FK 506 is currently used as the primary immunosuppressant in over 800 organ transplant patients at the University of Pittsburgh. FK 506 is a macrolide and is chemically very different from any other immunosuppressive drug used currently in transplant patients. However, FK 506 is very similar to cyclosporine (CyA) in a number of physicochemical properties and its mechanism of action (Table 1).1,2 The objective of the present study is to determine the pharmacokinetics of FK 506 after oral administration, to compare its kinetic properties with CyA, and to discuss the clinical relevance of the differences in the kinetic properties.
Table 1. Physicochemical Properties of FK 506 and CyA.
| Property | Cyclosporine | FK 506 |
|---|---|---|
| Source | Tolypocladium inflatum (fungal byproduct) | Streptomyces tsukubaensis (fungal byproduct) |
| Molecular weight | 1202 | 822 |
| Poorly soluble | Water, hexane | Water, hexane |
| Very soluble | Ethanol, methanol, propylene glycol, polyethylene glycol | Ethanol, methanol, propylene glycol, polyethylene glycol |
Dosage Form
FK 506 was administered to patients as an IV infusion over 2 to 4 hours or as a continuous infusion over 24 hours. For oral administration, a hard gelatin capsule containing a 20% solid dispersion of FK 506 in hydroxy propyl methyl cellulose and HCHO 60 was used.
Drug Analysis
For the kinetic study, multiple blood samples were obtained over a dosing interval of 12 or 24 hours. Blood samples were incubated at 37°C for 1 hour and plasma was separated at 37°C. FK 506 concentration in plasma was analyzed by a monoclonal antibody (MAb) based enzyme-linked immunosorbent assay (ELISA) as described earlier.3
Pharmacokinetics
The pharmacokinetics of FK 506 after IV administration has previously been reported.4 Similar pharmacokinetic parameters were also obtained in the present study. Following IV infusion, FK 506 concentration declines rapidly initially and at a slower rate after reaching distribution equilibrium (Fig 1). The half-life ranges from 3.5 to 40.5 hours with a mean value of 11.3 hours (n = 31). The plasma clearance of FK 506 ranges from 5.8-103 mL/min/kg, indicating the drug to be a high clearance drug. Higher half-lives and lower clearance values were observed in patients with impaired hepatic function. The large volume of distribution (10,751) indicates extensive distribution of the drug outside the plasma compartment.
Fig 1.
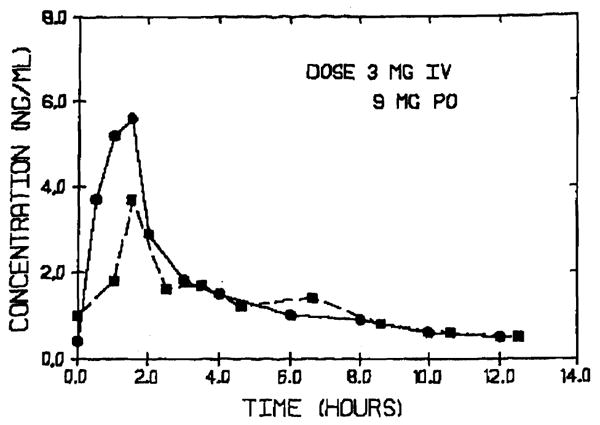
FK 506 plasma concentration vs time curve after 3 mg IV (●) and 9 mg oral (■) administration in a liver transplant patient.
Pharmacokinetics of FK 506 was studied in 14 patients on 31 occasions after oral doses of 2 to 18 mg. FK 506 was absorbed fairly rapidly in most of the subjects with peak plasma concentrations being reached within 1 hour. However, in certain patients peak concentrations were reached only at 4 hours. In nearly half of the patients studied there was sustained plasma concentration throughout the entire dosing interval. This may be the result of the slow dissolution of FK 506 from the solid dispersion in the gastric fluids. The dose normalized peak plasma concentration ranged from 0.1 to 0.5 ng/mL/mg. Sufficient data were available to calculate oral bioavailability in 14 studies. The absolute bioavailability ranged from 6% to 56% (mean, 25%) in transplant patients with various degrees of liver function.
