Abstract
Background.
The optimal blood pressure level to minimize the risk of ischemic stroke (IS) in older adults is undetermined. Cerebral white matter lesions (WML), prevalent in older adults, may be a marker for vulnerability to IS. We aimed at determining the relationship between diastolic blood pressure (DBP) levels and IS in the presence of WML.
Methods.
The Cardiovascular Health Study population (N = 3,345, age ≥ 65 years, N = 3,345) was followed between 1989 and 2002 for IS incidence. Survival analysis included quintiles of DBP analyzed within WML levels controlling for age and cardiovascular disease.
Results.
DBP had no effect on IS incidence in low WML levels but had a marginally significant J-curve relationship with IS in high WML levels: the adjusted hazard ratio for IS in the lowest (<63 mmHg) and highest (≥80) DBP quintiles compared with the third (nadir, 69–73 mmHg) was 1.64 (95% confidence interval: 0.93–2.9) and 1.83 (95% confidence interval: 1.06–3.15), respectively.
Conclusions.
In older adults with low-grade WML, low DBP may not pose a risk for IS. However, in high-grade WML, IS risk may increase in DBP less than 69 mmHg but is highest more than 80 mmHg. People with high-grade WML may be at risk of IS in high and low DBP.
Keywords: Ischemic stroke, White matter lesions, Blood pressure, Cardiovascular Health Study,
THE debate about the blood pressure (BP) range that would minimize the risk of stroke is ongoing. Although some studies describe a linearly decreasing risk of stroke with decreasing BP, others suggest a J-shaped or U-shaped relationship. Optimal BP levels for older adults (≥65 years)—the most prone to stroke population [75% of all strokes (1)]—may differ from those of younger people (2,3). It has been argued that some 85 years and older people may fare better with higher BP than with “normal” BP (4), possibly due to reduced vascular compliance and cerebral blood flow (5). Additionally, diurnal BP fluctuations and severe nocturnal BP drops tend to increase among older adults (6). Nocturnal BP drops of more than 20% (“extreme dipping”) have been associated with hypoperfusion and ischemic stroke (IS) (7,8).
Hypoperfusion is characterized by transient moderate drops in regional cerebral blood flow that induce an incomplete form of cerebral infarction (9). Over time, this process may clinically manifest as rarefactions in the brain’s white matter or “white matter lesions” (WML) (10). People with WML had a 38% reduction in cerebral blood flow compared with those who did not have them (13.40 vs 21.74 mL/min/100 grams of tissue, p = .002) (11). WML are prevalent mostly after age 60 (9,12,13) and have been shown to increase considerably as perfusion declines (10,13). They can be reliably detected by magnetic resonance imaging (MRI) (14) and have been shown to predict stroke (15).
Using MRI-detected WML as a marker for cerebral ischemic disease and poor perfusion (10,11,16), we considered the possibility that older adults with such ischemic injuries could be more vulnerable to BP lowering compared with those without this condition. Such individuals may be likely, therefore, to experience an IS, if their BP is reduced below a certain threshold, still considered tolerable by people without such ischemic vulnerabilities. We hypothesized that (a) in older adults with little or no WML, the risk of IS will increase linearly with increasing BP. On the other hand, (b) the relationship between BP and IS incidence among older adults with moderate-to-severe grades of WML would be J-shaped, after holding confounding variables constant.
J-curved relationships have been previously described mostly between diastolic blood pressure (DBP) and heart disease or cardiovascular disease (CVD) (17–21). Fewer studies described a J-curve relationship of BP with stroke (22,23), and none had information on WML. Although the association between WML and stroke has been demonstrated (12,24), the relationship between BP level and IS, in older adults, in the presence of WML, is yet to be elucidated.
METHODS
Study Population
Our hypotheses were examined within the Cardiovascular Health Study, a prospective study of cardiovascular risk factors and outcomes among community-dwelling older adults (25). Full details of the selection process of the Cardiovascular Health Study cohort and its core characteristics are described elsewhere (25). Briefly, eligible men and women were sampled from Medicare eligibility lists in four U.S. counties: Forsyth, North Carolina; Sacramento, California; Washington, Maryland; and Pittsburgh, Pennsylvania. An initial sample of 5,201 participants was recruited during 1989/1990 and an additional sample of 687 African Americans was recruited during 1992/1993. Eligible persons were 65 years or older at the time of examination, noninstitutionalized, expected to remain in the area for the next 3 years, able to give informed consent, and did not require a proxy respondent at baseline. Individuals who were wheelchair bound in the home at baseline or were receiving hospice treatment, radiation therapy, or chemotherapy for cancer were excluded.
