SUMMARY
Bacillus anthracis kills through a combination of bacterial infection and toxemia. Anthrax toxin working via the CMG2 receptor mediates lethality late in infection, but its roles early in infection remain unclear. We generated myeloid-lineage specific CMG2-deficient mice to examine the roles of macrophages, neutrophils, and other myeloid cells in anthrax pathogenesis. Macrophages and neutrophils isolated from these mice were resistant to anthrax toxin. However, the myeloid-specific CMG2-deficient mice remained fully sensitive to both anthrax lethal and edema toxins, demonstrating that targeting of myeloid cells is not responsible for anthrax toxin-induced lethality. Surprisingly, the myeloid-specific CMG2-deficient mice were completely resistant to B. anthracis infection. Neutrophil depletion experiments suggest that B. anthracis relies on anthrax toxin secretion to evade the scavenging functions of neutrophils to successfully establish infection. This work demonstrates that anthrax toxin uptake through CMG2 and the resulting impairment of myeloid cells specifically neutrophils, is essential to anthrax infection.
Keywords: Anthrax toxin, CMG2, edema toxin, lethal toxin, TEM8, neutrophils, macrophages, myeloid cells
INTRODUCTION
The symptoms of many bacterial diseases are due to the actions of toxic proteins released by the bacteria. Bacillus anthracis is such a pathogen, causing anthrax through a combination of bacterial infection and toxemia (Moayeri and Leppla, 2009). Anthrax infections are initiated when B. anthracis spores enter a potential host organism by ingestion, inhalation, or skin abrasion. The spores then germinate and replicate as vegetative bacteria, overcome the host innate immune responses, and ultimately enter the circulation leading to a systemic infection. In the bloodstream, B. anthracis multiplies rapidly and secretes the anthrax toxins, consisting of three components: protective antigen (PA), lethal factor (LF), and edema factor (EF). PA is a receptor-binding moiety that generates a protein-conducting channel for delivering EF and LF into the cytosol to exert their cytotoxic effects. EF, which combines with PA to form edema toxin (ET), is a calmodulin-dependent adenylate cyclase that elevates intracellular cAMP levels, thereby mediating diverse cAMP-induced cellular effects and death of experimental animals (Firoved et al., 2005; Leppla, 1982). LF, which combines with PA to form lethal toxin (LT), is a Zn+2-dependent metalloproteinase that cleaves and inactivates mitogen-activated protein kinase kinases (MAPKKs or MEKs) 1–4, 6 and 7 (Duesbery et al., 1998; Vitale et al., 1998; Vitale et al., 2000). This profoundly affects the many cellular functions that depend on the ERK, p38, and JNK mitogen-activated protein kinase (MAPK) signaling pathways, and is sufficient to kill experimental animals (Moayeri et al., 2003) through mechanisms that are still not well understood. PA binds to two cell surface receptors, tumor endothelium marker-8 (TEM8, also named anthrax toxin receptor 1 (ANTXR1)) and capillary morphogenesis protein-2 (CMG2, also named anthrax toxin receptor 2 (ANTXR2)) (Bradley et al., 2001; Scobie et al., 2003). We recently showed that CMG2 is the major receptor mediating lethality at late stages of anthrax infection (Liu et al., 2009), but the roles that anthrax toxin and its cellular receptors play in early stages of infection remain unclear.
Long before MEKs were identified as the specific targets of LF, it had been found that macrophages from certain mouse strains are uniquely lysed by LT within 90 min, whereas other mouse strains have macrophages that are totally resistant to the LT-induced rapid lysis. This finding directed much early work toward understanding the behavior of this single cell type, which was suspected of having a key role in pathogenesis (Friedlander, 1986; Friedlander et al., 1993; Moayeri et al., 2004; Moayeri and Leppla, 2009). The identification of this distinctive phenotype, with all mouse and rat macrophages falling into either “sensitive” or “resistant” groups based on their response to LF, allowed the gene controlling this phenotype to be mapped to Nlrp1b, for which at least five polymorphic alleles have been described in both mice and rats (Boyden and Dietrich, 2006; Newman et al., 2010). However, the LT-induced rapid macrophage lysis is not linked in a simple way to anthrax disease, because mice with “resistant” macrophages can also be killed by LT, although sometimes with a slower rate than sensitive mice (Moayeri et al., 2004). In fact, tests of human macrophages have not shown them to undergo an LT-induced rapid lysis. “Resistant” macrophages are able to bind and internalize LT, leading to MEK cleavage, and in some circumstances, to a slow apoptotic death (Park et al., 2002). Paradoxically, mice with “resistant” macrophages succumb faster than mice with “sensitive” macrophages when infected with B. anthracis spores (Terra et al., 2010; Welkos et al., 1986). For these reasons, it remains important to determine the contribution that LT targeting of macrophages plays in pathogenesis in mice, including those harboring “resistant” macrophages.
