Summary
The vascular endothelium may develop a pro-inflammatory profile with aging, but evidence is limited in humans. Expression of inflammatory proteins was determined in vascular endothelial cells (EC) obtained from peripheral veins of 24 young (23±1 years, mean±SE) and 36 older (63±1) healthy men and women using quantitative immunofluorescence. The older subjects had lower vascular endothelium-dependent dilation (forearm blood flow responses to acetylcholine, P<0.05), and higher plasma concentrations of C-reactive protein, interleukin-6 (IL-6) and oxidized low-density lipoprotein (all P<0.05), but not tumor necrosis factor-α (TNF-α). Total (O: 0.52±0.04 vs Y: 0.33±0.05 NFκB /HUVEC intensity P<0.05) and nuclear (O: 0.59±0.04 vs Y: 0.41±0.04) expression of nuclear factor κ B p65 (NFκB), a proinflammatory gene transcription factor, was greater in EC from the older subjects (P<0.05). EC expression of the inhibitor (of nuclear translocation) of NFκB (IκBα) was lower in the older subjects (O: 0.16±0.02 vs Y: 0.24±0.03; P<0.05), whereas IκB kinase was not different. EC expression of the proinflammatory proteins IL-6 (O: 0.42±0.06 vs Y: 0.29±0.03, P<0.05), TNF-α (O: 0.52±0.06 vs Y: 0.33±0.05, P<0.05) and monocyte chemoattractant protein 1 (MCP-1) (O: 0.59±0.06 vs Y: 0.38±0.02, P<0.05) was greater in the older subjects, whereas cyclooxygenase 2 and the receptor for advanced glycation endproducts did not differ. These findings indicate that impaired function with aging in healthy adults is associated with the development of a proinflammatory phenotype in the vascular endothelium that could be caused in part by reduced IκB-mediated activation of NFκB.
Keywords: endothelium-dependent dilation, IL-6, TNF-α, MCP-1, RAGE, COX-2
Cardiovascular diseases (CVD) are the leading cause of morbidity and mortality in modern societies, and the vascular endothelium is a key site influencing the development of CVD (Bonetti et al., 2003; Widlansky et al., 2003). Vascular endothelial dysfunction, defined as a generalized alteration in endothelial cell (EC) function, is associated with increased risk of CVD (Vita & Keaney, 2002; Widlansky et al., 2003). Aging is strongly associated with the development of both vascular endothelial dysfunction (Mitchell et al., 2004) and CVD (Lakatta & Levy, 2003). However, the molecular mechanisms underlying the effects of aging on the vascular endothelium of humans are not well understood.
Vascular endothelial dysfunction and CVD are associated with systemic and vascular inflammation (Libby et al., 2002). Patients with CVD or major risk factors for CVD demonstrate increased circulating pro-inflammatory cytokines and acute phase proteins and vascular endothelial dysfunction (Fichtlscherer et al., 2000; Brevetti et al., 2003). There is accumulating evidence that aging also may be associated with systemic and vascular inflammation in the absence of risk factors or clinical disease (Ungvari et al., 2004; Chung et al., 2006). Circulating concentrations of certain inflammatory cytokines and acute phase proteins are increased in some older adults (Harris et al., 1999; Cushman et al., 2005) and are associated with an increased risk of CVD (Cesari et al., 2003). Expression of inflammatory proteins is increased in vascular tissue of older Fischer 344 rats (Csiszar et al., 2003, 2004) (Chung et al., 2006; Zou et al., 2006) and non-human primates (Coe, 2004), and in the intima of normal aortas obtained from older compared with young subjects after death from trauma (Wang et al., 2007).
One mechanism that is believed to play an important role in inflammation mediated vascular endothelial dysfunction is the activation of the nuclear factor κ B (NFκB) (Ungvari et al., 2004; de Winther et al., 2005; Csiszar et al., 2006; Guzik & Harrison, 2007). NFκB is a transcription factor expressed in EC and other tissues that regulates the induction of genes that produce inflammatory molecules such as interleukin-6 (IL-6) tumor necrosis factor-α (TNF-α) and monocyte chemoattractant protein 1 (MCP-1) (de Winther et al., 2005; Guzik & Harrison, 2007). NFκB activation is suppressed by the inhibitor of NFκB (IκB), which prevents the translocation of NFκB from the cytosol to the nucleus (de Winther et al., 2005). Phosphorylation of IκB by IκB kinase (IκK) marks IκB for polyubiquitination and proteosomal degradation, allowing NFκB to enter the nucleus and bind to its target genes (Karin, 1999).