The annual examination cycles began in June 1989. Semiannual interim contacts were scheduled to ascertain and verify the incidence of CVD events, frequency of recurrent events, and sequelae of CVD. Extensive physical and laboratory evaluations were performed at baseline to identify the presence and severity of CVD risk factors. Evaluation included standardized questionnaires, BP measurements in upper and lower extremities, anthropometric measurements, 12-lead resting electrocardiogram, fasting lipid analyses, glucose and insulin measurements, pulmonary function tests, carotid sonography, and M-mode echocardiography. BP was measured in the right arm of seated participants after a 5-minute rest, using an appropriately sized cuff and a conventional mercury sphygmomanometer. The average of two measurements of the first (systolic) and fifth (diastolic) Korotkoff sounds was used for analysis (25).
MRI Evaluation
MRI was utilized to provide information on the prevalence and severity of WML. The first cranial MRI was implemented between years 2 and 5 of the study (1991–1994), providing data for this study while evaluating 3,660 participants, of whom 3,630 had a complete white matter grading. MRI was performed on General Electric or Picker 1.5-T scanners at three field centers and on a 0.35-T Toshiba instrument at the fourth center. Imaging data were archived on magnetic tape and sent to a single reading center for interpretation by neuroradiologists trained in the Cardiovascular Health Study protocol and blinded to participants’ identifying information. A method was developed to quantify white matter findings and grade them from barely detectable (Grades 0–1) to extensive and confluent (Grades 8–9). The inter-reader intra-class correlation coefficient for grade was 0.76 and the intra-reader coefficient was 0.89 (12,26).
Stroke Evaluation
The outcome measure was incident IS between the first MRI evaluation time and Year 12 (2002) of the study (over 9 years of follow-up). Stroke was defined as an abrupt onset of a new neurological deficit, lasting at least 24 hours, with specific localizing findings confirmed by unequivocal physical examination or laboratory data and without evidence for underlying nonvascular cause (25). Stroke events were identified semiannually and were adjudicated by a committee of neurologists, neuroradiologists, and internists, with information from patient interviews, medical records, and brain imaging studies (26). Stroke events were classified as ischemic when there was evidence of focal neurological deficit occurring without evidence of hemorrhage by neuroimaging, lumbar puncture, or autopsy and as hemorrhagic if there were evidence of blood in the subarachnoid space, ventricles, or parenchyma by neuroimaging that was not due to secondary hemorrhage into an infarct, or upon autopsy findings of bloody spinal fluid or evidence of hemorrhage (27).
Of the 3,630 participants with complete WML grading, 285 had a stroke or transient ischemic attack prior to the first MRI evaluation and were excluded from this research, leaving 3,345 qualifying participants. To obtain reliable estimates of IS risk (15), the 10 WML grades were collapsed into two categories of severity: low-grade WML = Grade 2 or lower and high-grade WML = Grade 3 or higher.
Analysis
Univariable survival analysis was applied to study the crude incidence of IS by the two WML categories. IS was also regressed separately on systolic blood pressure (SBP), DBP, and pulse pressure, as continuous variables. The effect of WML on the relationship between each BP index and IS was then estimated. During multivariable analysis, interaction terms, quadratic terms, and categorical variables for BP and age were implemented to detect nonlinearity. Proportionality assumption was verified (STATA tvc and stphtest options) by ruling out interaction of time-dependent covariates with log (time). Survival analysis models were used to describe the relationship between BP and IS, controlling for sociodemographic and cardiovascular risk factors. The fit of the models to the data was evaluated by generating the Cox–Snell residuals and then a cumulative hazard function (Nelson–Aalen), with the residuals at the abscissa. The graphed cumulative hazard function line created a 45° angle with the residuals indicating a good fit of the models to the data.
The following demographic and cardiovascular risk factors, traditionally associated with stroke and BP, reported at the clinic visit closest to the first MRI were initially treated as potential confounders: age (<74, 75–79, ≥80), gender, race (black/white), education (≤12 years, >12 years), current smoking status, weekly alcoholic beverages consumption (none, ≤2, >3), fasting serum cholesterol level (continuous millimeters per deciliter), body mass index (continuous), angina pectoris, serum creatinine (continuous), myocardial infarction, atrial fibrillation, orthostatic hypotension, and diabetes (impaired fasting glucose [blood glucose 110–126 mg/dL]) or a diagnosis of diabetes (blood glucose ≥126 mg/dL or insulin intake or oral hypoglycemic intake).
Potential confounders were excluded if their (two sided) p values were more than .10, unless they had a large coefficient (>1.5). Forward stepwise regression was used as an exploratory tool. Some age categories were not statistically significant in the high-grade WML group but were kept in the models due to the significance of age as a sociodemographic indicator and an established risk factor of stroke. Gender, race, education, alcohol consumption, and current smoking status were not informative at the outset and were removed from further models.
