Abstract
The effects of a daytime nap on inter-session habituation to aversive visual stimuli were investigated. Healthy young adult volunteers viewed repeated presentations of highly negative and emotionally neutral (but equally arousing) International Affective Picture System (IAPS) photographs during two afternoon sessions separated by 2.5 hrs. Half of the photographs were shown at both sessions (Repeated Sets) and half differed between sessions (Novel Sets). For each stimulus presentation, evoked skin conductance response (SCR), heart rate deceleration (HRD) and corrugator supercilii EMG response (EMG), were computed and range corrected using respective maximum session-1 responses. Following each presentation, subjects rated each photograph on dimensions of pleasantness and arousability. During the inter-session interval, Nap subjects had a 120-min polysomnographically monitored sleep opportunity, whereas Wake subjects watched a non-stimulating video. Nap and Wake subjects did not differ in their subjective ratings of photographs. However, for Repeated-Set photographs, Nap subjects demonstrated greater inter-session habituation in SCR and EMG but a trend toward lesser inter-session habituation in HRD. These group differences were absent for Novel-Set photographs. Group differences across all measures were greater for negative stimuli. Occurrence of SWS during the nap was associated with greater inter-session habituation of EMG whereas occurrence of REM was associated with lesser inter-session habituation of SCR to negative stimuli. Sleep may therefore promote emotional adjustment at the level of somatic responses. Physiological but not subjective inter-session habituation to aversive images was enhanced by a daytime nap.
Keywords: habituation, emotion, sleep, napping, emotion regulation, psychophysiology
1. Introduction
It is often hypothesized that sleep plays an emotional regulatory role in healthy humans (Walker, 2009; Walker and van der Helm, 2009) and that disruption of sleep negatively impacts mood (Dinges, Pack, Williams, Gillen, Powell, Ott, Aptowicz, and Pack, 1997; Haack and Mullington, 2005; Zohar, Tzischinsky, Epstein, and Lavie, 2005). Moreover, it is widely suggested that sleep disruption and waking emotion mutually interact in the etiology and perpetuation of mood and anxiety disorders (Franzen and Buysse, 2008; Germain, Buysse, and Nofzinger, 2008; Kim and Dimsdale, 2007; Mellman, 2006; Nielsen and Levin, 2007; Peterson and Benca, 2006). In humans, sleep may play a role in moderating emotional aspects of higher cognitive functions such as resolution of intrapersonal conflict (Cartwright, Luten, Young, Mercer, and Bears, 1998), affectively guided decision making (Killgore, Balkin, and Wesensten, 2006), moral reasoning (Killgore, Day, Li, Kamimori, Balkin, and Killgore, 2006) and recognizing of facial expressions of emotion (van der Helm, Gujar, and Walker, 2010). Similarly, sleep may modulate the impact of emotion on human declarative memory (Payne, Stickgold, Swanberg, and Kensinger, 2008; Walker, 2009). However, sleep may also moderate emotions by phylogenetically primitive learning mechanisms such as habituation and extinction (Pace-Schott, Milad, Orr, Rauch, Stickgold, and Pitman, 2009). Moreover, sleep may be essential for the homeostatic equilibration of stress, reward, autonomic and neuroendocrine systems that is essential to maintaining a euthymic waking mood (McEwen, 2006; Mullington, Haack, Toth, Serrador, and Meier-Ewert, 2009).
Habituation is an evolutionarily ancient mechanism of neuronal plasticity and non-associative learning whereby behavioral and physiological responses during initial exposure to a stimulus diminish with repeated presentations of that stimulus (Grissom and Bhatnagar, 2009; Leussis and Bolivar, 2006; Thompson and Spencer, 1966). Habituation studies in rodents differentiate between intra-session habituation, the decline in a behavior during an exposure session, and inter-session habituation, maintained low or further reduced responses from one session to the next (Leussis and Bolivar, 2006). Both processes are believed to reflect memory phenomena that are to some extent dissociable by their responses to experimental lesions (e.g., hippocampal) and pharmacological manipulations as well as by their genetic determinants (Bolivar, 2009; Leussis and Bolivar, 2006).
Habituation occurs with both biologically salient and neutral stimuli (McSweeney and Murphy, 2009). In humans as well as animals, habituation occurs to aversive stimuli on both behavioral and neuroendocrine levels (Grissom and Bhatnagar, 2009) and it has been argued that such “affective habituation” functions to promote behavioral flexibility by preventing maladaptive panic-like states (Dijksterhuis and Smith, 2002). In addition, subjective and behavioral habituation to emotional stimuli is accompanied by decreasing activity in areas involved in processing and regulating emotion, especially the amygdala and prefrontal cortex (Milad, Wright, Orr, Pitman, Quirk, and Rauch, 2007; Mutschler, Wieckhorst, Speck, Schulze-Bonhage, Hennig, Seifritz, and Ball, 2010; Wilson and Rolls, 1993; Wright, Fischer, Whalen, McInerney, Shin, and Rauch, 2001). Habituation, like extinction, may be a mechanism contributing to the efficacy of exposure therapies (Averill, Malmstrom, Koriat, and Lazarus, 1972; Craske, Kircanski, Zelikowsky, Mystkowski, Chowdhury, and Baker, 2008; Foa and Kozak, 1986; Jaycox, Foa, and Morral, 1998). Uniquely human cognitive mechanisms of emotion regulation, such as re-attribution of the meaning of events, may recruit the neural circuitry of these more basic mechanisms (Delgado, Nearing, Ledoux, and Phelps, 2008; Schiller and Delgado, 2010).
Sleep contributes to the consolidation of emotional memory (Hu, Stylos-Allan, and Walker, 2006; Nishida, Pearsall, Buckner, and Walker, 2009; Wagner, Degirmenci, Drosopoulos, Perras, and Born, 2005; Wagner, Gais, and Born, 2001; Wagner, Hallschmid, Rasch, and Born, 2006; Walker, 2009). Effects of sleep on emotional memory extend to adaptive modifications of relationships between different memory traces. For example, sleep enhances a trade-off in visual memory insofar as memory for emotional (but not neutral) objects is preferentially remembered (Payne et al., 2008) and sleep promotes generalization of extinction of conditioned fear (Pace-Schott et al., 2009). REM sleep may be especially important for the consolidation and modulation of emotional memory (Levin and Nielsen, 2007; Nishida et al., 2009; Wagner et al., 2001).
Two prior studies investigated sleep effects on emotional habituation using the International Affective Picture System (IAPS) (Lang, Bradley, and Cuthbert, 2008) along with self-ratings of valence (pleasantness vs. aversiveness) and arousability using the Self Assessment Manikin (SAM) (Bradley and Lang, 1994). Wagner et al. (Wagner, Fischer, and Born, 2002) examined SAM valence ratings of repeated (pre-sleep exposed) vs. novel (previously unseen) negative IAPS stimuli and compared these repeated vs. novel ratings differences between groups that had either slept or remained awake across an intersession interval. The group that slept was further divided into subgroups with early night (with SWS-rich sleep) and late night (with REM-rich sleep) sleep opportunities, each with its own wake control over the same time period. After an early-night period, ratings of repeated stimuli were less negative than those of novel images following sleep but more negative following wake. In contrast, after a late-night period, repeated stimuli were more negative following both sleep and wake but this difference was greater following sleep.
Similarly, Lara-Carrasco et al. (Lara-Carrasco, Nielsen, Solomonova, Levrier, and Popova, 2009) compared emotional reactivity to IAPS photographs seen for the second time following overnight sleep during which subjects were either minimally or substantially deprived of REM sleep. As in Wagner et al.’s 2002 study, greater reactivity was observed following the high- vs. low-REM condition. Neither study, however, employed the physiological measures that are known to capture covert or non-conscious expression of emotional responses (Bradley and Lang, 2007).
The current study examined the impact of an afternoon nap on habituation to emotionally aversive IAPS stimuli using physiological as well as subjective measures of emotion. A greater degree of intra-session habituation in comparison to studies cited above was established via repeated presentations of the same stimuli at the first session. It was hypothesized that napping vs. waking intervening between repeated viewings of the same stimuli would lead to enhanced inter-session habituation of emotional responses. It was further hypothesized that sleep facilitation of inter-session habituation would be (1) greater for negatively valenced stimuli, and (2), because habituation is a form of memory, would be specific to previously viewed stimuli rather than being generalized diminished reactivity to all stimuli.
2. Methods
2.1 Participants
Candidate participants were recruited via internet and screened by telephone. Exclusion criteria were self-report of current neurological or medical conditions, use of sleep-altering drugs, night shift work or inability to keep a regular sleep schedule, excessive caffeine (> 5 cups/day) or alcohol (> 12 drinks/week) consumption and average sleep per night < 6 or > 10 hours, as well as any current or history of neurological disorder, significant head trauma, DSM-IV-TR Axis 1 disorder (including alcohol or drug abuse), sleep disorder or use of psychiatric medication.
Forty-six paid volunteers (31 female), mean age = 20.78 (SD 2.25, range 18–27) were pseudorandomly assigned to Nap or Wake groups. Two Nap-group subjects’ data were not analyzed because they failed to sleep more than 10 minutes. One Wake-group subject’s data were not analyzed due to curtailed sleep the previous night. The final sample contained 22 Nap (15 females) and 21 Wake (15 females) subjects. Because extinction of fear conditioning varies with phase of the menstrual cycle (Milad, Goldstein, Orr, Wedig, Klibanski, Pitman, and Rauch, 2006), female participants were scheduled for study on a date they estimated would fall during the early follicular phase (day 1–7) of their cycle (a scheduling goal that was successful in 17 of 30 females). All procedures were approved by the Beth Israel Deaconess Medical Center Committee on Clinical Investigation and all subjects provided written informed consent.
Sample size was estimated from subjective data in Wagner et al. (2002) in which early-night sleep vs. wake difference was a trend with an effect size of d = .81 that would require 25 subjects to detect a difference at alpha = .05 with 80% power. The sleep vs. wake difference across late night was significant, with d = .84 and requiring 18 subjects per group. Our sample size fell between these figures.