Using a novel technique that combines acquisition of EC from peripheral blood vessels of healthy adults with measurements of protein expression using quantitative immunofluorescence (Colombo et al., 2002), we recently reported that total NFκB is increased in EC of older healthy men (Donato et al., 2007). Here we extend these previous findings by first determining if the increase in total EC NFκB protein with aging is associated with greater nuclear translocation. We then determined if greater nuclear NFκB is associated with reductions in IκB, as would be expected, and if expression of IκK is altered with aging. Finally, we sought to gain insight into the proinflammatory proteins that may be upregulated in EC as a consequence of age-related activation of NFκB in healthy subjects. To do so, we measured the expression of several proteins that can be increased by NFκB activation in EC including IL-6, TNF-α, MCP-1, cyclooxygenase 2 (COX-2) and the receptor for advanced glycation endproducts (RAGE). We hypothesized that nuclear abundance of NFκB would be greater in older subjects and that this would be associated with reduced IκB. We also hypothesized that the activation and nuclear translocation of NFκB in older subjects would be associated with increased EC expression of one or more of these inflammatory proteins. Finally, we phenotyped a subset of the subjects for endothelium-dependent and – independent dilation in order to confirm a selective impairment in vascular endothelial function in the older adults.
Results
Subject Characteristics (Table 1)
Table 1.
Subject Characteristics
| Young | Older | |
|---|---|---|
| N (Female) | 24 (9) | 36 (15) |
| Age (years) | 23±1 | 63±1* |
| Mass (kg) | 83±3 | 82±2 |
| Body mass index (kg/m2) | 26±1 | 28±1 |
| Percent body fat | 28±2 | 35±2* |
| Lesiure time activity (MET hrs/wk) | 25±6 | 25±4 |
| Systolic blood pressure (mmHg) | 110±2 | 126±2* |
| Diastolic blood pressure (mmHg) | 70±2 | 78±1* |
| Fasting blood glucose (mg/100 mL) | 91±1 | 93±1 |
| Fasting insulin (μU/mL) | 7.6±0.8 | 7.5±0.7 |
| Total cholesterol (mg /100 mL) | 177±8 | 202±6* |
| HDL cholesterol (mg /100 mL) | 45±2 | 53±2* |
| LDL cholesterol (mg /100 mL) | 107±7 | 125±5* |
| Triglycerides (mg/100 mL) | 122±10 | 121±9 |
Data are mean ± S.E. MET, metabolic equivalent; VO2max, maximal oxygen consumption; HDL, high density lipoprotein; LDL, low density lipoprotein.
P < 0.05 vs. young
The older subjects had higher total body fat, systolic and diastolic blood pressure, and plasma total, LDL and HDL cholesterol (all P<0.05); however, blood pressure and cholesterol values were within the normal ranges for both groups. Body mass, body mass index, leisure time physical activity, and fasting plasma glucose and triglycerides were not different.
Plasma Markers of Oxidative Stress, Antioxidants and Inflammation (Table 2)
Table 2.
Plasma markers of oxidative stress, antioxidants and inflammation.
| Young | Older | |
|---|---|---|
| Oxidized LDL (IU/L) | 48.6 ± 5.3 | 64.3 ± 2.8* |
| Total antioxidant status (mmol/L) | 1.39 ± 0.05 | 1.30 ± 0.04* |
| IL-6 (mg/mL) | 0.85±0.12 | 1.37 ± 0.15* |
| TNF-α (pg/mL) | 1.86±0.27 | 1.54 ± 0.12 |
| CRP (mg/L) | 0.80 ± 0.14 | 1.23 ± 0.15* |
Data are mean ± S.E. LDL, low density lipoprotein; IL-6, interleukin-6; TNFα, tumor necrosis factor α; CRP, C reactive protein.