Some variables had a fraction (<3%) of their values missing and were estimated through single imputations (28). The data were analyzed using STATA statistical software: STATA Corporation 2003, Release 8.1 (College-Station, TX).
RESULTS
Descriptive Information
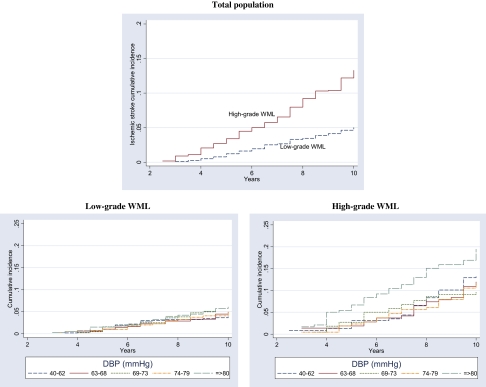
A total of 287 (8.58%) adjudicated incident strokes occurred among the 3,345 qualifying participants. Of them, 236 (82.2%) were ischemic, 34 (11.8%) hemorrhagic, and 17 (6%) unclassified. The unclassified strokes were included as ISs, generating a total of 253 (88.2%) outcomes. The MRI results indicated that 2,215 participants had WML grades of 0–2. They were classified as the “Low-Grade WML” group and experienced 108 (4.9%) incident ISs. The remaining 1,096 individuals had WML grades of 3–9 and were classified as the “High-Grade WML” group with 145 (13.2%) incident ISs (Supplementary Appendix 1). Comparative information on the two groups is displayed in Table 1. Figure 1 describes the cumulative incidence of IS by WML category, which was 13% and 5% in the high-grade vs low-grade WML categories, respectively, in the presence of competing risks (nonstroke death and hemorrhagic stroke). The incidence rates of IS events per 1,000 person years in high-grade and low-grade WML were 14.6 and 5.1, respectively.
Table 1.
Characteristics of Cardiovascular Health Study Participants by WML Grade, 1989–2002
| Continuous values | |||||||||||
| Risk factor | Low-grade WML (N = 2,215) |
High-grade WML (N = 1,096) |
p | ||||||||
| M | SD | Median | Percentile |
M | SD | Median | Percentile |
||||
| 5th | 95th | 5th | 95th | ||||||||
| Age | 74.1 | 4.7 | 73 | 68 | 83 | 76.8 | 5.4 | 76 | 69 | 87 | <.001 |
| BMI | 22.1 | 3.9 | 21.9 | 16.3 | 28.9 | 21.4 | 3.7 | 21.1 | 15.9 | 27.8 | <.001 |
| SBP | 132.5 | 20 | 131 | 103 | 168 | 138.7 | 21.1 | 137 | 106 | 178 | <.001 |
| DBP | 70.4 | 10.3 | 70 | 55 | 88 | 71.3 | 11.1 | 71 | 53 | 89 | .014 |
| SBP in ISH* (N = 1,001) | 146.2 (N = 663) | 18.7 | 144 | 119 | 179 | 149.4 (N = 438) | 19.3 | 148 | 120 | 184 | .004 |
| Pulse pressure | 62.2 | 17.1 | 60 | 38 | 93 | 67.4 | 18.9 | 66 | 38 | 93 | <.001 |
| Depression† | 4.8 | 4.6 | 4 | 0 | 14 | 5.4 | 4.9 | 4 | 0 | 15 | .003 |
| Person years | 9.4 | 1.3 | 10 | 6.5 | 10 | 8.9 | 1.7 | 10 | 5 | 10 | <.001 |
| Dichotomous values | |||||
| Risk factor | Low-grade WML (N = 2,215) | High-grade WML (N = 1,096) | p | ||
| % | n/N‡ | % | n/N‡ | ||
| White race | 84.11 | 1,863/2,215 | 84.3 | 924/1,096 | .883 |
| Female gender | 57.8 | 1,281/2,215 | 62.2 | 682/1,096 | .007 |
| Income <$50,000 | 83.4 | 1,743/2,090 | 90 | 916/1,018 | <.001 |
| Smokers | 10.3 | 227/2,215 | 7.9 | 87/1,096 | .027 |
| Angina pectoris | 16.1 | 356/2,215 | 19.4 | 213/1,096 | .016 |
| Atrial fibrillation | 2.3 | 51/2,215 | 3.3 | 36/1,096 | .096 |
| Myocardial infarction | 6.7 | 148/2,215 | 9.2 | 101/1,096 | .009 |
| Congestive heart failure | 3.8 | 83/2,215 | 5.1 | 56/1,096 | .080 |
| Orthostatic hypotension | 14.2 | 314/2,208 | 18.1 | 198/1,096 | .004 |
Notes: BMI = body mass index; DBP = diastolic blood pressure; ISH = Isolated Systolic Hypertension; SBP = systolic blood pressure; WML = white matter lesions.
