Abstract
A simple, selective and sensitive reversed-phase high performance liquid chromatography method for simultaneous analysis of hydrochlorothiazide and reserpine in human urine was developed and subjected to primary pharmacokinetic study. After a simple protein precipitation using methanol and extraction with ethyl acetate, the analytes were separated on an Elite C18 column at a flow rate of 0.8 mL min−1. The mobile phase was composed of acetonitrile (A) and 0.2% ammonium chloride solution (B) for a gradient elution starting at A:B at 30:70, v/v for 0~6 min, linearly raising the percent of A from 30% to 50% (6~9 min) and ending at 50:50, v/v (9~25 min). The standard curves were linear over the range of 0.05–20 µg mL−1 for hydrochlorothiazide and 0.02–5.0 µg mL−1 for reserpine, respectively (r > 0.999). The limit of detection (LOD) and the limit of quantification (LOQ) were 5.5 ng mL−1 and 18.2 ng mL−1 for hydrochlorothiazide, and 7.1 ng mL−1 and 23.6 ng mL−1 for reserpine, respectively. The recoveries for both analytes were above 89.0±1.35%. The intra-day and inter-day precision for hydrochlorothiazide were less than 1.91% and 1.38%, and those for reserpine were below 1.61% and 2.64%, respectively. The method indicated good performance in terms of specificity, linearity, detection and quantification limits, precision and accuracy, and it was employed successfully for the simultaneous determination of hydrochlorothiazide and reserpine in human urine samples.
Keywords: Hydrochlorothiazide, reserpine, sample pre-treatment, LC, urine
Introduction
Hydrochlorothiazide is one of the most commonly used thiazide diuretics to treat moderate hypertension [1]. It is usually administered in combination with another antihypertensive agent, reserpine, which is also extensively used for the treatment of hypertension.
Simultaneous determination of both drugs in biological samples is desirable as it would allow more efficient generation of clinical data and could be more effective than separate assays. However, as to our knowledge there has been no report for simultaneous determination of hydrochlorothiazide and reserpine in biological samples even though several chromatographic methods have been reported for the analysis of either hydrochlorothiazide or reserpine. Analytical methods for hydrochlorothiazide individually or combined with other antihypertensive drugs in tablets or urine have been reported based on capillary electrophoresis method [8], high performance liquid chromatography (LC) with UV, electrochemical detection [6] or mass spectrometry (LC-MS), and liquid chromatography coupled with tandem mass spectrometry (LC/MS/MS) [2–8]. A variety of methods also have been applied for assaying reserpine, such as spectrofluorimetric [9–12], GC and HPLC methods with electrochemical detectors [13, 14], and LC/MS/MS with a chelating agent [15]. Most of these methods required specific detectors, such as an electrochemical detector [14], while others required complicated sample pre-treatment [15, 16]. It is tedious to determine the analytes separately for each sample using two different LC conditions or two different detection methods. There was also a literature on simultaneous determination of both analytes in tablets with LC [17], where the linear range can not meet the determination requirement in the present study, the pre-treatment and LC method was not suitable for biological samples.
In this paper, a simple and sensitive LC assay for simultaneous analysis of two target compounds in human urine was developed without using other conventional techniques such as potentiometry and capillary electrophoresis. The present pre-treatment---liquid-liquid extraction with ethyl acetate after protein precipitation was simple and effective. This method was successfully applied for simultaneous determination of hydrochlorothiazide and reserpine in urine samples from healthy volunteers after a combined oral administration of 9.0 mg of hydrochlorothiazide and 1.0 mg of reserpine.
Materials and methods
Materials and reagents
Reserpine and hydrochlorothiazide were purchased from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China), and rifampicin (internal standard, IS) from J&K (USA). Distilled water was prepared from demineralized water and used throughout the present study. Ethyl acetate was of analytical grade while all other chemicals were of HPLC grade.
