Abstract
Background.
In the HORMA (Hormonal Regulators of Muscle and Metabolism in Aging) Trial, supplemental testosterone and recombinant human growth hormone (rhGH) enhanced lean body mass, appendicular skeletal muscle mass, muscle performance, and physical function, but there was substantial interindividual variability in outcomes.
Methods.
One hundred and twelve men aged 65–90 years received testosterone gel (5 g/d vs 10 g/d via Leydig cell clamp) and rhGH (0 vs 3 vs 5 μg/kg/d) in a double-masked 2 × 3 factorial design for 16 weeks. Outcomes included lean tissue mass by dual energy x-ray absorptiometry, one-repetition maximum strength, Margaria stair power, and activity questionnaires. We used pathway analysis to determine the relationship between changes in hormone levels, muscle mass, strength, and function.
Results.
Increases in total testosterone of 1046 ng/dL (95% confidence interval = 1040–1051) and 898 ng/dL (95% confidence interval = 892–904) were necessary to achieve median increases in lean body mass of 1.5 kg and appendicular skeletal muscle mass of 0.8 kg, respectively, which were required to significantly enhance one-repetition maximum strength (≥30%). Co-treatment with rhGH lowered the testosterone levels (quantified using liquid chromatography–tandem mass spectrometry) necessary to reach these lean mass thresholds. Changes in one-repetition maximum strength were associated with increases in stair climbing power (r = .26, p = .01). Pathway analysis supported the model that changes in testosterone and insulin-like growth factor 1 levels are related to changes in lean body mass needed to enhance muscle performance and physical function. Testosterone’s effects on physical activity were mediated through a different pathway because testosterone directly affected Physical Activity Score of the Elderly.
Conclusions.
To enhance muscle strength and physical function, threshold improvements in lean body mass and appendicular skeletal muscle mass are necessary and these can be achieved by targeting changes in testosterone levels. rhGH augments the effects of testosterone. To maximize functional improvements, the doses of anabolic hormones should be titrated to achieve target blood levels.
Keywords: Testosterone, Growth hormone, Lean body mass, Muscle performance, Physical function
LOSS of skeletal muscle mass (sarcopenia) contributes to declines in muscle performance and physical function during aging. Substantial losses in muscle strength may result in difficulty rising from a chair, climbing stairs, generating gait speed, maintaining balance, and frailty (1,2). The levels of endogenous anabolic hormones also decline during the aging process (3). Indeed, 25%–30% of men aged older than 60 years have low levels of serum testosterone levels (4) that may be associated with sarcopenia and muscle weakness (3,5). Restoring testosterone to youthful levels increases synthesis of myofibrillar proteins, total body cell mass, and muscle strength (6,7). Declines in growth hormone (GH) and insulin-like growth factor 1 (IGF-1) may also contribute to comorbidities in older men with normal testosterone levels (3,8).
To better understand the relative contributions of testosterone and GH/IGF-1 axes in older persons at risk for sarcopenia, we conducted the HORMA (Hormonal Regulators of Muscle and Metabolism in Aging) Trial to test our hypothesis that endogenous testosterone and GH are important independent but complementary regulators of skeletal muscle mass and function even into advanced age (9). Total lean body mass (LBM), appendicular skeletal muscle mass (ASMM), muscle performance, and stair climbing power increased significantly with testosterone and changes appeared to be enhanced by recombinant human growth hormone (rhGH) (9). However, there was considerable variability in anabolic responses as well as in changes in testosterone and IGF-1 levels during treatment. This provided the opportunity to examine relationships of a broad range of hormone changes, including declines in levels as may occur in clinical practice, and their effects on changes in lean tissue mass, muscle strength, performance, and physical function. We used pathway analysis to test the hypothesis that testosterone and rhGH affected muscle mass directly and that a threshold change in lean tissue mass was needed to generate significant improvements in muscle performance and physical function. Additionally, we used bootstrap analysis to determine target hormone levels associated with threshold changes in whole-body and appendicular lean mass that would be necessary for improving muscle performance and functional outcomes.
MATERIALS AND METHODS
Study Design
The HORMA study was a randomized double-masked investigation of testosterone and rhGH supplementation for 16 weeks in older community-dwelling men with testosterone and IGF-1 levels typical for older men (9). Eligible participants were randomized (factorial design) to one of two physiological doses of testosterone during a Leydig cell clamp as well to placebo or one of two physiological doses of rhGH.