Systolic BP in people with ISH (millimeter of mercury).
Center for Epidemiological Studies–Depression scale of 1–28.
Number of people with the characteristic (n) over number in WML category (N).
Figure 1.
Cumulative incidence of ischemic stroke by white matter lesions (WML) and diastolic blood pressure (DBP) categories 1989–2002, Cardiovascular Health Study participants with cranial magnetic resonance imaging screening.
The crude hazard ratios (HRs) of IS associated with DBP, SBP, and pulse pressure, in the presence and absence of WML, are presented in Table 2. DBP had the highest HR with IS, followed by SBP, both before and after stratifying by WML compared with pulse pressure, and the first two were implemented in further modeling. DBP was also the only index with a statistically significant quadratic term throughout. High-grade WML was associated with about a threefold crude increase in the risk of IS (HR = 2.92, 95% confidence interval [CI]: 2.3–3.7) compared with low-grade WML.
Table 2.
Models of Crude Hazard Ratios of Ischemic Stroke by WML and Blood Pressure (per 1 mmHg)
| Univariable models | |||
| Model | Predictor | HR | 95% CI (p)* |
| 1 | WML | 2.920 | 2.277–3.748 (<.001) |
| 2 | DBP | 1.019 | 1.007–1.031 (.002) |
| 3 | SBP | 1.017 | 1.011–1.022 (<.001) |
| 4 | PP | 1.016 | 1.009–1.023 (<.001) |
| 5 | DBP | 1.015 | 1.005–1.026 (.005) |
| DBP2 | 1.001 | 1.000–1.001 (.002) | |
| 6 | SBP | 1.013 | 0.948–1.051 |
| SBP2 | 1.000 | 1.000–1.000 | |
| 7 | PP | 1.018 | 1.004–1.031 (.010) |
| PP2 | 1.000 | 1.000–1.000 | |
| Bivariable models | |||
| 1 | DBP | 1.016 | 1.004–1.028 (.007) |
| WML | 2.874 | 2.240–3.690 (<.001) | |
| 2 | SBP | 1.014 | 1.008–1.020 (<.001) |
| WML | 2.694 | 2.095–3.465 (<.001) | |
| 3 | PP | 1.014 | 1.008–1.020 (<.001) |
| WML | 2.694 | 2.095–3.465 (<.001) | |
| Univariable models by WML grade | |||||
| Model | Predictor | Low-grade WML | High-grade WML | ||
| HR | 95% CI (p)* | HR | 95% CI (p)* | ||
| 1 | DBP | 1.014 | 1.000–1.030 | 1.018 | 1.000–1.030 |
| 2 | DBP | 1.013 | 1.000–1.030 | 1.013 | 1.000–1.030 (.05) |
| DBP2 | 1.0006 | 1.000–1.002 | 1.0009 | 1.000–1.002 (.007) | |
| 3 | SBP | 1.011 | 1.000–1.020 (.014) | 1.015 | 1.010–1.020 (.001) |
| 4 | SBP | 1.009 | 0.990–1.020 | 1.009 | 0.990–1.020 |
| SBP2 | 1.000 | 1.000–1.000 | 1.000 | 1.000–1.000 | |
Notes: DBP = diastolic blood pressure centered at 72 mmHg, SBP = systolic blood pressure centered at 120 mmHg, PP = pulse pressure centered at 48 mmHg, DBP2 = quadratic term for diastolic blood pressure, SBP2 = quadratic term for systolic blood pressure. CI = confidence interval; HR = hazard ratios; WML = white matter lesions.
p Value given in parenthesis is two sided and specified when ≤0.05.
BP indices were evenly distributed across age with a few extreme values that were clinically plausible. DBP had values as low as 40 mmHg across all age groups. This value was assigned when Korotkoff Phase 5 persisted during the BP measurement. The mean SBP among people with low-grade and high-grade WML was 132.5 and 138.7 and the mean DBP was 70.3 and 71.3 mmHg, respectively (Table 1). All BP indices were normally distributed. DBP ranged from 40 to 110 mmHg in the low-grade WML group and from 40 to 115 mmHg in the high-grade WML group. Mean DBP differed significantly between WML groups in the lowest quintiles (Supplementary Appendix 2). A 1-mmHg linear increase in DBP was associated with a 1.4% (p = .126, 95% CI: 1–1.03) crude average increase in the HR in the low-grade WML group and a 1.8% (HR = 1.018, p = .02, 95% CI: 1–1.03) increase in the high-grade WML group (Table 2).