P < 0.05 vs. young
Plasma oxidized LDL concentrations were higher, and total antioxidant status was slightly lower in the older subjects (both P<0.05). Plasma concentrations of IL-6 and CRP were higher in the older subjects (P<0.05), but there was no difference in TNF-α.
Endothelium-Dependent and –Independent Dilation (Figure 1)
Figure 1.
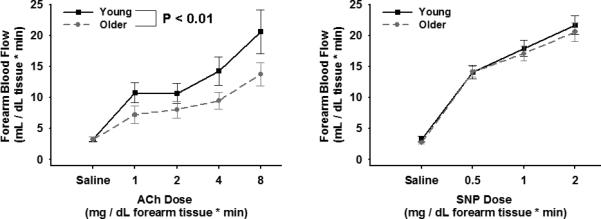
Left panel: Endothelium-dependent dilation (forearm blood flow to intrabrachial infusion of Ach; 1.0, 2.0, 4.0 and 8.0 μg per 100ml of forearm tissue) and Right panel: endothelium–independent dilation (forearm blood flow to intrabrachial infusion of SNP; 0.5, 1.0 and 2.0 μg per 100ml of forearm tissue) in young and older men. Values are mean ± SE.
The forearm blood flow responses to acetylcholine were smaller in the older compared with the young subjects (P<0.01). In contrast, the forearm blood flow responses to sodium nitroprusside were similar in the 2 groups (P=0.65). These results confirm a selective impairment in endothelium-dependent dilation in the older adults.
Vascular EC Protein Expression
Expression of total NFκB p65 was 57% greater in EC from the older subjects (O: 0.52±0.04 vs. Y: 0.33±0.05 NFκB/HUVEC intensity, P<0.05) and was associated with a 43% greater nuclear abundance of NFκB p65 (O: 0.59±0.04 vs. Y: 0.41±0.04 nuclear NFκB/HUVEC intensity, P<0.05) (Figure 2). EC expression of IκBα was 33% lower in the older subjects (O: 0.16±0.02 vs. Y: 0.24±0.03 IκBα/HUVEC intensity, P<0.05), whereas IκKβ was not different in the 2 groups (O: 0.35±0.04 vs. Y: 0.33±0.04 IκKβ/HUVEC intensity, P>0.05) (Figure 3). EC expression of IL-6 (O: 0.42±0.06 vs. Y: 0.29±0.03 IL-6/HUVEC intensity, P<0.05), TNF-α (O: 0.52±0.06 vs Y: 0.33±0.05 TNF-α/HUVEC intensity, P<0.05) and MCP-1 (O: 0.59±0.06 vs Y: 0.38±0.02 MCP-1/HUVEC intensity, P<0.05) was 45-58% greater in the older subjects, whereas COX-2 (O: 0.40±0.04 vs Y: 0.43±0.05 COX-2/HUVEC intensity, P>0.05) (Figure 4) and RAGE (O: 0.43±0.07 vs Y: 0.41±0.06 RAGE /HUVEC intensity, P>0.05) did not differ (Figure 5).
Figure 2.
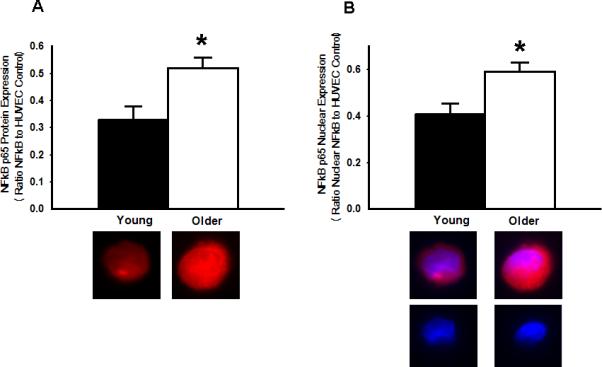
Increased NFκB p65 activation in endothelial cells (EC) of older adults. A: Whole cell expression of NFκB p65 is increased in EC of older compared with young adults. Representative images shown below the summary graph. B: Nuclear expression of NFκB p65 also is increased in EC obtained from the antecubital vein of older compared with young adults. Representative staining for NFκB p65 (top panels) and DAPI nuclear staining (bottom panels) are shown below the summary graph. Values are mean ± SE. HUVEC: human umbilical vein endothelial cell. * P < 0.05 vs. young
Figure 3.
