Abstract
Background:
Exhaled breath condensate (EBC) pH is 2 log orders below normal during acute asthma exacerbations and returns to normal with antiinflammatory therapy. However, the determinants of EBC pH, particularly in stable asthma, are poorly understood. We hypothesized that patients with severe asthma would have low EBC pH and that there would be an asthma subpopulation of patients with characteristically low values.
Methods:
We studied the association of EBC pH with clinical characteristics in 572 stable subjects enrolled in the Severe Asthma Research Program. These included 250 subjects with severe asthma, 291 with nonsevere asthma, and 31 healthy control subjects.
Results:
Overall, EBC in this population of stable, treated study subjects was not lower in severe asthma (8.02; interquartile range [IQR], 7.61-8.41) or nonsevere asthma (7.90; IQR, 7.52-8.20) than in control subjects (7.9; IQR, 7.40-8.20). However, in subjects with asthma the data clustered below and above pH 6.5. Subjects in the subpopulation with pH < 6.5 had lower fraction of exhaled NO (FeNO) values (FeNO = 22.6 ± 18.1 parts per billion) than those with pH ≥ 6.5 (39.9 ± 40.2 parts per billion; P < .0001). By multiple linear regression, low EBC pH was associated with high BMI, high BAL neutrophil counts, low prebronchodilator FEV1 ratio, high allergy symptoms, race other than white, and gastroesophageal reflux symptoms.
Conclusion:
Asthma is a complex syndrome. Subjects who are not experiencing an exacerbation but have low EBC pH appear to be a unique subpopulation.
The airway can be acidified in a variety of inflammatory lung diseases.1-10 The airway epithelium has a pH buffering function analogous to that of the renal tubular epithelium: Acid originating in the airway, or inhaled, is buffered by pH regulatory enzymes.2,6,11,12 In turn, these enzymes are regulated. For example, airway buffering by glutaminase may be inhibited by T helper cells 1 cytokines, possibly for innate host defense by airway acid.11-13 In cystic fibrosis, the airway is chronically somewhat acidic.8,10 In the case of asthma, the proximal airway is acidified minimally, if at all, at baseline,1,14,15 and it becomes more acidic12,13 in the context of acute exacerbations caused by intercurrent viral infections.1,16 Here, we tested the hypothesis that there is a subpopulation of stable, severe asthma patients with low exhaled breath condensate (EBC) pH. This primary outcome variable was studied in a large cohort of subjects in the Severe Asthma Research Program (SARP).
Determinants of EBC pH are multifactorial.1,11,17-19 Changes in deaerated EBC pH reflect changes in subglottic airway acid production and buffering.2,9,17,20 For example, EBC pH values measured through an endotracheal tube after intubation are the same as those measured through a mouthpiece before intubation,20 and subjects with asthma intubated for respiratory failure have low EBC pH that normalizes with corticosteroid treatment.9 Of note, EBC pH values do not vary with bronchodilator administration, hyperventilation, or hypoventilation.14,20
Asthma is likely not exclusively one homogeneous disease but is a collection of different biochemical and immune responses to environmental challenges that have, as a common pathway, airway inflammation and reversible bronchoconstriction.21,22 We report that low pH is associated with phenotypic features such as high BMI, low baseline predrug FEV1, high airway neutrophil percent identified by BAL, non-white race, and gastroesophageal reflux (GER) symptoms. Further, we confirm1,5,23 that there is a cluster of subjects with EBC pH values < 6.5 and that these have a low fraction of exhaled nitric oxide (FeNO) and, particularly in severe asthma, increased allergy symptoms. We conclude that low EBC pH may ultimately be useful as a biomarker to identify (1) specific subpopulations of asthma patients, and (2) exacerbations of asthma.1,16 EBC pH does not discriminate between severe and nonsevere subgroups of stable asthma, but rather is one biomarker that may be useful for identifying an asthma subpopulation.
Materials and Methods
The Severe Asthma Research Program is a multicenter collaborative study sponsored by the National Heart, Lung, and Blood Institute.24 The goal of the study is to characterize as fully as possible the distinguishing features of severe asthma, as defined by the American Thoracic Society Consensus Meeting and adopted by the SARP Steering Committee.24,25 All subjects with severe asthma are maintained long-term on high-dose inhaled and/or systemic corticosteroid treatment and remain symptomatic when compared with a large cohort of subjects with nonsevere asthma and with a smaller cohort of control subjects enrolled for comparison.24,25 The methods of evaluation have been published.24 All subjects undergo lung function testing, including maximum bronchodilator challenge; they also undergo an extensive, questionnaire-based history, including allergy history, and those subjects who do not have a specific contraindication undergo methacholine challenge and flexible bronchoscopy. Subjects also undergo allergen skin testing (Greer; Lenoir, North Carolina) for tree mix (white ash, American beech, red birch, American elm, shagbark hickory, red oak, cottonwood), timothy grass, ragweed, Alternaria, Cladosporium, Aspergillus, Dermatophagoides farinae, Dermatophagoides pteronyssinus, weed mix (cocklebur, lambs quarter, pigweed, English plantain, Russian thistle), dog, cat, and cockroach. Most subjects in the first phase of SARP, including all patients at the Wake Forest, North Carolina; Emory, Georgia; Cleveland, Ohio; Virginia; and Wisconsin sites, where there was a specific interest in EBC analysis, underwent EBC analysis. The protocol was approved by the Institution Review Board of each participating institution.
