation durations (ms) for image motion that simulated the whole waveform of IN image motion (filled circles) or only the simulated foveations (unfilled circles). The six panels show data for different number of cycles of stimulus presentation, represented by the value of N. Data shown are averaged across the three observers. Error bars represent ± 1 SE. The smooth curve drawn through each set of data represents the best-fit exponential function fit (see text for details).
There are several key findings revealed in Fig. 2. Consistent with previous studies,12–15 visual acuity generally improves with longer simulated foveation durations (repeated-measures ANOVA: F(df = 4,8) = 43.21, Greenhouse-Geisser corrected p = 0.004), regardless of whether the T-stimulus was presented throughout the whole waveform of image motion or during the simulated foveation period only, and regardless of the number of cycles of stimulus presentation. Averaged across the different number of cycles of stimulus presentation (data shown in different panels), acuity improves by 0.25 ± 0.02 [SE] logMAR as the duration of the simulated foveation duration increases from 20 to 120 ms for the whole-waveform condition and 0.17 ± 0.02 logMAR for the foveation-only condition.
Figure 2 also shows that visual acuity in the foveation-only condition is consistently better than in the whole-waveform condition (repeated-measures ANOVA: F(df = 1,2) = 25.28, Greenhouse-Geisser corrected p = 0.037), implying that the high-velocity image motion during the simulated nystagmus waveform is detrimental to visual acuity. Averaged across the different number of cycles of stimulus presentation, visual acuity is approximately 0.1 logMAR better for the foveation-only than for the whole-waveform condition. The difference in acuity between the whole-waveform and foveation-only conditions generally becomes less as the simulated foveation duration becomes longer, decreasing to 0.029 logMAR ± 0.019 [SE] when the simulated foveation duration is 120 ms. However, the interaction between waveform condition and foveation duration does not reach statistical significance (F(df = 4,8) = 4.34, p = 0.108).
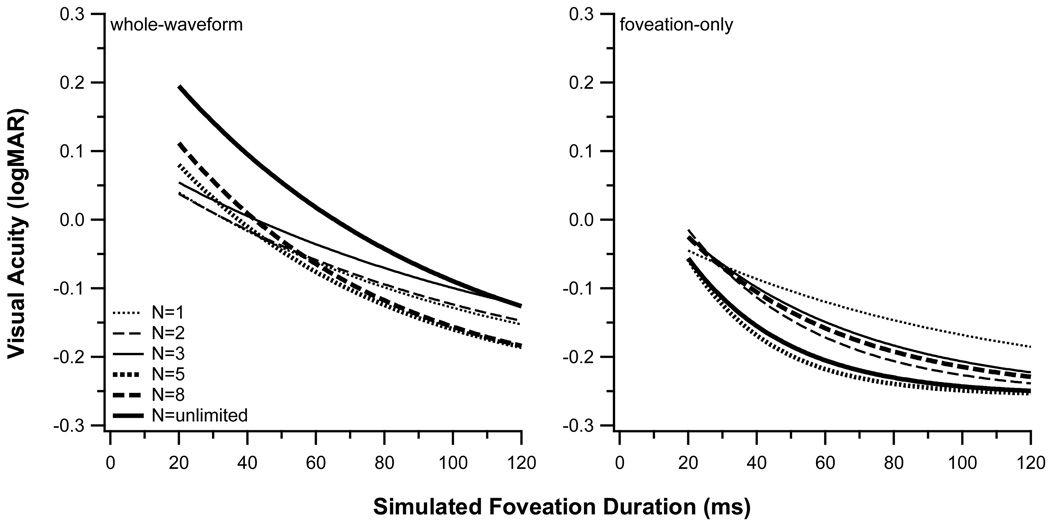
In the whole-waveform condition, acuity is poorer when the number of cycles of stimulus presentation is unlimited, compared with the conditions with fewer numbers of cycles. In contrast, acuity tends to improve with the number of cycles in the foveation-only condition (Fig. 3). The difference in acuity between the two waveform conditions increases significantly with the number of cycles (paired t(df = 4) = 4.04, p = 0.016). Taken together, these observations suggest that summation across multiple cycles produces better visual acuity in the foveation-only condition, but not in the whole-waveform condition.
