SUMMARY
Iron is required for bacterial proliferation and Staphylococcus aureus steals this metal from host hemoglobin during invasive infections. This process involves hemoglobin binding to the cell wall of S. aureus, heme extraction, passage through the cell envelope, and degradation to release free iron. Herein we demonstrate an enhanced ability of S. aureus to bind hemoglobin derived from humans as compared to other mammals. Increased specificity for human hemoglobin (hHb) translates into an improved ability to acquire iron and is entirely dependent on the staphylococcal hemoglobin receptor IsdB. This feature affects host-pathogen interaction as demonstrated by the increased susceptibility of hHb expressing mice to systemic staphylococcal infection. Interestingly, enhanced utilization of human hemoglobin is not a uniform property of all bacterial pathogens. These results suggest a step in the evolution of S. aureus to better colonize the human host and establish hHb expressing mice as a model of S. aureus pathogenesis.
INTRODUCTION
Vertebrates sequester nutrients required for microbial multiplication as a defense against infection in a process termed nutritional immunity (Bullen, 1981). In turn, pathogens have evolved intricate mechanisms to overcome this host defense and acquire needed nutrients. Iron is one such nutrient that is vital to the host-pathogen interaction (Crosa et al., 2004). In vertebrates, the majority of iron is bound by intracellular iron-binding proteins. Iron that is released upon cell lysis is bound by circulating transferrin ensuring that the level of available iron in the serum is incompatible with bacterial replication. In order to grow within vertebrates, bacterial pathogens must liberate intracellular iron and compete with host iron-sequestering proteins.
S. aureus has multiple iron acquisition systems including proteins involved in the acquisition of both siderophore-iron and heme-iron. The most abundant source of iron within vertebrates is hemoglobin (Drabkin, 1951). To access this iron source, S. aureus lyses erythrocytes through the secretion of hemolytic toxins (Torres et al., 2010). Upon erythrocyte lysis, S. aureus binds hemoglobin on the surface of the bacterial cell wall. The iron-containing heme co-factor is then extracted from hemoglobin and passed through the cell envelope into the cytoplasm where heme is degraded to release iron. This entire process is carried out by members of the Iron-regulated Surface Determinant system (Isd), which are up-regulated during conditions of iron starvation (Mazmanian et al., 2003; Muryoi et al., 2008; Pishchany et al., 2009; Reniere and Skaar, 2008; Reniere et al., 2007; Reniere et al., 2010; Skaar et al., 2004; Torres et al., 2006; Zhu et al., 2008). The importance of the Isd system to iron acquisition and staphylococcal pathogenicity has been demonstrated using murine models of infection (Cheng et al., 2009; Mazmanian et al., 2000; Pishchany et al., 2009; Torres et al., 2006).
A critical step in heme-iron acquisition is the capture of hemoglobin by the hemoglobin receptor IsdB (Mazmanian et al., 2003; Pishchany et al., 2009; Torres et al., 2006). This process is required for pathogenesis as demonstrated by the decreased proliferation of S. aureus strains inactivated for isdB in murine models. Notably, the primary amino acid sequence of hemoglobin differs across species and variation within hemoglobin primarily localizes to surface exposed residues that are likely recognized by IsdB (Figure S1A). Therefore, interspecies variation within hemoglobin may affect its capture by S. aureus and subsequently impact host range. In this paper we report that S. aureus IsdB preferentially recognizes human hemoglobin as compared to hemoglobins from other animal species. This preferential recognition results in enhanced bacterial proliferation and increased susceptibility of mice carrying human hemoglobin to staphylococcal infection.