2.2 Pre-study week
During the week before the experiment, subjects were asked to maintain a regular sleep schedule with ≥ 7 hours in bed each night and bedtime no later than 2:00 a.m. During this week, subjects were asked to refrain entirely from alcohol, recreational drugs and daytime napping and they filled out a nightly sleep diary (Pace-Schott, Stickgold, Muzur, Wigren, Ward, Hart, Clarke, Morgan, and Hobson, 2005). Subjects were asked not to use caffeine or nap on the study day. Before their study, subjects completed the Pittsburgh Sleep Quality Index (PSQI, Buysse, Reynolds, Monk, Berman, and Kupfer, 1989), Epworth Sleepiness Scale (ESS, Johns, 1994), Spielberger State-Trait Anxiety Inventory-Trait portion (STAI, Spielberger, Gorsuch, and Lushene, 1990) and the Revised NEO Personality Inventory (NEO-PI-R, Costa and McCrae, 1992). The first 4 subjects in each group were not given the NEO-PI-R due to delayed incorporation of this inventory into the experimental protocol.
2.3 Procedure
Participants arrived at 11:00 a.m. and had electrodes applied for PSG, skin conductance response (SCR), electrocardiography (ECG) and corrugator supercilii electromyography (EMG). All subjects were instrumented for PSG whether or not they were to nap in order that Wake-group participants experienced the same physical interventions, with any consequent discomfort, as Sleep-group participants. Note, however that electrode recordings were not obtained from the Wake group. They were then read a scripted introduction, beginning You will be viewing a number of slides some of which contain images of a very disturbing nature such as might be seen by a medical examiner or in a war zone and you may stop your participation at any point. Subjects then practiced making keyboard SAM rating responses and donned headphones before beginning the protocol (Figure 1).
Figure 1.
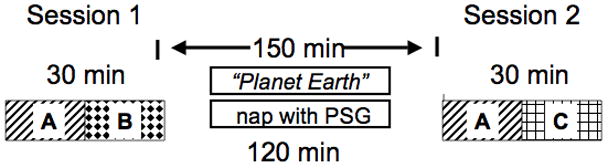
Experimental protocol showing sessions and inter-session intervals in the Nap and Wake groups. Designations A, B and C refer to the stimulus sets (Table 2) and this example shows one of 6 alternate versions that uses stimulus set A as the Repeated Set, B as the Session-1 Novel Set and C as the Session-2 Novel Set.
Time-of-day of sessions was standardized because Hot et al. (Hot, Leconte, and Sequeira, 2005; Hot, Naveteur, Leconte, and Sequeira, 1999) have demonstrated diurnal variation of SCRs to emotional pictures. Subjects first viewed a series of IAPS photographs at Session 1. All Nap-group subjects except the initial 4 (18 of 21) were then given a 120-min sleep opportunity (of the the initial 4, 3 were allowed 100 min and 1 only 55 min due to fire alarm) with polysomnography (PSG). Wake subjects watched non-arousing videos; 17 watched Planet Earth (by BBC, segments: “Seasonal Forest and” “Ocean Deep”). The remaining 4 Wake-group subjects viewed all of one and sometimes a portion of another non-violent popular film (e.g., Austin Powers, Royal Tennenbaums, Shreck, and Lion King). Subjects were checked regularly to ensure that they remained awake and they were aware of this visual monitoring. No instance of sleep was observed nor did any subject appear to be having difficulty remaining awake.
After a 2.5-hr inter-session interval, subjects viewed a second series of IAPS photographs at Session 2. Table 1 shows sessions’ mean start time, end time and duration as well as nap durations. Immediately before beginning and after ending each session, subjects completed the Stanford Sleepiness Scale (SSS, Hoddes, Zarcone, Smythe, Phillips, and Dement, 1973) and the Positive and Negative Affect Schedule (PANAS, McNair, Lorr, and Droppleman, 1981). Following the second session, subjects were debriefed and had electrodes removed.
Table 1.
Demographic, habitual sleep and sleepiness, pre-study subjective sleep duration, psychological trait and session times and durations for Nap and Wake group.
| Characteristic | Nap Group (SD) | Wake Group (SD) | p (t-test) |
|---|---|---|---|
| Age | 20.1 (1.5) | 21.3 (2.7) | 0.09 |
| Gender ratio (M:F) | 7:15 | 6:15 | |
| ESS | 6.81 (3.06) | 7.67 (4.41) | 0.45 |
| PSQI | 4.00 (2.81) | 3.19 (1.86) | 0.27 |
| STAI-Trait | 54.23 (3.80) | 53.67 (3.12) | 0.60 |
| * NEO-PI-R Neuroticism | 85.50 (23.05) | 84.00 (27.51) | 0.86 |
| NEO-PI-R Extraversion | 117.28 (23.74) | 113.41 (36.11) | 0.71 |
| NEO-PI-R Openness | 123.00 (24.13) | 124.71 (19.55) | 0.82 |
| NEO-PI-R Agreeableness | 122.22 (20.98) | 117.82 (20.12) | 0.53 |
| NEO-PI-R Conscientiousness | 116.61 (23.81) | 116.88 (24.82) | 0.97 |
| Mean diary sleep prior 1 wk (min) | 513 (49) | 514 (37) | 0.94 |
| Mean Diary sleep prior night (min) | 494 (50) | 485 (57) | 0.61 |
| Session 1 beginning (24-hr) | 12:42 (0:27) | 12:41 (0:26) | |
| Session 1 duration (min) | 34.27 (4.33) | 32.57 (2.87) | 0.14 |
| Session 2 beginning (24-hr) | 15:50 (0:29) | 15:50 (0:31) | |
| Session 2 duration (min) | 31.59 (3.85) | 33.09 (5.58) | 0.31 |
| ** Inter-session duration (min) | 152.72 (10.44) | 156.14 (9.34) | 0.27 |
| Nap duration (min) | 118 (17) |
N=35 (18 Nap, 17 Wake),
Inter-session duration from end of Session 1 to beginning of Session 2.
Stimuli were presented using SuperLab 2.0 (Cedrus Corporation, San Pedro, CA) on a Compaq PC with 17-inch monitor. Subjects sat in a comfortable chair with their dominant arm resting on a pillow and their dominant hand positioned over the keyboard keypad for SAM responses. Subjects were alone in the study room and were monitored via web-cam.
2.4 Stimuli and rating scales
Stimuli were chosen from the IAPS (Lang et al., 2008), a collection of emotion-eliciting color photographs standardized on large samples of healthy subjects using 9-point rating scales, of “Valence” (pleasant to unpleasant) and “Arousal” (arousing to unarousing). Six highly negatively rated stimuli (“Negative”) and 6 stimuli of neutral to mildly positive valence (“Neutral”) were matched for standardized IAPS arousal ratings and divided into three groups of 4 stimuli each of which contained 2 different Negative and 2 different Neutral stimuli (Table 2). Using these 4-stimulus groups, 3 series of 20 photographs each were created (stimulus sets A, B and C) in which each of the 2 Negative and 2 Neutral photographs were repeated 5 times. Within each stimulus set, stimuli were pseudorandomly intermingled with no particular stimulus occurring more than once in succession and no more than 2 Negative or Neutral stimuli occurring in succession.
Table 2.
IAPS Valence and Arousal ratings of negative and neutral stimuli.
| Set | Image (IAPS #) | IAPS valence | set mean | IAPS arousal | set mean |
|---|---|---|---|---|---|
| Negative | |||||
| A | Injury (3266) | 1.56 | 1.44 | 6.79 | 6.85 |
| A | BurnVictim (3053) | 1.31 | 6.91 | ||
| B | Mutilation (3069) | 1.70 | 1.59 | 7.03 | 7.13 |
| B | Mutilation (3080) | 1.48 | 7.22 | ||
| C | BabyTumor (3170) | 1.46 | 1.52 | 7.21 | 7.09 |
| C | Mutilation (3130) | 1.58 | 6.97 | ||
| MEAN (SD) | 1.52 (0.13) | 7.02 (0.17) | |||
| Neutral | |||||
| A | Bungee (8179) | 6.48 | 6.91 | 6.99 | 7.17 |
| A | Skier (8030) | 7.33 | 7.35 | ||
| B | RockClimber (8160) | 5.07 | 6.32 | 6.97 | 7.12 |
| B | Skydivers (8185) | 7.57 | 7.27 | ||
| C | Cliffdiver (8178) | 6.50 | 6.76 | 6.82 | 6.83 |
| C | Skysurfer (8186) | 7.01 | 6.84 | ||
| MEAN (SD) | 6.66 (0.89) | 7.04 (0.22) |
Six alternate versions of these 3 stimulus sets were counterbalanced across subjects. Two stimulus sets (40 stimuli) were shown at each of Sessions 1 and 2 (80 stimuli across both sessions). In each alternate version, one of the three stimulus sets (“Repeated Set”) was shown at the beginning of both Session 1 and Session 2 (Figure 1) and each stimulus set was the Repeated Set in 2 of the 6 alternate versions. One of the remaining two stimulus sets followed the Repeated Set at Session 1 and the other at Session 2 (“Novel Sets”). When shown at Session 2, the Repeated Set contained either a negative or neutral stimulus at the same ordinal positions as in Session 1. However, to minimize expectancy based on memory of stimulus order, we reversed the order of the 2 negative and 2 neutral stimuli across each of their 5 repetitions.
To habituate subjects’ initial orienting response to IAPS stimuli, Session 1 began with 5 IAPS stimuli rated neutral on valence and low on arousal scales (mean valence 5.14, mean arousal 2.49). A different set of 5 stimuli began Session 2 (valence 5.17, arousal 2.83). Before the first IAPS stimulus appeared and at the very end of each session, a 10-msec 80 dB white-noise startle sound was delivered through headphones. This startle noise provided non-specific stimulation at an intensity sufficient to elicit strong SCRs via a combination of orienting and startle responses (Turpin, Schaefer, and Boucsein, 1999) in order to test sympathetic reactivity unrelated to IAPS stimuli prior to and after viewing IAPS stimuli.