Figure 3.
Visual acuity (logMAR) is plotted as a function of simulated foveation durations (ms) for (left) the whole-waveform and (right) the foveation-only condition. In each panel, the different curves represent the acuity vs. duration functions fit to the data for different number of cycles of stimulus presentation. These curves are the same as those shown in Fig. 2.
The temporal integration period represents the duration beyond which acuity shows no further improvement, and can be approximated by three times the time constant (τ) of the fitted exponential function.14,15 Table 1 lists the values of the parameters of the best-fit exponential function to each set of data shown in Figs. 2 and 3, as well as the estimated temporal integration period. The estimated temporal integration period is approximately the same when acuity is determined for one cycle of the foveation-only condition and one cycle of the whole-waveform condition (275 vs. 286 ms). When the acuity target is visible for more cycles of the simulated IN waveform, the estimated temporal integration period remains approximately the same in the whole-waveform condition (average = 254 ± 32 [SE] ms), but decreases significantly in the foveation-only condition to an average value of 114 ± 15 ms (paired tdf=4 = 5.05, p = 0.007). One possible interpretation of this outcome is that temporal integration continues across successive foveation periods in the foveation-only condition but that the intercalated periods of motion blur prevent any useful integration across cycles in the whole-waveform condition.
Table 1.
Parameters (±1 SE) of the fitted exponential functions and the estimated temporal integration period for each of the fitted curve shown in Fig. 2.
| Waveform condition | Number of cycles of target presentation (N) |
A (logMAR) | Time constant, τ (ms) |
Estimated temporal integration period (ms) |
|---|---|---|---|---|
| Foveation-only | 1 | 0.263±0.082 | 91.7±51.3 | 275.0 |
| 2 | 0.408±0.125 | 38.2±13.0 | 114.5 | |
| 3 | 0.341±0.078 | 51.9±19.2 | 155.6 | |
| 5 | 0.453±0.228 | 24.3±9.3 | 73.0 | |
| 8 | 0.355±0.093 | 46.7±16.3 | 140.1 | |
| unlimited | 0.395±0.138 | 29.4±11.1 | 88.2 | |
| Whole-waveform | 1 | 0.365±0.042 | 95.3±26.1 | 285.8 |
| 2 | 0.359±0.072 | 100.6±38.6 | 301.7 | |
| 3 | 0.368±0.025 | 117.0±14.5 | 351.0 | |
| 5 | 0.460±0.066 | 64.1±16.4 | 192.3 | |
| 8 | 0.509±0.067 | 61.5±10.8 | 184.4 | |
| unlimited | 0.579±0.068 | 80.4±18.3 | 241.1 | |
DISCUSSION
The principal goal of this study was to examine if the retinal smear that arises as a result of the non-foveating phases of nystagmus-like image motion affects visual acuity. We addressed this goal by comparing visual acuity during a simulated nystagmus waveform (the whole-waveform condition) with the acuity during simulated foveation periods alone (foveation-only condition). Our data show two important differences between visual acuity in these conditions — acuity is better in the foveation-only condition and generally improves at a faster rate as the simulated foveation duration increases, as compared with the whole-waveform condition.
What accounts for the difference in visual acuity between these two conditions? It is well known that acuity is degraded in the presence of fast retinal image motion.1,3,24–26 Because the retinal image velocity is high during the slow and fast-return phases of the simulated IN waveform, a degradation of acuity during these phases of the simulated nystagmus waveform is expected. However, it is unclear how the degraded visual information from the slow and fast-return phases of the simulated waveform combines with the information from the simulated foveation periods, when the target is stationary and acuity is expected to be better.