EBC Collection
EBC was collected over 5 to 10 min at baseline on the initial visit of the SARP protocol as previously described. Samples were collected using the R-tube (Respiratory Research, Inc; Charlottesville, Virginia) at a collection temperature of −20°C. For this study, EBC was shipped on dry ice at variable time intervals to the University of Virginia for deaeration (at ambient temperature26) and pH measurement by investigators blinded to the identity of characteristics of the subject. Note that deaeration and storage at −20°C yields reproducible pH results, even in intercontinental shipping,20,27,28 across pH range 5-8 (n = 15); repeat assays give an r2 = 0.975.27 Samples were deaerated using argon as previously described for 7 min, and stable pH recorded as previously described.1
Data Analysis
All SARP data are collected, stored, and analyzed in the SARP Data Coordinating Center at National Jewish Hospital, Denver, Colorado. Data were further analyzed in the Department of Public Health Sciences at University of Virginia, Charlottesville, Virginia.
By univariate analysis, we studied the relationship between a continuous pH value and risk factors. We computed the correlation between pH value and continuous risk factors (eg, FEV1 and BMI, numbers of positive skin tests, presence of BAL neutrophils). We carried out t test and nonparametric rank-sum test for two-sample comparison (eg, GER) and analysis of variance for categorical variables with more than two levels (eg, allergy symptoms in spring and winter). We then used multiple linear regression to study the independent relationship of the continuous pH value with these risk factors, adjusting for other confounding variables. We studied variables associated with dichotomized pH determined by (1) the strongest positive and negative predictive value for identifying GER symptoms (identified by receiver operator curve analysis), and (2) distributions observed to be derived at pH 6.5. The statistical analyses were carried out in SAS 9.1 (SAS Institute Inc; Cary, North Carolina). The plots were drawn in S-Plus software (Insightful Corp; Seattle, Washington). Results are presented as mean ± SD or median and range.
Results
EBC was collected from 573 subjects aged 6 through 70 years between July 2003 and April 2007. This cohort included 249 subjects with severe asthma (aged 37.8 ± 15.6 years; 39% men), 293 subjects with nonsevere asthma (aged 32.2 ± 13.4 years; 32% men), and 31 healthy control subjects (aged 28.7 ± 9.6 years; 32% men) (e-Tables 1, 2).
In this cohort, EBC pH has a median of 7.94, with interquartile range (IQR) 7.55 to 8.30. Because the pH value is not normally distributed, we used the Wilcoxon rank-sum test to perform the two-sample comparison. The deaerated pH of subjects with asthma (median, 7.94; IQR, 7.56-8.30) did not differ from that of control subjects (7.90; IQR, 7.40-8.20; P = .80); pH in subjects with nonsevere asthma (7.90; IQR, 7.52-8.20) was lower than in subjects with severe asthma (8.02; IQR, 7.61-8.41). Patients with asthma had a bimodal distribution, with a subpopulation having low pH (Figs 1-4).
Figure 1.
Exhaled breath condensate pH population distribution in normal control subjects (green line), subjects with stable mild to moderate asthma (blue dashed line), and subjects with stable severe asthma (red dotted line). Note the distribution of some subjects with subjects substantially below pH 6.5. Individual subjects are shown as hash marks on the x-axis.
Figure 4.
The relationship between pH and response to GERD symptoms by questionnaire (yes/no). Asthma severity is denoted by symbols: red, normal; blue, not severe; green, severe. GERD = gastroesophageal reflux disease.
Figure 3.
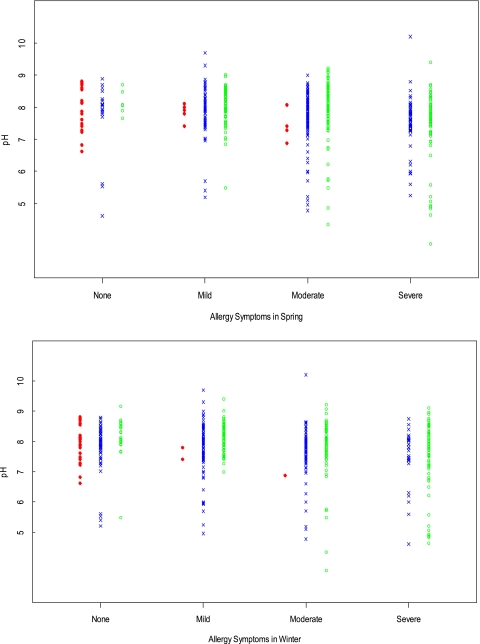
The relationship between pH and allergy symptoms in spring and winter. Asthma severity is denoted by symbols: red dot, normal; blue X, not severe; green circle, severe.