Genetics has proven to be a powerful tool for the functional dissection of toxin-receptor interactions (Liu et al., 2009). In this study, we generated myeloid-specific CMG2-null mice, in which both macrophages and neutrophils are unaffected by anthrax toxin due to lack of its binding and subsequent uptake. This allowed us to examine the role of macrophages and other myeloid cells in anthrax toxin pathogenesis, as well as in anthrax infection. We found that CMG2 is the principal anthrax toxin receptor on both macrophages and neutrophils. The myeloid-specific CMG2-null mice retained full sensitivity to both LT and ET, demonstrating that targeting of macrophages, neutrophils, and other myeloid cells is not required for the lethality induced by anthrax toxin. Surprisingly, these myeloid-specific CMG2-null mice were completely resistant to infection by the toxinogenic, non-encapsulated B. anthracis Ames strain. It follows that B. anthracis depends on anthrax toxin to cripple the innate immune defense function provided by myeloid cells so as to establish a successful infection.
RESULTS
Generation of myeloid-specific CMG2-null mice
We previously generated both TEM8 and CMG2 null (TEM8−/− and CMG2−/−) mice by deleting the transmembrane (TM) domain exons in the TEM8 and CMG2 genomic loci (Liu et al., 2009). To determine which receptor is preferentially expressed in mouse macrophages, we analyzed mouse bone-marrow derived macrophages (BMDMs) from CMG2−/− and TEM8−/− mice. TEM8−/− BMDMs were just as susceptible as C57BL/6 wildtype (WT) BMDMs to PA plus FP59 (Figure 1). FP59 is a fusion protein of LF amino acids 1-254 and the catalytic domain of Pseudomonas aeruginosa exotoxin A that kills cells by ADP-ribosylation and thus inactivation of elongation factor 2 (EF-2) after delivery to the cytosol by PA (Arora and Leppla, 1993; Liu and Leppla, 2003b). In contrast, CMG2−/− BMDMs were completely resistant to the toxin (Figure 1), demonstrating that CMG2 is the major anthrax toxin receptor on mouse macrophages.
Figure 1. CMG2-null mouse macrophages are completely resistant to PA + FP59 while macrophages from TEM8-null mouse are sensitive.
BMDMs from various genotypes as indicated were treated with varying concentrations of PA in the presence of FP59 (100 ng/ml) for 48 h. Cell viability was then evaluated by MTT assay. Data are reported as mean viability ± S.D.
To explore the role of macrophages in anthrax toxin pathogenesis, we generated myeloid-specific CMG2 knockout mice (CMG2flox/flox/LysM-Cre) in the C57BL/6 genetic background (Figure 2A). This was achieved by breeding CMG2flox/flox mice (having LoxP sites flanking the exon encoding the TM domain of CMG2) with LysM-Cre targeted mice (having cre recombinase expression under the control of the myeloid-specific M lysozyme endogenous promoter), followed by intercrossing of the resulting CMG2+/flox/LysM-Cre mice. The LysM-Cre strain provides a validated tool for generating mutants in the myeloid cell linage, including mature macrophages and neutrophils (Clausen et al., 1999; Mauer et al., 2010). Analysis of the resulting mice by RT-PCR showed that the CMG2 TM domain was efficiently deleted in both BMDMs and neutrophils, but remained largely intact in other tissues including brain, heart, kidney, liver, lung, muscles, spleen, and thymus in the CMG2flox/flox/LysM-Cre mice (Figure 2B). The small amount of the intact allele in neutrophils in Figure 2B can be attributed to contamination of the neutrophil preparation by a small amount of lymphocytes (Figure S1). The small fraction of the deleted allele which was observed in other tissues reflects the presence of myeloid cells residing in these tissues, with a higher fraction in the lymphoid organs such as the spleen and thymus (Figure 2B). RT-PCR detected only traces of TEM8 expression in BMDMs and neutrophils (Figure 2B), indicating that CMG2 is the major anthrax toxin receptor in both these cell types. As expected, the BMDMs from CMG2flox/flox/LysM-Cre mice lost the ability to bind PA, because these cells were highly resistant to PA/FP59, while BMDMs from WT control mice were sensitive (Figure 2C). Since primary neutrophils can only survive a few hours in culture, not allowing a similar cytotoxicity assay, we performed PA/LF binding and internalization analyses on neutrophils. Neutrophils of WT mice bound and proteolytically processed PA to the PA63 heptamer, which was then internalized into endocytic vesicles to form the well-characterized SDS/heat-resistant heptamer (Liu and Leppla, 2003a; Liu et al., 2007), resulting in LF delivery to the cytosol and MEK2 cleavage (Figure 2D). In contrast, CMG2flox/flox/LysM-Cre neutrophils had much less PA binding and heptamer formation, and no evidence of LF internalization and MEK2 cleavage (Figure 2D). The trace amounts of PA binding and heptamer formation may be attributed to the low level expression of TEM8 by neutrophils and/or contamination of lymphocytes during neutrophil isolation. The above results demonstrate that CMG2 is the major anthrax toxin receptor expressed on mouse macrophages and neutrophils.