Multivariable Analysis
Low-grade WML population.—
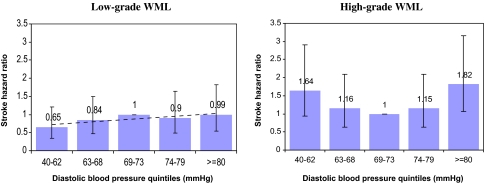
In an initial analysis of this population, both the systolic and DBP indices were included as continuous variables. Angina pectoris was somewhat correlated with a history of myocardial infarction (ρ = .41), whereas the latter was a stronger predictor of risk and was kept in further modeling. Orthostatic hypotension (p = .12, 95% CI: 0.9–2.36) and body mass index (p = .94, 95% CI: 0.95–1.06) were excluded due to large p values. DBP was centered at 72 mmHg, and a quadratic term for DBP in this group was not statistically significant (p = .49, 95% CI: 0.999–1.001). Modeling quintiles of DBP in low-grade WML (Table 3 and Figure 2) in the presence of SBP indicated a monotonic relationship with IS. The respective HR’s by quintiles were 0.65, 0.84, 1.0, 0.9, and 0.99. Atrial fibrillation was the strongest predictor of risk, almost tripling the hazard of IS in people with the condition, compared with those free of it. Increasing age was the next strongest predictor followed by myocardial infarction (MI).
Table 3.
HR of Incident Ischemic Stroke by Two WML Grade Levels Among Cardiovascular Health Study Participants, 1989–2002
| Low-Grade WML |
High-Grade WML |
|||||
| Risk Factor | HR | p | 95% CI | HR | p | 95% CI |
| DBP 40–62 | 0.65 | .17 | 0.35–1.2 | 1.64 | .088 | 0.93–2.90 |
| DBP 63–68 | 0.84 | .55 | 0.47–1.49 | 1.16 | .63 | 0.64–2.10 |
| DBP 69–73 | 1 | — | — | 1 | — | — |
| DBP 74–79 | 0.9 | .74 | 0.49–1.65 | 1.15 | .64 | 0.64–2.10 |
| DBP ≥80 | 0.99 | .96 | 0.54–1.82 | 1.83 | .03 | 1.06–3.15 |
| SBP per 1 mmHg increase | 1.006 | .25 | 1.00–1.017 | 1.014 | .001 | 1.01–1.02 |
| Atrial fibrillation | 2.86 | .013 | 1.25–6.58 | 5.02 | <.001 | 2.80–8.90 |
| Myocardial infarction | 2.09 | .006 | 1.23–3.55 | 2.23 | <.001 | 1.40–3.40 |
| Age 65–74 y | 1 | — | — | 1 | — | — |
| Age 75–80 y | 2.02 | .002 | 1.30–3.14 | 1.06 | .79 | 0.70–1.60 |
| Age >80 y | 2.50 | <.001 | 1.54–4.10 | 1.40 | .10 | 0.92–2.10 |
| Cholesterol/1 mm/dL increase | 1.004 | .015 | 1.001–1.008 | 1.003 | .148 | 0.999–1.007 |
| Diabetes mellitus | 1.81 | .013 | 1.13–2.89 | 1.17 | .502 | 0.74–1.85 |
Note: CI = confidence interval; DBP = diastolic blood pressure; HR = hazard ratios; SBP = systolic blood pressure; WML = white matter lesions.
Figure 2.
Hazard ratios of ischemic stroke by white matter lesions (WML) grade and diastolic blood pressure quintiles 1989–2002, Cardiovascular Health Study participants with cranial magnetic resonance imaging screening. Controlled for systolic blood pressure, atrial fibrillation, history of myocardial infarction, diabetes mellitus, total serum cholesterol, and age
High-grade WML population.—
The same confounders considered for the low-grade WML model were included in the high-grade WML model (Table 3). However, considerable heterogeneity in the predictors of IS was noted between the two populations, especially with regard to DBP, age, and diabetes mellitus. In addition, when DBP was included as a continuous variable (model not shown), the quadratic term was statistically significant (p = .013, 95% CI: 1.001–1.002).