Study Eligibility
Participants were screened at the University of Southern California, Tufts University, and Washington University after providing institutional review board–approved informed consent. Men were aged 65–90 years with morning total testosterone in the lower portion of the adult range (150–550 ng/dL) and IGF-1 in the lower adult tertile (<167 ng/mL). For screening, total testosterone was measured by automated platform immunoassays in the local clinical laboratories and IGF-1 at Quest Diagnostics (San Juan Capistrano, CA). Eligibility criteria included prostatic specific antigen ≤4.0 ng/mL, hematocrit ≤50%, and fasting blood glucose <126 mg/dL (9).
Study Interventions
Participants were treated monthly for 12 weeks with a long acting gonadotropin-releasing hormone agonist (leuprolide acetate depot, 7.5 mg intramuscularly; TAP Pharmaceuticals, Lake Forest, IL) and either 5 g or 10 g/d of 1% testosterone transdermal gel (Solvay Pharmaceuticals, Marietta, GA) daily for 16 weeks. The 5 g and 10 g doses of testosterone were chosen to produce a spectrum of serum levels via the Leydig cell clamp (to fully suppress endogenous production of testosterone) that would be in the low normal range typical of older men or mid-to-high normal levels typical of younger men, respectively (10). Participants also self-administered 0, 3, or 5 μg/kg of rhGH (Genentech, Foster City, CA) each evening. The 3 μg/kg dose of rhGH was chosen because 3.3 but not 2.0 μg/kg/d increased whole-body protein synthesis in GH-deficient adults (11). The 5 μg/kg/d dose was chosen to produce a greater anabolic stimulus.
Outcome Measures
Hormone assays.—
Testosterone and IGF-1 levels were determined at baseline and week 16. Total testosterone was measured using liquid chromatography–tandem mass spectrometry (12), free testosterone by equilibrium dialysis (13), and IGF-1 by a chemiluminescence immunoassay (9).
Body composition.—
Whole-body and regional lean mass were quantified by dual energy x-ray absorptiometry, calibrated using a soft tissue phantom. Scans were analyzed at the USC Reading Center by a dual energy x-ray absorptiometry–certified masked bionutritionist. Lean mass of the four extremities was summed to obtain ASMM.
Skeletal muscle performance and physical function.—
Upper and lower body muscle strength was determined by the one-repetition maximum (1-RM) method for the bilateral leg press, leg extension, leg flexion, latissimus pull-down, and chest press (14). Because different equipment was used at the testing centers, changes in muscle strength are presented as percentage change from baseline for the composite (sum) of the five exercises. Margaria stair climbing power was calculated from time (measured by photocells) to ascend the middle four steps in a 12-step staircase to quantify maximum power at steady state because there is an early acceleration to overcome inertia and gravity and there may be late deceleration due to fatigue (15). Physical activity was assessed with the Physical Activity Scale of the Elderly (PASE). VO2peak by cycle ergometry was determined during the baseline electrocardiogram stress test to assure that it was safe to conduct 1-RM testing.
Statistical Considerations
Paired t tests were used to assess within group effects. Pathway analyses using structural equation modeling (16) were conducted to examine the direct and indirect effects of the changes in hormone levels (predictors) on changes in LBM, ASMM, and 1-RM strength (mediators), Margaria stair climbing power (outcome), and physical activity by PASE (outcome). All relationships in the pathway model were assumed to be linear.
In addition, we compared the average change in total LBM and in ASMM between dichotomous groups defined by low (below-median) and high (above-median) changes in total testosterone, free testosterone, and IGF-1 for the participants who only received testosterone and those who received both testosterone and rhGH. To determine the combined effect of change in testosterone and IGF-1 on lean mass, changes in these hormones were dichotomized (low vs high) at their medians and participants were categorized into four groups: low/low, low/high, high/low, and high/high, respectively. One-way and two-way analysis of variance was used to examine the changes and the potential interaction of testosterone and IGF-1 levels on changes in total LBM and ASMM. Linear trends were determined by Wald analysis.
To determine the magnitude of change in testosterone with and without rhGH that is associated with 1.5 kg change in LBM and 0.8 kg change in ASMM, we used the bootstrapping method with 1000 iterations in which each bootstrap sample contained 90% of the original sample sets (without replacement) for the 39 men receiving only testosterone and 73 men receiving testosterone plus rhGH. Statistical analyses were carried out using the Statistical Analysis System 9.1 (Cary, NC).