Figure 2. Generation and anthrax toxin sensitivity of the myeloid-specific CMG2-null mice.
(A) Schematic targeting strategy. Diagram of the CMG2flox allele having exon 12 flanked by LoxP sites (“Floxed”), and the myeloid-specific CMG2-null allele (CMG2flox/LysM-Cre). The homozygous myeloid-specific CMG2-null mice (CMG2flox/flox/LysM-Cre) were obtained by the intercrossing of CMG2+/flox/LysM-Cre mice. The red arrowheads indicate LoxP sites.
(B) RT-PCR analyses of the CMG2 TM domain deletion in various tissues, macrophages, and neutrophils in a CMG2flox/flox/LysM-Cre mouse. Macrophages and neutrophils from a WT mouse were also included as controls. See Figure S1 for neutrophil isolation.
(C) Toxin susceptibility of BMDMs from WT and myeloid-specific CMG2-null mice. BMDMs were treated with various concentrations of PA plus FP59 (100 ng/ml) for 48 h. Cell viability was evaluated by MTT assay. Data are reported as mean viability ± S.D.
(D) Binding and processing of PA on mouse neutrophils. Neutrophils isolated from mouse bone marrow were incubated with 1 μg/ml PA and 0.5 μg/ml LF at 37 oC for 0–3 h. Cells were washed, lysed, and cell lysates subjected to Western blotting using either an anti-PA antiserum or a MEK2 antibody. A non-specific cross-reactive band indicated by the arrow head at the lower left of the blot serves as protein loading control.
(E-F) Sensitivity of myeloid-specific CMG2-null mice to the anthrax toxins. CMG2flox/flox/LysM-Cre mice and their control mice were treated with 100 μg PA plus 100 μg LF (intraperitoneally) (E) or with 50 μg PA plus 50 μg EF (intravenously) (F) and survival monitored for 2 weeks. Whole body CMG2-null mice (CMG2−/−) in the same genetic background (C57BL/6) were included as additional controls.
Targeting of mouse macrophages and neutrophils does not contribute to the lethality induced by anthrax toxin
Since the discovery in 1986 that macrophages from certain mouse strains are quickly lysed by LT (Friedlander, 1986), studies of anthrax toxin’s role in vivo have often focused on this single cell type, in some cases leading to the suggestion that release of cytokines from lysing macrophages is the cause of lethality. To assess the role that anthrax toxin targeting of mouse macrophages and neutrophils plays in toxin action in mice, we challenged the myeloid-specific CMG2-null mice with both LT (100 μg PA + 100 μg LF) and ET (50 μg PA + 50 μg EF). We found that these mice were as susceptible as WT control mice to both LT and ET (Figures 2E and 2F), demonstrating that anthrax toxin-mediated lethality is not due to targeting of macrophages and neutrophils.
Myeloid-specific CMG2-null mice are resistant to B. anthracis infection
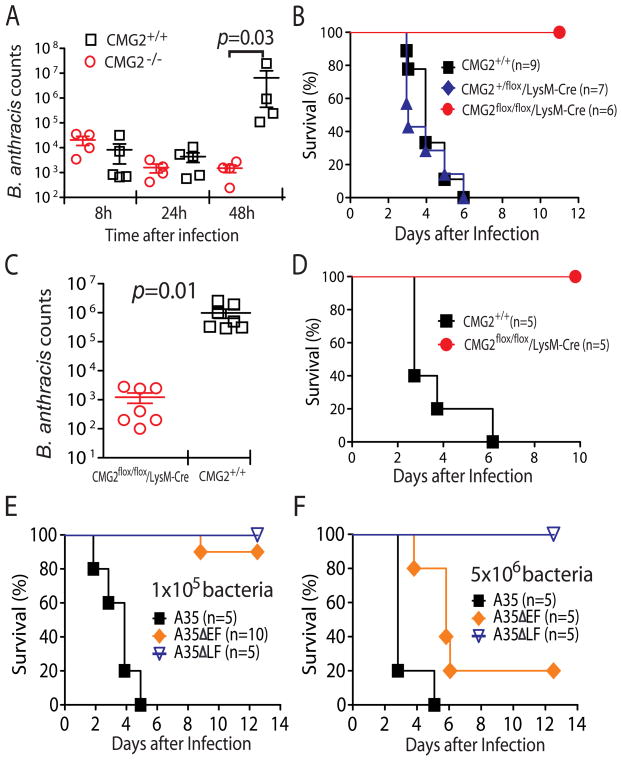
We previously showed that CMG2−/− mice are completely resistant to infection by toxinogenic, non-encapsulated B. anthracis Ames (A35) spores, while TEM8−/− mice remain susceptible (Liu et al., 2009). To further characterize the infectious process, we compared B. anthracis dissemination in these (whole-body) CMG2−/− and control mice. Bacterial counts in the organs during the initial 24 h after subcutaneous B. anthracis A35 spore infection were low and comparable in both CMG2−/− and the WT control mice (Figure 3A). However, at 48 h after infection, while the counts in CMG2−/− mice remained low, the counts in the WT control mice were 3~4 orders of magnitude higher (and death usually followed a few hours later in these mice) (Figure 3A). These data suggest that B. anthracis bacteria need to overcome the host innate immune response through targeting CMG2 to establish a successful infection.