Instrumentation
Chromatographic analyses were performed with a Shimadzu system that was equipped with a 20A pump and a diode-array detector (DAD). Chromatographic separation was achieved on the Elite ODS C18 column (250 mm × 4.6 mm, 5 µm), with a pre-column (Shimadzu ODS C18, 10 mm × 4.6 mm, 5 µm). The mobile phase was acetonitrile (A) and 0.2% ammonium chloride solution (B), 0~6 min, A: B (30:70, v/v); 6~9 min, the percent of A raised from 30% to 50%; 9~25 min, A: B (50:50, v/v). The flow rate was maintained at 0.8 mL min−1. Eluted peaks were detected at 268 nm.
Reference standard solution preparation
Since reserpine would be decomposed by oxidation under illumination, it should be protected from light exposure throughout the procedure. Stock solutions of reserpine (100 µg mL−1), hydrochlorothiazide (100 µg mL−1) and rifampicin for IS (1 mg mL−1) were prepared separately with acetonitrile: water (50:50, v/v) by sonicating for 30 min at room temperature. These solutions were further diluted with an appropriate amount of blank urine obtained from healthy volunteers to get a series of standards with concentrations of 0.05, 0.1, 0.2, 0.5, 1.0, 5.0, 10, and 20 (µg mL−1) for hydrochlorothiazide, and 0.02, 0.05, 0.1, 0.2, 0.5, 1.0 and 2.0 (µg mL−1) for reserpine, respectively.
Sample collection
Urine samples were collected from healthy volunteers every 1 h after oral administration of 9.0 mg of hydrochlorothiazide and 1.0 mg of reserpine. After the total volume was measured, about 10.0 mL of urine was stored in sealed containers at −20 °C.
Sample preparation