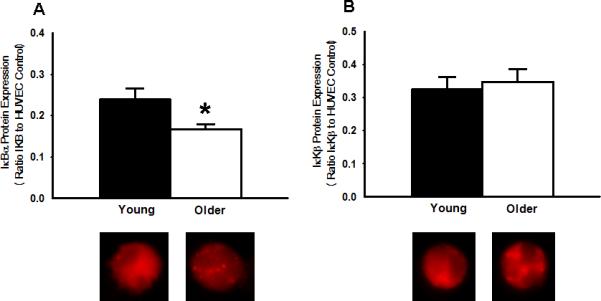
A: Reduced expression of the inhibitor of NFκB, IκBα, in EC from older compared with young adults. B: EC Expression of IκKβ, an NFκB activator, was not different between older and young adults. Representative images shown below the summary graphs. Values are mean ± SE. * P < 0.05 vs. young
Figure 4.
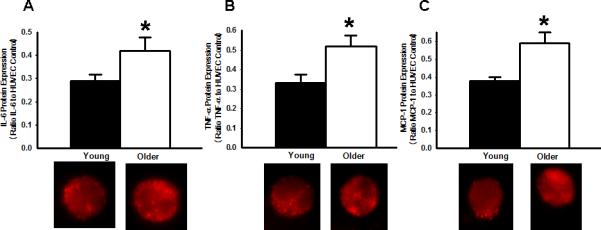
Increased expression of NFκB-modulated proinflammatory proteins in EC from older compared with young adults. A: Interleukin-6 (IL-6). B: Tumor necrosis factor-α (TNF-α). C: Monocyte chemoattractant protein-1 (MCP-1). Representative images shown below the summary graph. Values are mean ± SE. HUVEC: human umbilical vein endothelial cell. * P < 0.05 vs. young.
Figure 5.
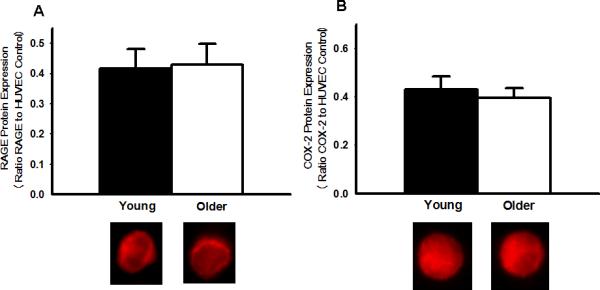
A: Protein expression for the receptor for advanced glycation endproducts (RAGE) in EC from young and older adults. B: Protein expression for cycloxygenase-2 (COX-2) in EC from young and older adults. Representative images shown below the summary graph. Values are mean ± SE. HUVEC: human umbilical vein endothelial cell. * P < 0.05 vs. young.
Of the CVD risk factors that differed in the young and older subjects (Table 1), the only significant correlations with EC proteins were between % body fat and total NFκB (r=0.42, P<0.05), as reported previously (Silver et al., 2007), and % body fat and MCP-1 (r=0.46, P<0.05). After accounting for the effects of % body fat (ANCOVA), the age group differences remained borderline significant (NFκB, P=0.05) or significant (MCP-1, P=0.04) for those 2 proteins. Thus, age-associated differences in CVD risk factors did not explain differences in EC protein expression between the young and older subjects.
Discussion
The novel findings of this study are as follows. First, the increase in total NFκB p65 expression in EC in healthy older subjects with impaired vascular endothelial function is associated with a greater nuclear content of this protein. Second, the greater nuclear NFκB p65 in these older adults is associated with reduced EC expression of IκBα in the absence of changes in IκKβ protein. Finally, the apparent activation and greater nuclear translocation of NFκB in EC of older subjects is associated with increases in expression of the proinflammatory cytokines IL-6 and TNF-α, and the chemokine MCP-1, but not in COX-2 or RAGE. Overall, our results provide new experimental support for the concept that vascular endothelial dysfunction with aging is associated with reduced IκB-mediated, NFκB activation-related increases in selective proinflammatory proteins in the vascular endothelium of healthy adults.