Figure 3. Myeloid-specific CMG2-null mice are resistant to B. anthracis infection.
(A) B. anthracis bacteria are efficiently cleared in whole body CMG2-null mice. CMG2−/− mice and their control mice were injected subcutaneously with 1×108 B. anthracis spores. At 8, 24, or 48 h after the infection, B. anthracis bacteria were counted in the pooled tissue lysates (which include heart, lung, kidney, spleen and liver) by dilution plating on LB agar plates.
(B) Myeloid-specific CMG2-null mice are resistant to B. anthracis spore infection. CMG2flox/flox/LysM-Cre mice and their littermate control mice were subcutaneously injected with 2×107 B. anthracis A35 spores and monitored for signs of malaise and survival for 2 weeks. See also Figure S2.
(C) B. anthracis bacteria are efficiently cleared in myeloid-specific CMG2-null mice. CMG2flox/flox/LysM-Cre mice and their littermate control mice were subcutaneously injected with 1×108 B. anthracis A35 spores. At 48 h after infection, B. anthracis bacteria were counted as described in A.
(D) Myeloid-specific CMG2-null mice are resistant to vegetative B. anthracis A35 infection. Mice were intravenously injected with 1×106 B. anthracis A35 vegetative bacteria and monitored for survival for 2 weeks.
(E and F) The anthrax toxins, in particular LT, are crucial for B. anthracis to overcome innate immune defense. C57BL/6 mice (WT) were challenged with 1×105 (E) or 5×106 (F) vegetative B. anthracis A35 or mutant strains lacking LF (ΔLF ) or EF (ΔEF) subcutaneously and monitored for signs of malaise and survival for 2 weeks. See also Figure S3.
To further explore this hypothesis, the myeloid-specific CMG2-null mice were challenged with B. anthracis spores. While all their littermate control mice succumbed to the infection within 6 days, all CMG2flox/flox/LysM-Cre mice survived infection (Figure 3B). The B. anthracis bacterial loads in CMG2flox/flox/LysM-Cre mice were also much lower than those in the WT control mice 48 h after infection (103-fold lower than in WT mice, Figure 2C). Furthermore, B. anthracis bacteria could not be detected in CMG2flox/flox/LysM-Cre mice 2 weeks after the infection (data not shown). These results demonstrate that the myeloid-specific CMG2-null mice are able to completely clear the B. anthracis infection. In this subcutaneous B. anthracis infection model, following injection of spores in the mouse upper dorsal area, a subcutaneous edema is usually visible as early as 8 h after infection, indicating the occurrence of spore germination accompanied with anthrax toxin secretion in the local area. The edematous lesion usually progresses to extend to the entire upper dorsal area (Figure S2), followed by a systemic infection and death within 6 days after infection. We did observe this characteristic local edema in CMG2flox/flox/LysM-Cre mice 1~3 days after infection, indicating that spore germination and toxin secretion did occur in these mice. However, the edema then gradually subsided and mice remained symptom-free throughout a two-week observation period (Figure S2), indicating the failure of the B. anthracis to disseminate when the mice contained anthrax toxin-resistant myeloid cells. These results demonstrate that B. anthracis uses anthrax toxin as an essential weapon to target myeloid cells and thereby blunt the host innate immune response.
To investigate whether the myeloid cell-based defense against B. anthracis infection operates in situations other than the subcutaneous spore challenge, we also infected mice with vegetative B. anthracis A35 bacteria intravenously. Just as in the previous model, the CMG2flox/flox/LysM-Cre mice were completely resistant to the infection while the WT control mice were sensitive (Figure 3D). This shows that the host innate immune defense conferred by myeloid cells is also able to eliminate B. anthracis vegetative bacteria from the blood stream and organs, as well as from the subcutaneous tissues infected in the previous infection model.