IAPS stimuli each appeared for 6 seconds followed by a 3-sec black screen after which SAM Valence and Arousal scales appeared. The SAM scales consist of two series of cartoon-like figures depicting 5 levels of valence and arousal that, with intermediate steps, generate 9-point, non-verbal rating scales (Bradley and Lang, 1994). Subjects selected their responses to the preceding stimulus using the keyboard number pad. The Valence scale appeared first and, immediately after its selection was made, the Arousal scale appeared. (Note: the negative pole in the current Valence SAM was 9 vs. the “1” in the IAPS standard ratings shown in Table 2.) After arousal ratings, a black screen reappeared for an inter-stimulus interval (ISI) varying between 17 and 29 sec (in 3-sec increments) before the next IAPS stimulus appeared. The final startle sound followed the last ISI.
2.5 Psychophysiological measurements
SCR, ECG and EMG were recorded using the MP35 data acquisition unit (BIOPAC Systems, Inc., Goleta, CA) and BIOPAC Student Lab Pro 3.7.2 software. SuperLab-triggered square pulse outputs immediately preceded each IAPS stimulus (or startle sound) and were transmitted to the MP35 using a BIOPAC STP100 optical interface. Square pulse signals were acquired synchronously with ongoing recording allowing precise alignment of each stimulus onset with physiological data. Sampling rate was 2000 Hz.
Skin conductance level (SCL), a reliable index of sympathetic activation (Dawson, Schell, and Filion, 2007), was recorded using 2 BIOPAC EL504 disposable adhesive sensors, separated by 10 mm, attached to the hypothenar surface of the non-dominant hand. SCR was computed by subtracting the mean SCL in microSiemens (μS) during the 2 seconds preceding stimulus onset from the maximum SCL during seconds 2–6 following stimulus onset. SCRs were square-root transformed and, if the untransformed SCR was negative, the negative sign was retained after calculating the square root of the SCR’s absolute value (Orr, Metzger, Lasko, Macklin, Peri, and Pitman, 2000).
ECG was recorded using two BIOPAC EL503 electrodes attached to the torso over the first intercostal space (right) and below the lowest rib the subject could feel (left). Heart rate (beats-per-minute; BPM) was calculated by the software and heart-rate deceleration (HRD), a replicated phasic response to negative IAPS stimuli (Bradley, Codispoti, Cuthbert, and Lang, 2001; Bradley and Lang, 2007; Lang, Bradley, and Cuthbert, 1997; Moratti, Keil, and Stolarova, 2004) that has been shown to persist across the entire 6 sec of IAPS viewing (Bradley et al., 2001; Moratti et al., 2004), was computed by subtracting the mean BPM during the 2 seconds preceding stimulus onset from the minimum BPM during the 6-sec stimulus presentation.
Corrugator supercilli EMG, a reliable index of both overt and covert negative emotion (Bradley and Lang, 2007), was recorded using two BIOPAC EL254S Ag-AgCl 4-mm TP shielded recording electrodes filled with CG04 Saline Base Signa Gel. Electrode attachment followed Fridlund and Cacioppo (1986). Impedance was maintained below 10 KOhm. EMG was integrated over a 250-msec time constant and response to each stimulus was calculated by subtracting the mean signal amplitude (in μV) during the 2 seconds preceding stimulus onset from the maximum amplitude during the 6-sec stimulus presentation.
2.6 Polysomnography (PSG)
Using a Grass Colleague system, PSG was recorded digitally with 4 channels of EEG (C3, C4, F3, and F4), 2 channels of EOG (both outer canthi, one above and one below the eye), and 1 channel of submental EMG. EEG and EOG were referenced to contralateral mastoids (A1, A2). Impedance was maintained below 10 KOhm. Sampling rate was 256 Hz with 0.3 Hz high-pass, 35 Hz low-pass and 60 Hz notch filters. Records were visually scored by an expert polysomnographer (M.T.) following new American Academy of Sleep Medicine criteria (Iber and al., 2007).
2.7 Outcome variables and habituation indices
Psychophysiological (SCR, HR, EMG) responses and SAM Valence and Arousal ratings were the main outcome variables. Within each Session (Session 1 and 2) each variable’s data were first sorted by stimulus set (Repeated and Novel Sets), then by Valence (Negative, Neutral), then by the order in which they were seen (first to fifth “Trials”). Lastly, responses to the 2 different negative and 2 different neutral stimuli were averaged for each Trial. Individual dependent-variable data points always refer to such per-Trial, 2-stimulus means. To control for inter-individual variability, each subject’s psychophysiological responses from both sessions were range-corrected (Lykken and Venables, 1971) using their respective maximum response to an IAPS stimulus during the first half of Session 1 (Repeated Set) or the second half of Session 1 (Novel Sets).
One subject in the Wake group was missing the last of five trials (2-stimulus means) for Negative and Neutral stimuli in the Novel Set at Session 2. These two missing values for each outcome variable were replaced by the mean of their respective preceding four Negative or Neutral responses respectively. In the Novel Sets, one sleep group subject’s SCR data and one Wake group subject’s EMG data were not analyzed due to extreme values.1. EMG data from 2 subjects and HRD data from 1 subject were not analyzed due to poor quality recording. The final number of subjects analyzed for each outcome variable is shown in Tables 3 and S1–S4.
Table 3.
ANOVA results for SCR responses to Negative and Neutral Stimuli in Repeated and Novel Sets.
| Variable/Effect (N Nap, N Wake) | df | F | p | Interpretation |
|---|---|---|---|---|
| SCR Repeated Negative (22, 21) | ||||
| Session 1 Trial main effect | 4,164 | 19.80 | <.0001 | Session 1 intra-session habituation |
| Session 1 Group main effect | 1,41 | 1.06 | .31 | No baseline group difference |
| Session main effect | 1,41 | 10.29 | .003 | Inter-session habituation |
| Group × Session | 1,41 | 4.80 | .034 | Inter-session habituation Group difference |
| SCR Repeated Neutral (22,21) | ||||
| Session 1 Trial main effect | 4,164 | 3.80 | .008 | Session 1 intra-session habituation |
| Session 1 Group main effect | 1,41 | .14 | .71 | No baseline group difference |
| Session main effect | 1,41 | .27 | .61 | No inter-session habituation |
| Group × Session | 1,41 | 1.16 | .29 | No inter-session habituation Group difference |
| SCR Novel Negative (21*, 21) | ||||
| Session 1 Trial main effect | 4,160 | 9.90 | <.0001 | Session 1 intra-session habituation |
| Session 1 Group main effect | 1,40 | .29 | .59 | No baseline group difference |
| Session main effect | 1,40 | 3.09 | .088 | Inter-session generalized habituation trend |
| Group × Session | 1,40 | 1.49 | .23 | No inter-session generalized habituation Group diff |
| SCR Novel Neutral (21, 21) | ||||
| Session 1 Trial main effect | 4,160 | 3.85 | .009 | Session 1 intra-session habituation |
| Session 1 Group main effect | 1,40 | .01 | .93 | No baseline group difference |
| Session main effect | 1,40 | .23 | .64 | No inter-session generalized habituation |
| Group × Session | 1,40 | .42 | .52 | No inter-session generalized habituation Group diff |
One Nap participant excluded because Session 1 maximum raw SCR abnormally small resulting in artifactually high Session 2 range corrected SCR.
For correlative analyses, a Habituation Index (HI) was created for each outcome variable for Negative stimuli only of the Repeated Set. For SCR, HRD and EMG, a percentage-based inter-session HI was adapted from an analogous extinction retention index (Milad et al. 2007) as follows: HI = 100% minus the mean of all 5 trials at Session 2 divided by the mean of all 5 trials at Session 1 expressed as a percent. For SCR HI, negative values were first converted to zero to avoid ambiguous percentages. For SAM Valence ratings, an inter-session HI was the mean of all five ratings at Session 2 subtracted from the mean of all five ratings at Session 1 whereas for SAM Arousal ratings, this subtraction was reversed. Therefore, for each HI, greater inter-session habituation was represented by larger numbers.
2.8 Statistical Analyses
Two omnibus methods were first used to analyze large portions of these data prior to the more detailed analyses below in order to initially minimize the number of analyses and the risk of Type I error. First, each outcome variable was analyzed separately in Repeated and Novel Sets with pooled Negative and Neutral stimuli using 4-factor mixed ANOVA (between-subjects factor: Group; within-subject factors: Trial nested in Session nested in Valence) (Table S1). Secondly, MANOVA was performed on Habituation Indices for the three physiological variables (that were all similarly computed as percentages).
Separate analyses for each outcome variable were carried out on Session-1 data for Negative and Neutral stimuli in Repeated and Novel Sets using 2-factor mixed analyses of variance (ANOVA). The between-subjects variable was Group (Nap, Wake) and the within-subject variable was Trial (Trials 1–5). Intra-session habituation at Session 1 was measured by the main effect of Trial and the Group factor tested for baseline group differences. Both sessions’ data were then similarly analyzed for inter-session habituation to Negative and Neutral stimuli in Repeated and Novel Sets using 3-factor mixed ANOVA, nesting Trial within an additional within-subject variable, Session (Sessions 1 and 2). In Novel Sets, because stimuli differed between sessions, diminished responding at Session 2 is termed inter-session “generalized habituation”. Overall inter-session habituation (or generalized habituation) was measured by the main effect of Session and group differences in inter-session habituation (or generalized habituation) were determined from Group × Session interactions. Significant interactions were then decomposed by Group. The Greenhouse-Geisser correction for sphericity was applied to within-subject main effects and their interactions.
Relationships between PSG variables and inter-session habituation to Negative stimuli in the Sleep group were measured in two ways. First, for each outcome variable, presence vs. absence of SWS (SWS, no SWS) and REM (REM, no REM) were separately added as between-subjects factors to 3-factor mixed ANOVAs with REM (or SWS) vs. no REM (no SWS) as a between subjects factor and Trial nested in Session as within-subject factors. Second, regressions were performed using 7 sleep parameters (sleep efficiency and minutes of total, N2, SWS and REM sleep as well as sleep and REM latencies) as predictors of each variable’s HI. Regression also analyzed relationships of psychological-state, sleep/sleepiness as well as trait measures with HI.