Modeling quintiles of DBP in high-grade WML (Table 3) indicated a J-shaped trend in relation to IS, after controlling for all other covariates. The risk in the fifth (DBP > 80 mmHg) quintile was significantly higher (HR = 1.83, p = .031, 95% CI: 1.06–3.15) than that in the third (reference, nadir), followed by the first quintile (HR = 1.64, p = .088). The respective HRs by quintiles were 1.64, 1.16, 1.0, 1.15, and 1.83 (Figure 2). The HRs of IS in the first DBP quintile in high WML were qualitatively different from those in the corresponding low WML (1.64 vs 0.65, respectively, Figure 2), and their confidence intervals barely overlapped (0.93–2.9 vs 0.35–1.2, respectively). SBP was linearly associated with IS risk (HR = 1.014 per each 1 mmHg BP increase, p = .001, 95% CI: 1.006–1.02). Atrial fibrillation increased the hazard of stroke fivefold (p < .001, 95% CI: 2.8–8.9). MI was the next strongest IS predictor (HR = 2.23, p < .001, 95% CI: 1.4–3.4). Unlike in the low-grade WML population, increasing age was not a predictor of IS in this group.
DISCUSSION
This study focused on IS only as a subtype of stroke different from hemorrhagic stroke. This may help explain the emphasized role of DBP as a possible risk factor in IS compared with SBP in people with WML. However, achieved DBP and SBP were studied without taking into account the effect of HTN treatment, which may introduce a limitation.
The relationship between DBP and IS incidence in this population of older adults differed by the presence of WML. DBP had a monotonic effect on IS risk in low-grade WML but had a J-curve with borderline statistical significance in high-grade WML, after controlling for demographic and cardiovascular covariates. DBP less than 63 mmHg was not associated with risk in low-grade WML but indicated a possible hazard in high-grade WML (qualitative difference). DBP was a predictor of IS only in the presence of high-grade WML. SBP was informative in high-grade WML, where the proportion of people with isolated systolic hypertension was higher than in low-grade WML. The main predictors of IS among individuals with low-grade WML were atrial fibrillation, age, and diabetes.
It is plausible that high-grade WML would be associated with conditions conducive to increased IS incidence when DBP is low. WML may represent regions of the brain vulnerable to reduced perfusion pressure more likely to occur during diastole. In a healthy brain, the autoregulatory mechanism is able to adjust low or high mean (between 50 and 150 mmHg) arterial BP fast enough to suit the needs of the various cerebral regions. In the presence of cerebral ischemic disease, this protective mechanism may become inadequate below a certain pressure level, considered normal for a healthy brain (29–31). Perfusion-compromised brain regions exposed to further hemodynamic insufficiency may eventually experience a full-blown IS. Microemboli are often found in patients with severe arterial stenosis, and one accepted explanation is that hypoperfusion promotes formation of emboli (32,33). It has also been postulated that decreased perfusion reduces the clearance (washout) of emboli that have entered the vascular bed of hypoperfused regions (32).
A recent Framingham study (34) associated low DBP with excess cardiovascular risk among people with isolated systolic hypertension and explained the finding by wide pulse pressure. Increased stroke risk in low DBP has been explained by the presence of CVD (35). However, in the Rotterdam study, stroke risk in older adults with the lowest quartile of DBP (<65 mmHg) was still twice as high as in those with DBP of 65–74 mmHg, after controlling for CVD (3). Our findings suggest explaining the association between low DBP and IS, independently of overt CVD, by the presence of high-grade WML. The phenomenon of decreasing stroke risk with moderately increasing DBP (curve’s nadir) has been described before (3,36). Previous studies that investigated the issue of optimal DBP suggested a range between 69 and 90 mmHg depending on the population and the cardiovascular outcome (37–40). The Hypertension Optimal Treatment study (participants’ age ≥50 years) investigators found that the optimal DBP for prevention of major incident cardiovascular events was 82.5 mmHg and that a reduction of DBP up to 70 mmHg was still safe (37). The Systolic Hypertension in the Elderly Program study (participants’ age ≥60 years) found that the lowest safe DBP level for stroke prevention was 69 mmHg (38). Both studies were not able to demonstrate that reduction less than 69 mmHg was safe. The findings in this study suggest that DBP less than 69 mmHg may still be safe in older adults with low-grade WML and may not be beneficial (and possibly associated with increased risk) only among people with high-grade WML. The finding of optimal DBP level of 69–73 mmHg is in agreement with the recommendations of the seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of high BP (41).
At DBP ≥80 mmHg (right arm of the J-curve), the increase in IS risk is the steepest in older adults with high-grade WML. In atherosclerotic vessels, high BP may form turbulence—especially at bifurcation points—which precipitates the formation of fresh thrombi and advances dislodged emboli into areas with narrowed vessels and compromised perfusion, increasing the risk of infarcts (32).