RESULTS
S. aureus preferentially recognizes human hemoglobin
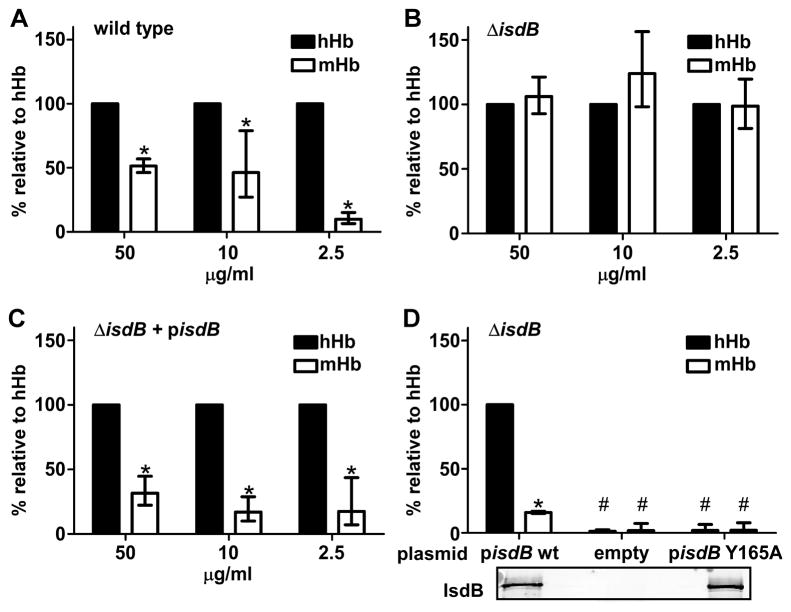
Many bacterial pathogens acquire nutrient iron from hemoglobin during infection. Interspecies variation in the primary amino acid sequence of hemoglobin suggests that bacterial pathogens may differentially recognize hemoglobin from distinct animals. Due to the extensive use of mice as animal models of S. aureus infections, we sought to compare the efficiencies with which S. aureus recognizes human (hHb) and mouse (mHb) hemoglobin. Hemoglobin was purified from fresh human or mouse blood and incubated with iron-starved S. aureus expressing the Isd system. Bound hemoglobin was then eluted and the relative amounts of hHb and mHb associated with the surface of S. aureus were compared. These experiments revealed that S. aureus binds hHb more effectively than mHb across a range of concentrations (Figures 1A and S1B). To test whether this preferential binding is dependent on IsdB we measured relative quantities of hHb and mHb bound by an isogenic isdB mutant (ΔisdB). S. aureus ΔisdB fails to bind increased quantities of hHb compared to mHb and this phenotype is fully complemented by providing a full length copy of isdB in trans (Figures 1B,C and S1B). Cell wall expression of a mutant version of isdB containing an alanine substitution in place of the absolutely conserved tyrosine residue at position 165 eliminates hemoglobin binding, establishing this residue as being critical for hemoglobin recognition by IsdB (Figure 1D). These results demonstrate that S. aureus has evolved to bind hHb through IsdB with increased efficiency compared to mHb. The hHb preference of S. aureus is evident amongst hemoglobins from a variety of animal species suggesting that S. aureus has evolved to most efficiently recognize hemoglobin from its human host (Figure S1C).
Figure 1. S. aureus displays increased binding of hHb as compared to mHb.
(A-D) Iron-starved S. aureus strain Newman were incubated with hemoglobin at the indicated concentrations and washed. Captured hemoglobin was eluted, subjected to SDS-PAGE, and silver stained. Representative images are shown in Figure S1B. Bound hemoglobin was quantified based on the relative intensity of Hb bands. Relative quantities of cell-wall bound hemoglobin are expressed as percent of hHb bound by (A) wild type, (B) ΔisdB, and (C) ΔisdB + pisdB. (D) ΔisdB harboring the indicated plasmids were incubated with hemoglobin at 10 μg/ml. Insert below panel is an image of an anti-IsdB immunoblot, demonstrating cell wall IsdB expression. Means and statistical significance were calculated based on logarithmically transformed fractions. Error bars represent confidence intervals (α = 0.05); asterisks denote quantities of bound mHb statistically different from hHb supplemented at the same conditions (Student’s two-tailed t-test, P<0.05). In panel (D) # denotes quantities that are significantly different from hHb and mHb bound by ΔisdB + pisdB (wt). Each graph is a result of three independent experiments.
The IsdB-dependent requirement for the preferential binding of hHb to the surface of S. aureus suggests that IsdB binds hHb with an increased affinity as compared to mHb. To test this hypothesis, we measured the affinity of recombinant IsdB (rIsdB) for hHb and mHb by biolayer interferometry (Figure S2). In support of our in vivo findings, the KD of the rIsdB-hHb interaction (5.5 × 10−8 M) is significantly stronger than the KD of the rIsdB-mHb interaction (9.8 × 10−7 M). Notably, this calculated affinity for the interaction of IsdB and hHb is consistent with previously published findings (Dryla et al., 2007). This result indicates that the preferential binding of hHb to the cell wall of S. aureus is achieved through a stronger interaction with the Hb receptor IsdB.