We initially studied the relationship between a continuous pH value and other risk factors by univariate analysis. We found that low pH value was significantly correlated with GER symptoms (P = .007), race (nonwhite; P = .002), history of pneumonia (P = .04), allergic symptoms without cold or flu (P = .04), acute or recurrent sinusitis (P = .03), nasal polyps (P = .01), not taking any form of cortico steroid (P = .02), receiving anti-IgE therapy (P = .04), and more severe allergy symptoms, particularly in the spring (P = .0001). Low pH value was also associated with high BMI (P = .0009), low baseline prebronchodilator FEV1 (P = .04), low Asthma Quality of Life Questionnaire (AQLQ) Emotion Score, and low AQLQ Environmental Score (P = .002).
We then adopted multiple linear regression to study the relationship between the continuous pH level and risk factors (Table 1). We included all the variables that were significant in the univariate analysis. In this multiple linear regression model including all subjects who underwent bronchoscopy (n = 161; overall R2 = 0.32), we found that pH value was negatively associated with BAL neutrophil count, BMI, baseline prebronchodilator FEV1, and GER symptoms, and positively associated with prebronchodilator FEV1. White patients had a 0.42 U higher pH value than other races (Table 1) (P = .0006). These differences were not affected by a priori National Heart, Lung, and Blood Institute guidelines asthma severity assignment. Of note, the association of low pH with GER symptoms was confounded by a strong association between GER symptoms and BMI (P < .0001). In terms of pathophysiology, it is reasonable to speculate that in some patients, high BMI could lead to GER, which, in turn, could contribute to low EBC pH.
Table 1.
—Relation of pH and Risk Factors Using Multiple Linear Variable Analysis
| Risk Factor | Estimatea | SE | P Value |
| Baseline prebronchodilator FEV1 | 0.16 | 0.06 | .001 |
| BAL neutrophils, % | −0.037 | 0.010 | .0005 |
| BMI | −0.018 | 0.008 | .03 |
| GER symptoms | −0.41 | 0.15 | .009 |
| Race (white vs non-white) | 0.42b | 0.12 | .0006 |
N = 161; overall R2 = 0.32. GER = gastroesophageal reflux.
Slope of relationship (continuous variables) or difference (dichotomous variables).
White pH was 0.42 units higher than non-white (primarily black).
In secondary analyses, we observed natural clusters of EBC pH in asthma above and below 6.5 (Figs 1, 2). This distribution had been previously reported.1,5,23 We therefore also compared patient characteristics dichotomized above and below pH 6.5 (Figs 1-4; Tables 2, 3). We found that FeNO was lower in those subjects with EBC pH < 6.5 (P < .0001), consistent with a previous report.23 Very low EBC pH was also associated with low provocative concentration of methacholine causing a 20% fall in FEV1 (PC20) (P = .03) and, with severe asthma, allergic symptoms in the spring (P = .006) and winter (P = .03) (Fig 4). There was a trend toward association with number of positive allergy skin tests, but this did not achieve significance (Table 2).
Figure 2.
The relation between pH and phenotypic features. A, BMI. B, Fraction of BAL neutrophils. C, FEV1. D, GER symptoms. Asthma severity is denoted by the following: green dot, control; blue X, not severe; red circle, severe. Note that there is a natural distribution of these data above and below pH 6.5. GER = gastroesophageal reflux.
Table 2.