A 0.50 mL aliquot of the collected urine samples was pipetted into a centrifuge tube to which the internal standard solution (5 µL, 0.1 mg mL−1) and 2 mL of methanol were added. After vortexing for 3 min, the tube was centrifuged at 4000×g for 20 min. Then 0.5 mL of ethyl acetate was added to the supernatant, the tube was vortexed for 30 s and allowed to stand at room temperature for 15 min. The upper layer was transferred into a new borosilicate glass tube and evaporated to complete dryness under a nitrogen stream at 40 °C. The residue was reconstituted in 0.5 mL of 50% acetonitrile-water, and a 20 µL aliquot was injected into the HPLC system immediately.
Result and Discussion
Chromatographic selectivity
In order to achieve good separation of the examined drugs, a variety of reverse phase and gradient mobile phase LC systems were examined. The optimum wavelength for detection was 268 nm, at which good detection responses were achieved. A series of different buffer systems were studied and ammonium chloride solution was found to be superior. The pH of ammonium chloride was restricted to the range 3–6 with HCl. It was found that changing of pH had no obvious influence on the retention and absorbance of samples. Since isocratic elution can not simultaneously separate all components, gradient elution (see section “Instrumentation”) was adopted.
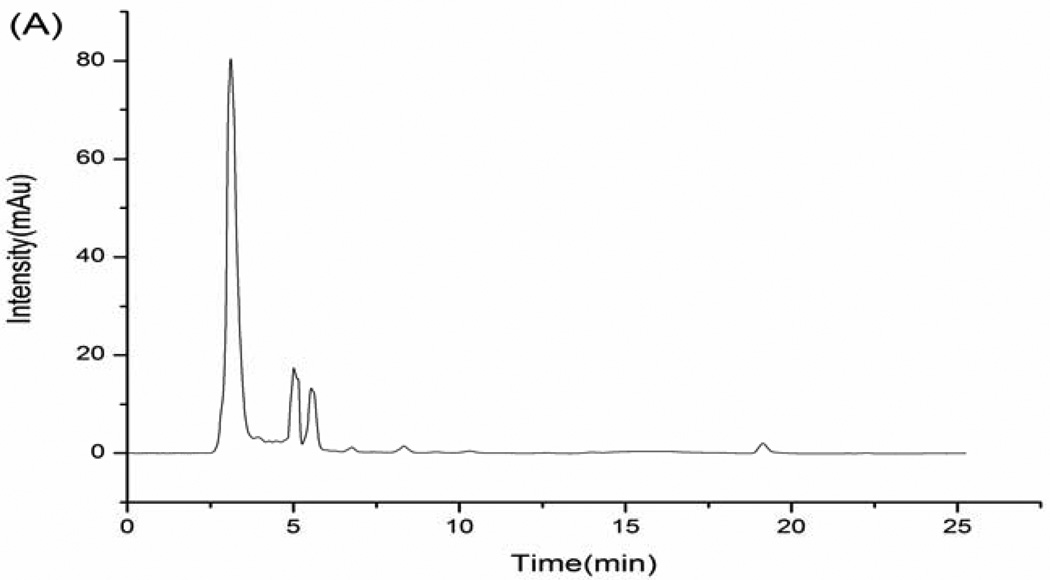
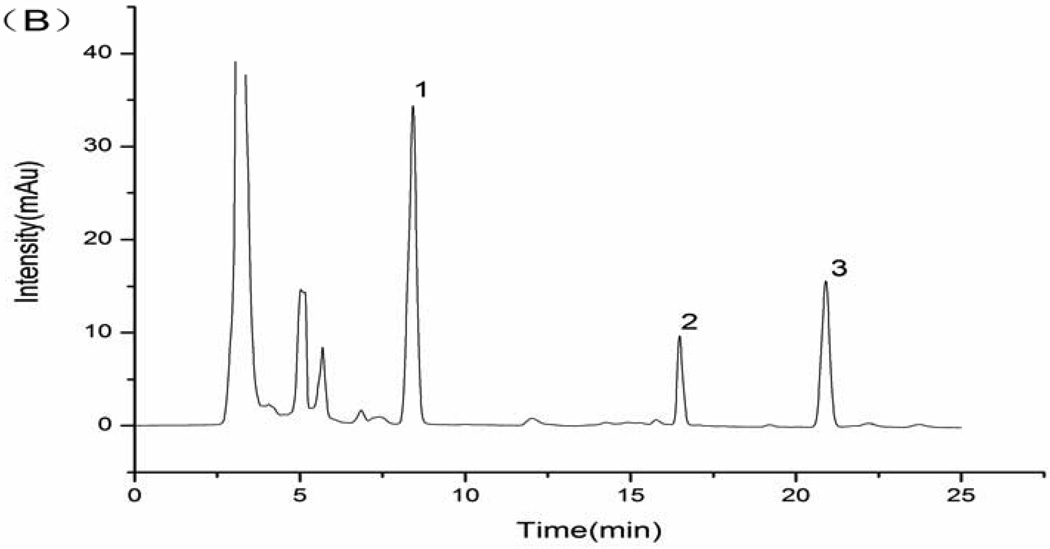
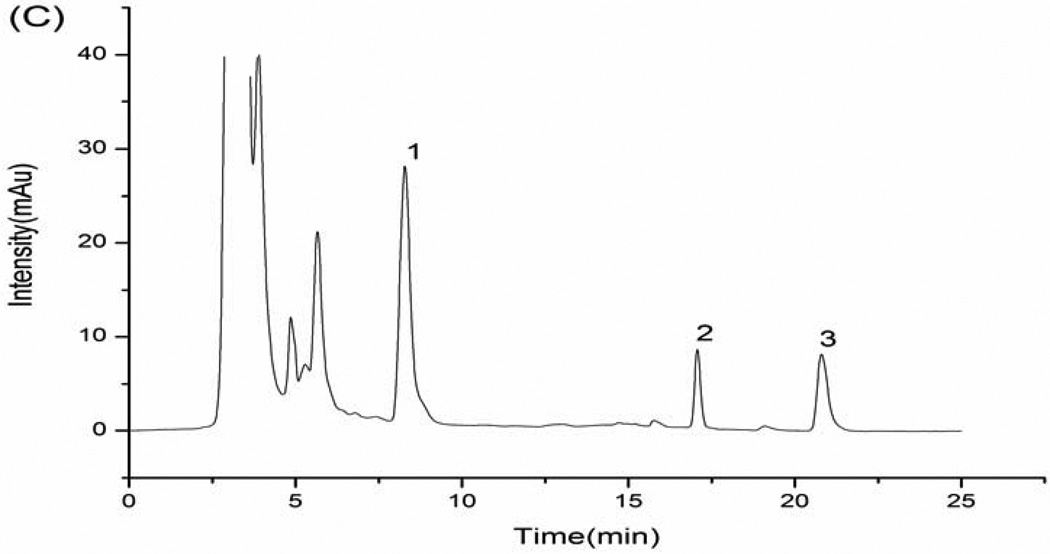
This simple procedure afforded efficient separation and purification of hydrochlorothiazide and reserpine in human urine without interference of peaks of endogenous constituents from urine and reagents. Fig. 1 shows chromatograms of the blank urine, blank urine spiked with standard substances (5.0 µg mL−1 hydrochlorothiazide and 5.0 µg mL−1 reserpine), and a urine sample obtained from a volunteer 1 h after a single oral dose of 9.0 mg hydrochlorothiazide and 1.0 mg reserpine. The retention times for the internal standard and investigated drugs were found to be 16.8 min (IS), 8.3 min (hydrochlorothiazide) and 20.8 min (reserpine).