When no conclusive outcome with regard to the existence of the J-curve was reached in the Hypertension Optimal Treatment study, Kaplan suggested in a commentary that “the J-curve has not been burnt off by the Hypertension Optimal Treatment study” (40,42). In response to the Hypertension Optimal Treatment and Systolic Hypertension in the Elderly Program findings, Hansson (43) concluded in a January 2000 review that, biologically, the J-curve has to exist, and the threshold is not above DBP of 70 mmHg but remains to be identified. In 1999, Voko and colleagues (3) described a J-shaped relationship between DBP and stroke incidence among a population of Rotterdam suburbs whose minimal age was 55 in 1990.
Conclusions
In older adults with low-grade WML, no threshold for IS risk was identified as DBP decreased. In comparison, in older adults with high-grade WML, there was no benefit in lowering DBP less than 69 mmHg and the optimal DBP level was 69–73 mmHg. There is moderate evidence to suggest increased risk of incident IS in DBP less than 69 mmHg only in high-grade WML. In DBP more than 80 mmHg, the risk of IS was highest in the presence of WML. People with high-grade WML may therefore differ from people with low-grade WML with regard to IS risk and may be at increased risk for this event in the presence of high as well as low DBP.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at http://biomed.gerontologyjournals.org.
FUNDING
Contracts N01-HC-35129, N01-HC-45133, N01-HC-75150, N01-HC-85079 through N01-HC-85086, N01 HC-15103, N01 HC-55222, and U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of participating Cardiovascular Health Study investigators and institutions can be found at http://www.chs-nhlbi.org.
References
- 1.Sudlow CLM, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. Stroke. 2003;28:491–499. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 2.Forette F, Lechowski L, Rigaud AS, Seux ML, Dessi F, Forette B. Does the benefit of antihypertensive treatment outweigh the risk in very elderly hypertensive patients? J Hypertens Suppl. 2000;18:S9–S12. [PubMed] [Google Scholar]
- 3.Voko Z, Bots ML, Hofman A, Koudstaal PJ, Witteman JC, Breteler MM. J-shaped relation between blood pressure and stroke in treated hypertensives. Hypertension. 1999;34:1181–1185. doi: 10.1161/01.hyp.34.6.1181. [DOI] [PubMed] [Google Scholar]
- 4.Mattila K, Haavisto M, Rajala S, Heikinheimo R. Blood pressure and five year survival in the very old. Br Med J (Clin Res Ed) 1988;296:887–889. doi: 10.1136/bmj.296.6626.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer J, Lechner H, Reivich M, Ott EO. Four Year Longitudinal Prospective Analysis of Age-Related Changes in Cerebral Blood Flow Measured in Normal Healthy and Risk Factored Volunteers. Amsterdam, The Netherlands: Excerpta Medica; 1983. pp. 15–21. [Google Scholar]
- 6.Pickering TG. The clinical significance of diurnal blood pressure variations. Dippers and nondippers. Circulation. 1990;81:700–702. doi: 10.1161/01.cir.81.2.700. [DOI] [PubMed] [Google Scholar]
- 7.Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. doi: 10.1161/hy1001.092640. [DOI] [PubMed] [Google Scholar]
- 8.Kario K, Shimada K, Pickering TG. Abnormal nocturnal blood pressure falls in elderly hypertension: clinical significance and determinants. J Cardiovasc Pharmacol. 2003;41(suppl 1):S61–S66. [PubMed] [Google Scholar]
- 9.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 10.Kawamura J, Terayama Y, Takashima S, et al. Leuko-araiosis and cerebral perfusion in normal aging. Exp Aging Res. 1993;19:225–240. doi: 10.1080/03610739308253935. [DOI] [PubMed] [Google Scholar]
- 11.Markus HS, Lythgoe DJ, Ostegaard L, O’Sullivan M, Williams SC. Reduced cerebral blood flow in white matter in ischaemic leukoaraiosis demonstrated using quantitative exogenous contrast based perfusion MRI. J Neurol Neurosurg Psychiatry. 2000;69:48–53. doi: 10.1136/jnnp.69.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longstreth WT, Jr., Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 13.Meyer JS, Rauch G, Rauch RA, Haque A. Risk factors for cerebral hypoperfusion, mild cognitive impairment, and dementia. Neurobiol Aging. 2000;21:161–169. doi: 10.1016/s0197-4580(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 14.Bhadelia RA, Anderson M, Polak JF, et al. Prevalence and associations of MRI-demonstrated brain infarcts in elderly subjects with a history of transient ischemic attack. The Cardiovascular Health Study. Stroke. 1999;30:383–388. doi: 10.1161/01.str.30.2.383. [DOI] [PubMed] [Google Scholar]