S. aureus has evolved to acquire nutrient iron from hHb more efficiently than from mHb
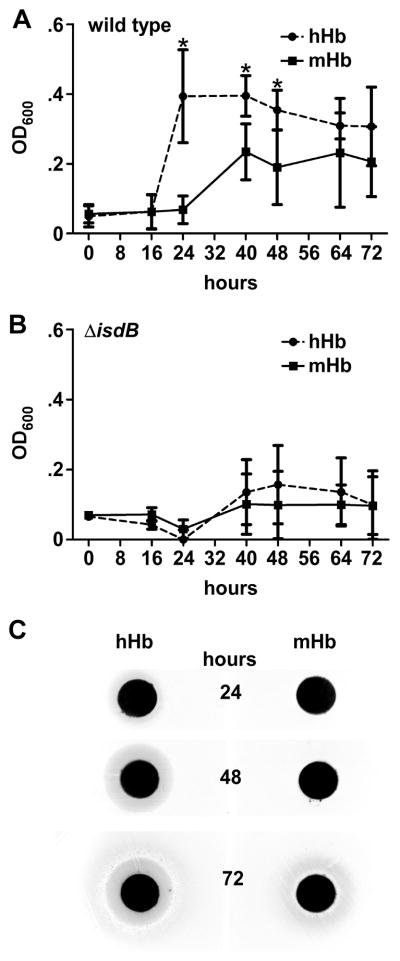
In iron-limiting conditions such as those encountered during infection (Pishchany et al., 2009; Reniere and Skaar, 2008), hemoglobin is a preferred source of iron that is sufficient to provide S. aureus with iron necessary for growth (Skaar et al., 2004; Torres et al., 2006). Therefore, we sought to determine whether preferential binding of hHb correlates with an improved ability to utilize hHb as an iron source. To test this hypothesis we measured the capacity of hHb and mHb to support S. aureus proliferation in an otherwise iron-deficient medium. Iron-starved S. aureus were inoculated into medium containing either hHb or mHb as a sole source of iron and bacterial replication was monitored over time as a function of either optical density or enumeration of colony forming units. S. aureus supplemented with mHb displayed a significant delay in growth as compared to hHb supplementation (Figure 2A and S3). These results reveal that S. aureus more efficiently utilizes hHb as an iron source as compared to mHb. The enhanced growth of S. aureus in the presence of hHb is dependent on IsdB, as indicated by ΔisdB exhibiting similar growth rates on either hHb or mHb (Figure 2B and S3). Notably, ΔisdB does not display an altered growth pattern when non-hemoglobin sources of iron are available (Torres et al., 2006).
Figure 2. hHb promotes S. aureus replication in iron-limiting conditions.
(A and B) Growth of S. aureus Newman wild type (A) and ΔisdB (B) in liquid medium supplemented with 5 μg/ml hemoglobin as a sole source of iron was measured based on optical density at 600 nm (OD600) over 72 hours. The graphs represent a mean of three independent experiments. Error bars represent standard deviation; asterisks denote OD600 values upon hHb supplementation significantly different from values upon mHb supplementation at the same time point (Student’s two-tailed t-test, P<0.05). (C) Petri dishes containing iron-restrictive agar were streaked with bacterial cultures. Disks impregnated with 10 μg of hemoglobin were placed on top of the agar and S. aureus growth surrounding the disks was monitored over 72 hours. Opaque gray zones around disks indicate zone of growth. The images are a representative of five independent experiments.
To further evaluate the efficiency of hHb-iron utilization, we monitored the ability of S. aureus to grow on solid medium where either hHb or mHb was the sole iron source. S. aureus was spread on iron-deficient medium containing discs impregnated with either mHb or hHb. The zone of growth around the disks was recorded as a measure of the ability of S. aureus to utilize Hb as an iron source. Growth around disks containing hHb was observed by 24 hours and continued to expand over the course of the experiment. In contrast, growth was not detectable around the mHb containing disc until approximately 72 hours after inoculation (Figure 2C). These findings demonstrate that S. aureus has evolved to acquire nutrient iron from hHb more efficiently than from mHb, and the enhanced recognition of hHb is mediated by the hemoglobin receptor IsdB.
Human hemoglobin exacerbates S. aureus infection in a murine model
Hemoglobin binding is a prerequisite for heme-iron acquisition during infection and as such, plays a critical role during S. aureus infection (Pishchany et al., 2009; Torres et al., 2006). In order to test whether the increased specificity for hHb benefits S. aureus during infection, we examined the susceptibility of transgenic αHβA mice that express normal adult human hemoglobin to systemic staphylococcal infection (Romero et al., 2004). S. aureus were inoculated intravenously into wild type or αHβA mice and the infection was allowed to proceed for 96 hours. Following this time course, mice were sacrificed, organ tissues were removed and homogenized, and bacterial counts were enumerated. In accordance with an increased ability of S. aureus to utilize hHb as an iron source, αHβA mice were more efficiently colonized as compared to wildtype animals (Figure 3, top). The presence of human hemoglobin does not affect infection by ΔisdB as demonstrated by the similar susceptibility of wildtype and αHβA mice to ΔisdB (Figure 3, bottom). Thus, the increased susceptibility of αHβA mice to systemic S. aureus infection is fully dependent on hemoglobin binding by IsdB. These results demonstrate that the enhanced specificity of S. aureus for hHb translates into increased colonization and establishes αHβA as a humanized mouse that exhibits increased susceptibility to S. aureus infections. Importantly, αHβA mice express approximately equal levels of both hHb and mHb suggesting that the effects observed here may underestimate the contribution of hHb to staphylococcal infection in humans. In addition, total Hb concentration does not differ between αHβA and wild type animals therefore variations in susceptibility are not due to hemoglobin abundance (data not shown). Knock-in mice that express exclusively hHb have been previously generated. However, hHb knock-in mice are notoriously difficult to breed and are therefore unsuitable for infection models, which require high numbers of subjects in order to evaluate statistical significance (Ye et al., 2008).