Previously, we presented evidence that the degradation of acuity in the presence of fast retinal image motion can be attributed to a shift in the size of the spatial filters that are used by the visual system to analyze moving compared with stationary visual targets.25,26 This explanation implies the spatial information that is extracted during the foveating and non-foveating phases of the simulated nystagmus waveform should be analyzed by separate spatial filters, with little or no interaction between their outputs. However, the uniformly poorer visual acuities measured during the whole-waveform condition indicate that information from the slow and/or the fast-return phases of the simulated nystagmus waveforms degrades the information that is available during the simulated foveation periods. Clearly, the visual system is not able to ignore the blurred spatial information from the non-foveating phases of the whole-waveform condition.
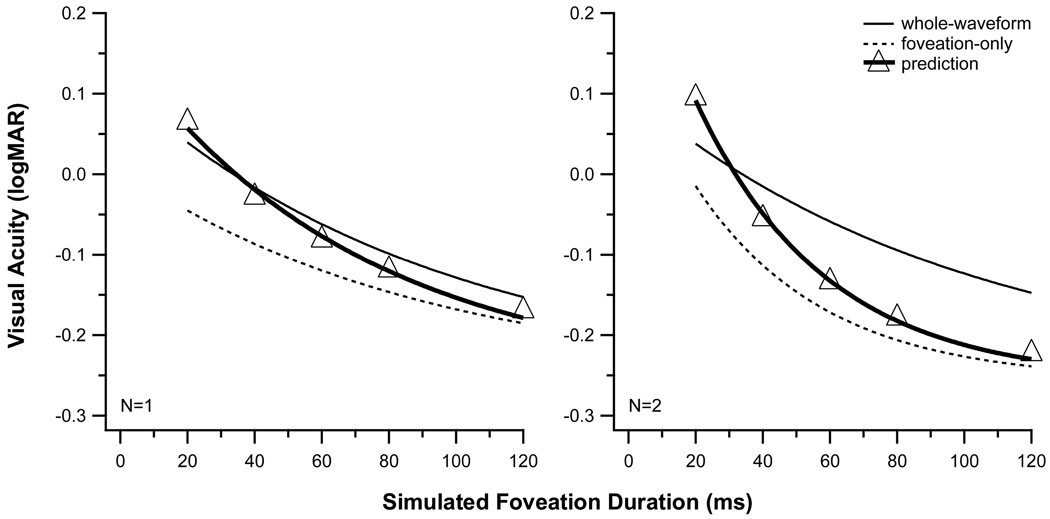
One possibility is that the acuity measured during the whole-waveform condition represents a weighted average of the acuities that would be obtained separately during the foveating and the non-foveating phases of the simulated nystagmus waveform.a To evaluate this possibility, we used the following procedure to estimate the relative weights and visual acuities during the foveating and non-foveating phases of the waveform. First, we assigned a weight to the visual acuity during the foveating phase that was equal to the duration of the foveation period. Next, we estimated the acuity during the foveating phase from the exponential function that was fit to the data from the foveation-only condition, which essentially represents how visual acuity improves with duration. To determine a weight for the acuity during non-foveating phases of the nystagmus waveform, we assumed that spatial and/or temporal interactions with the stationary image during the foveation period, akin to crowding or masking, occur only during the initial part of the simulated slow phase.b The basis for this assumption is that the moving, blurred image from the whole-waveform condition is in the same spatial vicinity as the stationary image from the foveation period, which we designate the spatial interaction zone, only during the initial part of the simulated slow phase. The weight that we assigned to the acuity during the non-foveating phases of the waveform was the time that the acuity target remains in this spatial interaction zone, while moving at the initial ramp velocity of the simulated slow phase (range, for different foveation durations = 17 – 31°/s). Finally, we estimated the acuity during the initial ramp velocity of the simulated nystagmus slow phase using the relationship between logMAR acuity and velocity that was reported by Demer and Amjadi3.