Our finding of greater total NFκB p65 expression in EC of older compared with young healthy subjects confirms a recent observation from our laboratory (Donato et al., 2007). The present results extend this finding by showing for the first time that the nuclear content of NFκB p65 also is greater in EC of older adults. This is important given that translocation of NFκB to the nucleus is required for it to exert its actions on gene transcription. We also found that EC expression of IκBα, a protein that inhibits the translocation of NFκB from the cytoplasm to the nucleus, was reduced in older adults, consistent with this being a key molecular mechanism involved in the greater nuclear presence of NFκB. We did not find any difference in expression of IκKβ. However, it is the activation of IκK by inflammatory stimuli that leads to phosphorylation and subsequent proteosomal degradation of IκB (de Winther et al., 2005). As such, greater content of IκK protein is not required to mediate a reduction in IκB in EC with aging. Indeed, the present findings in vascular EC obtained from young and older subjects are consistent with previous findings of unchanged IκK protein with age in rat liver (Helenius et al., 2001). Thus, the present results do not support an increase in EC IκK with healthy aging in humans, but rather point to an increased state of IκK activity. Currently, there is no information available on the latter possibility.
Our findings also provide new insight into the upregulation of proinflammatory factors associated with this activation of NFκB in EC of older subjects. We examined 5 inflammatory proteins that can be produced as a result of NFκB induced gene transcription in EC (de Winther et al., 2005; Guzik & Harrison, 2007). We found increases in IL-6, TNF-α, MCP-1 in EC of older compared with young subjects, but no differences in COX-2 or RAGE. These observations suggest that NFκB activation is associated with a selective induction of proinflammatory genes in EC of older subjects. This is presumably the result of an increase in NFκB binding to the promoters of specific inflammatory genes, in this case, those for IL-6, TNF-α and MCP-1. The molecular events involved in such a specific upregulation of inflammatory factors with aging in EC from healthy adult humans remain to be determined. We are unaware of previous data on changes in these proinflammatory proteins in EC with aging in humans.
Our findings are consistent with earlier work showing increased IL-6 expression in the coronary arteries and IL-6 production in aortas of older compared with young rats (Belmin et al., 1995; Csiszar et al., 2004). A recent unique and detailed study of small groups (n=5) of apparently healthy men who died of traumatic causes found increased MCP-1 and several other proinflammatory morphological and biochemical changes in the intima of the aortic wall of the older men (Wang et al., 2007). The present findings extend these observations by demonstrating age-associated increases in several inflammatory proteins in vascular EC per se of larger groups of healthy subjects.
To our knowledge, the present data are the first to report data on RAGE protein expression with aging in vascular tissue. A previous study found increased RAGE protein in heart muscle of older patients undergoing cardiac bypass surgery (Simm et al., 2004). AGE and RAGE appear to be elevated most clearly and consistently in pre-diabetic or diabetic states/models (Ramasamy et al., 2005). The fact that our older adults had normal fasting blood glucose may contribute to a lack of age-associated differences in RAGE. The absence of greater RAGE protein in EC of older adults in the present study, however, does not rule out the possibility of increased RAGE signaling, as AGE and other agonists for RAGE may be greater with aging in serum and vascular tissue (Qiu et al., 2007; Uribarri et al., 2007). The lack of difference in COX-2 protein expression in EC of young and older healthy adult humans in our study is in agreement with previous observations in coronary arteries from young and older rats (Csiszar et al., 2002). Greater COX-2 mRNA gene expression was reported in endothelial, but not vascular smooth muscle cells with aging in a recent study in rats (Tang & Vanhoutte, 2008).
The results of the present study do not provide definitive insight into the direction or chronology of these events. It is proposed (Guzik & Harrison, 2007) that a variety of stimuli including circulating inflammatory cytokines, lipids, angiotensin II and mechanical forces acting on the vascular endothelial wall activate transmembrane receptors. This stimulates intra-cellular signaling pathways leading to IκK mediated phosphorylation and degradation of IκB, and translocation of the NFκB p50/p65 heterodimer to the nucleus where it binds to promoters of genes that increase the expression of inflammatory proteins (Guzik & Harrison, 2007). Our findings provide support for several of these steps in mediating increased expression of proinflammatory molecules in the vascular endothelium with aging. Circulating concentrations of CRP and IL-6 were increased in the older subjects, as well as total and LDL cholesterol and oxidized LDL, a marker of oxidative stress, although plasma concentrations of TNF-α and triglycerides did not differ between the groups. EC IκB was reduced, whereas nuclear content of NFκB was increased, and this was associated with increased EC expression of IL-6, TNF-α, MCP-1. Thus, although the present findings on EC obtained from young and older humans do not provide direct cause and effect evidence, they are at least consistent with the series of mechanistic events described above.
