Abstract
In people with advanced chronic kidney disease (CKD), secondary hyperparathyroidism is associated with high-turnover bone disease. A low serum PTH may not necessarily be due to hypodynamic bone but another facet of the malnutrition-inflammation cachexia-syndrome (MICS). A recent 5-year cohort study in 748 stable hemodialysis outpatients showed that after removing the confounding by the MICS, the moderately low PTH in 100 to 150 p/ml range was associated with the greatest survival. Survival data from Japanese dialysis patients show similar survival advantages of lower PTH range. Low serum PTH appears associated with markers of protein-energy wasting and inflammation, and this association may confound the relationship between serum PTH and alkaline phosphatase. PTH stimulates lipogenesis through influx of calcium into the adipocytes. PTH secretion is suppressed by interleukin-1 beta and interleukin-6, which are pro-inflammatory cytokines associated with poor outcome in dialysis patients. These cytokines inhibits PTH secretion in cultured parathyroid tissue slices. In this article we review the association of a low serum PTH with the MICS in CKD patients and suggest avoiding over-interpretation of low serum PTH as an indicator of low-turnover bone disease.
Keywords: Parathyroid hormone (PTH), adynamic bone disease, malnutrition-inflammation complex, alkaline phosphatase, cytokines
Low Serum PTH
In individuals who have chronic kidney disease (CKD), secondary hyperparathyroidism (serum PTH >65 pg/ml) is associated with high-turnover bone disease, high fracture rates and higher death rates.1,2 In dialysis patients, a serum PTH between 150 to 300 pg/ml is considered a reasonable target zone.3 A low serum PTH below 150 pg/ml, however, may not necessarily be due to the so-called “hypodynamic bone” but another facet of the malnutrition-inflammation cachexia-syndrome (MICS).2 An observational study of hemodialysis patients by Dukkipati et al 2 studied the relationship between low PTH and protein-energy malnutrition and inflammation and found that patients with low PTH in the range of 150–200 pg/ml had the greatest survival in this cohort. Furthermore, there are other conditions associated with low PTH including calcium based binders, calcium rich diet, higher calcium concentration in dialysate bath, metabolic syndrome, diabetes mellitus, oxidative stress, peritoneal dialysis, advanced age, and caucasian race.4 In this review we focus on role of the MICS to this end.
PTH and Malnutrition-Inflammation Complex
The Kidney Disease Outcome Quality Initiative (KDOQI) and Kidney Disease Initiative Global Outcomes (KDIGO) guidelines suggest that serum PTH should be maintained above 150 pg/ml or 130 pg/ml, respectively, in chronic dialysis patients in order to avoid adynamic bone disease, while the Japanese Society of Dialysis Therapy (JSDT) recommends a target PTH range between 60 and 180 pg/ml.4 However, significant discrepancies may exist between the histopathologic diagnosis of the adynamic bone and the biochemical detection by means of “low PTH”.4 Notwithstanding the known histopathologic features of the adynamic bone disease, this condition might indeed be a secondary phenomenon and a consequence of the MICS,5 which per se is commonly associated with increased cardiovascular disease and death in dialysis patients.6 PTH stimulates lipogenesis through influx of calcium into the adipocytes,7 it is reasonable to suggest that a low PTH may prevent the accumulation of adipose tissue which may serve as a mechanism for protein energy wasting in low PTH states. A recent study by Kovesdy et al showed high PTH in obese patients with non-dialysis dependent chronic kidney disease.8
A recent study in 44 chronic peritoneal dialysis patients showed that low serum albumin was associated with adynamic bone.9 In another study, the in vitro PTH secretion was suppressed by IL-6,10 a strong pro-inflammatory cytokine that is associated with poor outcome in maintenance dialysis patients.11 Interleukin-1 beta (IL-1β), another pro-inflammatory cytokine, inhibits PTH secretion in cultured parathyroid tissue slices.12 This effect may be mediated through the specific IL-1 receptors that up regulate calcium-sensing receptor mRNA leading to apparent low bone turnover.12 Indeed, in the foregoing study, the inhibitory effect of IL-1β could be counteracted by the IL-1 receptor antagonist (IL-1ra),12 indicating that the inflammation induced suppression of PTH can potentially be overcome by treatment of malnutrition-inflammation complex in individuals with CKD.