Figure 3. Mice expressing human hemoglobin exhibit increased susceptibility to S. aureus.
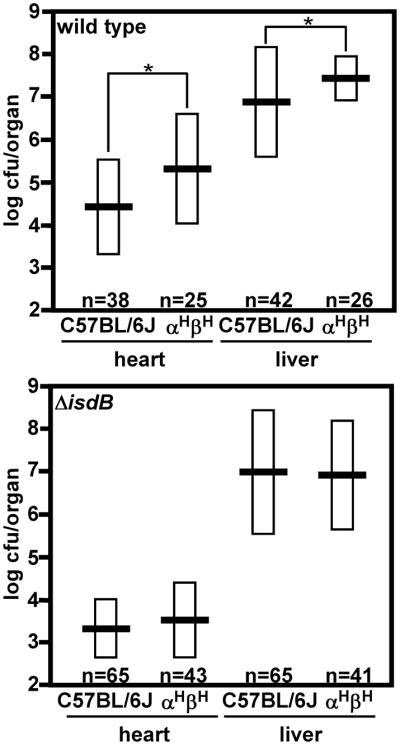
Number of colony forming units (CFU) of S. aureus Newman isolated from organs of systemically infected C57BL/6J and αHβA mice 96 hours post-inoculation as determined by serial dilution. Data were logarithmically transformed prior to statistical analyses. Horizontal bars represent the average values of CFU/organ, boxes represent standard deviation. Asterisks denote significantly different values (Student’s two-tailed t-test, P<0.05). The graphs represent combined data acquired from multiple independent experiments.
Preference for human hemoglobin varies across pathogens
The experiments described above were performed using S. aureus Newman, a commonly studied laboratory strain. To assess the ability of other laboratory and clinically relevant S. aureus isolates to acquire iron from mHb and hHb we tested the Hb preference of a panel of staphylococcal strains. As demonstrated in Figure 4A, all tested S. aureus isolates display increased binding of hHb as compared to mHb. Isogenic ΔisdB mutants of clinically relevant USA300 and the common laboratory strains RN4220 and RN6390 lost the ability to bind increased quantities of hHb as observed with strain Newman (Figures 4B and 1A). In support of the role of IsdB in increased hHb binding, S. aureus do not differentiate between hHb and mHb when grown under iron-replete conditions that prohibit isdB expression (Figure S4). Further, disk diffusion assays demonstrated increased proliferation using hHb as a sole iron source as compared to mHb for all tested S. aureus strains (Figure 4C). These results demonstrate that the preferential utilization of hHb as an iron source by IsdB is conserved among tested S. aureus isolates.
Figure 4. Bacterial pathogens vary in preference of hHb over mHb.
(A and B) Binding of hHb and mHb by S. aureus strains was assessed as in Figure 1. (C and D) Petri dishes containing iron-restrictive agar were streaked with (C) strains of S. aureus, and (D) other bacterial pathogens. Disks impregnated with 10 μg of hHb or mHb were placed on top of the agar and bacterial growth surrounding the disks was measured. The graphs depict growth on mHb as a percentage of growth on hHb in the same conditions (growth on hHb = 100%). The graphs represent a mean of three to four independent experiments. Means and statistical significance were calculated based on logarithmically transformed fractions. Error bars represent confidence intervals (α= 0.05); asterisks denote growth on mHb that is statistically different from growth on hHb supplemented at the same conditions (Student’s two-tailed t-test, P<0.05).
Numerous bacterial pathogens express hemoglobin receptors and utilize hemoglobin as an iron source during infection (Crosa et al., 2004). In keeping with this, we evaluated the ability of a number of bacterial species to grow in the presence of hHb and mHb. Many organisms that do not express hemoglobin receptors were unable to proliferate in the presence of either hHb or mHb, including Escherichia coli DH5α, Staphylococcus haemolyticus, Staphylococcus epidermidis and Shigella flexneri (data not shown). In contrast, Staphylococcus lugdunensis, Staphylococcus simulans and Corynebacterium diphtheriae displayed a preference for hHb similar to S. aureus (Figure 4D). Finally, Acinetobacter baumannii, Pseudomonas aeruginosa, Bacillus anthracis and Bacillus cereus utilized mHb and hHb with equal efficiency. These results demonstrate that the preferential utilization of hHb as an iron source is conserved across some bacterial pathogens while others do not discriminate between hHb and mHb.
DISCUSSION
S. aureus is a commensal organism that colonizes the anterior nares of approximately 30% of the human population (Weems, 2001). S. aureus is also capable of breaching these sites of initial colonization leading to significant morbidity and mortality (Klevens et al., 2007; Kuehnert et al., 2005). The host and bacterial factors that mediate this switch from commensal colonization to invasive disease are not understood. Herein, we demonstrate that human hemoglobin is a factor that impacts the host susceptibility to S. aureus through its interaction with the hemoglobin receptor IsdB. The significant affinity of IsdB for human hemoglobin permits the efficient utilization of hHb as an iron source leading to increased colonization and disease. Importantly, all tested clinically relevant strains displayed increased iron acquisition from hHb. By exploiting these observations, we have established αHβA mice as an improved murine model for studies into the pathogenesis of staphylococcal infections. These findings raise the exciting possibility that human hemoglobin polymorphisms may have implications regarding individual susceptibility to bacterial infections (Hardison et al., 2002).