RESULTS
Study Population
Of 122 eligible participants, 112 were randomized and completed 16 weeks of study medication. For testosterone treatments, 58 participants were randomized to 5 g of transdermal gel daily and 54 received 10 g/d. For rhGH treatments, 39 participants received placebo, 36 received 3.0 μg/kg, and 37 received 5.0 μg/kg daily. Table 1 summarizes baseline characteristics of the participants.
Table 1.
Baseline Characteristics of the Study Population Prior to Treatment
| Characteristic |
N = 112 Participants |
|
| Mean ± SD or N (%) | Median (range) | |
| Age, years | 70.2 ± 4.2 | 69.0 (64.0–85.0) |
| BMI, kg/m2 | 27.2 ± 3.3 | 26.8 (20.1–34.8) |
| Non-Hispanic Caucasian | 96 (86) | N/A |
| On treatment for hypertension | 30 (27) | N/A |
| History of smoking | 41 (37) | N/A |
| On treatment for dyslipidemia | 39 (35) | N/A |
| History ischemic heart events | 13 (12) | N/A |
| PASE | 147 ± 59 | 143 (30–369) |
| Hemoglobin, g/dL | 14.6 ± 0.9 | 14.8 (11.1–17.2) |
| Serum creatinine, mg/dL | 1.0 ± 0.16 | 1.0 (0.7–1.5) |
| Albumin, g/dL | 4.1 ± 0.3 | 4.1 (3.5–5.4) |
| Total testosterone, ng/dL* | 363 ± 97 | 361 (155–546) |
| Total testosterone, ng/dL† | 493 ± 170 | 473 (111–961) |
| IGF-1, ng/mL | 111 ± 29 | 110 (31–167) |
| Total lean body mass, kg | 58.2 ± 6.9 | 57.5 (41.6–78.0) |
| Appendicular lean mass, kg | 25.5 ± 3.3 | 25.5 (16.7–34.0) |
| VO2peak test, mL/kg/min | 24.6 ± 4.9 | 24.3 (9.2–36.8) |
Notes: BMI = body mass index; IGF-1 = insulin-like growth factor 1; PASE = Physical Activity Scale for the Elderly; SD = standard deviation.
By automated platform immunoassays for screening.
By liquid chromatography–tandem mass spectrometry (batch testing after study).
Changes in Serum Testosterone and IGF-1 Levels
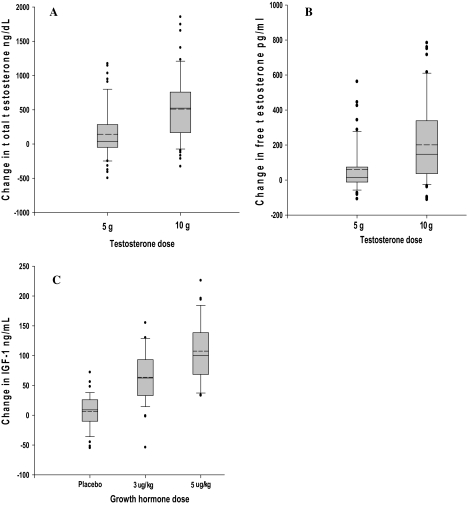
Total testosterone levels by liquid chromatography–tandem mass spectrometry increased by 143 ± 379 ng/dL (p = .006) with the 5 g dose and by 510 ± 503 ng/dL (p < .0001) with the 10 g dose (for between-dose comparison p < .0001; Figure 1A). Testosterone levels declined in 21 participants receiving the 5 g dose and in eight receiving the 10 g dose. Free testosterone increased by 60 ± 136 pg/mL (p = .001) in men receiving 5 g/d testosterone gel and by 201 ± 231 pg/mL (p < .0001) in those receiving 10 g/d (for between-dose comparison p < .0001; Figure 1B); 31 participants had decreases from 0 to −111.5 pg/mL compared with baseline.
Figure 1.