—Relation Between Dichotomous pH (Cutpoint 6.5) and Continuous Risk Factors
| Risk Factor | pH ≤ 6.5, Mean (SD) n = 39 | pH > 6.5, Mean (SD) n = 533 | P Value |
| BAL pure neutrophils, % | 11.4 (15.3)a | 2.6 (4.1)b | .18 |
| BAL eosinophils, % | 0.99 (1.18)a | 0.97 (2.13)b | .98 |
| BMI | 32.1 (11.4) | 28.6 (8.1) | .07 |
| Baseline prebronchodilator FEV1 | 2.30 (0.74) | 2.45 (0.92) | .24 |
| Baseline prebronchodilator FEV1/FVC ratio | 0.73 (0.10) | 0.72 (0.12) | .34 |
| Methacholine PC20 | 4.1 (5.1) | 6.8 (13.5) | .03 |
| AQLQ emotion score | 4.3 (1.7) | 4.7 (1.7) | .21 |
| AQLQ environmental score | 4.1 (1.5) | 4.6 (1.6) | .08 |
| IgE | 253 (436) | 344 (761) | .29 |
| Log eosinophils: blood | −0.72 (0.51) | −0.67 (0.50) | .63 |
| No. positive skin tests | 2.4 (2.9) | 3.2 (3.0) | .09 |
| Age at diagnosis of asthma, y | 13.1 (11.6) | 13.7 (14.0) | .78 |
| Age of onset of allergic symptoms without cold or flu, y | 9.8 (8.1) | 11.3 (10.9) | .32 |
| Average FeNO value | 22.6 (18.1) | 39.8 (40.2) | < .0001 |
AQLQ = asthma quality of life questionnaire; FeNO = fraction of exhaled nitric oxide; PC20 = provocative concentration of methacholine causing a 20% fall in FEV1.
Only seven subjects had data available.
Only 157 subjects had data available.
Table 3.
—Relation Between Dichotomous pH (Cutpoint 6.5) and Discrete Risk Factors
| Risk Factor | pH ≤ 6.5, No. (%)(n = 39) | pH > 6.5, No. (%)(n = 533) | P Value |
| Pneumonia history | 20 (53) | 218 (42) | .23 |
| Visited ED for breathing problems in last 12 mo | 10 (26) | 161 (30) | .72 |
| Use theophylline | 2 (5) | 40 (8) | .76 |
| Asthma symptoms caused by physical activity | 23 (61) | 307 (61) | > .99 |
| Asthma symptoms caused by animal exposure | 24 (63) | 301 (62) | > .99 |
| Asthma symptoms caused by physical exercise | 26 (70) | 416 (83) | .07 |
| Asthma symptoms caused by respiratory infections | 37 (95) | 451 (90) | .41 |
| Allergic symptoms without cold or flu | 35 (90) | 455 (90) | > .99 |
| GER symptoms | 10 (28) | 109 (21) | .40 |
| History of cats in the home | 8 (21) | 104 (20) | .84 |
| History of rodents in the home | 0 (0) | 12 (2) | > .99 |
| History of acute or recurrent sinusitis | 18 (47) | 205 (39) | .31 |
| History of CPAP or bilevel pressure ventilation use | 1 (3) | 15 (3) | > .99 |
| History of immunotherapy for asthma | 2 (5) | 23 (5) | .69 |
| History of nasal polyp | 5 (14) | 63 (12) | .79 |
| Contraceptive use | 3 (13) | 74 (24) | .31 |
| Herbal or natural supplement treatment | 0 (0) | 25 (6) | .24 |
| Allergy symptoms in spring | .006 | ||
| None | 3 (8) | 43 (8) | |
| Mild | 4 (10) | 148 (29) | |
| Moderate | 15 (38) | 218 (42) | |
| Severe | 17 (44) | 109 (21) | |
| Allergy symptoms in winter | .03 | ||
| None | 5 (13) | 110 (21) | |
| Mild | 8 (21) | 160 (31) | |
| Moderate | 11 (28) | 152 (29) | |
| Severe | 15 (38) | 94 (18) | |
| Taking steroid | 31 (79) | 394 (74) | .57 |
| Taking oral steroid | 9 (24) | 102 (20) | .54 |
| Anti-IgE therapy | 3 (8) | 23 (4) | .41 |
| Use leukotriene modifiers | 14 (37) | 190 (37) | > .99 |
| Any long-acting β-agonist use | 25 (66) | 333 (66) | > .99 |
| Race | .23 | ||
| White | 21 (54) | 336 (63) | |
| Black | 16 (41) | 150 (28) | |
| Other | 2 (5) | 48 (9) |
No normal control patients have pH ≤ 6.5. CPAP = continuous positive airway pressure. See Table 1 for expansion of other abbreviation.
Discussion
Asthma is increasingly appreciated to be a multifactorial symptom complex.21,22,29 Genetic and metabolomic markers may ultimately be important tools for patient classification leading to more targeted treatment. For example, it is envisioned that response to therapies such as β2-agonists, corticosteroids, and leukotriene modifiers may be at least in part genetically determined. Optimal treatment could be targeted based on genetics and/or biochemical markers for responsiveness to these treatments.29
EBC pH is a metabolomic marker that is low (acidic) during acute asthma exacerbations. Further, decreased airway pH recapitulates many features of asthma.1,2,7,30-33 Airway pH appears to be normally distributed in the general population, measured at a retail outlet store,34 though outliers with low pH might be explained if some of the subjects had unreported asthma, GER, or intercurrent infections.11,12,16,19 Here, we report that EBC pH in a large cohort with well-characterized severe and nonsevere asthma is, on the whole, normal. In fact, values for subjects with severe asthma were higher than those previously reported for normal black subjects,23 and could represent a corticosteroid treatment effect. Consistent with a previous report,33 EBC pH was higher in whites than non-whites.