Comparison of FK 506 and CyA
Absorption
The pharmacokinetic parameters for FK 506 and CyA are listed in Table 2. FK 506 appears to be absorbed more rapidly than CyA; however, both compounds are absorbed to a similar extent. This indicates that three to four times higher oral doses are required, as compared with IV doses, to maintain similar plasma concentration time profiles for both drugs. In a number of patients FK 506 is absorbed over many hours with less fluctuations in plasma concentration vs time curve over a dosing interval.
Table 2. Pharmacokinetic Parameters of CyA and FK 506.
| Parameter | CyA | FK 506 |
|---|---|---|
| Absorption | ||
| Time to peak | 2-4 h | 0.5-4 h |
| Extent of absorption | 30% | 25% |
| Distribution | ||
| Blood/plasma | 1-2 | >4 |
| Lipoprotein | High | Low |
| Lipoprotein free plasma | Low | High |
| Volume of distribution (plasma data) | 2,501 | 10,751 |
| Elimination | ||
| Metabolism | >98% | >98% |
| Urinary excretion of unchanged drug | <2% | <2% |
| Metabolite excretion | Biliary | Biliary |
| Half-life | 5-12 h | 4-14 h |
| Clearance | 10 mL/min/kg | 43 mL/min/kg |
Distribution
Both FK 506 and CyA are highly distributed into red blood cells. While the blood to plasma concentrations of CyA is about 1.5, that of FK 506 is greater than 4. Within plasma, CyA is primarily associated with lipoproteins while FK 506 is associated with lipoprotein deficient plasma.5
Elimination
CyA and FK 506 are primarily eliminated by hepatic metabolism.4,6 Most of the metabolites are excreted in the bile. These observations indicate that hepatic dysfunction will impair the elimination of these drugs.7 The elimination of the drug is also susceptible to induction and inhibition of hepatic drug metabolizing enzymes. Such drugs should, therefore, be used with caution in patients receiving FK 506 or CyA. Less than 2% of the drug is excreted in the urine as unchanged drug. This indicates that changes in renal function are not likely to affect the elimination of FK 5068 or CyA.9
Effect of Certain Clinical Events on Kinetics
T-Tube Clamping
T-tube clamping is well known to increase CyA concentrations in liver transplant patients.10 Since very little CyA is excreted unchanged in the bile, enterohepatic circulation is not responsible for this observation. Increased availability of bile facilitates CyA absorption resulting from solubilization of CyA. On the other hand, clamping of the T-tube does not alter the area under the plasma concentration vs time curve for FK 5067 (Fig 2). This indicates that bile is not as essential for FK 506 absorption as it is for CyA absorption. Therefore, no dosing changes are indicated when the T-tube is clamped in liver transplant patients (Table 3).
Fig 2.
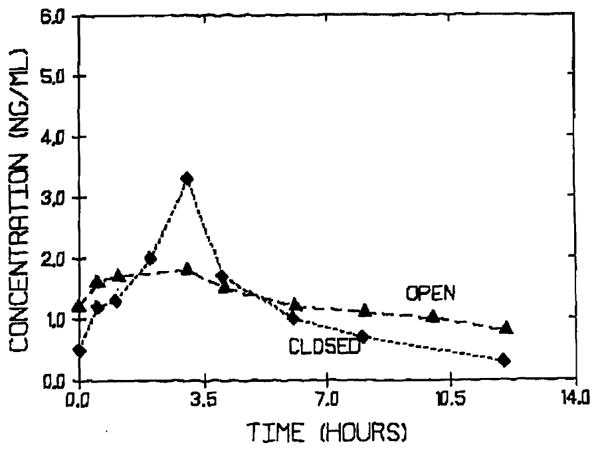
FK 506 plasma concentration vs time curve in a liver transplant patient with open (▲) and closed (◆) T-tube.
Table 3. Dosing Recommendations for Clinical Events.
| CyA | FK 506 | |
|---|---|---|
| T-Tube Clamping | Decrease | No change |
| Liver dysfunction | ||
| IV | Decrease | Decrease |
| PO | IV? | Increase |
| Renal dysfunction | No change | No change |
| Dialysis | No change | No change |
Impaired Liver Function
The oral absorption of CyA is decreased in patients with liver disease.9 In dogs with experimentally induced cholestasis CyA absorption is significantly reduced11 (Fig 3). Liver dysfunction does not decrease the oral absorption of FK 506 in liver transplant patients.7 In dogs with experimentally induced cholestasis the oral bioavailability of FK 506 is in fact increased as compared with control dogs12 (Fig 3). This indicates that more FK 506 reaches systemic circulation in the absence of bile. Presently additional studies are being conducted in dogs with choledochoureterostomy to determine the effect of bile on FK 506 absorption. Independent of the mechanism involved it is clear that while CyA doses have to be increased or if the patient has to be converted to IV CyA therapy in presence of hepatic dysfunction, a dose reduction is necessary for FK 506 under similar circumstances8 (Table 3).