Changes in serum testosterone and insulin-like growth factor 1 (IGF-1) levels by dose assignment. The box plots represent the distribution of changes from baseline to week 16 for testosterone participants who received 5 or 10 g/d doses and IGF-1 for participants who received rhGH at 0, 3, and 5 μg/kg/d. The solid line within each box represents the median and hatched line the mean of the distribution change. The upper and lower boundaries of the boxes represent the 75th and 25th percentiles of the distribution, respectively. The upper and lower whiskers represent the 90th and 10th percentiles, respectively. Dots above and below the 90th and 10th percentiles, respectively, are individual values outside this range. For Panel A (total testosterone) and Panel B (free testosterone), within dose group changes were significant (p < .0001) as were differences between groups (p < .0001). For Panel C (IGF-1), within group changes were significant for the 3 and 5 μg/kg doses (p < .0001) as was the Wald trend across the groups (p < .0001).
Treatment with rhGH (0, 3, and 5 μg/kg/d) increased IGF-1 levels by 6 ± 28 (p = .16), 64 ± 44 (p < .0001), and 108 ± 51 ng/mL (p < .0001), respectively (Figure 1C). IGF-1 levels declined in 20 participants receiving placebo, three receiving 3 μg/kg/d, but none receiving 5 μg/kg/d of rhGH.
Primary Outcomes
After 16 weeks of treatment, total LBM increased by 1.8 ± 1.9 kg (interquartile range = 0.6–2.8 kg, maximum = 7.5 kg, p < .0001) and ASMM by 0.8 ± 1.2 kg (interquartile range = 0.0–1.5 kg, maximum = 4.4 kg, p < .0001, N = 112). Composite maximal voluntary 1-RM strength increased by 24 ± 33% (interquartile range = 2.3%–43.8%, maximum = 117%, p < .0001, N = 95) and Margaria stair climbing power by 63 ± 210 W (interquartile range = −11 to +143 W, maximum = 1248 W, p = .003, N = 112).
Pathway Analysis
Before construction of the pathway model, we examined the relationships between changes in hormone levels, lean tissue mass, muscle strength, and physical function. These analyses revealed that improvements in LBM and ASMM were correlated with increases in 1-RM strength (LBM r = .32, p = .001; ASMM r = .30, p = .003). However, change in 1-RM strength was not related to changes in testosterone (r = .12, p = .24) or IGF-1 levels (r = −.01, p = .90). Regression analysis indicated that LBM had to increase by 1.5 kg and ASMM by 0.8 kg to achieve meaningful changes in muscle strength and the associated improvements in physical function. For participants achieving ≥1.5 kg increases in LBM (n = 58), 1-RM strength increased 30.2 ± 33.0% compared with participants (n = 54) accruing <1.5 kg LBM (16.2 ± 32.3%, p = .04). Similarly, for participants achieving ≥0.8 kg increases in ASMM (n = 58), 1-RM strength increased 31.0 ± 32.4% compared with participants (n = 54) accruing <0.8 kg LBM (14.0 ± 32.3%, p = .01).
Margaria stair climbing power increased by 63 ± 210 W (p < .0001). Change in Margaria power was correlated to change in 1-RM strength (r = .26, p = .01) but not to changes in LBM (r = −.03, p = .77), ASMM (r = −.06, p = .53), testosterone (r = .05, p = .61), or IGF-1 (r = .06, p = .54)—data not shown. PASE differed in being directly correlated to changes in testosterone (r = .18, p = .08) but not to the other parameters.
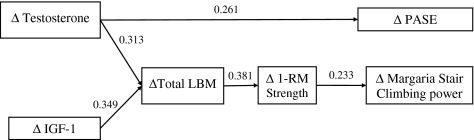
These initial analyses led us to propose the pathway model shown in Figure 2. We included LBM rather than ASMM in the model because tests of upper body strength—chest press and latissimus pull-down—are dependent on chest and back muscles as well as the muscles of the shoulder and arm (only the latter are measured as ASMM). The model shows that the primary determinant of change in LBM is the change in testosterone and IGF-1 level (regression coefficients of 0.313 and 0.349, respectively). Change in LBM was highly correlated to change in ASMM (r = .79, p < .0001), and both were associated significantly with the change in 1-RM strength, with a regression coefficient of 0.381. Finally, change in Margaria stair climbing power, a measure of physical function, was positively related to change in 1-RM strength (regression coefficient of 0.233). Changes in hormones were not significantly correlated to measures of muscle performance or physical function.
Figure 2.
Pathway analysis. This figure shows the result of the pathway analysis based on our hypotheses. Numbers associated with arrows are the estimated regression coefficients of the predictors/mediators with their corresponding mediators and outcomes; each is significant at p < .05. Predictors are changes (Δ) in serum testosterone and insulin-like growth factor 1 (IGF-1) levels; mediators are changes in lean body mass (LBM) and one-repetition maximum (1-RM) strength; and outcomes are changes in Physical Activity Score of the Elderly (PASE) and Margaria stair climbing power.