LT and ET are crucial for B. anthracis to cause infection
To explore the roles that LT and ET play in impairing the functions of innate immunity, we infected WT C57BL/6 mice subcutaneously with B. anthracis A35 strains lacking EF or LF (i.e., ΔEF and ΔLF strains). We found that mice survived infections initiated with either 1×105 or 5×106 A35ΔLF vegetative bacteria, but all succumbed to these doses of A35 bacteria (Figures 3E and 3F). Interestingly, the mice challenged with the A35ΔLF strain developed similar degrees of edema as those challenged with the A35 strain during the first 1~2 days after infection, indicating successful initiation of infection by both strains (Figure S3). However, the edematous lesions caused by the A35ΔLF strain gradually subsided within 5 days whereas the edema in A35-challenged mice progressed quickly, followed by death within 5 days after infection. Thus, infections with the A35ΔLF strain can be successfully contained, attesting to the importance of LT in impairing the functions of the myeloid cells so as to facilitate the infection. The A35ΔEF was also attenuated in that 90% of the mice survived infection with 1×105 A35ΔEF bacteria (Figure 3E). Although the mice did largely succumb (80%) to infection with 5 ×106 A35ΔEF bacteria, the mice died at later times than the A35 treated mice (Figure 3F). These experiments suggest that EF may also play a role (although a lesser role than LT) in overcoming the host innate immune response. Edematous lesions did not occur in A35ΔEF infected mice (Figure S3), indicating that these lesions resulted from the action of EF. Taken together, the above results suggest that LF and EF may coordinate to disable the innate immune functions of the myeloid cells to facilitate anthrax infections.
Neutrophils are the major cell type that control B. anthracis infection
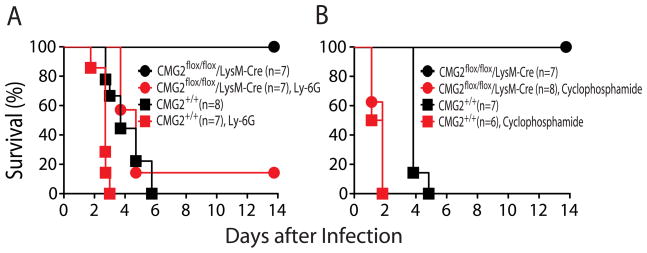
During the acute phase of a bacterial infection, the neutrophils normally located in the blood stream are one of the first types of inflammatory cells to migrate toward the site of infection. To determine whether the CMG2-null neutrophils are the major innate immune cells responsible for the resistance of the CMG2flox/flox/LysM-Cre mice to B. anthracis infection, we depleted neutrophils from these mice prior to infection. This was accomplished using either the neutrophil-specific anti-Ly-6G monoclonal antibody 1A8, which selectively depleted only neutrophils (Daley et al., 2008) (Figure S4), or with cyclophosphamide, which depleted neutrophils as well as other white blood cells (Zuluaga et al., 2006) (Table S1). In both cases, CMG2flox/flox/LysM-Cre mice, which are normally completely resistant to the infection (as per Figure 3B), became strikingly sensitized to spore infection, with 80% (5/6) succumbing to infection when neutrophils were depleted with anti-Ly-6G antibody and all the mice (6/6) succumbing when neutrophils were depleted with cyclophosphamide (Figures 4A and 4B). Neutrophil depletion also sensitized WT mice to the infection (Figures 4A and 4B). These results demonstrate that neutrophils are a major component of the host defenses that control B. anthracis infections, and that B. anthracis production of LT and ET impairs the neutrophil’s function.
Figure 4. Depletion of neutrophils sensitizes myeloid-specific CMG2-null mice to B. anthracis infection.
(A and B) Mice were depleted of neutrophils by either cyclophosphamide (A) or Ly-6G 1A8 antibody (B), and then challenged with 5 ×107 B. anthracis A35 spores subcutaneously and monitored for signs of malaise and mortality for 2 weeks. See also Figure S4 and Table S1.
DISCUSSION
In the present study, to explore the in vivo role of macrophages and neutrophils in anthrax pathogenesis, we generated myeloid-specific CMG2-null mice. We found that CMG2 is the major receptor for anthrax toxin in both macrophages and neutrophils because both cell types from myeloid-specific CMG2-null mice were defective in toxin binding. However, the myeloid-specific CMG2-null mice remained fully sensitive to both LT and ET, while whole body CMG2-null mice were resistant. These results demonstrate that the lethality caused by anthrax toxin is not due to targeting of macrophages, neutrophils, or other myeloid cells.
Macrophages and neutrophils are important components of host innate immune defenses against bacterial infections (Tournier et al., 2009a). Although these myeloid cells are dispensable in anthrax toxin-mediated lethality, overcoming host innate immune defenses provided by them may be crucial for B. anthracis infections. To test this hypothesis, we infected the myeloid-specific CMG2-null mice with B. anthracis and compared them to WT mice. Surprisingly, the myeloid-specific CMG2-null mice were completely resistant to both the B. anthracis spore and vegetative bacterium infections while the WT mice were highly susceptible. In contrast to the WT mice, the myeloid-specific CMG2-null mice cleared the bacteria. These results reveal that targeting of myeloid cells by anthrax toxin through the CMG2 receptor is crucial for B. anthracis to establish a successful infection. We further showed that LT and ET are likely to coordinate to cripple the myeloid cell-mediated innate immune functions, because LF- and EF-deficient B. anthracis mutant strains had greatly reduced abilities to establish systemic anthrax infections.