In secondary analysis, we found that among subjects with asthma there was a cluster of subjects with pH < 6.5. There was also the previously observed threshold at 7.8, but this was not nearly as distinct in our population of closely monitored, heavily treated patients as that at 6.5. This threshold at pH 6.5 has also previously been noted.1,5,23 It could reflect the point between pKas (the logarithm to the base 10 of the proton concentration in solution at which the acid is 50% protonated) of weak acids and bases. Strikingly, the variable most strongly associated with pH < 6.5 was low FeNO, consistent with a previous report that FeNO decreases with pH < 6.5.23 FeNO has been used as a nonspecific marker of inflammation, so the robust association of low FeNO with low pH may be counterintuitive. However, this association is not completely surprising for two reasons. First, pH < 6.5 approaches the pKa (the logarithm to the base 10 of the proton concentration in solution at which the acid is 50% protonated) of nitrite.7 In vitro and in vivo, low airway lining fluid pH can lead to nitrite protonation, forming nitrous acid, which rapidly leads to nitric oxide formation.1,7,10 Although this will acutely increase FeNO,7 the result of chronically low pH will be depletion of nitrite and other airway nitrogen oxides.1,7,35 Additionally, S-nitrosoglutathione (GSNO) metabolism increases FeNO acutely in vivo but depletes airway GSNO levels long-term, decreasing FeNO; indeed, GSNO metabolism is increased in many patients with asthma.32,33,36-39 Single nucleotide polymorphisms in GSNO metabolic enzyme, GSNO reductase, are predictive of asthma,38,40 and mice deficient in GSNO reductase are protected from experimental asthma.41 Note in this regard that GSNO reductase activity can be induced by allergen exposure and can result in increased methacholine responsiveness.36 Both increased allergic symptoms in general (note that samples were obtained in and out of season) and low methacholine PC20 were characteristics of this low-pH, low-FeNO phenotype. Increased GSNO reductase activity in asthma can be associated not only with decreased airway nitric oxide levels but also with increased production of formic acid, an important determinant of asthmatic airway acidification.18,42,43 Therefore, the subset of patients with increased GSNO reductase activity might be predicted to have low FeNO and low EBC pH. One way or another, this subset appears to be biochemically unique and may be a subset in which the metabolomically targeted therapy (for example, with inhaled base7,35) may be particularly beneficial.
It should be noted that airway EBC pH was unrelated to inhaled corticosteroid dose. However, the use of corticosteroids will raise EBC pH1 acutely during exacerbation. The severe asthmatic group, defined by high-dose inhaled or systemic corticosteroid use, had and overall tendency to have a slightly higher pH.
EBC pH was lower in patients with GER symptoms. A subpopulation of patients with chronic cough who have consistently low EBC pH on serial studies at home appear to be those patients whose cough can be successfully treated with high-dose proton pump inhibitor therapy.18 However, the finding is based on a subjective questionnaire. Prospective studies will be needed to determine whether a specific EBC pH will be useful in predicting responses to antacid-class therapies in asthma.
In multiple linear regression, low pH was also independently associated with high BAL neutrophil counts, high BMI, low log blood eosinophil count, and low baseline predrug FEV1. With regard to the association between EBC pH and BAL neutrophilia, note that this relationship (1) suggests but does not prove that distal airway/alveolar lung lining fluid may influence EBC pH values, and (2) suggests that EBC pH is influenced by neutrophils. Evidence suggests that the distal airway, which is sampled by BAL, may be more acidic than the proximal airway.35 In the aggregate, subjects with allergies and allergy symptoms could theoretically receive more corticosteroid treatments—and/or have better adherence—leading to higher BMI, more GER, lower lung function, and/or relatively more airway neutrophilia. A chronic, undetected infection could also play a role in the neutrophilic inflammation and low pH.9,12,16 Low EBC pH was associated with modest evidence for flow limitation, consistent with a previous report,5 although a decrease in FEV1 per se does not cause a change in EBC pH,41 and the negative relationship between pH and FEV1/FVC ratio suggests that low pH does not predict obstruction. The determinants of airway pH in stable asthma appear to be multifactorial.
It will be important prospectively to study the associations identified in this study, particularly including the subpopulations with (1) low EBC and low FeNO, and (2) low EBC pH and increased BAL neutrophils. These subpopulations may be amenable to targeted, steroid-sparing therapy. Indeed, it may be worthwhile to consider interventional trials—for example, with antacid therapy, inhaled alkaline buffer, inhaled nitrogen oxides, and/or exercise and weight loss—in which patients are stratified by EBC pH.