These analyses were consistent with the following pathway model: changes in testosterone and IGF-1→change in LBM→change in muscle strength→improvement in measures of physical function (Figure 2). The proposed model yielded a goodness-of-fit chi-square value of 2.16 with 8 degrees of freedom and p = .98, indicating that the data strongly support the pathway model. All regression coefficients for the model were significant at p < .05.
The change in physical activity (PASE) was associated only with increases in testosterone levels (regression coefficient 0.261), suggesting that testosterone might improve this outcome through a different mechanistic pathway.
Relationship of Change in Study Hormones and Lean Mass
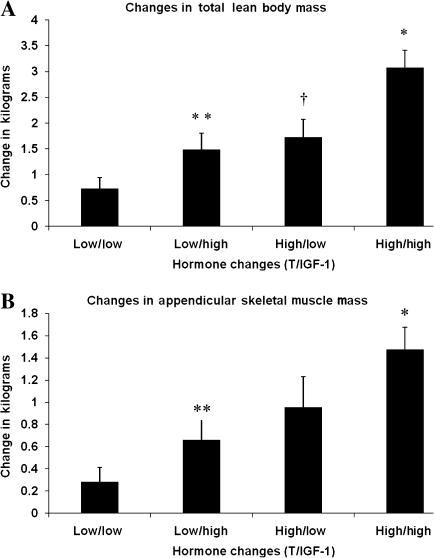
Changes in LBM and ASMM were significantly greater for participants whose changes in total or free testosterone and IGF-1 levels were greater than the median at week 17 (Table 2). However, there was a linear trend for participants with low levels (below medians) of both total testosterone and IGF-1 to the cohort with high levels (above medians) for both hormones in producing changes in total LBM (one-way analysis of variance, p < .0001; Wald trend, p < .0001; Figure 3). Although two-way analysis of variance of high versus low changes (at median) did not show a significant interaction between total testosterone and IGF-1 levels in affecting changes in total LBM (p = .35) or ASMM (p = .72) (data not shown), changes in total LBM (3.1 ± 2.0 vs 0.7 ± 1.3 kg) and ASMM (1.5 ± 1.2 vs 0.3 ± 0.8 kg) were significantly greater in the high/high than low/low groups.
Table 2.
Changes in Lean Body Mass Related to Changes in Hormone Levels Dichotomized at Week 16 Medians
| N = 73 (Testosterone + rhGH) | Change in Serum Total Testosterone Levels |
||
| Low* (−497 to 244 ng/dL), n = 37 | High (244–1854 ng/dL), n = 36 | p | |
| Change in total LBM† | 1.37 ± 1.73‡ | 2.75 ± 2.06 | .003 |
| Change in ASMM† | 0.53 ± 0.93 | 1.36 ± 1.30 | .003 |
| Change in Serum Free Testosterone Levels | |||
| Low (−103 to 56 pg/mL), n = 36 | High (60–784 pg/mL), n = 36 | p | |
| Change in total LBM | 1.06 ± 1.40 | 3.06 ± 2.08 | <.0001 |
| Change in ASMM | 0.43 ± 0.89 | 1.44 ± 1.27 | .002 |
| Change in Serum IGF-1 Levels | |||
| Low (−54 to 82 ng/mL), n = 38 | High (83–226 ng/mL), n = 35 | p | |
| Change in total LBM | 1.43 ± 1.86 | 2.73 ± 1.97 | .005 |
| Change in ASMM | 0.56 ± 1.15 | 1.34 ± 1.13 | .005 |
| N = 39 (Testosterone only) | Change in Serum Total Testosterone | ||
| Low (−377 to 106 ng/dL), n = 19 | High (111–1655 ng/dL), n = 20 | p | |
| Change in total LBM | 0.85 ± 1.27 | 1.74 ± 1.71 | .08 |
| Change in ASMM | 0.28 ± 0.82 | 1.07 ± 1.14 | .02 |
| Change in Serum-Free Testosterone Levels | |||
| Low (−111 to 50 pg/mL), n = 19 | High (58–759 pg/mL), n = 20 | p | |
| Change in total LBM | 0.53 ± 1.19 | 2.02 ± 1.56 | .002 |
| Change in ASMM | 0.27 ± 0.81 | 1.12 ± 1.16 | .01 |
Notes: ASMM = appendicular skeletal muscle mass; LBM = lean body mass.