We believe that our findings may have important clinical implications. Systemic and vascular inflammation are central features of CVD, the prevalence of which increases markedly with age. However, the molecular mechanisms linking aging to CVD in humans are not well understood. The present findings provide further evidence that in subjects free of major coronary risk factors and clinical disease, aging is associated with the development of a pro-inflammatory phenotype in the vascular endothelium that could predispose to the development of vascular endothelial dysfunction and CVD.
The use of EC obtained from veins rather than arteries is a limitation of present study as it is difficult to invasively obtain sufficient arterial EC from large numbers of older subjects to study age-associated differences in multiple proteins. However, we (Donato et al., 2007) and others (Colombo et al., 2002) have shown that age- and CVD-related group differences in arterial EC expression of NFκB, COX-2 and other proteins are consistently reflected in EC obtained from veins. Therefore, we do not believe that the use of EC sampled from veins affect the primary conclusions of this study.
In summary, the results of the present study provide the first evidence for greater nuclear translocation of NFκB in EC of healthy older humans with impaired vascular endothelial function, and that this is associated with lower IκB and increased expression of the proinflammatory proteins IL-6, TNF-α, and MCP-1. Along with other recent observations (Donato et al., 2007; Wang et al., 2007), our findings indicate that aging is associated with the development of a proinflammatory phenotype in the vascular endothelium of healthy adults. Our results also provide novel insight into the molecular mechanisms by which NFκB may play a key role in the etiology of age-associated vascular inflammation, endothelial dysfunction and CVD in humans.
Experimental Procedures
Subjects
Data were obtained on 83 healthy men and women: 24 young (aged 18-34 years; 9 women and 15 men) and 36 older (55-70 years; 15 women and 21 men). All subjects had resting blood pressure < 140/90 mmHg, and were free of cardiovascular diseases (CVD), diabetes, and other clinical disorders as assessed by medical history, physical examination, ankle brachial index and blood chemistries. Subjects > 40 years of age were further screened for CVD using electrocardiogram and blood pressure responses to incremental treadmill exercise performed to volitional exhaustion. Subjects were non-smokers, not taking medications or dietary supplements (including antioxidants), and not regularly exercising. All procedures were approved by the Human Research Committee of the University of Colorado at Boulder. The nature, benefits, and risks of the study were explained to the volunteers and their written informed consent was obtained prior to participation.
Study Procedures
All measurements were performed at the University of Colorado at Boulder General Clinical Research Center after an overnight fast and a 24-hour abstention from alcohol and physical activity.
Subject characteristics
Body mass index was determined from measurement of height and body mass, and percent body fat was determined by dual X-ray absorptiometry (Eskurza et al., 2005). Arterial blood pressure and resting heart rate were measured over the brachial artery during seated rest using a semi-automated device (Dynamap XL, Johnson and Johnson). Leisure time activity was estimated to provide a measure of total physical activity (Eskurza et al., 2002). Fasting plasma metabolic factors were determined by standard assays.
Plasma markers of oxidative stress, antioxidants and inflammation
Plasma samples were analyzed for oxidized low-density lipoprotein, a marker of systemic oxidative stress, and serum samples were analyzed for total antioxidant status, a measure of systemic antioxidant defenses, as previously described (Moreau et al., 2005). Serum concentrations of TNF-α and IL-6 were measured using a high-sensitivity ELISA, whereas C-reactive protein was measured by an Olympus AU400e Chemistry Analyzer.
Endothelium-dependent and –independent dilation
In a subgroup of the subjects (9 young/7M, 11 older/8M), endothelium-dependent and -independent dilation were determined by the increases in forearm blood flow in response to intra-brachial artery infusion of incremental doses of acetylcholine and sodium nitroprusside, respectively, as described previously (DeSouza et al., 2000; Pierce et al., 2008; Donato et al., in press).