3. Results
3.1 Group characteristics and sex differences
Using un-paired t-tests, Wake and Nap groups did not significantly differ in age, sex ratio (Chi-squared), ESS, PSQI, STAI, subscales of the NEO-PI-R, mean diary-recorded sleep duration over the prior week and on the night before study, start time and duration of sessions or the inter-session interval (Table 1). Effects on HI of Sex (Male, Female), phase of menstrual cycle in females (Early follicular, all others) and of specific Repeated Sets (A,B,C) were computed by unpaired t-tests and 1-way ANOVA respectively. The only significant difference among the HI for the 5 outcome variables was greater HI in females for SAM Valence ratings [F(1,41) = 5.19, p = .028]. Therefore, sex differences were unlikely to have altered the observed pattern of group differences.
3.2 Habituation of physiological and subjective responses to IAPS stimuli
Omnibus tests provided clear evidence for overall group differences among individual physiological but not subjective (SAM) measures. First, a four-factor mixed ANOVA (see Statistical Analyses above) showed a significant Group × Session interaction for Repeated Sets SCR [F(1,41) = 4.08, p = .05] and EMG [F(1,39) = 6.66, p = .014] as well as a trend for HRD [F(1,40) = 2.84, p = .10] (Table S1). These interactions were, however, not observed in Novel Sets: SCR [F(1,40) = .00, p = .95], HRD [F(1,40) = .55, p = .46] or EMG [F(1,38) = 1.81, p = .19].
Second, MANOVA showed a trend for overall difference between Habituation Indices for physiological variables in the two groups (Wilks Lambda = .808, F(3,34) = 2.69, p = .062) with significant post-hoc ANOVAs for SCR HI [F(1,36) = 5.40, p = .026] and EMG HI [F(1,36) = 4.69, p = .037] but not HRD HI [F(1,36) = 1.23, p = .27]. [Sample size was reduced (Nap N = 21, Wake N = 17) because EMG and HRD data were unusable for 2 and 1 participants respectively and because Session-1 mean SCRs to Negative stimuli of 2 participants, being negative numbers, became zero denominators in the formula for SCR HI.]
Table 3 (SCR results) in the following section illustrates the ANOVA analyses performed on each outcome variable. Tables S1-S5 in Supplementary Materials provide complete ANOVA analyses of each additional outcome variable along with brief interpretation of each result. Analyses excluding the first 8 participants are also provided in Supplementary Materials.
3.2.1 SCR
At Session 1, Repeated Set SCR showed strong intra-session habituation and no baseline group differences for both Negative and Neutral stimuli (Table 3). In the Repeated Set, SCR to Negative stimuli also showed significant overall inter-session habituation as well as a significant Group × Session interaction indicating a group difference in inter-session habituation (Table 3, Figure 2a). Decomposing this interaction by Group revealed inter-session habituation (significant main effect of Session) in the Nap group [F(1,21)=13.65, p=.001] but not in the Wake group [F(1,20)=.56, p=.46] (Figure 2a). However, Repeated-Set Neutral stimuli showed neither overall inter-session habituation nor a group difference in inter-session habituation (Table 3, Figure 2a). The HI for SCR trended higher in Nap vs. Wake groups, [F(1,39) = 3.46, p = .07].
Figure 2.
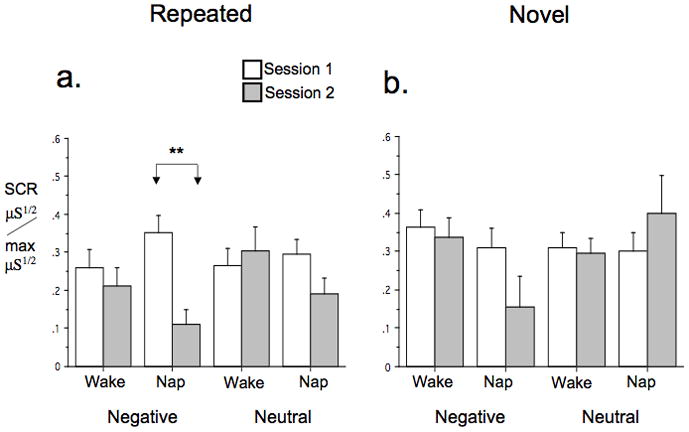
SCR to IAPS stimuli in Nap and Sleep groups. a. Repeated Set, b. Novel Sets. Bars indicate standard error of the mean (SEM). Significance levels shown only when Group × Session interaction within a specific valence (Negative or Neutral) was significant or a trend. μS1/2/max μS1/2 = range-corrected SCR, **p < .01.
At Session 1, Novel Set SCR also showed strong intra-session habituation and no baseline group differences for both Negative and Neutral stimuli (Table 3). However, for Novel Sets SCR, neither showed overall inter-session generalized habituation nor a group difference in generalized habituation for either Negative or Neutral stimuli (Table 3, Figure 2b).
In addition to inter-session habituation, sleep-related changes in non-specific sympathetic reactivity might have influenced SCR. Therefore, SCR to the 4 startle sounds were also compared between groups with 3-factor mixed ANOVA using the between-subjects-factor Group and the within-subject factors, Position (Beginning of session, End of session) nested in Session. There was no Group main effect [F(1,402.) = .06, p = .81], Group × Session interaction [F(1,40) = .40, p = .53) or Group × Session × Position interaction [F(1,40) = .65, p = .42]. Therefore the SCR Group × Session interaction was specific to IAPS stimuli and napping diminished SCR only to previously seen, Negative stimuli.
3.2.2 HRD
At Session 1, Repeated Set HRD showed neither intra-session habituation nor baseline group differences for both Negative and Neutral stimuli (Table S2). There was also no overall inter-session habituation of HRD to Negative or Neutral stimuli nor was there an overall inter-session habituation group difference to Repeated Set Neutral stimuli. There was, however, an inter-session habituation group difference (Group × Session interaction) trend for HRD to Repeated Set Negative stimuli [F(1,40) = 3.67, p=.062] (Figure 3a). Decomposing this interaction by Group revealed significant HRD inter-session habituation in the Wake [F(1,19)=4.39, p=.05] but not the Nap group [F(1,21) = .07, p=.80] (Figure 3a). The HI for HRD also trended higher in Nap vs. Wake groups, [F(1,40) = 3.37, p = .074].
Figure 3.
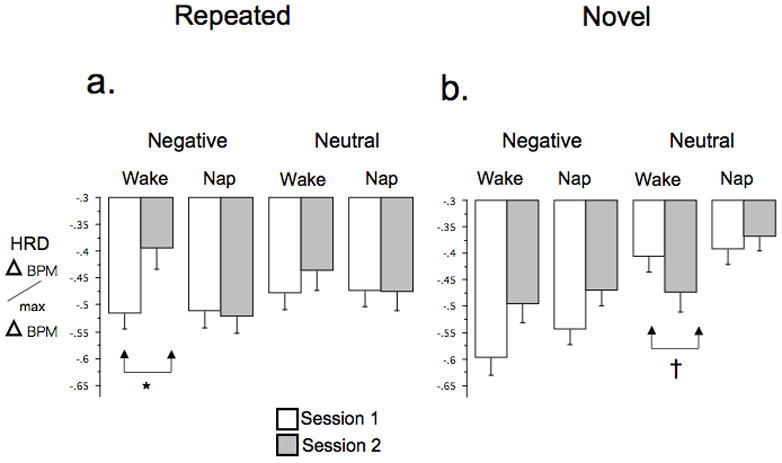
HRD to IAPS stimuli in Nap and Sleep groups. a. Repeated Set, b. Novel Sets. Bars indicate SEM. Significance levels shown only when Group × Session interaction within a specific valence was significant or a trend. Delta BPM/max Delta BPM = range-corrected HRD. BPM, beats per minute, † p < .1
At Session 1, Novel Sets HRD showed intra-session habituation that was a trend for Negative stimuli and significant for Neutral stimuli. However, neither Negative nor Neutral stimuli showed baseline group differences (Table S2). Novel Sets HRD to Negative but not Neutral stimuli showed overall inter-session generalized habituation (Table S2). In addition, Novel Sets HRD showed an inter-session generalized habituation group difference (Group × Session interaction) trend for HRD response to Neutral but not Negative stimuli [F(1,40) = 3.53, p=.068] (Figure 3b). Decomposing by group showed no significant inter-session generalized habituation in the Nap group [F(1,21) = .49, p=.49] but a near-trend toward an HRD increase (generalized sensitization) [F(1,19) = 2.87, p=.11] in the Wake group (Figure 3b).
3.2.3 Corrugator EMG
At Session 1, Repeated Set EMG showed an intra-session habituation trend for Negative but not Neutral stimuli and neither Negative nor Neutral stimuli showed a baseline group difference (Table S3). Neither Negative nor Neutral stimuli showed overall inter-session habituation, however Negative stimuli showed a group difference for inter-session habituation as a significant Group × Session interaction [F(1,39) = 5.69, p = .022] that was also a trend for Neutral stimuli [F(1,38) = 3.67, p = .062] (Figure 4a). Decomposing this interaction by Group in the Repeated Set Negative stimuli showed significant inter-session habituation in the Nap group [F(1,20) = 7.80, p = .011] but not the Wake group [F(1,19) = 1.73, p=.20] (Figure 4a). Similarly, in the Neutral stimuli, there was inter-session habituation in the Nap group [F(1,20) = 8.73, p = .008] but not the Wake group [F(1,19) = 1.44, p = .24] (Figure 4a). Notably, however, whereas the direction of change in EMG across sessions in the Nap group was a decrease, indicating habituation, in the Wake group it was a non-significant increase, indicating sensitization (Figure 4a). The HI for EMG was also significantly higher in Nap vs. Wake groups, [F(1,39) = 5.60, p = .023].
Figure 4.