Fig. 1.
(A) blank urine; (B) blank urine spiked with 5.0 µg mL−1 hydrochlorothiazide and 5.0 µg mL−1 reserpine; (C) urine sample obtained from a volunteer 1 h after a single oral dose of 9.0 mg of hydrochlorothiazide and 1.0 mg of reserpine. (1) hydrochlorothiazide; (2) IS; (3) reserpine
Linearity and LOD, LOQ
Calibration standards of seven concentrations of hydrochlorothiazide (0.05, 0.1, 0.2, 0.5, 1.0, 5.0, 10.0, and 20.0 µg mL−1) and reserpine (0.02, 0.05, 0.1, 0.2, 0.5, 1.0, 2.0 and 5.0 µg mL−1) were extracted and assayed. The calibration curve was obtained by plotting the peak area ratio of hydrochlorothiazide to IS and reserpine to IS. Calibration curves were linear over the concentration range of 0.05–20 µg mL−1 for hydrochlorothiazide and 0.02–5.0 µg mL−1 for reserpine, respectively.
Typical equations of calibration curves were as follows:
Here, Y=peak-area ratio (component/internal standard) and X=component concentration (ng mL−1).
The limit of detection (LOD) and the limit of quantification (LOQ) of hydrochlorothiazide and reserpine were calculated using the following equations:
where N is the standard deviation of the peak areas (six injections) in the baseline signal of drug-free urine samples, m is the slope of the corresponding calibration curve. The limit of detection and the limit of quantification were 5.5 ng mL−1 and 18.2 ng mL−1 for hydrochlorothiazide, and 7.1 ng mL−1 and 23.6 ng mL−1 for reserpine, respectively.
Recovery tests
QC samples were prepared as described under the section “sample preparation”, where three concentrations (0.2, 0.5, 1.0 µg mL−1) were prepared and analyzed for each analyte, corresponding to the lower limit, middle point and upper limit of the linearity curve. The extraction recovery was calculated by the ratio of the areas obtained from the spiked samples and the areas obtained from the standard solutions. As illustrated in Table 1, the lowest recovery was 89.0%, which showed a good performance on the accuracy of the method.
Table 1.
Recovery of assay for hydrochlorothiazide and reserpine (n=5)
| Hydrochlorothiazide | reserpine | ||
|---|---|---|---|
| Added(ng mL−1) | Mean ± S.D.(%) | Added( ng mL−1) | Mean ± S.D.(%) |
| 200 | 97.9±2.23 | 200 | 89.2±1.72 |
| 500 | 96.2±1.13 | 500 | 89.0±1.35 |
| 1000 | 97.1±1.75 | 1000 | 95.1±1.27 |
Precision studies
The assays described under “recovery tests” were repeated five times within the same day to ensure reproducibility (intra-day precision) and six times over three different days to obtain inter-mediate precision (inter-day precision), both expressed as RSD values. In As shown in Table 2, all values of the RSD of intra-day precision were less than 2.0%, while those of inter-day precision were less than 3.0%. The results showed that the precision of this method was satisfactory.
Table 2.