A Japanese study involving over 15,000 dialysis patients by Akizawa and collaborates 13 reported an increased odds ratio of low PTH (<60 pg/ml) in presence of low serum albumin and urea nitrogen concentrations. Avram and collaborates 14,15, also, reported positive correlation between serum iPTH and serum albumin, creatinine, prealbumin and total cholesterol concentrations. Fukagawa et al 16 advanced the hypothesis that relative hypoparathyroidism reflects a state of malnutrition and contributes to the poor prognosis of dialysis patients, which may be, at least in part due to unknown mechanisms related to PTH deficiency, or from other abnormalities that suppress PTH secretion. Though the association between malnutrition and inflammation in CKD patients is well described, 17 the study by Dukkipati et al2 is the first one to indicate an association between malnutrition-inflammation complex and low serum PTH in CKD patients. It is important to note that a study by Mehrtora et al.18 showed that age related decline of PTH in dialysis patients is independent of inflammation or malnutrition.
If additional studies can verify findings by Dukkipati et al2, interventions that can improve hypoalbuminemia and the “Kidney Disease Wasting” and/or inflammation may be more promising approaches for the management of the “biochemical” adynamic bone (low PTH<150 pg/ml) rather than decreasing the dose of or withholding activated vitamin D analogs or calcimimetics.19
Low PTH and Outcomes
A recent epidemiologic study in non-dialysis dependent CKD patients found that the lower the PTH the greater the survival.20 The U-shaped PTH-survival association found in a dialysis cohort study 3 may be due to iatrogenic factors, e.g. as a result of the guidelines that recommend withholding active vitamin D and calcimimetics when PTH is below 150 pg/ml.21 The latter intervention may contribute to increased death risk in individuals with low PTH, leaving the artificial association between low PTH and increased death risk.5 Measured serum PTH level may also be confounded by such non-bone related factors such as obesity. 8 Epidemiologic study by Regidor et al found that even among dialysis patients with an intact PTH below 150 pg/ml, a high serum alkaline phosphatase (>120 U/L) was associated with higher death risk compared to lower alkaline phosphatase levels.22 In Japan, where the PTH target is 60–180 pg/ml per JSDT guidelines, the highest dialysis patient survival is reported with the lowest PTH level.4 Dreschler et al23. reported that PEW modifies the the association of PTH with adverse outcomes in diabetic dialysis patients‥
PTH Link with Cytokines
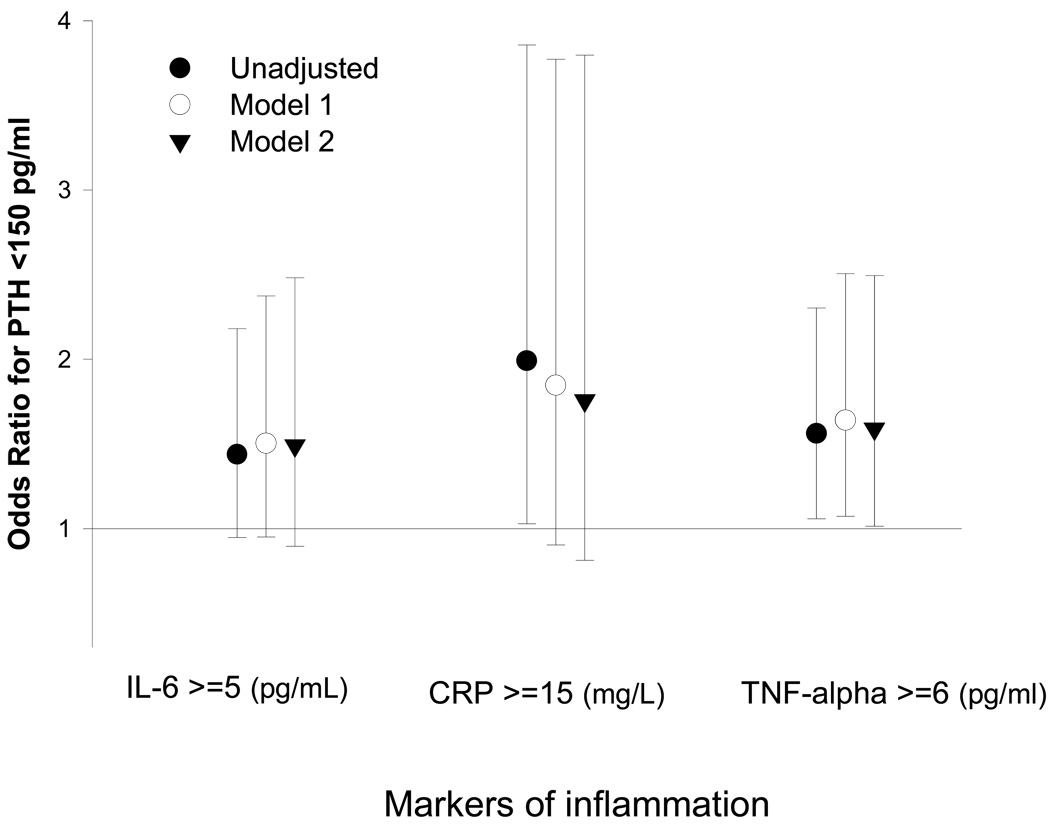
The recently discovered PTH-nutrition association may also explain as to why African American dialysis patients, who usually have a better nutritional status and greater survival 24 have relatively high PTH values.25 The MICS, a constellation of markers of malnutrition and inflammation, 26,27 is negatively associated with low serum PTH, and this association significantly modifies the expected association between serum PTH and alkaline phosphatase in high ranges of PTH.2 Hence, it may be speculated that malnutrition-inflammation complex plays a primary role in suppressing PTH level even in the setting of normal or high turnover bone status. The lack of any apparent or confounded associations between serum PTH and coronary artery calcification or bone mineral density may indicate to that end.2 As shown in Figure 1, higher levels of three inflammatory markers, IL-6, CRP and TNF-alpha are associated with the likelihood of a low serum intact PTH <150 pg/ml, which is often misinterpreted as adynamic bone disease.2