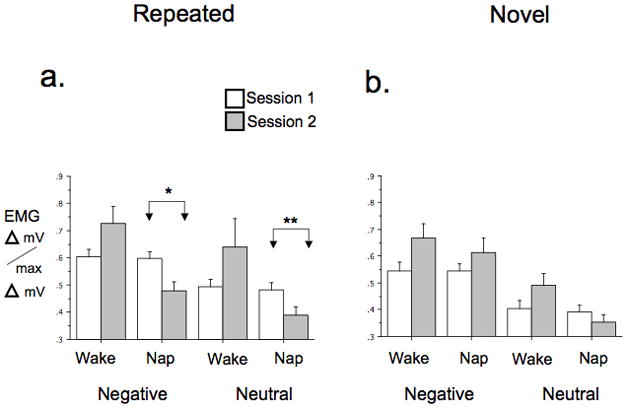
Corrugator EMG response to IAPS stimuli in Nap and Wake groups. a. Repeated Set, b. Novel Sets. Bars indicate SEM. Significance levels shown only when Group × Session interaction within a specific valence was significant or a trend. Delta mV/max Delta mV = range-corrected EMG. *p < .05 **p < .01.
At Session 1, Novel Set EMG showed strong intra-session habituation for Negative but not Neutral stimuli and neither showed a baseline group difference (Table S3). There was also an overall inter-session generalized habituation trend for Negative but not Neutral stimuli. For Neutral stimuli there was also a trend toward a Group × Session interaction [F(1,38) = 3.67, p = .063] indicating a group difference in inter-session generalized habituation (Figure 4b). Decomposing this interaction showed no inter-session generalized habituation in the Nap group [F(1,20) = .93, p = .35] but non-significant generalized sensitization [F(1,18) = 2.31, p = .15] in the Wake group (Figure 4b).
3.2.1 SAM valence and arousal ratings
In the Repeated Set, SAM Valence ratings showed Session 1 intra-session habituation for Neutral but not Negative stimuli, the latter of which showed only a near trend (Table S4). Neither Repeated Set Neutral nor Neutral stimuli showed a baseline group difference. The Repeated Set showed strong overall inter-session habituation for both Negative and Neutral stimuli but lack of a Group × Session interaction for Negative stimuli indicated similar inter-session habituation in the two groups. Among Neutral stimuli, however, a Group × Session interaction trend [F(1,41) = 4.0, p = .052] reflected a main effect of Session in the Wake [F(1,20) = 10.05, p = .005] but not the Nap group [F(1,21) = .83, p =.37]. This interaction resulted from non-significantly more positive Session 1 ratings of Neutral stimuli in the Wake group followed by similar Session-2 Valence ratings between both groups. Novel Sets showed Session-1 intra-session habituation and no baseline group difference for both Negative and Neutral stimuli (Table S4). There was neither overall inter-session generalized habituation nor a group difference in inter-session generalized habituation.
Repeated Set SAM Arousal ratings showed nearly identical results as SAM Valence ratings except that both Negative and Neutral stimuli showed Session-1 intra-session habituation and neither Negative nor Neutral stimuli showed a group difference in inter-session habituation (Table S5). In the Novel Sets, SAM Arousal ratings showed an identical pattern to SAM Valence ratings (Table S5).
3.3 Comparison of SSS and PANAS between groups and correlations between HI and subjective state, sleep/sleepiness and trait variables
SSS was analyzed in the same manner as SCR response to the startle (see above) and showed a Group × Session × Position interaction [F(1,41)=5.81, p=.021]. Decomposing by Group, the Nap group showed a significant Session × Position interaction [F(1,41)=14.25, p=.001]. Decomposing this interaction and comparing the Beginning and End of each session (2 comparisons, critical alpha = .025), the Nap group revealed a significant increase in sleepiness across Session 1 (F=9.49, p=.006) but a trend decrease across Session 2 (F=5.10, p=.035). In contrast, in the Wake group, there was no Session × Position interaction but a Position main effect trend [F(1,20)=3.14, p=.092] indicated a sleepiness increase across both sessions.
For PANAS a third within-subjects factors, Valence (Positive, Negative) was nested in Position that was in turn nested in Session. Neither the 3-way interaction, Group × Session × Valence [F(1,41)=.82, p=.37] nor the 4-way interaction Group × Session × Valence × Position [F(1,41)=.00, p=.97] were significant, indicating similar changes in overall positive and negative mood across sessions in the two groups.
There were no correlations at p < .05 between each of the 5 outcome variables’ HI and SSS or PANAS scores at the beginning of Session 2. Similarly, each variable’s HI did not correlate with individuals’ habitual, self-reported sleep quality (PSQI) and sleepiness (ESS). Lastly, there were no significant correlations between each variable’s HI and the STAI, NEO-PI-R Neuroticism or NEO-PI-R Extraversion trait variables.
3.4 Polysomnography (Nap group, Repeated Set and Negative stimuli only)
Table 4 shows sleep parameters for the Nap group. Among the 22 Nap subjects, 14 (63.6%) entered SWS, 15 (68.2%) entered REM and 9 (41%) entered both sleep stages.
Table 4.
Sleep parameters of the Nap group
| Mean | SD | Max | Min | count | |
|---|---|---|---|---|---|
| Time in bed | 115.64 | 11.08 | 124.00 | 78.50 | 22 |
| Total sleep time | 84.25 | 25.84 | 111.50 | 34.00 | 22 |
| Sleep latency | 5.16 | 3.50 | 12.50 | 1.00 | 22 |
| Latency to N2 | 8.84 | 4.05 | 16.50 | 3.50 | 22 |
| Sleep efficiency | 72.34 | 19.88 | 92.89 | 28.69 | 22 |
| N1 | 15.77 | 8.68 | 30.00 | 4.50 | 22 |
| N2 | 41.36 | 12.27 | 60.50 | 19.50 | 22 |
| SWS (N3) | 15.14 | 18.39 | 64.50 | 0.00 | 22 |
| SWS (N3)* | 23.79 | 18.02 | 64.50 | 1.50 | 14 |
| REM (R) | 11.98 | 10.17 | 31.00 | 0.00 | 22 |
| REM (R)** | 17.57 | 7.07 | 31.00 | 7.00 | 15 |
| REM latency | 66.37 | 17.31 | 103.00 | 42.00 | 15 |
| WASO | 25.36 | 22.45 | 82.50 | 2.00 | 22 |
Excluding those with no SWS,
Excluding those with no REM
For EMG, the SWS factor (SWS vs. no SWS) showed a significant SWS × Session interaction [F(1,19) = 4.84, p = .04]. Decomposing by SWS showed a highly significant reduction in EMG at Session 2 among who entered [F(1,13) = 12.79, p = .003] but not those who failed to enter [F(1,6) = .00, p = .97] SWS (Figure 5a). Notably, HI for EMG was significantly higher for subjects entering SWS [F(1,19) = 4.67, p = .044]. No other dependent variable showed a significant interaction of Session with the SWS factor.
Figure 5.
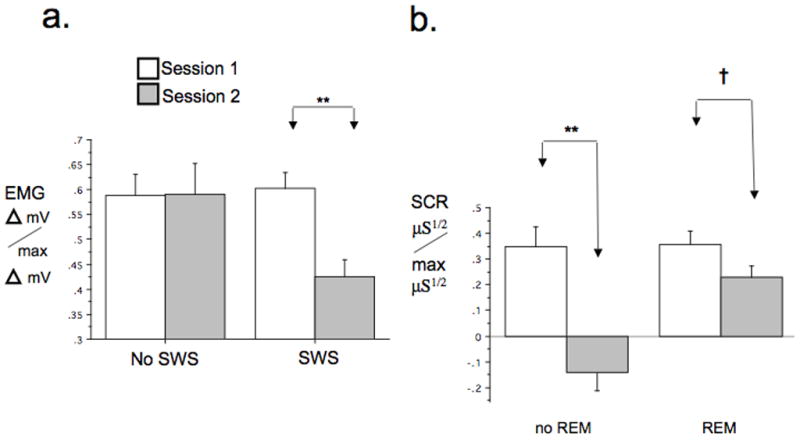
Sleep stages during nap associated with of inter-session habituation to Negative stimuli. a. Positive association of entry into SWS with inter-session habituation of EMG to Negative stimuli. b. Negative association of entry into REM with inter-session habituation of SCR to Negative stimuli. Delta mV/max Delta mV = normalized EMG, μS1/2/max μS1/2 = normalized SCR, † p < .1, **p<.01, .
For SCR, the REM factor (REM vs. no REM) showed a significant REM × Session interaction [F(1,20)=9.19, p=.007]. Decomposing this 2-way interaction by the REM factor, showed a highly significant Session main effect [F(1,6) = 31.68, p = .001] among subjects not entering REM whereas, in subjects entering REM, this was only a trend [F(1,14) = 3.25, p=.093] (Figure 5b). The HI for SCR trended higher for subjects not entering REM [F(1,20) = 3.74, p = .067]. No other dependent variable showed a significant interaction of Session with the REM factor.
Regression models predicting each of 5 HI’s with each of 7 sleep variables individually and in a step-wise manner yielded no findings at the p < .05 level (except 2 relationships attributable to outlying values). Notably, however, a positive correlation of HI for EMG with minutes of SWS (which also showed the categorical effect above) was a near trend (R = .36, p = .11). This was not the case, however, for a negative correlation between HI for SCR and minutes of REM (R = .25, p = .26). There were no correlations between any HI measure and self-reported amount of sleep on the night before or means of the week before the study day.
4. Discussion
A nap versus an equal duration of waking, following repeated intra-session exposure to highly negative and equally arousing, neutral IAPS pictorial stimuli, resulted in greater inter-session habituation to previously seen stimuli in SCR and corrugator EMG responses but not in subjective ratings. EMG responses to previously seen stimuli were, in fact, augmented (sensitized) in the Wake but not the Nap group, a phenomenon that has been previously reported in waking subjects (Bradley, Cuthbert, and Lang, 1996). HRD behaved oppositely with greater inter-session habituation to previously seen stimuli in the Wake group. Novel (non-repeated) stimuli did not display significantly different inter-session group effects. Therefore, significant effects of napping on inter-session habituation were specific to previously seen stimuli. In the Nap group, occurrence of SWS during napping was associated with more inter-session habituation of EMG to Negative stimuli whereas occurrence of REM was associated with less inter-session habituation of SCR to Negative stimuli.