Precision of assay for hydrochlorothiazide and reserpine (n=5)
| Conc.added (ng mL−1) | Intra-day | Inter-day | ||
|---|---|---|---|---|
| Mean Caculated | RSD (%) | Mean Caculated | RSD (%) | |
| hydrochlorothiazide | ||||
| 200 | 195.7 | 1.91 | 191.6 | 1.22 |
| 500 | 480.6 | 0.98 | 460.1 | 1.35 |
| 1000 | 979.3 | 1.49 | 952.5 | 1.38 |
| reserpine | ||||
| 200 | 178.4 | 1.61 | 166.7 | 2.12 |
| 500 | 445.0 | 1.26 | 444.3 | 2.53 |
| 1000 | 949.2 | 1.11 | 936.4 | 2.64 |
Stability
Stock solutions of hydrochlorothiazide and reserpine were stable for at least 20 days when stored at 4 °C. Short-term stability of hydrochlorothiazide and reserpine in urine samples at room temperature was determined within 24 h. Long-term stability was studied by assaying samples within 10 days of storage in −20 °C. The results were expressed by the percentage of initial content of hydrochlorothiazide and reserpine in the freshly treated urine samples. In short-term stability, no significant change was found in reserpine for 12 h and in hydrochlorothiazide for 24 h. Long-term stability was 10 days for hydrochlorothiazide and 2 days for reserpine. Furthermore, hydrochlorothiazide and reserpine were stable through at least two freeze-thaw cycles.
Application to urine samples
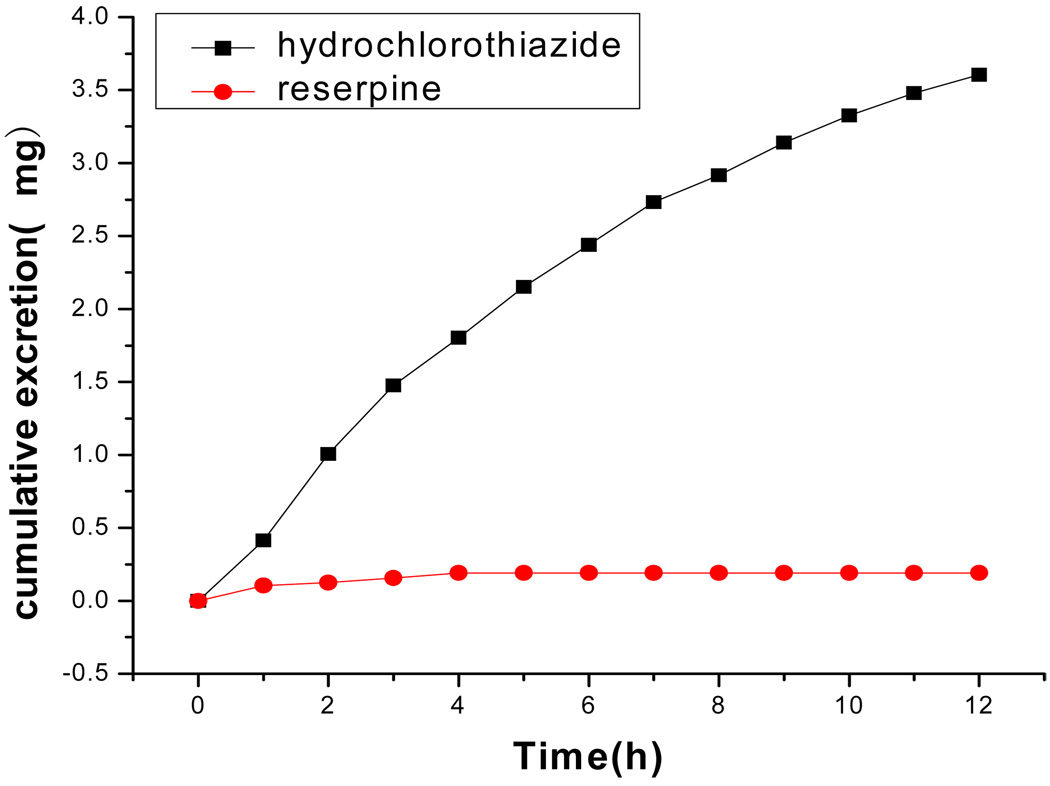
The method described above was applied to assay hydrochlorothiazide and reserpine in human urine samples. The urine samples were processed as described previously and assayed immediately. Fig. 2 showed the cumulative amount of hydrochlorothiazide and reserpine excreted in urine within 12 h. There was a quick urinary excretion within 1 h for both hydrochlorothiazide and reserpine. The fraction of the oral dose excreted in urine was 40.05% for hydrochlorothiazide and 10.37% for reserpine in 12 h. This result of pharmacokinetic study was consistent with the literature data.