Figure 1.
Unadjusted and adjusted association between three inflammatory markers and having adynamic bone disease defined as parathyroid hormone (PTH) <150 pg/ml in 748 maintenance hemodialysis patients with PTH <300 pg/ml. Model 1: Adjusted for age, gender, race/ethnicity, diabetes, log vintage, primary insurance (medicare), modified Charlson comorbidity score, dialysis dose (Kt/v). Model 2: Adjusted for the variables of the model 1 and serum albumin, creatinine, phosphorus, total iron binding capacity, bicarbonate, lymphocyte percentage, body mass index, and log Paricalcitol. Data adapted from Dukkipati et al.2
Concluding Remarks
In recent years increasing attention has also been devoted to the so-called “adynamic bone disease”, which is associated with attenuated osteoclastic and osteoblastic activity and other features of low turnover bone including low serum parathyroid hormone (PTH). 28,29 Adynamic bone disease is reported to develop more frequently among diabetic patients and those with advanced age, non-black race and undergoing peritoneal dialysis. 30,31 In addition to adynamic bone disease, there may be other factors associated with a low serum PTH concentration including hypercalcemia, high calcium load and administration of vitamin D products, active vitamin D analogs and/or calcium sensing receptor antagonists (calcimimetics). 32–34 The KDOQI guidelines recommend the target range of 150 to 300 pg/ml for serum PTH in CKD stage 5 and suggest withholding active vitamin D and calcimimetics if serum PTH is below 150 pg/ml.21 More surprisingly, the recent KDIGO guidelines recommend a target PTH area of 130 to 580 pg/ml. These recommendations are set forth despite the fact that the normal range of the serum PTH in the general population serum is below 65 pg/ml and despite data from Japan showing superior survival of low PTH ranges. However, serum intact PTH below 150 pg/ml was associated with surrogates of malnutrition-inflammation complex in a cohort of 748 hemodialysis patients. Serum PTH was not associated with coronary artery calcification, nor bone mineral density, among 167 randomly selected hemodialysis patients who underwent EBCT and DEXA.2 These findings lead us to speculate that when PTH is in range of 100–200 pg/ml attention should focus on screening for MICS and treating it if present. If MICS is not present, then withdrawal of vitamin D and calcimimetic may still be considered if other causes of low PTH can be excluded. Appropriate interventions aiming at improving nutritional and inflammatory status of CKD patients may help implement better management strategies for low PTH and presumed adynamic bone disease. Additional prospective observational and interventional studies are needed to carefully examine the accuracy of our findings and the nature of the associations between malnutrition-inflammation complex, kidney bone disease and survival in CKD patients.