Neutrophils are phagocytes patrolling in blood and represent the first responders to acute infection. Human neutrophils can kill B. anthracis spores (Mayer-Scholl et al., 2005). To determine the roles of neutrophils in early B. anthracis infections, we depleted neutrophils from both WT mice and myeloid-specific CMG2-null mice by two different methods prior to infection, using either cyclophosphamide or the neutrophil-specific anti-Ly-6G monoclonal antibody 1A8. Remarkably, the myeloid-specific CMG2-null mice were strikingly sensitized to spore infection following neutrophil depletion. These results demonstrate that neutrophils are a major cell type that contributes to control of B. anthracis infection, and it is likely the toxin-mediated impairment of their function, acting through the CMG2 receptor, that is essential for successful infection. The roles of macrophages and other myeloid cells play in anthrax pathogenesis remains an open question. Although macrophages are another major cell type in innate immunity and may play an important role in controlling anthrax infections, the immune activities of macrophages and other myeloid cells appear insufficient to protect against anthrax infection when neutrophils are depleted.
Anthrax toxin-based vaccines or anti-toxin therapies can efficiently protect experimental animals from B. anthracis infection in various animal models (Tournier et al., 2009b). The results obtained in this study suggest that the protective antibodies produced in response to vaccination against anthrax toxin may work at early stages of infections by protecting neutrophils from LT’s inhibitory action, thus allowing neutrophils to perform their normal surveillance function in containing infections at an early stage. It has been argued previously that antibody protection against many bacterial infections, including those diseases in which potent secreted toxins play a key role, is achieved at the earliest stages of an infection by assuring that phagocytes can destroy the inoculum (Robbins et al., 1995). This principle may also apply here.
The results presented in this study suggest that the key roles of anthrax toxin in anthrax infections are those summarized below: At early stages of infection, B. anthracis spores germinate locally and secrete LT and ET, which act through binding to CMG2 receptors and incapacitate myeloid cells (neutrophils and probably also macrophages). This allows the expanding population of vegetative cells to evade the powerful anti-bacterial functions of myeloid cells. At later stages, following dissemination and establishment of a systemic infection, B. anthracis multiplies to high numbers in the blood, producing sufficient amounts of the anthrax toxins and other virulence factors to kill infected animals by inducing irreversible damage to non-myeloid tissues and cells types. Candidate target cells may include cardiomyocytes and endothelial cells (Moayeri et al., 2009; Warfel et al., 2005).
This study also provides strong evidence extending the prior data (Liu et al., 2009) showing that CMG2 is the physiologically relevant receptor for anthrax infection in vivo.
EXPERIMENTAL PROCEDURES
Generation of myeloid-specific CMG2-null mice
Generation of mice having the CMG2 TM domain exon 12 flanked by loxP sites (a “floxed” allele), namely, the CMG2flox/flox mice, and their use to make CMG2-null mice (CMG2−/−) mice were described previously (Liu et al., 2009). To generate mice having CMG2 deleted only in the myeloid cell lineage, the CMG2flox/flox mice were first mated with LysM-Cre targeted mice (Clausen et al., 1999) (Jackson Laboratories). Myeloid-specific CMG2-null mice (CMG2flox/flox/LysM-Cre) were obtained by the subsequent intercrossing of the resulting CMG2+/flox/LysM-Cre mice. Genotyping was performed by PCR using mouse tail DNA. In analyzing CMG2 expression, total RNA isolated from various mouse tissues using TRIZOL reagent (Invitrogen, Carlsbad, CA) was reverse transcribed using the SuperScript III Reverse Transcriptase (Invitrogen). The 451-bp TM-containing CMG2 cDNA fragment was amplified from WT tissues using a forward and reverse primer pair, 5’-GGAAGAGCAGTCACGTCGATCAGTCA-3’ and 5’-GACCTCCGTAGTAGGAAGCGT-3’. These primers amplify a 355-bp TM-deleted CMG2 cDNA fragment from CMG2 TM domain deleted tissues. CMG2-null mice as well as the myeloid-specific CMG2-null mice were in the C57BL/6 genetic background. All animal studies were carried out in accordance with protocols approved by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee.
BMDM and neutrophil isolation and cytotoxicity assay
BMDMs were isolated as described previously (Newman et al., 2010). BMDMs were cultured in L929 conditioned DMEM with 10% fetal bovine serum (FBS). Mouse neutrophils were isolated from bone marrow using density gradient centrifugation with Histopaque 1119 and 1077 according to the manufacturer’s manual (Sigma-Aldrich). Neutrophil purity was ~90%, with a small fraction of lymphocyte contamination (Figure S1). For cytotoxicity assays, BMDMs were grown in 96-well plates and treated with serial dilutions of PA combined with 100 ng/ml FP59 for 48 h. Cell viability was then assayed by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) as described previously (Liu and Leppla, 2003a).