4.1 Differing nap effects on objective vs. subjective responses
Recent findings suggest a possible explanation for discrepant effects of napping on physiological and subjective habituation. D-cycloserine (DCS), a partial N-methyl d-aspartate (NMDA) agonist, augments extinction of conditioned fear in rodents and in anxiety disordered patients but not healthy humans (Grillon, 2009). Grillon, (2009) posits that human extinction learning involves both high-level, cognitive processes and autonomically instantiated learning mechanisms that humans share with other mammals. These processes become dysregulated in anxiety disorders and Grillon suggests that DCS augments learning and memory only in these lower-level systems. Certain NMDA-dependent processes are dependent on sleep (Kopp, Longordo, Nicholson, and Luthi, 2006) and therefore, like DCS, sleep may enhance the plasticity underlying habituation and extinction in limbic and central autonomic circuits. Notably, key structures in autonomic and emotional responses such as the hypothalamus, amygdala and midbrain also take part in sleep regulatory circuits (Pace-Schott and Hobson, 2002). In contrast, cortical systems associated with cognitively mediated emotion regulation (Ochsner and Gross, 2005) may be less sensitive to both sleep and pharmacological effects on NMDA-dependent processes involved in somatovisceral learning. Nonetheless, an alternative possibility remains that the 9-point, Likert-style SAM scales, albeit non-verbally mediated, may not elicit sufficient introceptive discrimination to detect subtle changes in somatovisceral responses.
4.2 Opposing effects of Nap on HRD vs SCR and corrugator EMG
HRD in response to negatively valenced stimuli is a widely replicated phenomenon (Bradley and Lang, 2007) that habituates with repeated stimulus presentation in some (Averill et al., 1972) but not all experimental paradigms (Bradley, Lang, and Cuthbert, 1993). Habituation of HRD across inter-session waking but not napping might be explained by differential influences of sleep on parasympathetic vs. sympathetic control of physiological responses. HRD to external stimuli is mediated by parasympathetic mechanisms (Porges, 2007). NREM sleep, and particularly SWS, is known to reduce sympathetic and increase parasympathetic influence on cardiovascular parameters (Brandenberger, Ehrhart, Piquard, and Simon, 2001; Trinder, Kleiman, Carrington, Smith, Breen, Tan, and Kim, 2001). Moreover, brief naps have been shown to increase the high frequency component of heart rate variability indicative of increased parasympathetic influence (Takahashi, Fukuda, and Arito, 1998). Therefore, a nap may up-regulate central parasympathetic outflow, augment the capacity of a stimulus to mediate an HRD response and thereby counteract any prior habituation of HRD. In contrast, following continuous waking, HRD habituation would remain un-opposed. During the latter half of Session 2, nap-related augmentation of parasympathetic outflow may have dissipated thereby allowing habituation to be expressed by the time novel stimuli were presented.
4.3 Neural mechanisms for sleep augmentation of habituation
How might sleep augment physiological habituation to previously encountered stimuli? Negative IAPS stimuli activate limbic and paralimbic brain regions associated with processing negative emotions (e.g., fear) such as the amygdala (Grimm, Schmidt, Bermpohl, Heinzel, Dahlem, Wyss, Hell, Boesiger, Boeker, and Northoff, 2006; Hariri, Mattay, Tessitore, Fera, and Weinberger, 2003) as well as networks associated with disgust including the striatum and anterior insular and lateral prefrontal cortices (Caseras, Mataix-Cols, An, Lawrence, Speckens, Giampietro, Brammer, and Phillips, 2007; Mataix-Cols, An, Lawrence, Caseras, Speckens, Giampietro, Brammer, and Phillips, 2008). These structures are part of the midline limbic and paralimbic regions that undergo marked changes across the different sleep stages including prominent deactivation in SWS (Braun, Balkin, Wesenten, Carson, Varga, Baldwin, Selbie, Belenky, and Herscovitch, 1997; Dang-Vu, Desseilles, Laureys, Degueldre, Perrin, Phillips, Maquet, and Peigneux, 2005; Kaufmann, Wehrle, Wetter, Holsboer, Auer, Pollmacher, and Czisch, 2006) and reactivation to levels equaling or exceeding waking during REM (Braun et al., 1997; Maquet, Peters, Aerts, Delfiore, Degueldre, Luxen, and Franck, 1996; Nofzinger, Mintun, Wiseman, Kupfer, and Moore, 1997).
During habituation to emotional stimuli in humans, initially high activity in the amygdala declines over repeated stimulus presentations (Breiter, Etcoff, Whalen, Kennedy, Rauch, Buckner, Strauss, Hyman, and Rosen, 1996; Wright et al., 2001). The amygdala, in concert with the hippocampus, mediates the processing (McGaugh, 2004; Phelps, 2004) as well as the expression of emotional memories, the latter under regulation by the ventromedial prefrontal cortex (vmPFC) (Milad, Rauch, Pitman, and Quirk, 2006). Both the amygdala and vmPFC are part of strongly sleep-modulated midline regions (Nofzinger, Mintun, Wiseman, Kupfer, and Moore, 1997) and sleep deprivation augments amygdala response to negative IAPS stimuli while diminishing its functional connectivity with the vmPFC (Yoo, Gujar, Hu, Jolesz, and Walker, 2007). Therefore, sleep may enhance consolidation of inter-session habituation by regulating interactions between the amygdala and vmPFC. Such consolidation may, in turn, modulate forebrain influence on autonomic control centers such as the hypothalamus (Barbas, Saha, Rempel-Clower, and Ghashghaei, 2003), and somatic expressions of emotion they control, when habituated stimuli are again encountered.
4.4 Sleep stages associated with habituation, relevance to emotion regulation
In the Nap group, lower SCR habituation in subjects who entered REM suggests that this sleep stage, one that is more sympathetically active than SWS (Trinder et al., 2001), may oppose inter-session SCR habituation. In contrast, greater EMG habituation in subjects entering SWS, a stage more parasympathetically active than REM (Trinder et al., 2001), suggests that SWS may enhance inter-session habituation of corrugator EMG responses to negative stimuli. It is tempting then to speculate that REM, a highly brain-activated state, may act to adaptively reinforce emotional salience (see also Wagner et al., 2002 and Lara-Carrasco et al., 2009) and thereby reduce habituation, whereas SWS may promote return to emotional homeostasis. The latter role of SWS may be analogous to its proposed homeostatic roles related to sleep need (Borbely and Achermann, 2005) as well as to the role of sleep in moderating allostatic load (McEwen, 2006).
4.5 Can peripherally measured affective habituation reflect memory processes?
Changes in peripheral autonomic responses to specific stimuli (e.g., conditioning, extinction, habituation) reflect central neuroplasticity induced by previous exposure to such stimuli (Rankin, Abrams, Barry, Bhatnagar, Clayton, Colombo, Coppola, Geyer, Glanzman, Marsland, McSweeney, Wilson, Wu, and Thompson, 2009; Schiller and Delgado, 2010). For example, plastic changes in basolateral amygdala support fear conditioning and its extinction while plasticity in vmPFC (infralimbic cortex in rat) subsequently consolidates extinction memory and supports its later expression via inhibition of amygdala output to autonomic effectors (Quirk and Mueller, 2008). Notably, functional responses in similar prefrontal and amygdala regions have been shown to accompany the acquisition of emotional habituation in humans (Milad et al., 2007; Mutschler et al., 2010; Wright et al., 2001).
In the case of conditioning and extinction in humans, an autonomic response (SCR) has been the gold-standard peripheral measure of fear conditioning and extinction (reviewed in Sehlmeyer, Schoning, Zwitserlood, Pfleiderer, Kircher, Arolt, and Konrad, 2009). Habituation can be similarly measured not only by decreased overt behavioural responses but also by simple reflexes, autonomic changes such as SCR, motor-neuron potentials and neuroendocrine responses (Rankin et al., 2009). For example, habituation in the hypothalamic-pituitary adrenal (HPA) axis to repeated stress displays memory phenomena such as long-term maintenance, spontaneous recovery, contextual sensitivity and generalization, the same phenomena observed in conditioning and extinction (Grissom and Bhatnagar, 2009). These latter investigators argue that HPA habituation involves lasting memory traces that are dependent on plasticity in forebrain limbic circuits similar to those that support other forms of emotional learning. The phenomena of dishabituation (whereby a different strong stimulus restores responding to an habituated stimulus), stimulus specificity (whereby a change in stimulus restores responding to an habituated stimulus), and spontaneous recovery similarly distinguish habituation from sensory adaptation, fatigue, endocrine negative-feedback mechanisms and other memory-independent processes (Grissom and Bhatnagar, 2009; Rankin et al., 2009).
Additional evidence suggesting that habituation involves memory processes comes from fear conditioning experiments where a habituation procedure is added to extinction—the repeated presentation of a conditioned stimulus (CS) in the absence of the unconditioned stimulus (US). In rats, habituation to a US by its repeated presentation, termed “devaluation of the US”, additively diminishes conditioned responding to an extinguished CS (Storsve, McNally, and Richardson, 2010). Moreover, devaluation of the US shares with extinction context specificity, reinstatement phenomena and sensitivity to NMDA antagonism (Storsve et al., 2010).
It should be noted that corrugator EMG responses, the variable for which we noted greatest Nap-dependent facilitation of inter-session habituation, is not an autonomic response, but rather the output of central regions that underlie expression of negative affect (Diesch and Flor, 2007; Lanteaume, Khalfa, Regis, Marquis, Chauvel, and Bartolomei, 2007; Lee, Josephs, Dolan, and Critchley, 2006). Habituation of corrugator EMG (or its sensitization as occurred in the Wake group) to aversive images is presumably initiated by experienced-based neuroplastic changes in cortical (e.g., insula) and subcortical (e.g., amygdala) limbic areas that receive input from visual areas and send output to premotor and motor areas that support CNS control of facial musculature.