Fig. 2.
Cumulative urinary excretion profile of hydrochlorothiazide and reserpine after a single oral administration of 9.0 mg of hydrochlorothiazide and 1.0 mg of reserpine. Total cumulative excretion within 12 h.
Conclusion
A simple, selective and sensitive LC method has been developed for the simultaneous quantitation of hydrochlorothiazide and reserpine in human urine. The major advantages of this method are the simple sample preparation, and high precision and accuracy. This method has been successfully applied to analyze hydrochlorothiazide and reserpine in urine samples of eight healthy volunteers following the administration of a single oral dose of 9.0 mg of hydrochlorothiazide and 1.0 mg of reserpine.
References
- 1.Liu F, Xu Y, Gao S, zhang J, Guo Q. J. Pharm. Biomed. Anal. 2007;44:1187–1191. doi: 10.1016/j.jpba.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Huang T, He Z, Yang B, Shao L, Zheng X, Duan G. J. Pharm. Biomed. Anal. 2006;41:644–648. doi: 10.1016/j.jpba.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Tutunji MF, Ibrahim HM, Khabbas MH, Tutunji LF. J. Chromatogr. B. 2009;877:1689–1697. doi: 10.1016/j.jchromb.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Yan T, Li H, Deng L, Guo Y, Yu W. J. P. Fawcett, J. Pharm. Biomed. Anal. 2008;48:1225–1229. doi: 10.1016/j.jpba.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Wang Y, Jiang Y, Tang Y. J. Chromatogr. B. 2007;852:436–442. doi: 10.1016/j.jchromb.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Richter K, Oertel R, Kirch W. J. Chromatogr. A. 1996;729:293–296. doi: 10.1016/0021-9673(95)00900-0. [DOI] [PubMed] [Google Scholar]
- 7.Abdel Razak Omayma. J.Pharm.Biomed.Anal. 2004;34:433–440. doi: 10.1016/S0731-7085(03)00497-7. [DOI] [PubMed] [Google Scholar]
- 8.Hillaert S, Grauwe KD, Bossche WV. J. Chromatogr. A. 2001;924:439–449. doi: 10.1016/s0021-9673(01)00714-2. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, Chen H, Guo X, Zhao Y. Analytica Chimica Acta. 1995;302:207–214. [Google Scholar]
- 10.Aly FA, El-Brashy A, Belal F. Analytica Chimica Acta. 1994;291:141–145. [Google Scholar]
- 11.Calatayud JM, Benito CG. Analytica Chimica Acta. 1991;245:101–107. [Google Scholar]
- 12.Chen H, He Q. Talanta. 2000;53:463–469. doi: 10.1016/s0039-9140(00)00512-9. [DOI] [PubMed] [Google Scholar]
- 13.Khayyal SE, Ayad MM, Girgis AN. J. Chromatogr. A. 1984;285:495–499. doi: 10.1016/s0021-9673(01)87791-8. [DOI] [PubMed] [Google Scholar]
- 14.Wang Joseph, Bonakdar Mojtaba. J. Chromatogr. B. 1986;382:349–354. doi: 10.1016/s0378-4347(00)83541-0. [DOI] [PubMed] [Google Scholar]
- 15.Ke J, Yancey M, Zhang S, Lowes S, Henion J. J. Chromatogr. B. 2000;742:369–380. doi: 10.1016/s0378-4347(00)00186-9. [DOI] [PubMed] [Google Scholar]
- 16.Varma SR, MartinezCalatayud J, Mottola HoracioA. Analytica Chimica Acta. 1990;233:235–241. [Google Scholar]
- 17.Cieri, Ugo R. Journal of AOAC International. 1994;77(5):1104–1108. [Google Scholar]