EC protein expression
The procedures used for collection of EC and measurement of EC protein expression were described and validated originally by Feng et al. (Feng et al., 1999) and Colombo et al., (Colombo et al., 2002) and more recently by our laboratory (Eskurza et al., 2006; Donato et al., 2007; Silver et al., 2007). Briefly, J-wires were advanced into the antecubital vein ~ 4 cm beyond the tip of the catheter and withdrawn, and cells were recovered by washing and centrifugation. Collected cells were fixed with 3.7% formaldehyde and plated on slides. After blocking non-specific binding sites with 5% donkey serum (Jackson Immunoresearch) cells were incubated with monoclonal antibodies for one of the following: nuclear factor κ B p65 (NFκB) (Novus), IκKβ (Santa Cruz), IκBα (Santa Cruz), TNF-α (Abcam), IL-6 (Santa Cruz), COX-2 (Cayman Chemical) and RAGE (Santa Cruz). Cells were next incubated with Alexaflour555-conjugated secondary antibodies (Invitrogen).
Slides were systematically scanned to identify EC (positive staining of von Willebrand factor) and nuclear integrity was confirmed using DAPI (4',6'-diamidino-2-phenylindole hydrochloride) staining. EC images were captured and analyzed using Metamorph Software (Universal Imaging Corp, Downingtown, PA) to quantify the intensity of Alexaflour 555 staining (i.e., average pixel intensity). Values are reported as ratios of EC protein expression/HUVEC. Reporting ratios minimizes the possible confound of differences in intensity of staining among different staining sessions. One technician analyzed all batches of slides and was blinded to subject identity during the staining and analysis procedures. The nuclear regions of 30 EC were identified by DAPI staining, overlayed on the same cell's Alexaflour 555 image, and analyzed for fluorescent intensity as an estimate of nuclear NFκB p65 (Figure 6). Nuclear NFκB p65 was normalized to a HUVEC control. The reproducibility of our measurements of EC expression of NFκB-related proteins was determined in 4 subjects (ages 19-61) in whom EC were collected at least a week apart. Reproducibility was defined as the absolute difference in EC mean fluorescent intensity in trial 1 vs. trial 2 divided by average mean fluorescent intensity for the trials. Individual and mean values were NFκB: 4%-8% (6.3%), nuclear NFκB: 2-11% (7.1%), IκBα: 0.4%-15% (6.8%) and IκKB: 5%-22% (11.1%).
Figure 6.
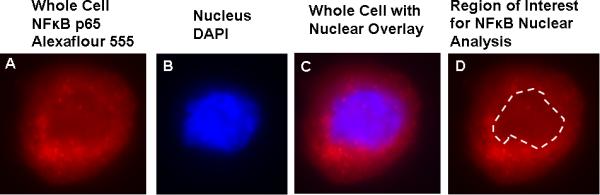
Measurement of endothelial cell (EC) nuclear protein expression. A: Representative EC immunostained for the p65 subunit of the nuclear transcription factor NFκB and visualized by fluorescent microscopy. B: Nucleus of the same cell is visualized by DAPI nuclear stain. C: Overlay of nuclear stain with that of total EC NFκB p65. The border of the nucleus is outlined using this overlay image. D: Fluorescent intensity of the cell within the border of the nucleus is measured to determine nuclear NFκB p65 protein expression.
Data Analysis
Statistical analyses were performed with SPSS (version 11.0.3). Group differences were determined by t-tests for independent sample comparisons. Pearson correlation analysis was used to determine bivariate relations of interest. Analysis of covariance (ANCOVA) was used to account for the effects of CVD risk factors on age group differences in EC protein expression. Statistical significance for all analyses was set at P < 0.05.
Acknowledgements
We thank Rhea Chiang and Brooke Lawson for their efforts in endothelial cell collections and endothelial cell analysis.
Sources of Funding
Supported by National Institutes of Health awards AG006537, AG013038, AG022241, AG029337 and RR00051.