“Low” refers to the participants with changes (baseline to end of 16 weeks of study therapy) below the median, including those with values lower than before treatment. “High” refers to participants with changes above the median.
Kilograms.
Mean ±1 SD.
Figure 3.
Changes in total lean body and appendicular skeletal muscle mass related to changes in testosterone and insulin-like growth factor 1 (IGF-1) levels. Panels A and B shows changes in lean tissue mass for four groups of participants for whose change in total testosterone (T) and IGF-1 levels from baseline to week 16 were dichotomized as “high” (greater than median) or “low” (below median). For Panel A (change in total lean body mass), the Wald trend across the four groups was <0.0001. For pairwise comparisons with Tukey adjustment, asterisk represents difference (p < .0001) between high/high and low/low groups, double asterisks represent difference (p = .004) between high/high and low/high groups, and dagger represent difference (p = .02) between high/high and high/low groups. For Panel B (change in appendicular skeletal muscle mass), the Wald trend across the four groups was p = .0001. For pairwise comparisons with Tukey adjustment, asterisk represents difference (p < .0001) between high/high and low/low groups and double asterisk represent difference (p = .04) between high/high and low/high groups.
Increases in lean mass above the median change of 1.5 kg for total LBM and above 0.8 kg for ASMM were associated with significant increases in muscle strength and improvements in physical function. Accordingly, we determined the magnitude of change in testosterone levels (including participants who had declines in levels below baseline values) necessary to increase LBM by 1.5 kg and ASMM by 0.8 kg by using bootstrap analyses. For participants who received only testosterone (n = 39, rhGH placebo), increases in total testosterone of 1046 ng/dL (95% confidence interval = 1040–1051 ng/dL) and 898 ng/dL (95% confidence interval = 892–904 ng/dL) were needed to increase LBM by 1.5 kg and ASMM by 0.8 kg (Table 3). Changes in free testosterone of 477 pg/mL (95% confidence interval = 474–480 pg/mL) and 397 pg/mL (95% confidence interval = 394–399 pg/mL) were necessary to achieve these threshold increases in LBM and ASMM. Changes in total or free testosterone levels necessary to achieve improvements in LBM were significantly lower when rhGH was coadministered (3 or 5 μg/kg/d).
Table 3.
Bootstrap Analysis to Determine Changes in Testosterone Levels Necessary to Augment Lean Mass
| Total LBM Target = 1.5 kg | ASMM Target = 0.8 kg | |
| Change in testosterone (T) alone, ng/dL, N = 39 | 1046* (1040–1051)† | 898 (892–904) |
| Change in T with any dose of rhGH, N = 73 | 944 (938–949) | 912 (906–919) |
| p Value | <.0001 | .002 |
| Change in free testosterone alone, pg/mL, N = 38 | 477 (474–480) | 397 (394–399) |
| Change in free T with any dose of rhGH, N = 72 | 303 (301–304) | 275 (273–276) |
| p Value | <.0001 | <.0001 |
Notes: ASMM = appendicular skeletal muscle mass; LBM = lean body mass.
Mean.
95% confidence interval.
DISCUSSION
The pathway analysis confirmed our hypothesis that increases in testosterone and IGF-1 concentrations in older men were robustly associated with gains in total lean body and ASMM but were not directly related to the demonstrable improvements in muscle performance or physical function. In contrast, the global enhancements in maximal voluntary strength of the major muscle groups of the upper and lower body along with significant increases in Margaria stair climbing power were directly related to increases in lean tissue mass. Independently, changes in testosterone levels were directly related to change in PASE scores, but the pathway analysis indicated that the latter was not related to changes in muscle mass or performance per se and thus may be related to improved state of well-being or to other mechanisms including central regulation of mood and blood flow to different areas of the brain (17).