The current findings are consistent with Walker’s model of sleep-mediated regulation of emotional memory whereby emotionally labeled memories are concurrently strengthened in terms of the core memory but the affective charge associated with the memory is weakened (Walker, 2009; Walker and van der Helm, 2009). The current findings clearly support the latter postulate, that the emotional charge may be reduced by sleep. However, limiting exposure to 12 IAPS stimuli precluded testing a benefit on the core memory due to ceiling effects. Interestingly, whereas Walker et al. postulate a specific role of REM in promoting emotion regulation, the current findings instead implicate SWS in moderating negative aspects of a previously encountered negative stimulus insofar as it is expressed by muscles of facial expression. In contrast, entry into REM appears to diminish affective habituation insofar is it is measured by SCR. Notably, these two measures appear to load on different factors with facial EMG responsive to the pleasant to unpleasant (valence) continuum and SCR reflecting the arousal dimension (Bradley and Lang, 2007; Lang, Greenwald, Bradley, and Hamm, 1993).
4.6 Methodological issues
The lack of a Group × Session × Valence interaction for HRD and corrugator EMG in the Repeated Set (see Table S1), despite a stronger Group × Session interaction among Negative vs. Neutral stimuli analyzed separately (Tables 3 and S2–S5), suggests loss of statistical power due to high variability in these data. In HRD data, sub-groups of normal subjects who have acceleratory (vs. deceleration) heart rate responses to negative IAPS stimuli (Sanchez-Navarro, Martinez-Selva, and Roman, 2006) may have further increased such variability. In the case of SCR, absence of this 3-way interaction is not surprising because general arousal is a greater determinant of SCR magnitude than valence (Bradley and Lang, 2007) and stimuli in the current study were matched on this dimension. Nonetheless, for SCR, Negative stimuli again showed a greater Group × Session interaction than Neutral stimuli.
Another limitation of this study involves the potential effects of viewing the videos on the Wake group. Although videos were selected to be neutral and unarousing by conventional standards, there undoubtedly exist individual differences in the strength and valence of emotional response to the Planet Earth segments (or, in the case of the first 4 Wake participants, to the particular movies watched). For certain individuals, such reactions to the videos might have impacted their response to subsequently shown IAPS images. Therefore, interpretation of results would have been aided if Wake participants had rated the degree to which they found the videos pleasant to unpleasant (valence) and arousing to unarousing (arousal) using the SAM or another similar rating scale. Such ratings performed at the beginning, middle and conclusion of the video could additionally capture any systematic change in emotional response across the awake inter-session interval.
An additional limitation is the low degree of Session 1 intra-session habituation for HRD and EMG. Group difference might have been more distinct and may have emerged in the subjective SAM ratings if Session-1 habituation had been more deeply established either by sequential (vs. interspersed) stimulus presentations or by a greater number of presentations. Notably, however, studies of exposure therapy have clearly shown that inter-session habituation is fully separable from and potentially independent of intra-session habituation (Craske et al., 2008; Jaycox et al., 1998). Moreover, as noted, animal studies have shown intra-session and inter-session habituation to be biologically dissociable processes (Bolivar, 2009; Leussis and Bolivar, 2006).
Although 120 minutes were allowed for the nap, only about 2/3 of Nap subjects achieved SWS or REM thereby reducing the number of individuals in which quantities of these two parameters could be directly correlated with habituation. Nonetheless, non-100% entry into SWS and REM across allowed individuals’ inter-session habituation to be differentiated on the categorical basis of exposure to these sleep states.
Five subjects had episodes of REM sleep without SWS raising the possibility that they might have been sleep-deprived. However, these individuals had neither higher PSQI or ESS scores nor did any of their SSS scores (before and after each session) differ from the remaining 17 Sleep-group subjects. Moreover, average REM latencies of the 5 who had REM but not SWS were longer (71 min, SD 13) than the remaining 17 (37 min, 36) (p=.057), the opposite than what would be expected if they rapidly entered REM and curtailed descent into SWS due to sleep deprivation. Additionally, REM latency did not correlate with ESS, PSQI or any of the SSS measures. Therefore, there is no evidence that these 5 individuals were abnormally sleep deprived.
5. Conclusion
A brief afternoon nap promotes inter-session habituation of SCR and corrugator EMG responses to arousing stimuli, an effect that was greater in negatively valenced stimuli. However, napping did not influence habituation of subjects’ subjective report of emotional responses to these same stimuli. SCR and EMG are known to reflect sympathetic arousal and covert negative emotion respectively (Bradley and Lang, 2007). The current study provides preliminary evidence for regulation, by sleep, of physiological aspects of emotional expression via augmented inter-session habituation. Greater sleep-mediated habituation of repeated vs novel stimuli suggests that such regulation may operate via sleep-dependent augmentation of non-associative memory. Findings suggest a potential mental-health benefit of daytime naps for moderating acute responses to unpleasant events.
Supplementary Material
Acknowledgments
Supported by NIH DA11744 and MH48832
The authors thank Alexander Dimov of BioPac Inc. and Drs. Scott P. Orr, Mauricio Codispoti and Matthew Walker for invaluable technical and scientific advice. We thank the National Institute on Drug Abuse (DA11744) and the National Institutes of Health (MH48832) for supporting this research.
Abbreviations
- ANOVA
analysis of variance
- BPM
beats-per-minute
- CS
conditioned stimulus
- DCS
D-cycloserine
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision
- EEG
electroencephalogram
- EMG
electromyogram
- ESS
Epworth Sleepiness Scale
- HRD
heart rate deceleration
- Hz
Hertz (cycles per second)
- IAPS
International Affective Picture System
- ISI
inter-stimulus interval
- KOhm
kilo Ohm
- μS
microSiemens
- μV
microVolts
- NEO-PI-R
Revised NEO Personality Inventory
- NMDA
N-methyl d-aspartate
- NREM
non-rapid eye movement sleep
- PANAS
Positive and Negative Affect Schedule
- PSQI
Pittsburgh Sleep Quality Index
- PSG
polysomnography
- REM
rapid eye movement sleep
- SAM
Self-Assessment Manikin
- SCL
skin conductance level
- SCR
skin conductance response
- SD
standard deviation
- SEM
standard error of the mean
- SSS
Stanford Sleepiness Scale
- STAI
Spielberger State-Trait Anxiety Index
- SWS
slow wave sleep (NREM stages 3 and 4)
- US
unconditioned stimulus
Footnotes
One Sleep group subject’s very low Session-1 maximum unadjusted SCR (.05 microSiemens) to Novel Sets stimuli was followed by more typical responses for Session-2, Trial-1 SCRs to Negative and Neutral stimuli (.778 and .346 microSiemens respectively). This resulted in adjusted Trial-1 SCRs at Session 2 that were 14 and 8 standard deviations respectively above the overall sample means for Novel-Set SCRs. Because Session 1’s low SCRs were likely due to a recording defect, this subject’s Novel-Set SCR data were not analyzed. Similarly in one Wake subject, adjusted EMG at Session 2 to Trial 1 of Neutral stimuli and Trial 4 of Negative stimuli were 13 and 6 standard deviations respectively above the overall sample means for Novel-Set EMGs.
Responses to the fourth startle sound were not available for the single subject whose data were missing responses to the last 2 stimuli of Novel Set Negative, Novel Set Neutral IAPS images (See Methods, 2.7 Outcome variables and habituation indices) as well as to the final startle sound.