The visual acuities predicted by this weighted-average model agree reasonably well with the measured values of acuity for one cycle of the whole-waveform condition if the spatial interaction zone is assumed to be approximately 5 arc min (Fig. 4). Specifically, for the one-cycle condition, the rms error of the predicted logMAR acuity is 0.022. However, the weighted-average model fails for multiple-cycle conditions, as the predicted visual acuity for long foveation durations is systematically better than the measured acuity in the whole-waveform condition. We conclude that a weighted average of the acuities during the foveating and non-foveating phases of the simulated nystagmus waveform is unable to account for our results.
Figure 4.
The visual acuities predicted from the weighted-average model that is described in the text (triangular symbols with thick solid line) are compared with the empirically determined acuities for the whole-waveform (thin solid line) condition. For reference, the empirical data for the foveation-only (dotted line) condition is shown also. The acuity vs. duration curves for the empirical data are the same as those shown in Fig. 2. For a single cycle of stimulus presentation (left panel), the predicted and empirical values match reasonably well. However, with an increase in the number of cycles of stimulus presentation (N=2, right panel), the model systematically underestimates the measured acuities at longer simulated foveation durations. Model predictions for larger numbers of cycles of the whole-waveform condition exhibit similar deviations from the empirical whole-waveform data (not shown).
The weighted-average model may fail for multiple cycles of the whole-waveform condition because the period of temporal integration differs for the whole-waveform and the foveation-only conditions. Implicitly, the weighted-average model assumes that the same period of temporal integration governs visual acuity in the two waveform conditions. Indeed, as shown in Table 1, we determined similar temporal integration periods of approximately 280 ms for one cycle of the whole-waveform and foveation-only conditions. This duration of temporal integration is in reasonable agreement with estimates from previous studies that varied the duration of a stationary visual acuity target21,27–29 and from studies that measured visual acuity in the presence of simulated nystagmus waveforms (for a summary, see Chung & Bedell14). Although the estimated integration period remains virtually unchanged for multiple cycles of the whole-waveform condition (mean = 262 ± 32 [SE] ms), the estimates of the integration period are significantly shorter for multiple cycles of the foveation-only condition. In the weighted-average model that is described above, a reduced period of temporal integration for multiple cycles of the foveation-only condition causes the predicted acuity for multiple cycles of the whole-waveform condition to improve too rapidly as the duration of the foveation period increases (see Fig. 4). But, what might account for the shorter estimated integration periods for multiple cycles of the foveation-only condition?c
Temporal summation for high spatial frequency targets includes a period of complete summation, which adheres to Bloch’s law, and a period of partial summation, which usually is attributed to probability summation.20,30,31 Our data suggest that probability summation occurs across cycles of the foveation-only condition, but not the whole-waveform condition. In the foveation-only condition, the probability summation that occurs across cycles and the probability summation that occurs when the foveation duration extends beyond the period of complete integration should exert similar effects on improving visual acuity. If we assume that the visual acuity achieved with a stationary target for an unlimited viewing duration represents the asymptotic value, this value should be attained more quickly when multiple cycles of the foveation-only condition are presented and the effects of within-cycle and across-cycle probability summation are combined. If acuity reaches the asymptotic value when the simulated foveation duration is briefer, then the estimated integration duration for this condition will be shorter.
In the whole-waveform condition, our data indicate that the information from the clear, stationary target and the moving, blurred target are combined, resulting in an elevation of acuity by approximately 0.1 logMAR. Unlike the foveation-only condition, the summation produced by extending the foveation duration within each individual cycle would be expected to benefit acuity more than probability summation for a partially blurred target across multiple cycles. Therefore, in the whole-waveform condition, summation across cycles is expected to add relatively little to the summation achieved by lengthening the foveation duration, and acuity should approach the asymptotic threshold at approximately the same rate as when only a single cycle is presented.