Increases in LBM of 1.5 kg and ASMM of 0.8 kg were associated with significant improvements in maximal voluntary strength (30%–31%) as determined by 1-RM testing, with maximal changes exceeding 115%. Physical function, as assessed by the Margaria stair climbing power, improved by an average of 63 W, which was correlated with increases in 1-RM strength. Because threshold increases in LBM and ASMM were associated with significant improvements in muscle performance and physical function, we deemed these changes in body composition to represent threshold targets for promyogenic agents that affect primarily muscle mass. In a meta-analysis, testosterone replacement in androgen-deficient men across all ages was associated with ∼1.2 kg gain in total LBM (18), similar to our minimal threshold for improvements in maximum voluntary muscle strength and function. Our analyses suggest that increments in LBM that exceed this threshold may be associated with improvements in muscle performance (9,19–22).
Increases in total testosterone of 1046 and 898 ng/dL were required for participants only receiving testosterone (rhGH placebo) to achieve threshold improvements in LBM and ASMM, respectively; these corresponded to increases in free testosterone of 477 and 397 pg/mL. These represent conservative estimates because they include men whose testosterone levels declined, as may occur during clinical treatment with testosterone, and as such delineate target testosterone levels needed to sufficiently enhance LBM and ASMM necessary to improve muscle strength and physical function. Our data may also help explain why some testosterone trials, which used relatively low fixed doses of testosterone and achieved small (if any) increments in testosterone levels, reported relatively modest LBM gains and little or no change in muscle strength or physical function (23–25). Our data highlight the need for dose titration to target testosterone levels in clinical trials of testosterone for anabolic applications.
Total testosterone levels by liquid chromatography–tandem mass spectrometry were on average ∼130 ng/dL higher than those obtained by the automated platform immunoassays. It is possible that serum level targets may differ when other assays are used to quantify serum testosterone levels. Changes in free testosterone performed comparable to total testosterone in their ability to predict changes in LBM or ASMM.
The addition of rhGH at either 3 or 5 μg/kg/d to transdermal testosterone generally lowered the concentrations of total and free testosterone needed to achieve the threshold gains of 1.5 kg increase in LBM and 0.8 kg increase in ASMM. When participants were partitioned according to whether they had high or low changes in testosterone and IGF-1 levels, there was a clear hormone concentration–dependent response for the combinations that related to improvements in LBM and ASMM. The changes in both total testosterone and IGF-1 above the median resulted in greater improvements in lean mass than in other groups. Thus, consistent with other studies, there are greater enhancements in LBM and protein synthesis when both hormones are coadministered (19,26).
The HORMA study provides the rationale to further investigate strategies that combine an androgen (eg, testosterone or selective androgen receptor modulator), anti-myostatin, or similar agent with either rhGH or GH secretagogue (eg, capromorelin or oral ghrelin mimetic) (27,28) for impaired populations with sarcopenia who are weaker than our study population or those with overt physical frailty. Our findings suggest that combination strategies using agents with different but complementary promyogenic actions may more effectively enhance muscle mass accrual. It will be important to determine if lower dosing with the combinations will augment muscle performance and function while decreasing worrisome potential side effects associated with either long-term testosterone treatment (29) or the early adverse musculoskeletal and metabolic effects that have been associated with rhGH treatments (30). Our data highlight the importance of titrating the dose of the anabolic therapy to achieve a target circulating hormone level necessary to induce threshold gains in skeletal muscle mass that adequately improve muscle function. Conversely, candidate molecules that fail to induce threshold gains in skeletal muscle mass are less likely to meaningfully improve muscle strength and physical function than those which do.
FUNDING
Support for this trial was provided by the National Institute of Aging (AG18169) and NCRR M0I RR000043 at USC and RR000036 at Washington University, grants AG22356 and AG031679 at Boston Medical Center, and USDA grant (58-1950-9-001) and NCRR RR000054 at Tufts University. Study therapies were provided by Solvay Pharmaceuticals Inc., Genentech Inc., and Tap Pharmaceutical Products Inc.
Acknowledgments
We thank the wonderful participants who volunteered for this investigation, Dr. Jagadish Ulloor for performing free testosterone assays, and Mr. Thomas Wright for measuring IGF-1 levels.