These results revealed that a variety of distinct pathogens display an enhanced ability to utilize hHb as an iron source, while others do not exhibit hemoglobin preference (Figure 4D). Notably, bacteria that primarily associate with humans (S. aureus, S. lugdunensis, S. simulans, C. diphtheria) display preference for hHb over mHb, whereas environmental bacteria that infect numerous hosts (A. baumannii, P. aeruginosa, B. anthracis and B. cereus ) grow at comparable levels on hHb and mHb. In this regard, S. aureus IsdB binds hHb with a much stronger KD value (5.5 × 10−8 M) than the B. anthracis hemoglobin binding protein IsdX1 (7.3 × 10−6 M) (Maresso et al., 2008). This supports the hypothesis that S. aureus IsdB is optimized to bind hHb in order to acquire iron and colonize humans.
Much of what has been learned regarding the pathogenesis of S. aureus infection has been obtained from murine models of infection. However, due to inherent differences between mice and humans, murine infection models do not perfectly recapitulate human disease. To improve on this shortcoming, significant effort has been devoted to the development of humanized mouse models that more accurately reflect human disease (Legrand et al., 2009; Shultz et al., 2007). To date, few humanized mouse models have been established that exploit non-immune host factors (Johansson et al., 2003; Lecuit et al., 2001; Sun et al., 2004). Our findings add the human hemoglobin expressing mouse to the list of humanized animals that are valuable tools for modeling infection. Moreover, these findings demonstrate that humanized mouse models can be created that exploit the nutrient requirements of bacterial pathogens. Importantly, many bacterial pathogens utilize hemoglobin as an iron source; therefore human hemoglobin expressing mice may be valuable for studies into a variety of infectious diseases (Crosa et al., 2004).
EXPERIMENTAL PROCEDURES
Bacterial strains and growth conditions
All experiments were carried out with S. aureus strain Newman (Duthie and Lorenz, 1952), or with mutants generated in its background, unless indicated otherwise. The following strains of other bacteria were used for growth assays: Acenitobacter baumannii 17978, Psuedomonas aeruginosa PAO1, E. coli DH5α, Staphylococcus lugdunensis HKU09-01, Staphylococcus simulans TNK3, Staphylococcus epidermidis NRS6, Bacillus cereus 569, Bacillus anthracis Sterne, Staphylococcus haemolyticus NRS9, Corynebacterium diphtheriae C7(−) and Shigella flexneri SC560 (an M90T derivative with a ΔicsA::ΩSpr mutation). All cultures were inoculated from a single colony and grown overnight (~20 hours) in 5 ml RPMI (Thermo) medium supplemented with 1% casamino acids (RPMI + CA) in 15 ml conical tubes at 37°C with shaking at 180 rotations per minute (rpm) unless noted otherwise. The isogenic variant lacking isdB (ΔisdB) has been described previously (Mazmanian et al., 2003). A complementing plasmid containing isdB has also been previously described (Torres et al., 2006). Alanine substitution mutations within isdB at position Y165 were generated using Pfu mutagenesis and confirmed by sequencing. In order to maintain the plasmids, the complemented strains were grown in the presence of chloramphenicol (10 μg/ml). RN6390ΔisdB has been described previously (Taylor and Heinrichs, 2002). Strains inactivated for isdB in RN4220 and USA300 were generated by transducing the ΔisdB::ermC allele from Newman ΔisdB using bacteriophage φ-85 (Mazmanian et al., 2003).
Purification of human and mouse hemoglobin
Erythrocytes were sedimented by centrifugation (1,500 × g, 20 minutes, 4°C) from fresh human or mouse blood supplemented with anticoagulant. Erythrocytes were then washed 3 times with 3 volumes of ice cold saline (0.9% NaCl). Hemoglobin was released from erythrocytes by gently resuspending red blood cells in 1.5 volumes of 10 mM Tris-HCl (pH8.0) and 20% toluene (v/v) overnight on a rotisserie at 4°C. Hemolysate was separated from insoluble cellular debris (pellet) and membranes (toluene, upper layer) by a single centrifugation (20,000 × g, 1 hour, 4 C°). Hemolysate was then passed through a 0.4 μm filter. Hemoglobin was purified using an HPLC anion exchange column (Varian, PL-SAX 1000Å 8μm, 150mm × 4.6mm). The mobile phase A was 10 mM Tris-HCl (pH 8.0) and mobile phase B was 10 mM Tris-HCl (pH 8.0) + 0.5 M NaCl. A 0-100% gradient of solvent B was run over 2 minutes at 2.0 ml/min flow rate. The eluant was monitored based on absorption (λ: 410 nm and 280 nm). Purified hemoglobin was dialyzed twice at 4°C against phosphate buffered saline (PBS). Final hemoglobin concentrations were measured by Drabkin’s reagent (Sigma). Purified hemoglobin was stored in liquid nitrogen.