Despite the clear degradation of normal acuity in the simulated whole-waveform condition, it is not certain that the retinal image motion during non-foveating phases has a similar deleterious effect on the visual acuity of individuals with IN. Individuals with IN sample visual information during the entire nystagmus waveform and not just during the foveation periods.32–34 Consequently, the reduction of image quality during non-foveating periods of the IN waveform would be expected to have an influence on visual acuity. However, as noted in the Introduction, observers with IN perceive substantially less motion smear than that seen by normal observers, during comparable motion of the retinal image during a simulated IN waveform.18,19 In normal observers, though, the extent of perceived motion smear depends critically on whether the motion of the retinal image occurs during an eye movement. Specifically, normal observers report that considerably less motion smear results from retinal image motion during pursuit, smooth vergence, or the slow phase of the vestibulo-ocular reflex, compared with when comparable image motion occurs during steady fixation.35–37 Also, normal observers exhibit higher contrast sensitivity when motion of the retinal image occurs during pursuit compared with fixation, especially for high-spatial frequency targets.38,39 Based on these results, it is plausible that the drastically reduced perception of motion smear during IN is associated with a greatly reduced influence of the non-foveating portions of the IN waveform on visual functions. If so, then individuals with IN might achieve better contrast sensitivity and visual acuity than would be expected from the parameters of their retinal image motion.
ACKNOWLEDGMENTS
This research was supported in part by research grant, R01-EY05068, short-term training grant, T35-EY07088, and core grant P30-EY07551 from the National Eye Institute. The preparation of this manuscript was also supported by research grant R01-EY012810.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A way to conceptualize this weighted-average model is that the contrast of an acuity target is effectively reduced by the motion blur that occurs during the non-foveating phases in the whole-waveform condition. The influence on acuity of this reduction of the effective target contrast can be approximated by averaging the acuity values that would be achieved for a stationary, high-contrast target (i.e., during the foveation-only condition) and for a moving, blurred target, each weighted by their relative duration.
In our experimental paradigm, it is difficult to separate the possible effects on visual acuity of crowding and various forms of masking because (1) the spatio-temporal separation between the moving and stationary target during the whole-waveform condition varies according to the velocity of the acuity target during the simulated IN slow phase and (2) multiple types of forward and backward masking could contribute to a decrease in visual acuity in the whole-waveform condition, except when only one cycle of the simulated IN waveform is presented. For simplicity, our analysis considers all of these spatio-temporal interactions as a single entity.
The similarity of the time constants fit to the data in the whole-waveform and foveation-only conditions for one cycle of the simulated IN waveform suggests at least that backward masking does not contribute to a difference in the temporal integration periods for these two conditions.
REFERENCES
- 1.Westheimer G, McKee SP. Visual acuity in the presence of retinal-image motion. J Opt Soc Am. 1975;65:847–850. doi: 10.1364/josa.65.000847. [DOI] [PubMed] [Google Scholar]