Acknowledgments
Funding Sources:
This study was supported by a research grant from the National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Disease grant # R21 DK077341 (for KKZ), a research grant from DaVita, Inc (KKZ), a philanthropist grant from Mr. Harold Simmons (for KKZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflict of Interests:
KKZ and/or CPK have received grants and/or honoraria from Amgen, Abbott, Fresenius, Genzyme and/or Shire.
References
- 1.Kalantar-Zadeh K, Shah A, Duong U, Hechter RC, Dukkipati R, Kovesdy CP. Kidney Bone Disease and Mortality in CKD: Revisiting the Role of Vitamin D, Alkaline Phosphatase and Minerals. Kidney Int Suppl. 2010 doi: 10.1038/ki.2010.189. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dukkipati R, Kovesdy CP, Colman S, et al. Association of Relatively Low Serum Parathyroid Hormone With Malnutrition-Inflammation Complex and Survival in Maintenance Hemodialysis Patients. J Ren Nutr. 2010 doi: 10.1053/j.jrn.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Shah A, Duong U, et al. Kidney Bone Disease and Mortality in CKD: Revisiting the Role of Vitamin D, Alkaline Phosphatase and Minerals. Kidney Int Suppl. 2010;78 Suppl 117:S10–S21. doi: 10.1038/ki.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Duong U, Miller JE, Rambod M, Dezfuli A, Kovesdy CP. Kidney Bone Disease and Mortality in CKD: The Role of Vitamin D, Alkaline Phosphatase and Minerals. Kidney Int Suppl. 2008 doi: 10.1038/ki.2010.189. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Kopple JD. Response to 'Adynamic Bone Disease and MICS'. Kidney Int. 2007;71:1327. [letter reply] [Google Scholar]
- 7.Ni Z, Smogorzewski M, Massry SG. Effects of parathyroid hormone on cytosolic calcium of rat adipocytes. Endocrinology. 1994;135:1837–1844. doi: 10.1210/endo.135.5.7525254. [DOI] [PubMed] [Google Scholar]
- 8.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Obesity is associated with secondary hyperparathyroidism in men with moderate and severe chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:1024–1029. doi: 10.2215/CJN.01970507. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Gonzalez MC, Lopez-Barea F, Bajo MA, Selgas R. Serum albumin levels, an additional factor implicated in hyperparathyroidism outcome in peritoneal dialysis: a prospective study with paired bone biopsies. Adv Perit Dial. 2006;22:198–202. [PubMed] [Google Scholar]
- 10.Carlstedt E, Ridefelt P, Lind L, Rastad J. Interleukin-6 induced suppression of bovine parathyroid hormone secretion. Biosci Rep. 1999;19:35–42. doi: 10.1023/a:1020146023812. [DOI] [PubMed] [Google Scholar]
- 11.Bologa RM, Levine DM, Parker TS, et al. Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis. 1998;32:107–114. doi: 10.1053/ajkd.1998.v32.pm9669431. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen PK, Rasmussen AK, Butters R, et al. Inhibition of PTH secretion by interleukin-1 beta in bovine parathyroid glands in vitro is associated with an up-regulation of the calcium-sensing receptor mRNA. Biochem Biophys Res Commun. 1997;238:880–885. doi: 10.1006/bbrc.1997.7207. [DOI] [PubMed] [Google Scholar]
- 13.Akizawa T, Kinugasa E, Kurihara R, et al. Risk factors for the development of parathyroid hormone deficiency in dialysis patients. J Am Soc Nephrol. 1998;9:561A. [Google Scholar]
- 14.Avram M, Sreedhara R, Oo K, et al. Prognostic value of enrollment nutritional markers including novel predictors PTH and prealbumin in hemodialysis patients: 12 years of follow-up. J Am Soc Nephrol. 1999;10:272A. [Google Scholar]
- 15.Avram M, Sreedhara R, Henry A, et al. Correlates of survival in peritoneal dialysis: 15 years of follow-up. J Am Soc Nephrol. 1999;10:311A. [Google Scholar]