Fig 3.
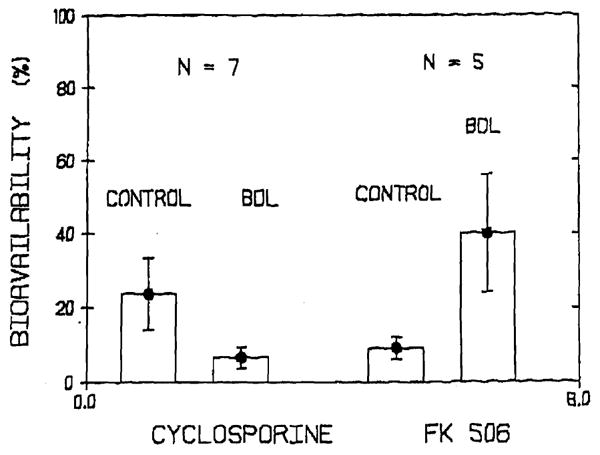
Bioavailability of CyA and FK 506 in dogs before and after experimental liver dysfunction induced by cholestasis.
Renal Dysfunction and Dialysis
Since very little CyA or FK 506 is excreted in the urine, no changes in the dosing regimen of FK 506 or CyA is necessary in patients with renal impairment or patients undergoing dialysis (Table 3).
CyA and FK 506 are similar in a number of respects such as their physicochemical and certain pharmacokinetic properties and mechanism of action. However, they are distinctly different with regards to the effect of T-tube clamping and the effect of bile and hepatic dysfunction on their absorption. These factors must be kept in mind in any dosing regimen changes of these drugs in transplant patients.
Acknowledgments
Supported in part by grant no. 5R01 DK 34475 from the National Institutes of Health, Bethesda, MD.
References
- 1.Honbo T, Kobayashi M, Hane K, et al. Transplant Proc. 1987;19(Suppl 6):17. [PubMed] [Google Scholar]
- 2.Zeevi A, Duquesnoy R, Eiras G, et al. Transplant Proc. 1987;19(Suppl 6):40. [PMC free article] [PubMed] [Google Scholar]
- 3.Tamura K, Kobayashi M, Hashimoto K, et al. Transplant Proc. 1987;19(Suppl 6):23. [PubMed] [Google Scholar]
- 4.Venkataramanan R, Jain A, Cadoff E, et al. Transplant Proc. 1990;22(Suppl 1):52. [PMC free article] [PubMed] [Google Scholar]
- 5.Warty V, Venkataramanan R, Zendehrouh P, et al. Transplant Proc. in press. [PMC free article] [PubMed] [Google Scholar]
- 6.Maurer M, Loosli H, Schreier E, et al. Drug Metab Dispos. 1984;12:120. [PubMed] [Google Scholar]
- 7.Jain A, Venkataramanan R, Cadoff E, et al. Transplant Proc. 1990;22(Suppl 1):57. [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Elmagd K, Fung J, Alessiani M, et al. Transplantation. submitted. [Google Scholar]
- 9.Ptachcinski R, Venkataramanan R, Burckart G, et al. Clin Pharmacokinet. 1986;11:107. doi: 10.2165/00003088-198611020-00002. [DOI] [PubMed] [Google Scholar]
- 10.Mehta M, Venkataramanan R, Burckart G, et al. Br J Clin Pharmacol. 1988;25:579. doi: 10.1111/j.1365-2125.1988.tb03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takaya S, Iwatsuki S, Starzl T, et al. Transplant Proc. 1988;20(Suppl 1):154. [PMC free article] [PubMed] [Google Scholar]
- 12.Imventarza O, Furukawa H, Venkataramanan R, et al. Transplantation. doi: 10.1097/00007890-199204000-00002. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]