- 15.Kuller LH, Longstreth WT, Jr., Arnold AM, Bernick C, Bryan RN, Beauchamp NJ., Jr. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 16.Meyer JS, Kawamura J, Terayama Y. White matter lesions in the elderly. J Neurol Sci. 1992;110:1–7. doi: 10.1016/0022-510x(92)90002-3. [DOI] [PubMed] [Google Scholar]
- 17.Cruickshank J. The J-curve in hypertension. Curr Cardiol Rep. 2003;5:441–452. doi: 10.1007/s11886-003-0105-1. [DOI] [PubMed] [Google Scholar]
- 18.Cruickshank JM. Antihypertensive treatment and the J-curve. Cardiovasc Drugs Ther. 2000;14:373–379. doi: 10.1023/a:1007856014581. [DOI] [PubMed] [Google Scholar]
- 19.Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J-curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? JAMA. 1991;265:489–495. [PubMed] [Google Scholar]
- 20.Samuelsson OG, Wilhelmsen LW, Pennert KM, Wedel H, Berglund GL. The J-shaped relationship between coronary heart disease and achieved blood pressure level in treated hypertension: further analyses of 12 years of follow-up of treated hypertensives in the Primary Prevention Trial in Gothenburg, Sweden. J Hypertens. 1990;8:547–555. doi: 10.1097/00004872-199006000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet. 1987;1:581–584. doi: 10.1016/s0140-6736(87)90231-5. [DOI] [PubMed] [Google Scholar]
- 22.Somes GW, Pahor M, Shorr RI, Cushman WC, Applegate WB. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med. 1999;159:2004–2009. doi: 10.1001/archinte.159.17.2004. [DOI] [PubMed] [Google Scholar]
- 23.Voko Z, Koudstaal P, Bots M, Hofman A, Breteler M. Aspirin use and risk of stroke in the elderly: the rotterdam study. Neuroepidemiology. 2001;20:40–44. doi: 10.1159/000054756. [DOI] [PubMed] [Google Scholar]
- 24.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 25.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 26.Manolio TA, Kronmal RA, Burke GL, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 27.Price TR, Psaty B, O’Leary D, Burke G, Gardin J. Assessment of cerebrovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:504–507. doi: 10.1016/1047-2797(93)90105-d. [DOI] [PubMed] [Google Scholar]
- 28.STATA Corporation. STATA Base Reference Manual. College Station, TX: Stata Press; 2003. Release 8.0[2] [Google Scholar]
- 29.Strandgaard S, Paulson OB. Cerebrovascular damage in hypertension. J Cardiovasc Risk. 1995;2:34–39. [PubMed] [Google Scholar]
- 30.Strandgaard S, Paulson OB. Cerebral blood flow in untreated and treated hypertension. Neth J Med. 1995;47:180–184. doi: 10.1016/0300-2977(95)00065-u. [DOI] [PubMed] [Google Scholar]
- 31.Meyer JS, Terayama Y, Takashima S. Cerebral circulation in the elderly. Cerebrovasc Brain Metab Rev. 1993;5:122–146. [PubMed] [Google Scholar]
- 32.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 33.Chimowitz MI, Furlan AJ, Jones SC, et al. Transcranial Doppler assessment of cerebral perfusion reserve in patients with carotid occlusive disease and no evidence of cerebral infarction. Neurology. 1993;43:353–357. doi: 10.1212/wnl.43.2.353. [DOI] [PubMed] [Google Scholar]
- 34.Kannel WB, Wilson PW, Nam BH, D’Agostino RB, Li J. A likely explanation for the J-curve of blood pressure cardiovascular risk. Am J Cardiol. 2004;94:380–384. doi: 10.1016/j.amjcard.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 35.Kannel WB, D’Agostino RB, Silbershatz H. Blood pressure and cardiovascular morbidity and mortality rates in the elderly. Am Heart J. 1997;134:758–763. doi: 10.1016/s0002-8703(97)70061-9. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan NM. Kaplan’s Clinical Hypertension. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 37.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 38.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 39.Hansson L. How far should we lower blood pressure in the elderly. Cardiovasc Drugs Ther. 2001;15:275–279. doi: 10.1023/a:1011928609508. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan N. J-curve not burned off by HOT study. Hypertension Optimal Treatment. Lancet. 1998;351:1748–1749. doi: 10.1016/s0140-6736(98)22024-1. [DOI] [PubMed] [Google Scholar]
- 41.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention Detection, Evaluation, and Treatment of High Blood Pressure. The JNC 7 Report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 42.Kaplan NM. What is goal blood pressure for the treatment of hypertension? Arch Intern Med. 2001;161:1480–1482. doi: 10.1001/archinte.161.12.1480. [DOI] [PubMed] [Google Scholar]
- 43.Hansson L. Antihypertensive treatment: does the J-curve exist? Cardiovasc Drugs Ther. 2000;14:367–372. doi: 10.1023/a:1007804030510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.