S. aureus hemoglobin binding assay
Hemoglobin was either purified from blood (Figures 1 and S1B) or purchased (Sigma) (Figure S1C). S. aureus was grown overnight in RPMI + CA supplemented with 0.5 mM of the iron chelator 2,2-dipyridyl (iron deplete) or 100 μM FeCl3 (iron replete). Bacterial numbers were normalized to an optical density at 600 nm (OD 600) of 2.0. One ml of each culture was sedimented by centrifugation (3,000 × g, 10 minutes), resuspended in 1 ml of PBS containing the indicated concentrations of hemoglobin and incubated at 37°C for 0.5 hour with shaking at 180 rpm. Upon completion of incubation, bacteria were washed 3 times with 1 ml of ice cold PBS, resuspended in 30 μl of 4% sodium dodecyl sulfate (SDS) 0.5M Tris-HCl (pH 8.0) and boiled for 5 minutes to release bound hemoglobin. S. aureus was then sedimented by centrifugation (16,000 × g, 5 minutes) and the supernatant containing hemoglobin was collected. Solubilized hemoglobin was subjected to 15% SDS-PAGE electrophoresis. Gels were silver stained (GE Healthcare Kit) and the relative abundance of bound hemoglobin was estimated based on the density of hemoglobin bands quantified by the Odyssey infrared imaging system (LI-COR) at 800 nm.
Immunoblotting of cell wall IsdB
Cell walls were solubilized by incubation of S. aureus in 20 μg/ml lysostaphin for 0.5 hour at 37 C°. Cell wall proteins were separated by 12% SDS-PAGE and transferred onto nitrocellulose membranes. Membranes were blocked with 5% milk made in TBS with 0.1% Tween 20 (TBST) from 1 h to overnight. The membranes were then incubated in milk plus primary rabbit anti-IsdB (1:10,000), washed three times with TBST, incubated in milk plus 0.1% sodium dodecyl sulfate plus secondary Alexa Fluor 680 goat anti-rabbit IgG(H+L) (1:25,000), and washed three times in TBST. Membranes were visualized using an Odyssey infrared imaging system (Li-Cor).
IsdB–hemoglobin affinity measurement
Purification of recombinant IsdB has been previously described (Mazmanian et al., 2003). Hemoglobin was biotinylated using EZ-Link NHS-LC-LC-Biotin (Pierce) at 1:2 protein:biotin ratio according to manufacturer’s recommendations. Unbound biotin was removed with Zeba Desalt Spin Columns (Pierce 89889). Binding kinetics were measured with an Octet QK (ForteBio, Inc., Menlo Park, CA) apparatus (Figure S2). Briefly, streptavidin high binding capacity FA biosensors (ForteBio 18-5019) were loaded with biotinylated hemoglobin at 25 μg/ml. Upon washing in PBS the sensors were transferred to rIsdB solution (5–10,000 nM) to allow association between rIsdB and hemoglobin. The sensors were then transferred to PBS to measure dissociation. Dissociation constants were calculated using Origin 7.5 SR6 software (OriginLab Corp., Northampton, MA) based on data acquired from three experiments using an automated curve fitting prompted by the Octet 4 software (ForteBio).
Growth in liquid medium
Single colonies of S. aureus were inoculated into RPMI + CA supplemented with 0.5 mM of the iron chelator ethylenediamine-di(o-hydroxyphenylacetic acid) (EDDHA, LGC Standards GmbH) and grown overnight. EDDHA was used in place of 2,2-dipyridyl due to the fact that EDDHA is less toxic to S. aureus in growth assays. One ml of overnight cultures was normalized to OD600 of 3.0, bacteria were sedimented (3,000 × g, 10 minutes), and resuspended in 1 ml NRPMI + 0.5 mM EDDHA. NRPMI was prepared in advance by treating RPMI + CA with Chelex 100 (Sigma) according to the manufacturer’s recommendations and supplementing the resulting ion-deficient medium with 25 μM ZnCl2, 25 μM MnCl2, 100 μM CaCl2 and 1 mM MgCl2. The resulting suspension of S. aureus was subcultured (1:100) into 1 ml of NRPMI + 0.5 mM EDDHA + hemoglobin at indicated concentrations. One ml cultures were incubated at 37°C in 15 ml conical tubes on a rotating wheel. OD600 measurements were taken at indicated time-points by mixing 10 μl aliquots of the culture with 90 μl PBS in 96 well plates. The number of colony forming units per milliliter of culture were quantified by serial dilution and plating on tryptic soy agar.