Supplementary Material
Acknowledgments
Author contributions: Dr Liu: contributed to data analysis and manuscript preparation.
Dr Teague: contributed to study design and data analysis.
Dr Erzurum: contributed to study design and manuscript preparation.
Dr Fitzpatrick: contributed to study design, data collection, and manuscript preparation.
Ms Mantri: contributed to data analysis and interpretation.
Dr Dweik: contributed to study design.
Dr Bleecker: contributed to study design.
Dr Meyers: contributed to study design and data analysis.
Dr Busse: contributed to study design and manuscript preparation.
Dr Calhoun: contributed to study design and data analysis.
Dr Castro: contributed to study design.
Dr Chung: contributed to study design and data analysis.
Dr Curran-Everett: contributed to study design, data collection, and data analysis.
Dr Israel: contributed to study design and data collection.
Dr Jarjour: contributed to study design and data collection.
Dr Moore: contributed to study design, data collection, and data analysis.
Dr Peters: contributed to study design and data analysis.
Dr Wenzel: contributed to study design and data analysis.
Dr Hunt: contributed to data collection, data analysis, and manuscript preparation.
Dr Gaston: contributed to study design, data analysis, and manuscript preparation.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Busse has provided consultancy/advisory board services to Alexon, AstraZeneca, Altair, Amgen, Abbot Laboratories, Asthmatx, Inc, Boehringer-Ingelheim, Bristol-Meyer Squibb Company, Centocor, Dainippon Sumitomo, Funxional Therapeutics, Ltd, GlaxoSmithKline, Pfizer, Genentech, Johnson & Johnson, Merck, Novartis, Schering-Plough, TEVA, and Wyeth; received lecture fees from Merck; received industry-sponsored grants from Novartis, MedImmune, AstraZeneca, Ception, and GlaxoSmithKline; and received National Institutes of Health (NIH) grant support from NIH-NIAID and NIH-NHLBI. Dr Chung has received grant funding from GlaxoSmithKline, MRC (UK), and Wellcome Trust, and has been renumerated for participation at advisory board meetings of GlaxoSmithKline, Merck, and Gilead. Dr Jarjour has received pharmaceutical company grant money from GlaxoSmithKline and Merck and has been a consultant to GlaxoSmithKline, Genentech, and Asthmatx. Dr Hunt is a shareholder in Respiratory Research, Inc and in Airbase Pharmaceuticals and is cofounder of Respiratory Research, Inc, which manufactures EBC collection equipment and has financial interest in EBC pH testing. He is an inventor of EBC pH assays, and his university owns IP on EBC pH testing. He speaks regarding EBC pH testing (no honorarium). He advises for Pulse Health, Inc, a company that studies exhaled breath for oxidant stress. He has received grant money from NIH and the UVA Philip Morris Tobacco Research Fund, and Respiratory Research, Inc. He is the founder of AirBase Therapeutics, LLC. Dr Gaston is a shareholder in Respiratory Research, Inc and in Airbase Pharmaceuticals. The remaining authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Additional information: The e-Appendix and e-Tables can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/139/2/328/suppl/DC1
Abbreviations
- AQLQ
Asthma Quality of Life Questionnaire
- EBC
exhaled breath condensate
- FeNO
fraction of exhaled nitric oxide
- GER
gastroesophageal reflux
- GSNO
S-nitrosoglutathione
- PC20
provocative concentration of methacholine causing a 20% fall in FEV1
- SARP
Severe Asthma Research Program
Footnotes
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
Funding/Support: This work was supported by the National Institutes of Health [Grants NIH/NHLBI: 2RO1, HL 69170 NHLBI Severe Asthma Research Program].
A complete list of study group participants is available in e-Appendix 1.