PA binding, LF translocation, and MEK cleavage assays
Neutrophils (1×106) were incubated with 1 ml DMEM with 10% FBS containing PA plus LF (1 μg/ml and 0.5 μg/ml, respectively) for 0–3h at 37oC, then washed five times with Hank’s Balanced Salt Solution (Biofluids, Rockville, MD) by centrifugation to remove unbound toxins. Cells were then lysed in modified RIPA lysis buffer containing protease inhibitors (Liu and Leppla, 2003a) and lysates were subjected to SDS-PAGE and Western blotting to detect cell-associated PA and MEK2 cleavage. Anti-PA polyclonal rabbit antiserum (#5308) was made in our laboratory. Anti-MEK2 was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
B. anthracis dissemination experiments
Mice were sacrificed at 8, 24, or 48 h after subcutaneous infection with B. anthracis spores (see below). Heart, lung, kidney, spleen and liver were pooled and homogenized in PBS, followed by dilution plating of tissue lysates on LB agar plates.
Toxin and B. anthracis challenge studies
In toxin challenge experiments, 8–10 week old male and female mice with various genotypes were injected with 100 μg PA plus 100 μg LF (in 0.5 ml PBS intraperitoneally) or with 50 μg PA plus 50 μg EF (in 0.2 ml PBS intravenously) and observed for signs of malaise twice daily for 2 weeks following injection. B. anthracis spores were prepared from the non-encapsulated toxinogenic B. anthracis Ames 35 (A35) strain (Pomerantsev et al., 2006). The EF- or LF-deficient A35 mutant strains were constructed using a LoxP/Cre-mediated deletion approach (Pomerantsev et al., 2006). The bacteria were grown on nutrient sporulation agar at 37°C for 3 days and spores were removed from plates by washing with sterile water and further purified by centrifugation (Hu et al., 2006). Spore counts were assessed by dilution plating and microscopy. For spore infections, mice were injected with 2 ×107 to 1×108 B. anthracis A35 spores subcutaneously. For vegetative B. anthracis A35 infection, mice were injected with 1×105 or 5×106 bacteria subcutaneously or 1×106 bacteria via tail vein. The challenged mice were monitored twice daily for 2 weeks post-infection for signs of malaise or mortality. In neutrophil depletion studies with cyclophosphamide, mice were injected intraperitoneally with 125 mg/kg of drug 72 and 48 h prior to infection, and with 200 mg/kg 24 h before and 3 h after infection. For neutrophil depletion with the Ly-6G 1A8 monoclonal antibody (BD Pharmingen), mice were injected intraperitoneally with 100 μg antibody 24 and 6 h before infection. PA, LF, and EF were purified as previously described (Liu et al., 2007; Gupta et al., 2008; Firoved et al., 2005). The LF used here contains the native amino-terminal sequence AGG (Gupta et al., 2008).
HIGHLIGHTS.
CMG2 is the physiologically-relevant receptor for anthrax toxin in vivo
Damaging myeloid cells is not required for the lethality induced by anthrax toxin
However, anthrax toxin targeting of myeloid cells is essential for B. anthracis infections
Anthrax toxin targets neutrophils to evade bacterial scavenging and allow infection to establish
Supplementary Material
Acknowledgments
This research was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank Rasem Fattah for assistance in protein purification. We also thank Zonglin Hu and Stephen Chow for construction of A35 mutant strains. None of the authors of this manuscript have a financial interest related to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Arora N, Leppla SH. Residues 1-254 of anthrax toxin lethal factor are sufficient to cause cellular uptake of fused polypeptides. J Biol Chem. 1993;268:3334–3341. [PubMed] [Google Scholar]
- Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- Duesbery NS, Webb CP, Leppla SH, Gordon VM, Klimpel KR, Copeland TD, Ahn NG, Oskarsson MK, Fukasawa K, Paull KD, Vande Woude GF. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- Firoved AM, Miller GF, Moayeri M, Kakkar R, Shen Y, Wiggins JF, McNally EM, Tang WJ, Leppla SH. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am J Pathol. 2005;167:1309–1320. doi: 10.1016/S0002-9440(10)61218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander AM. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J Biol Chem. 1986;261:7123–7126. [PubMed] [Google Scholar]
- Friedlander AM, Bhatnagar R, Leppla SH, Johnson L, Singh Y. Characterization of macrophage sensitivity and resistance to anthrax lethal toxin. Infect Immun. 1993;61:245–252. doi: 10.1128/iai.61.1.245-252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PK, Moayeri M, Crown D, Fattah RJ, Leppla SH. Role of N-terminal amino acids in the potency of anthrax lethal factor. PLoS ONE. 2008;3:e3130. doi: 10.1371/journal.pone.0003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Sa Q, Koehler TM, Aronson AI, Zhou D. Inactivation of Bacillus anthracis spores in murine primary macrophages. Cell Microbiol. 2006;8:1634–1642. doi: 10.1111/j.1462-5822.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- Leppla SH. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982;79:3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Crown D, Miller-Randolph S, Moayeri M, Wang H, Hu H, Morley T, Leppla SH. Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc Natl Acad Sci U S A. 2009;106:12424–12429. doi: 10.1073/pnas.0905409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Leppla SH. Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalization. J Biol Chem. 2003a;278:5227–5234. doi: 10.1074/jbc.M210321200. [DOI] [PubMed] [Google Scholar]