Growth on solid medium
Single colonies of bacteria were inoculated into RPMI + CA + EDDHA (500 μM for S. aureus strains and P. aeruginosa, 250 μM for B. anthracis, and S. lugdunensis, 100 μM for A. baumannii, S. haemolyticus, S. simulans, E. coli, and S. flexneri, 25 μM for S. epidermidis, 10 μM for B. cereus and none for C. diphtheriae) and grown overnight. Overnight cultures were spread with cotton swabs on NRPMI agar (NRPMI + 1.2% Bacto Agar) supplemented with EDDHA (500 μM for S. aureus strains, P. aeruginosa, B. anthracis, S. lugdunensis, and B. cereus, 100 μM for A. baumannii, S. haemolyticus, S. simulans, E. coli, S. flexneri, and S. epidermidis). C. diphtheriae were grown on RPMI + CA agar supplemented with 1 μM EDDHA. Sterile Whatman (d = 7 mm) disks were impregnated with 10 μl PBS-hemoglobin (1 mg/ml), placed onto agar and incubated at 37°C. Pictures were taken at a 72 hour time point except: USA500 at 96 hours, B. cereus at 14 hours, B. anthracis at 20 hours. Growth was measured by quantifying the distance between the edge of the disk and the edge of the zone of growth.
Systemic mouse infections
Seven week old C57BL/6J or human hemoglobin transgenic αHβA mice that were hemizygous for the transgene (Romero et al., 2004), but had no knock-outs or deletions, were infected retroorbitaly with ~107 CFU grown to mid-log phase in tryptic soy broth and resuspended in sterile PBS. Ninety-six hours post infection the mice were euthanized with forced inhalation of CO2. The hearts and livers were removed post mortem and homogenized in 1 ml sterile PBS. Organ suspensions were serially diluted, plated on tryptic soy agar and incubated overnight at 37°C. The following morning the numbers of CFU/organ were quantified. Animal experiments were approved by the institutional animal care and use committee of Vanderbilt University.
Highlights.
Staphylococcus aureus preferentially binds hemoglobin derived from humans (hHb).
S. aureus IsdB binds hHb with a stronger affinity than mouse Hb.
Increased binding of hHb facilitates iron acquisition by S. aureus.
Increased iron availability exacerbates S. aureus infection in hHb-expressing mice.
Supplementary Material
Acknowledgments
This work was supported by U.S. Public Health Service grants AI69233 and AI073843 from the National Institute of Allergy and Infection Diseases (E.P.S.) and American Heart Association Greater Southeast Affiliate Predoctoral Fellowship 09PRE2140221 (G.P.). E.P.S. is a Searle Scholar and holds an Investigator in Pathogenesis of Infectious Disease award from the Burroughs Wellcome Fund. We thank the members of the Skaar laboratory for critical reading of the manuscript, Dr. Kimberly Crimin for statistical advice, and Drs. Timothy Foster, Michael Schmitt, and Marcia Goldberg for providing strains. The authors have no conflicting financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bullen JJ. The significance of iron in infection. Rev Infect Dis. 1981;3:1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. Faseb J. 2009;23:3393–3404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa JH, Mey AR, Payne SM. Iron Transport in Bacteria. Washington, D.C: A.S.M. Press; 2004. [Google Scholar]
- Drabkin D. Metabolism of the Hemin Chromoproteins. Physiological Reviews. 1951;31:345–431. doi: 10.1152/physrev.1951.31.4.345. [DOI] [PubMed] [Google Scholar]
- Dryla A, Hoffmann B, Gelbmann D, Giefing C, Hanner M, Meinke A, Anderson AS, Koppensteiner W, Konrat R, von Gabain A, Nagy E. High-affinity binding of the staphylococcal HarA protein to haptoglobin and hemoglobin involves a domain with an antiparallel eight-stranded beta-barrel fold. J Bacteriol. 2007;189:254–264. doi: 10.1128/JB.01366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie ES, Lorenz LL. Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- Hardison RC, Chui DH, Giardine B, Riemer C, Patrinos GP, Anagnou N, Miller W, Wajcman H. HbVar: A relational database of human hemoglobin variants and thalassemia mutations at the globin gene server. Hum Mutat. 2002;19:225–233. doi: 10.1002/humu.10044. [DOI] [PubMed] [Google Scholar]
- Johansson L, Rytkonen A, Bergman P, Albiger B, Kallstrom H, Hokfelt T, Agerberth B, Cattaneo R, Jonsson AB. CD46 in meningococcal disease. Science. 2003;301:373–375. doi: 10.1126/science.1086476. [DOI] [PubMed] [Google Scholar]