No conflict of interest exist for authors of this report
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Averill JR, Malmstrom EJ, Koriat A, Lazarus RS. Habituation to complex emotional stimuli. Journal of Abnormal Psychology. 1972;80:20–28. doi: 10.1037/h0033309. [DOI] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ. Intrasession and intersession habituation in mice: from inbred strain variability to linkage analysis. Neurobiology of Learning and Memory. 2009;92:206–214. doi: 10.1016/j.nlm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4. Philadelphia: Elsevier; 2005. pp. 405–417. [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Picture media and emotion: effects of a sustained affective context. Psychophysiology. 1996;33:662–670. doi: 10.1111/j.1469-8986.1996.tb02362.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Emotion and motivation. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 3. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- Bradley MM, Lang PJ, Cuthbert BN. Emotion, novelty, and the startle reflex: habituation in humans. Behavioral Neuroscience. 1993;107:970–980. doi: 10.1037//0735-7044.107.6.970. [DOI] [PubMed] [Google Scholar]
- Brandenberger G, Ehrhart J, Piquard F, Simon C. Inverse coupling between ultradian oscillations in delta wave activity and heart rate variability during sleep. Clinical Neurophysiology. 2001;112:992–996. doi: 10.1016/s1388-2457(01)00507-7. [DOI] [PubMed] [Google Scholar]
- Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, Selbie S, Belenky G, Herscovitch P. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cartwright R, Luten A, Young M, Mercer P, Bears M. Role of REM sleep and dream affect in overnight mood regulation: a study of normal volunteers. Psychiatry Research. 1998;81:1–8. doi: 10.1016/s0165-1781(98)00089-4. [DOI] [PubMed] [Google Scholar]
- Caseras X, Mataix-Cols D, An SK, Lawrence NS, Speckens A, Giampietro V, Brammer MJ, Phillips ML. Sex differences in neural responses to disgusting visual stimuli: implications for disgust-related psychiatric disorders. Biological Psychiatry. 2007;62:464–471. doi: 10.1016/j.biopsych.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO five-factor inventory (NEO-FFI) Professional Manual. Odessa FL: Psychological Assessment Resources, Inc; 1992. [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Desseilles M, Laureys S, Degueldre C, Perrin F, Phillips C, Maquet P, Peigneux P. Cerebral correlates of delta waves during non-REM sleep revisited. Neuroimage. 2005;28:14–21. doi: 10.1016/j.neuroimage.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 3. New York: Cambridge University Press; 2007. pp. 159–181. [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesch E, Flor H. Alteration in the response properties of primary somatosensory cortex related to differential aversive Pavlovian conditioning. Pain. 2007;131:171–180. doi: 10.1016/j.pain.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis A, Smith PK. Affective habituation: subliminal exposure to extreme stimuli decreases their extremity. Emotion. 2002;2:203–214. doi: 10.1037/1528-3542.2.3.203. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychological Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10:473–481. doi: 10.31887/DCNS.2008.10.4/plfranzen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Med Rev. 2008;12:185–195. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C. D-Cycloserine Facilitation of Fear Extinction and Exposure-Based Therapy Might Rely on Lower-Level, Automatic Mechanisms. Biological Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Schmidt CF, Bermpohl F, Heinzel A, Dahlem Y, Wyss M, Hell D, Boesiger P, Boeker H, Northoff G. Segregated neural representation of distinct emotion dimensions in the prefrontal cortex-an fMRI study. Neuroimage. 2006;30:325–340. doi: 10.1016/j.neuroimage.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Grissom N, Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiology of Learning and Memory. 2009;92:215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119:56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Hot P, Leconte P, Sequeira H. Diurnal autonomic variations and emotional reactivity. Biological Psychology. 2005;69:261–270. doi: 10.1016/j.biopsycho.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Hot P, Naveteur J, Leconte P, Sequeira H. Diurnal variations of tonic electrodermal activity. International Journal of Psychophysiology. 1999;33:223–230. doi: 10.1016/s0167-8760(99)00060-4. [DOI] [PubMed] [Google Scholar]
- Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotional declarative memory. Psychol Sci. 2006;17:891–898. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- Iber C, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Jaycox LH, Foa EB, Morral AR. Influence of emotional engagement and habituation on exposure therapy for PTSD. Journal of Consulting and Clinical Psychology. 1998;66:185–192. doi: 10.1037//0022-006x.66.1.185. [DOI] [PubMed] [Google Scholar]
- Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17:703–710. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- Kaufmann C, Wehrle R, Wetter TC, Holsboer F, Auer DP, Pollmacher T, Czisch M. Brain activation and hypothalamic functional connectivity during human non-rapid eye movement sleep: an EEG/fMRI study. Brain. 2006;129:655–667. doi: 10.1093/brain/awh686. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Balkin TJ, Wesensten NJ. Impaired decision making following 49 h of sleep deprivation. Journal of Sleep Research. 2006;15:7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Day LM, Li CJ, Kamimori GH, Balkin TJ, Killgore DB. Moral reasoning is affected by sleep deprivation. Sleep. 2006;29 [Google Scholar]
- Kim EJ, Dimsdale JE. The effect of psychosocial stress on sleep: a review of polysomnographic evidence. Behav Sleep Med. 2007;5:256–278. doi: 10.1080/15402000701557383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C, Longordo F, Nicholson JR, Luthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. Journal of Neuroscience. 2006;26:12456–12465. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: affect, activation, and action. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and Orienting: Sensory and Motivational Processes. Hillsdale, NJ: Lawrence Erlbaum Associates; 1997. pp. 97–135. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. University of Florida; Gainesville, FL: 2008. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lanteaume L, Khalfa S, Regis J, Marquis P, Chauvel P, Bartolomei F. Emotion induction after direct intracerebral stimulations of human amygdala. Cerebral Cortex. 2007;17:1307–1313. doi: 10.1093/cercor/bhl041. [DOI] [PubMed] [Google Scholar]
- Lara-Carrasco J, Nielsen TA, Solomonova E, Levrier K, Popova A. Overnight emotional adaptation to negative stimuli is altered by REM sleep deprivation and is correlated with intervening dream emotions. Journal of Sleep Research. 2009;18:178–187. doi: 10.1111/j.1365-2869.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- Lee TW, Josephs O, Dolan RJ, Critchley HD. Imitating expressions: emotion-specific neural substrates in facial mimicry. Soc Cogn Affect Neurosci. 2006;1:122–135. doi: 10.1093/scan/nsl012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neuroscience and Biobehavioral Reviews. 2006;30:1045–1064. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Levin R, Nielsen TA. Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychological Bulletin. 2007;133:482–528. doi: 10.1037/0033-2909.133.3.482. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Venables PH. Direct measurement of skin conductance: a proposal for standardization. Psychophysiology. 1971;8:656–672. doi: 10.1111/j.1469-8986.1971.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Maquet P, Peters J, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, An SK, Lawrence NS, Caseras X, Speckens A, Giampietro V, Brammer MJ, Phillips ML. Individual differences in disgust sensitivity modulate neural responses to aversive/disgusting stimuli. European Journal of Neuroscience. 2008;27:3050–3058. doi: 10.1111/j.1460-9568.2008.06311.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism: Clinical and Experimental. 2006;55(Suppl 2):S20–23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1981. [Google Scholar]
- McSweeney FK, Murphy ES. Sensitization and habituation regulate reinforcer effectiveness. Neurobiology of Learning and Memory. 2009;92:189–198. doi: 10.1016/j.nlm.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Mellman TA. Sleep and anxiety disorders. Psychiatric Clinics of North America. 2006;29:1047–1058. doi: 10.1016/j.psc.2006.08.005. abstract x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, Rauch SL. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behavioral Neuroscience. 2006;120:1196–1203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biological Psychology. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Moratti S, Keil A, Stolarova M. Motivated attention in emotional picture processing is reflected by activity modulation in cortical attention networks. Neuroimage. 2004;21:954–964. doi: 10.1016/j.neuroimage.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Progress in Cardiovascular Diseases. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Speck O, Schulze-Bonhage A, Hennig J, Seifritz E, Ball T. Time Scales of Auditory Habituation in the Amygdala and Cerebral Cortex. Cerebral Cortex. 2010 doi: 10.1093/cercor/bhq001. [DOI] [PubMed] [Google Scholar]
- Nielsen T, Levin R. Nightmares: a new neurocognitive model. Sleep Med Rev. 2007;11:295–310. doi: 10.1016/j.smrv.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cerebral Cortex. 2009;19:1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofzinger EA, Mintun MA, Wiseman M, Kupfer DJ, Moore RY. Forebrain activation in REM sleep: an FDG PET study. Brain Research. 1997;770:192–201. doi: 10.1016/s0006-8993(97)00807-x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109:290–298. [PubMed] [Google Scholar]
- Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Milad MR, Orr SP, Rauch SL, Stickgold R, Pitman RK. Sleep promotes generalization of extinction of conditioned fear. Sleep. 2009;32:19–26. [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Stickgold R, Muzur A, Wigren PE, Ward AS, Hart CL, Clarke D, Morgan A, Hobson JA. Sleep quality deteriorates over a binge--abstinence cycle in chronic smoked cocaine users. Psychopharmacology. 2005;179:873–883. doi: 10.1007/s00213-004-2088-z. [DOI] [PubMed] [Google Scholar]
- Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychol Sci. 2008;19:781–788. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MJ, Benca RM. Sleep in mood disorders. Psychiatric Clinics of North America. 2006;29:1009–1032. doi: 10.1016/j.psc.2006.09.003. abstract ix. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney FK, Wilson DA, Wu CF, Thompson RF. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiology of Learning and Memory. 2009;92:135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Navarro JP, Martinez-Selva JM, Roman F. Uncovering the relationship between defence and orienting in emotion: cardiac reactivity to unpleasant pictures. International Journal of Psychophysiology. 2006;61:34–46. doi: 10.1016/j.ijpsycho.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci. 2010 doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS ONE. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory (Self-Evaluation Questionnaire) Palo Alto: Consulting Psychologists Press; 1990. [Google Scholar]
- Storsve AB, McNally GP, Richardson R. US habituation, like CS extinction, produces a decrement in conditioned fear responding that is NMDA dependent and subject to renewal and reinstatement. Neurobiology of Learning and Memory. 2010 doi: 10.1016/j.nlm.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Fukuda H, Arito H. Brief naps during post-lunch rest: effects on alertness, performance, and autonomic balance. Eur J Appl Physiol Occup Physiol. 1998;78:93–98. doi: 10.1007/s004210050392. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Trinder J, Kleiman J, Carrington M, Smith S, Breen S, Tan N, Kim Y. Autonomic activity during human sleep as a function of time and sleep stage. Journal of Sleep Research. 2001;10:253–264. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- Turpin G, Schaefer F, Boucsein W. Effects of stimulus intensity, risetime, and duration on autonomic and behavioral responding: implications for the differentiation of orienting, startle, and defense responses. Psychophysiology. 1999;36:453–463. [PubMed] [Google Scholar]
- van der Helm E, Gujar N, Walker MP. Sleep Deprivation Impairs the Accurate Recognition of Human Emotions. Sleep. 2010;33:335–342. doi: 10.1093/sleep/33.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Degirmenci M, Drosopoulos S, Perras B, Born J. Effects of cortisol suppression on sleep-associated consolidation of neutral and emotional memory. Biological Psychiatry. 2005;58:885–893. doi: 10.1016/j.biopsych.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Wagner U, Fischer S, Born J. Changes in emotional responses to aversive pictures across periods rich in slow-wave sleep versus rapid eye movement sleep. Psychosomatic Medicine. 2002;64:627–634. doi: 10.1097/01.psy.0000021940.35402.51. [DOI] [PubMed] [Google Scholar]
- Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learning and Memory. 2001;8:112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Hallschmid M, Rasch B, Born J. Brief sleep after learning keeps emotional memories alive for years. Biological Psychiatry. 2006;60:788–790. doi: 10.1016/j.biopsych.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Annals of the New York Academy of Sciences. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychological Bulletin. 2009;135:731–748. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FA, Rolls ET. The effects of stimulus novelty and familiarity on neuronal activity in the amygdala of monkeys performing recognition memory tasks. Experimental Brain Research. 1993;93:367–382. doi: 10.1007/BF00229353. [DOI] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep--a prefrontal amygdala disconnect. Current Biology. 2007;17:R877–878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Zohar D, Tzischinsky O, Epstein R, Lavie P. The effects of sleep loss on medical residents’ emotional reactions to work events: a cognitive-energy model. Sleep. 2005;28:47–54. doi: 10.1093/sleep/28.1.47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