- 2.Murphy BJ. Pattern thresholds for moving and stationary gratings during smooth eye movement. Vision Res. 1978;18:521–530. doi: 10.1016/0042-6989(78)90196-7. [DOI] [PubMed] [Google Scholar]
- 3.Demer JL, Amjadi F. Dynamic visual acuity of normal subjects during vertical optotype and head motion. Invest Ophthalmol Vis Sci. 1993;34:1894–1906. [PubMed] [Google Scholar]
- 4.Abadi RV, Worfolk R. Retinal slip velocities in congenital nystagmus. Vision Res. 1989;29:195–205. doi: 10.1016/0042-6989(89)90124-7. [DOI] [PubMed] [Google Scholar]
- 5.Abadi RV, Bjerre A. Motor and sensory characteristics of infantile nystagmus. Br J Ophthalmol. 2002;86:1152–1160. doi: 10.1136/bjo.86.10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dell'Osso LF. Fixation characteristics in hereditary congenital nystagmus. Am J Optom Arch Am Acad Optom. 1973;50:85–90. [PubMed] [Google Scholar]
- 7.Dell'Osso LF, Daroff RB. Congenital nystagmus waveforms and foveation strategy. Doc Ophthalmol. 1975;39:155–182. doi: 10.1007/BF00578761. [DOI] [PubMed] [Google Scholar]
- 8.Abadi RV, Pascal E. Visual resolution limits in human albinism. Vision Res. 1991;31:1445–1447. doi: 10.1016/0042-6989(91)90063-b. [DOI] [PubMed] [Google Scholar]
- 9.Sheth NV, Dell'Osso LF, Leigh RJ, Van Doren CL, Peckham HP. The effects of afferent stimulation on congenital nystagmus foveation periods. Vision Res. 1995;35:2371–2382. doi: 10.1016/0042-6989(94)00321-c. [DOI] [PubMed] [Google Scholar]
- 10.Simmers AJ, Gray LS, Winn B. The effect of abnormal fixational eye movements upon visual acuity in congenital nystagmus. Curr Eye Res. 1999;18:194–202. doi: 10.1076/ceyr.18.3.194.5374. [DOI] [PubMed] [Google Scholar]
- 11.Cesarelli M, Bifulco P, Loffredo L, Bracale M. Relationship between visual acuity and eye position variability during foveations in congenital nystagmus. Doc Ophthalmol. 2000;101:59–72. doi: 10.1023/a:1002702609387. [DOI] [PubMed] [Google Scholar]
- 12.Currie DC, Bedell HE, Song S. Visual acuity for optotypes with image motions simulating congenital nystagmus. Clin Vision Sci. 1993;8:73–84. [Google Scholar]
- 13.Chung STL, Bedell HE. Effect of retinal image motion on visual acuity and contour interaction in congenital nystagmus. Vision Res. 1995;35:3071–3082. doi: 10.1016/0042-6989(95)00090-m. [DOI] [PubMed] [Google Scholar]
- 14.Chung STL, Bedell HE. Velocity criteria for "foveation periods" determined from image motions simulating congenital nystagmus. Optom Vis Sci. 1996;73:92–103. doi: 10.1097/00006324-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Chung STL, Bedell HE. Congenital nystagmus image motion: influence on visual acuity at different luminances. Optom Vis Sci. 1997;74:266–272. [PubMed] [Google Scholar]
- 16.Dickinson CM. The elucidation and use of the effect of near fixation in congenital nystagmus. Ophthalmic Physiol Opt. 1986;6:303–311. [PubMed] [Google Scholar]
- 17.Hanson KS, Bedell HE, White JM, Ukwade MT. Distance and near visual acuity in infantile nystagmus. Optom Vis Sci. 2006;83:823–829. doi: 10.1097/01.opx.0000238650.33150.73. [DOI] [PubMed] [Google Scholar]
- 18.Bedell HE, Bollenbacher MA. Perception of motion smear in normal observers and in persons with congenital nystagmus. Invest Ophthalmol Vis Sci. 1996;37:188–195. [PubMed] [Google Scholar]
- 19.Bedell HE, Tong J. Asymmetrical perception of motion smear in infantile nystagmus. Vision Res. 2009;49:262–267. doi: 10.1016/j.visres.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Watson AB. Probability summation over time. Vision Res. 1979;19:515–522. doi: 10.1016/0042-6989(79)90136-6. [DOI] [PubMed] [Google Scholar]