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Jama. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- Kuehnert MJ, Hill HA, Kupronis BA, Tokars JI, Solomon SL, Jernigan DB. Methicillin-resistant-Staphylococcus aureus hospitalizations, United States. Emerg Infect Dis. 2005;11:868–872. doi: 10.3201/eid1106.040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit M, Vandormael-Pournin S, Lefort J, Huerre M, Gounon P, Dupuy C, Babinet C, Cossart P. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science. 2001;292:1722–1725. doi: 10.1126/science.1059852. [DOI] [PubMed] [Google Scholar]
- Legrand N, Ploss A, Balling R, Becker PD, Borsotti C, Brezillon N, Debarry J, de Jong Y, Deng H, Di Santo JP, et al. Humanized mice for modeling human infectious disease: challenges, progress, and outlook. Cell Host Microbe. 2009;6:5–9. doi: 10.1016/j.chom.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresso AW, Garufi G, Schneewind O. Bacillus anthracis secretes proteins that mediate heme acquisition from hemoglobin. PLoS Pathog. 2008;4:e1000132. doi: 10.1371/journal.ppat.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Liu G, Jensen ER, Lenoy E, Schneewind O. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc Natl Acad Sci U S A. 2000;97:5510–5515. doi: 10.1073/pnas.080520697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, Jelenska J, Joachmiak A, Missiakas DM, Schneewind O. Passage of heme-iron across the envelope of Staphylococcus aureus. Science. 2003;299:906–909. doi: 10.1126/science.1081147. [DOI] [PubMed] [Google Scholar]
- Muryoi N, Tiedemann MT, Pluym M, Cheung J, Heinrichs DE, Stillman MJ. Demonstration of the iron-regulated surface determinant (Isd) heme transfer pathway in Staphylococcus aureus. J Biol Chem. 2008;283:28125–28136. doi: 10.1074/jbc.M802171200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pishchany G, Dickey SE, Skaar EP. Subcellular localization of the Staphylococcus aureus heme iron transport components IsdA and IsdB. Infect Immun. 2009;77:2624–2634. doi: 10.1128/IAI.01531-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reniere ML, Skaar EP. Staphylococcus aureus haem oxygenases are differentially regulated by iron and haem. Mol Microbiol. 2008;69:1304–1315. doi: 10.1111/j.1365-2958.2008.06363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reniere ML, Torres VJ, Skaar EP. Intracellular metalloporphyrin metabolism in Staphylococcus aureus. Biometals. 2007;20:333–345. doi: 10.1007/s10534-006-9032-0. [DOI] [PubMed] [Google Scholar]
- Reniere ML, Ukpabi GN, Harry SR, Stec DF, Krull R, Wright DW, Bachmann BO, Murphy ME, Skaar EP. The IsdG-family of haem oxygenases degrades haem to a novel chromophore. Mol Microbiol. 2010;75:1529–1538. doi: 10.1111/j.1365-2958.2010.07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero JR, Suzuka SM, Nagel RL, Fabry ME. Expression of HbC and HbS, but not HbA, results in activation of K-Cl cotransport activity in transgenic mouse red cells. Blood. 2004;103:2384–2390. doi: 10.1182/blood-2003-01-0237. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O. Iron-source preference of Staphylococcus aureus infections. Science. 2004;305:1626–1628. doi: 10.1126/science.1099930. [DOI] [PubMed] [Google Scholar]
- Sun H, Ringdahl U, Homeister JW, Fay WP, Engleberg NC, Yang AY, Rozek LS, Wang X, Sjobring U, Ginsburg D. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science. 2004;305:1283–1286. doi: 10.1126/science.1101245. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Heinrichs DE. Transferrin binding in Staphylococcus aureus: involvement of a cell wall-anchored protein. Mol Microbiol. 2002;43:1603–1614. doi: 10.1046/j.1365-2958.2002.02850.x. [DOI] [PubMed] [Google Scholar]
- Torres VJ, Attia AS, Mason WJ, Hood MI, Corbin BD, Beasley FC, Anderson KL, Stauff DL, McDonald WH, Zimmerman LJ, et al. Staphylococcus aureus Fur regulates the expression of virulence factors that contribute to the pathogenesis of pneumonia. Infect Immun. 2010;78:1618–1628. doi: 10.1128/IAI.01423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J Bacteriol. 2006;188:8421–8429. doi: 10.1128/JB.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems JJ., Jr The many faces of Staphylococcus aureus infection. Recognizing and managing its life-threatening manifestations. Postgrad Med. 2001;110:24–26. 29–31, 35–26. doi: 10.3810/pgm.2001.10.1042. [DOI] [PubMed] [Google Scholar]
- Ye L, Chang JC, Lu R, Kan YW. High oxygen environment during pregnancy rescues sickle cell anemia mice from prenatal death. Blood Cells Mol Dis. 2008;41:67–72. doi: 10.1016/j.bcmd.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Zhu H, Xie G, Liu M, Olson JS, Fabian M, Dooley DM, Lei B. Pathway for heme uptake from human methemoglobin by the iron-regulated surface determinants system of Staphylococcus aureus. J Biol Chem. 2008;283:18450–18460. doi: 10.1074/jbc.M801466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.