- Liu S, Leppla SH. Retroviral insertional mutagenesis identifies a small protein required for synthesis of diphthamide, the target of bacterial ADP-ribosylating toxins. Mol Cell. 2003b;12:603–613. doi: 10.1016/j.molcel.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Liu S, Leung HJ, Leppla SH. Characterization of the interaction between anthrax toxin and its cellular receptors. Cell Microbiol. 2007;9:977–987. doi: 10.1111/j.1462-5822.2006.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer J, Chaurasia B, Plum L, Quast T, Hampel B, Bluher M, Kolanus W, Kahn CR, Bruning JC. Myeloid cell-restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PLoS Genet. 2010;6:e1000938. doi: 10.1371/journal.pgen.1000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Scholl A, Hurwitz R, Brinkmann V, Schmid M, Jungblut P, Weinrauch Y, Zychlinsky A. Human neutrophils kill Bacillus anthracis. PLoS Pathog. 2005;1:e23. doi: 10.1371/journal.ppat.0010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayeri M, Crown D, Dorward DW, Gardner D, Ward JM, Li Y, Cui X, Eichacker P, Leppla SH. The heart is an early target of anthrax lethal toxin in mice: a protective role for neuronal nitric oxide synthase (nNOS) PLoS Pathog. 2009;4:e1000456. doi: 10.1371/journal.ppat.1000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayeri M, Haines D, Young HA, Leppla SH. Bacillus anthracis lethal toxin induces TNF-á-independent hypoxia-mediated toxicity in mice. J Clin Invest. 2003;112:670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayeri M, Leppla SH. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol Aspects Med. 2009;30:439–455. doi: 10.1016/j.mam.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayeri M, Martinez NW, Wiggins J, Young HA, Leppla SH. Mouse susceptibility to anthrax lethal toxin is influenced by genetic factors in addition to those controlling macrophage sensitivity. Infect Immun. 2004;72:4439–4447. doi: 10.1128/IAI.72.8.4439-4447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman ZL, Printz MP, Liu S, Crown D, Breen L, Miller-Randolph S, Flodman P, Leppla SH, Moayeri M. Susceptibility to anthrax lethal toxin-induced rat death is controlled by a single chromosome 10 locus that includes rNlrp1. PLoS Pathog. 2010;6:e1000906. doi: 10.1371/journal.ppat.1000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Greten FR, Li ZW, Karin M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase Inhibition. Science. 2002;297:2048–2051. doi: 10.1126/science.1073163. [DOI] [PubMed] [Google Scholar]
- Pomerantsev AP, Sitaraman R, Galloway CR, Kivovich V, Leppla SH. Genome engineering in Bacillus anthracis using Cre recombinase. Infect Immun. 2006;74:682–693. doi: 10.1128/IAI.74.1.682-693.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins JB, Schneerson R, Szu SC. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2003;100:5170–5174. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terra JK, Cote CK, France B, Jenkins AL, Bozue JA, Welkos SL, Levine SM, Bradley KA. Cutting edge: Resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J Immunol. 2010;184:17–20. doi: 10.4049/jimmunol.0903114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JN, Rossi PS, Quesnel-Hellmann A, Baldari CT. Anthrax toxins: A weapon to systematically dismantle the host immune defenses. Mol Aspects Med. 2009a;30:456–466. doi: 10.1016/j.mam.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Tournier JN, Ulrich RG, Quesnel-Hellmann A, Mohamadzadeh M, Stiles BG. Anthrax, toxins and vaccines: a 125-year journey targeting Bacillus anthracis. Expert Rev Anti Infect Ther. 2009b;7:219–236. doi: 10.1586/14787210.7.2.219. [DOI] [PubMed] [Google Scholar]
- Vitale G, Bernardi L, Napolitani G, Mock M, Montecucco C. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem J. 2000;352(Pt 3):739–745. [PMC free article] [PubMed] [Google Scholar]
- Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, Montecucco C. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem Biophys Res Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- Warfel JM, Steele AD, D'Agnillo F. Anthrax lethal toxin induces endothelial barrier dysfunction. Am J Pathol. 2005;166:1871–1881. doi: 10.1016/S0002-9440(10)62496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welkos SL, Keener TJ, Gibbs PH. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect Immun. 1986;51:795–800. doi: 10.1128/iai.51.3.795-800.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuluaga AF, Salazar BE, Rodriguez CA, Zapata AX, Agudelo M, Vesga O. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC Infect Dis. 2006;6:55. doi: 10.1186/1471-2334-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.