- 21.Baron WS, Westheimer G. Visual acuity as a function of exposure duration. J Opt Soc Am. 1973;63:212–219. doi: 10.1364/josa.63.000212. [DOI] [PubMed] [Google Scholar]
- 22.Abadi RV, Dickinson CM. Waveform characteristics in congenital nystagmus. Doc Ophthalmol. 1986;64:153–167. doi: 10.1007/BF00159990. [DOI] [PubMed] [Google Scholar]
- 23.Wheeless LL, Jr, Boynton RM, Cohen GH. Eye-movement responses to step and pulse-step stimuli. J Opt Soc Am. 1966;56:956–960. doi: 10.1364/josa.56.000956. [DOI] [PubMed] [Google Scholar]
- 24.Ludvigh EJ. Visual acuity while one is viewing a moving object. Arch Ophthalmol. 1949;42:14–22. doi: 10.1001/archopht.1949.00900050017002. [DOI] [PubMed] [Google Scholar]
- 25.Chung STL, Bedell HE. Vernier and letter acuities for low-pass filtered moving stimuli. Vision Res. 1998;38:1967–1982. doi: 10.1016/s0042-6989(97)00327-1. [DOI] [PubMed] [Google Scholar]
- 26.Chung STL, Bedell HE. Velocity dependence of Vernier and letter acuity for band-pass filtered moving stimuli. Vision Res. 2003;43:669–682. doi: 10.1016/s0042-6989(02)00628-4. [DOI] [PubMed] [Google Scholar]
- 27.Kahneman D, Norman J. The time-intensity relation in visual perception as a function of observer’s task. J Exp Psychol. 1964;68:215–220. doi: 10.1037/h0046097. [DOI] [PubMed] [Google Scholar]
- 28.Alexander KR, Derlacki DJ, Fishman GA, Szlyk JP. Temporal properties of letter identification in retinitis pigmentosa. J Opt Soc Am (A) 1993;10:1631–1636. doi: 10.1364/josaa.10.001631. [DOI] [PubMed] [Google Scholar]
- 29.Niwa K, Tokoro T. Measurement of temporal summation of visual acuity with use of modified tachistoscope. Jpn J Ophthalmol. 1997;41:403–408. doi: 10.1016/s0021-5155(97)00082-8. [DOI] [PubMed] [Google Scholar]
- 30.Legge GE. Sustained and transient mechanisms in human vision: temporal and spatial properties. Vision Res. 1978;18:69–81. doi: 10.1016/0042-6989(78)90079-2. [DOI] [PubMed] [Google Scholar]
- 31.Watson AB, Nachmias J. Summation of asynchronous gratings. Vision Res. 1980;20:91–94. doi: 10.1016/0042-6989(80)90147-9. [DOI] [PubMed] [Google Scholar]
- 32.Jin YH, Goldstein HP, Reinecke RD. Absence of visual sampling in infantile nystagmus. Korean J Ophthalmol. 1989;3:28–32. doi: 10.3341/kjo.1989.3.1.28. [DOI] [PubMed] [Google Scholar]
- 33.Waugh SJ, Bedell HE. Sensitivity to temporal luminance modulation in congenital nystagmus. Invest Ophthalmol Vis Sci. 1992;33:2316–2324. [PubMed] [Google Scholar]
- 34.Woo S, Bedell HE. Beating the beat: reading can be faster than the frequency of eye movements in persons with congenital nystagmus. Optom Vis Sci. 2006;83:559–571. doi: 10.1097/01.opx.0000230272.10471.03. [DOI] [PubMed] [Google Scholar]
- 35.Bedell HE, Lott LA. Suppression of motion-produced smear during smooth pursuit eye movements. Curr Biol. 1996;6:1032–1034. doi: 10.1016/s0960-9822(02)00650-4. [DOI] [PubMed] [Google Scholar]
- 36.Bedell HE, Chung STL, Patel SS. Attenuation of perceived motion smear during vergence and pursuit tracking. Vision Res. 2004;44:895–902. doi: 10.1016/j.visres.2003.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong J, Patel SS, Bedell HE. The attenuation of perceived motion smear during combined eye and head movements. Vision Res. 2006;46:4387–4397. doi: 10.1016/j.visres.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schütz AC, Braun DI, Kerzel D, Gegenfurtner KR. Improved visual sensitivity during smooth pursuit eye movements. Nat Neurosci. 2008;11:1211–1216. doi: 10.1038/nn.2194. [DOI] [PubMed] [Google Scholar]
- 39.Schütz AC, Braun DI, Gegenfurtner KR. Improved visual sensitivity during smooth pursuit eye movements: temporal and spatial characteristics. Vis Neurosci. 2009;26:329–340. doi: 10.1017/S0952523809990083. [DOI] [PubMed] [Google Scholar]