References
- 1.Hunt JF, Fang K, Malik R, et al. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med. 2000;161(3 pt 1):694–699. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 2.Hunt JF, Gaston B, Ricciardolo F. Acid stress in the airway. J Allergy Clin Immunol. 2004;84(3):731–765. doi: 10.1016/j.jaci.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 3.Jack CI, Calverley PM, Donnelly RJ, et al. Simultaneous tracheal and oesophageal pH measurements in asthmatic patients with gastro-oesophageal reflux. Thorax. 1995;50(2):201–204. doi: 10.1136/thx.50.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karnad DR, Mhaisekar DG, Moralwar KV. Respiratory mucus pH in tracheostomized intensive care unit patients: Effects of colonization and pneumonia. Crit Care Med. 1990;18(7):699–701. doi: 10.1097/00003246-199007000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Kostikas K, Papatheodorou G, Ganas K, Psathakis K, Panagou P, Loukides S. pH in expired breath condensate of patients with inflammatory airway diseases. Am J Respir Crit Care Med. 2002;165(10):1364–1370. doi: 10.1164/rccm.200111-068OC. [DOI] [PubMed] [Google Scholar]
- 6.Paget-Brown A, Hunt JF, Gaston B. Tracheal fluid pH in human preterm infants. Eur Respir J. 2007;30(5):840–842. doi: 10.1183/09031936.00015507. [DOI] [PubMed] [Google Scholar]
- 7.Gaston B, Kelly R, Urban P, et al. Buffering airway acid decreases exhaled nitric oxide in asthma. J Allergy Clin Immunol. 2006;118(4):817–822. doi: 10.1016/j.jaci.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 8.Tate S, MacGregor G, Davis M, Innes JA, Greening AP. Airways in cystic fibrosis are acidified: detection by exhaled breath condensate. Thorax. 2002;57(11):926–929. doi: 10.1136/thorax.57.11.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh BK, Mackey DJ, Pajewski T, Yu Y, Gaston BM, Hunt JF. Exhaled-breath condensate pH can be safely and continuously monitored in mechanically ventilated patients. Respir Care. 2006;51(10):1125–1131. [PubMed] [Google Scholar]
- 10.Yoon SS, Coakley R, Lau GW, et al. Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite under conditions characteristic of the cystic fibrosis airway. J Clin Invest. 2006;116(2):436–446. doi: 10.1172/JCI24684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt JF, Erwin E, Palmer L, et al. Expression and activity of pH-regulatory glutaminase in the human airway epithelium. Am J Respir Crit Care Med. 2002;165(1):101–107. doi: 10.1164/ajrccm.165.1.2104131. [DOI] [PubMed] [Google Scholar]
- 12.Carraro S, Doherty J, Zaman K, et al. S-nitrosothiols regulate cell-surface pH buffering by airway epithelial cells during the human immune response to rhinovirus. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L827–L832. doi: 10.1152/ajplung.00406.2005. [DOI] [PubMed] [Google Scholar]
- 13.Gaston B, Adjei A, Amoah AG, et al. Breath condensate pH and ammonia levels are low in patients with active pulmonary tuberculosis. Conference of the Am Thorac Soc. 2005:A574. [Google Scholar]
- 14.Nicolaou NC, Lowe LA, Murray CS, Woodcock A, Simpson A, Custovic A. Exhaled breath condensate pH and childhood asthma: unselected birth cohort study. Am J Respir Crit Care Med. 2006;174(3):254–259. doi: 10.1164/rccm.200601-140OC. [DOI] [PubMed] [Google Scholar]
- 15.Coop C, Hagan LL, Dice JP. Exhaled breath condensate pH in the evaluation of asthma. Allergy Asthma Proc. 2008;29(1):51–54. doi: 10.2500/aap2008.29.3073. [DOI] [PubMed] [Google Scholar]
- 16.Hunt J, Ngamtrakulpanit L, Vaughan J, et al. Airway acid-base disturbance in rhinovirus cold. Eur Respir J. 2003;45(Suppl):566S. [Google Scholar]
- 17.Wells K, Vaughan J, Pajewski TN, et al. Exhaled breath condensate pH assays are not influenced by oral ammonia. Thorax. 2005;60(1):27–31. doi: 10.1136/thx.2003.020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenwald R, Fitzpatrick AR, Erzurum S, et al. The severe asthma phenotype is characterized by elevated levels of formate in exhaled breath condensate [abstract] Am J Respir Crit Care Med. 2009 [Google Scholar]
- 19.Hunt J, Yu Y, Burns J, et al. Identification of acid reflux cough using serial assays of exhaled breath condensate pH. Cough. 2006;2(1):3–6. doi: 10.1186/1745-9974-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughan J, Ngamtrakulpanit L, Pajewski TN, et al. Exhaled breath condensate pH is a robust and reproducible assay of airway acidity. Eur Respir J. 2003;22(6):889–894. doi: 10.1183/09031936.03.00038803. [DOI] [PubMed] [Google Scholar]
- 21.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368(9537):804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 22.Holgate ST, Polosa R. The mechanisms, diagnosis, and management of severe asthma in adults. Lancet. 2006;368(9537):780–793. doi: 10.1016/S0140-6736(06)69288-X. [DOI] [PubMed] [Google Scholar]