- 16.Fukagawa M, Akizawa T, Kurokawa K. Is aplastic osteodystrophy a disease of malnutrition? Curr Opin Nephrol Hypertens. 2000;9:363–367. doi: 10.1097/00041552-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Kalantar-Zadeh K. Recent advances in understanding the malnutrition-inflammation-cachexia syndrome in chronic kidney disease patients: What is next? Semin Dial. 2005;18:365–369. doi: 10.1111/j.1525-139X.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 18.Mehrotra R, Supasyndh O, Berman N, et al. Age-related decline in serum parathyroid hormone in maintenance hemodialysis patients is independent of inflammation and dietary nutrient intake. J Ren Nutr. 2004;14:134–142. doi: 10.1053/j.jrn.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Kovesdy CP, Kalantar-Zadeh K. Novel targets and new potential: developments in the treatment of inflammation in chronic kidney disease. Expert Opin Investig Drugs. 2008;17:451–467. doi: 10.1517/13543784.17.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakai S, Akiba T, Kazama J, et al. Effects of serum calcium, phosphorous, and intact parathyroid hormone levels on survival in chronic hemodialysis patients in Japan. Ther Apher Dial. 2008;12:49–54. doi: 10.1111/j.1744-9987.2007.00540.x. [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation: Kidney Disease-Dialysis Outcome Quality Initiative: K/DOQI Clinical Practice Guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S202. [PubMed] [Google Scholar]
- 22.Regidor D, Kovesdy C, Mehrotra R, et al. Mortality Predictability of Serum Alkaline Phosphatase in Maintenance Hemodialysis Patients. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2008010014. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drechsler C, Krane V, Grootendorst DC, et al. The association between parathyroid hormone and mortality in dialysis patients is modified by wasting. Nephrol Dial Transplant. 2009 doi: 10.1093/ndt/gfp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC. Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol. 2007;3:493–506. doi: 10.1038/ncpneph0570. [DOI] [PubMed] [Google Scholar]
- 25.Wolf M, Betancourt J, Chang Y, et al. Impact of activated vitamin D and race on survival among hemodialysis patients. J Am Soc Nephrol. 2008;19:1379–1388. doi: 10.1681/ASN.2007091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rambod M, Bross R, Zitterkoph J, et al. Association of Malnutrition-Inflammation Score with Quality of Life and Mortality in Maintenance Hemodialysis Patients: a 5-Year Prospective Cohort Study. Am J Kidney Dis. 2008 doi: 10.1053/j.ajkd.2008.09.018. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rambod M, Kovesdy CP, Kalantar-Zadeh K. Malnutrition-Inflammation Score for risk stratification of patients with CKD: is it the promised gold standard? Nat Clin Pract Nephrol. 2008;4:354–355. doi: 10.1038/ncpneph0834. [DOI] [PubMed] [Google Scholar]
- 28.Coen G. Adynamic bone disease: an update and overview. J Nephrol. 2005;18:117–122. [PubMed] [Google Scholar]
- 29.Mucsi I, Hercz G. Adynamic bone disease: pathogenesis, diagnosis and clinical relevance. Curr Opin Nephrol Hypertens. 1997;6:356–361. doi: 10.1097/00041552-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Haris A, Sherrard DJ, Hercz G. Reversal of adynamic bone disease by lowering of dialysate calcium. Kidney Int. 2006;70:931–937. doi: 10.1038/sj.ki.5001666. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez CP. Adynamic bone revisited: is there progress? Perit Dial Int. 2006;26:43–48. [PubMed] [Google Scholar]
- 32.Kovesdy CP, Kalantar-Zadeh K. Bone and mineral disorders in pre-dialysis CKD. Int Urol Nephrol. 2008;40:427–440. doi: 10.1007/s11255-008-9346-7. [DOI] [PubMed] [Google Scholar]
- 33.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int. 2008;73:1296–1302. doi: 10.1038/ki.2008.64. [DOI] [PubMed] [Google Scholar]
- 34.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008;168:397–403. doi: 10.1001/archinternmed.2007.110. [DOI] [PubMed] [Google Scholar]