References
- 1.Lindle RS, Metter EJ, Lynch NA, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20-93 yr. J Appl Physiol. 1997;83(5):1581–1587. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 2.Bassey EJ, Bendall MJ, Pearson M. Muscle strength in the triceps surae and objectively measured customary walking activity in men and women over 65 years of age. Clin Sci. 1988;74(1):85–89. doi: 10.1042/cs0740085. [DOI] [PubMed] [Google Scholar]
- 3.Abbasi AA, Drinka PJ, Mattson DE, Rudman D. Low circulating levels of insulin-like growth factors and testosterone in chronically institutionalized elderly men. J Am Geriatr Soc. 1993;41(9):975–982. doi: 10.1111/j.1532-5415.1993.tb06764.x. [DOI] [PubMed] [Google Scholar]
- 4.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107(2):123–136. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 6.Urban RJ, Bodenburg YH, Gilkison C, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269(5, pt 1):E820–E826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 7.Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab. 1992;75(4):1092–1098. doi: 10.1210/jcem.75.4.1400877. [DOI] [PubMed] [Google Scholar]
- 8.Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev. 1993;14(1):20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- 9.Sattler FR, Castaneda-Sceppa C, Binder EF, et al. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab. 2009;94(6):1991–2001. doi: 10.1210/jc.2008-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85(12):4500–4510. doi: 10.1210/jcem.85.12.7045. [DOI] [PubMed] [Google Scholar]
- 11.Lucidi P, Lauteri M, Laureti S, et al. A dose-response study of growth hormone (GH) replacement on whole body protein and lipid kinetics in GH-deficient adults. J Clin Endocrinol Metab. 1998;83(2):353–357. doi: 10.1210/jcem.83.2.4545. [DOI] [PubMed] [Google Scholar]
- 12.Sir-Petermann T, Codner E, Perez V, et al. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94(6):1923–1930. doi: 10.1210/jc.2008-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinha-Hikim I, Arver S, Beall G, et al. The use of a sensitive equilibrium dialysis method for the measurement of free testosterone levels in healthy, cycling women and in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 1998;83(4):1312–1318. doi: 10.1210/jcem.83.4.4718. [DOI] [PubMed] [Google Scholar]
- 14.Schroeder ET, Wang Y, Castaneda-Sceppa C, et al. Reliability of maximal voluntary muscle strength and power testing in older men. J Gerontol A Biol Sci Med Sci. 2007;62(5):543–549. doi: 10.1093/gerona/62.5.543. [DOI] [PubMed] [Google Scholar]
- 15.Margaria R, Aghemo P, Rovelli E. Measurement of muscular power (anaerobic) in man. J Appl Physiol. 1966;21(5):1662–1664. doi: 10.1152/jappl.1966.21.5.1662. [DOI] [PubMed] [Google Scholar]
- 16.Kline RB. Principles and Practice of Structural Equation Modeling. 2nd ed. New York: The Guilford Press; 2005. [Google Scholar]
- 17.Azad N, Pitale S, Barnes WE, Friedman N. Testosterone treatment enhances regional brain perfusion in hypogonadal men. J Clin Endocrinol Metab. 2003;88(7):3064–3068. doi: 10.1210/jc.2002-020632. [DOI] [PubMed] [Google Scholar]
- 18.Bhasin S, Calof OM, Storer TW, et al. Drug insight: testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab. 2006;2(3):146–159. doi: 10.1038/ncpendmet0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackman MR, Sorkin JD, Munzer T, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288(18):2282–2292. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 20.Ferrando AA, Sheffield-Moore M, Yeckel CW, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282(3):E601–E607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 21.Page ST, Amory JK, Bowman FD, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90(3):1502–1510. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 22.Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90(2):678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 23.Nair KS, Rizza RA, O’Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355(16):1647–1659. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 24.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299(1):39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 25.Giannoulis MG, Sonksen PH, Umpleby M, et al. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91(2):477–484. doi: 10.1210/jc.2005-0957. [DOI] [PubMed] [Google Scholar]
- 26.Huang X, Blackman MR, Herreman K, Pabst KM, Harman SM, Caballero B. Effects of growth hormone and/or sex steroid administration on whole-body protein turnover in healthy aged women and men. Metabolism. 2005;54(9):1162–1167. doi: 10.1016/j.metabol.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 27.White HK, Petrie CD, Landschulz W, et al. Effects of an oral growth hormone secretagogue in older adults. J Clin Endocrinol Metab. 2009;94(4):1198–1206. doi: 10.1210/jc.2008-0632. [DOI] [PubMed] [Google Scholar]
- 28.Nass R, Pezzoli SS, Oliveri MC, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149(9):601–611. doi: 10.7326/0003-4819-149-9-200811040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91(6):1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- 30.Molitch ME, Clemmons DR, Malozowski S, et al. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2006;91(5):1621–1634. doi: 10.1210/jc.2005-2227. [DOI] [PubMed] [Google Scholar]