- 23.Hauswirth DW, Sundy JS, Mervin-Blake S, et al. Normative values for exhaled breath condensate pH and its relationship to exhaled nitric oxide in healthy African Americans. J Allergy Clin Immunol. 2008;122(1):101–106. doi: 10.1016/j.jaci.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Moore WC, Bleecker ER, Curran-Everett DC, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenzel SE, Busse WW, National Heart, Lung, and Blood Institute’s Severe Asthma Research Program Severe asthma: lessons from the Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(1):14–21. doi: 10.1016/j.jaci.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Koczulla AR, Noeske S, Herr C, et al. Ambient temperature impacts on pH of exhaled breath condensate. Respirology. 2010;15(1):155–159. doi: 10.1111/j.1440-1843.2009.01664.x. [DOI] [PubMed] [Google Scholar]
- 27.Ngamtrakulpanit L, Yuanlin Y, Adjei A, Amoah G, Gaston B, Hunt J. Identification of intrinsic airway acidification in pulmonary tuberculosis. Glob J Health Sci. 2010;2(1):106–110. doi: 10.5539/gjhs.v2n1p106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kullmann T, Barta I, Horváth I. Effect of freezing on exhaled breath condensate pH. Respir Med. 2007;101(12):2566. doi: 10.1016/j.rmed.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Weiss ST, Litonjua AA, Lange C, et al. Overview of the pharmacogenetics of asthma treatment. Pharmacogenomics J. 2006;6(5):311–326. doi: 10.1038/sj.tpj.6500387. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed T, Ali JM, al-Sharif AF. Effect of alkali nebulization on bronchoconstriction in acute bronchial asthma. Respir Med. 1993;87(3):235–236. doi: 10.1016/0954-6111(93)90106-a. [DOI] [PubMed] [Google Scholar]
- 31.Holma B. Influence of buffer capacity and pH-dependent rheological properties of respiratory mucus on health effects due to acidic pollution. Sci Total Environ. 1985;41(2):101–123. doi: 10.1016/0048-9697(85)90181-0. [DOI] [PubMed] [Google Scholar]
- 32.Honma S, Tanaka H, Teramoto S, Igarashi T, Abe S. Effects of naturally-occurring acid fog on inflammatory mediators in airway and pulmonary functions in asthmatic patients. Respir Med. 2000;94(10):935–942. doi: 10.1053/rmed.2000.0816. [DOI] [PubMed] [Google Scholar]
- 33.Martínez D, Vermeulen M, von Euw E, et al. Extracellular acidosis triggers the maturation of human dendritic cells and the production of IL-12. J Immunol. 2007;179(3):1950–1959. doi: 10.4049/jimmunol.179.3.1950. [DOI] [PubMed] [Google Scholar]
- 34.Paget-Brown AO, Ngamtrakulpanit L, Smith A, et al. Normative data for pH of exhaled breath condensate. Chest. 2006;129(2):426–430. doi: 10.1378/chest.129.2.426. [DOI] [PubMed] [Google Scholar]
- 35.Shin H-W, Shelley DA, Fitzpatrick A, Gaston B, George SC. Airway nitric oxide is reduced after PBS inhalation in asthma. J Appl Physiol. 2007;102(3):1028–1033. doi: 10.1152/japplphysiol.01012.2006. [DOI] [PubMed] [Google Scholar]
- 36.Snyder AH, McPherson ME, Hunt JF, Johnson M, Stamler JS, Gaston B. Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis. Am J Respir Crit Care Med. 2002;165(7):922–926. doi: 10.1164/ajrccm.165.7.2105032. [DOI] [PubMed] [Google Scholar]
- 37.Gaston B, Sears S, Woods J, et al. Bronchodilator S-nitrosothiol deficiency in asthmatic respiratory failure. Lancet. 1998;351(9112):1317–1319. doi: 10.1016/S0140-6736(97)07485-0. [DOI] [PubMed] [Google Scholar]
- 38.Wu H, Romieu I, Sienra-Monge JJ, et al. Genetic variation in S-nitrosoglutathione reductase (GSNOR) and childhood asthma. J Allergy Clin Immunol. 2007;120(2):322–328. doi: 10.1016/j.jaci.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staab CA, Hellgren M, Höög J-O. Medium- and short-chain dehydrogenase/reductase gene and protein families: Dual functions of alcohol dehydrogenase 3: implications with focus on formaldehyde dehydrogenase and S-nitrosoglutathione reductase activities. Cell Mol Life Sci. 2008;65(24):3950–3960. doi: 10.1007/s00018-008-8592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore PE, Ryckman KK, Williams SM, Patel N, Summar ML, Sheller JR. Genetic variants of GSNOR and ADRB2 influence response to albuterol in African-American children with severe asthma. Pediatr Pulmonol. 2009;44(7):649–654. doi: 10.1002/ppul.21033. [DOI] [PubMed] [Google Scholar]
- 41.Que LG, Liu L, Yan Y, et al. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308(5728):1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henderson EM, Gaston B. SNOR and wheeze: the asthma enzyme? Trends Mol Med. 2005;11(11):481–484. doi: 10.1016/j.molmed.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Que LG, Yang Z, Stamler JS, Lugogo NL, Kraft M. S-nitrosoglutathione reductase: an important regulator in human asthma. Am J Respir Crit Care Med. 2009;180(3):226–231. doi: 10.1164/rccm.200901-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.