Footnotes
Disclosures
None
References
- Belmin J, Bernard C, Corman B, Merval R, Esposito B, Tedgui A. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995;268:H2288–2293. doi: 10.1152/ajpheart.1995.268.6.H2288. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- Brevetti G, Silvestro A, Di Giacomo S, Bucur R, Di Donato A, Schiano V, Scopacasa F. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg. 2003;38:374–379. doi: 10.1016/s0741-5214(03)00124-1. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circ. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- Coe CL. Biological and social predictors of immune senescence in the aged primate. Mech Ageing Dev. 2004;125:95–98. doi: 10.1016/j.mad.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Colombo PC, Ashton AW, Celaj S, Talreja A, Banchs JE, Dubois NB, Marinaccio M, Malla S, Lachmann J, Ware JA, Le Jemtel TH. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol. 2002;92:1331–1338. doi: 10.1152/japplphysiol.00680.2001. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart and Circ. 2006;291:H1694–1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards J, Kaminski P, Wolin M, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Research. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards J, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. The FASEB J. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards J, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, Polak JF, Tracy RP. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circ. 2005;112:25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:904–914. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circ. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Jablonski KL, Gano LB, Pierce GL, Seals DR. Cytochrome P450 2C9 Signaling Does Not Contribute to Age-Associated Vascular Endothelial Dysfunction in Humans. J App Physiol. doi: 10.1152/japplphysiol.90629.2008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Donato AJ, Moreau KL, Seals DR, Tanaka H. Changes in maximal aerobic capacity with age in endurance-trained women: 7-yr follow-up. J Appl Physiol. 2002;92:2303–2308. doi: 10.1152/japplphysiol.01124.2001. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol. 2006;571:661–668. doi: 10.1113/jphysiol.2005.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Stern DM, Pile-Spellman J. Human endothelium: endovascular biopsy and molecular analysis. Radiology. 1999;212:655–664. doi: 10.1148/radiology.212.3.r99au28655. [DOI] [PubMed] [Google Scholar]
- Fichtlscherer S, Rosenberger G, Walter DH, Breuer S, Dimmeler S, Zeiher AM. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circ. 2000;102:1000–1006. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Harrison DG. Endothelial NF-kappaB as a mediator of kidney damage: the missing link between systemic vascular and renal disease? Circ Res. 2007;101:227–229. doi: 10.1161/CIRCRESAHA.107.158295. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr., Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Helenius M, Kyrylenko S, Vehviläinen P, Salminen A. Characterization of aging-associated up-regulation of constitutive nuclear factor-kappa B binding activity. Antioxid Redox Signal. 2001;3:147–156. doi: 10.1089/152308601750100669. [DOI] [PubMed] [Google Scholar]
- Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J of Biol Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circ. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circ. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr., Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertens. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertens. 2005;45:1107–1112. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertens. 2008;52:72–79. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Depre C, Ghosh K, Resuello RG, Natividad FF, Rossi F, Peppas A, Shen YT, Vatner DE, Vatner SF. Mechanism of gender-specific differences in aortic stiffness with aging in nonhuman primates. Circ. 2007;116:669–676. doi: 10.1161/CIRCULATIONAHA.107.689208. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16R–28R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circ. 2007;115:627–637. doi: 10.1161/CIRCULATIONAHA.106.657486. [DOI] [PubMed] [Google Scholar]
- Simm A, Casselmann C, Schubert A, Hofmann S, Reimann A, Silber RE. Age associated changes of AGE-receptor expression: RAGE upregulation is associated with human heart dysfunction. Exp Gerontol. 2004;39:407–413. doi: 10.1016/j.exger.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics. 2008;32:409–418. doi: 10.1152/physiolgenomics.00136.2007. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Kaley G. Vascular inflammation in aging. Herz. 2004;29:733–740. doi: 10.1007/s00059-004-2625-x. [DOI] [PubMed] [Google Scholar]
- Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, Vlassara H. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci. 2007;62:427–433. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita JA, Keaney JF., Jr. Endothelial function: a barometer for cardiovascular risk? Circ. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertens. 2007;50:219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF, Jr., Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- Zou Y, Yoon S, Jung KJ, Kim CH, Son TG, Kim MS, Kim YJ, Lee J, Yu BP, Chung HY. Upregulation of aortic adhesion molecules during aging. J Gerontol A Biol Sci Med Sci. 2006;61:232–244. doi: 10.1093/gerona/61.3.232. [DOI] [PubMed] [Google Scholar]


