Abstract
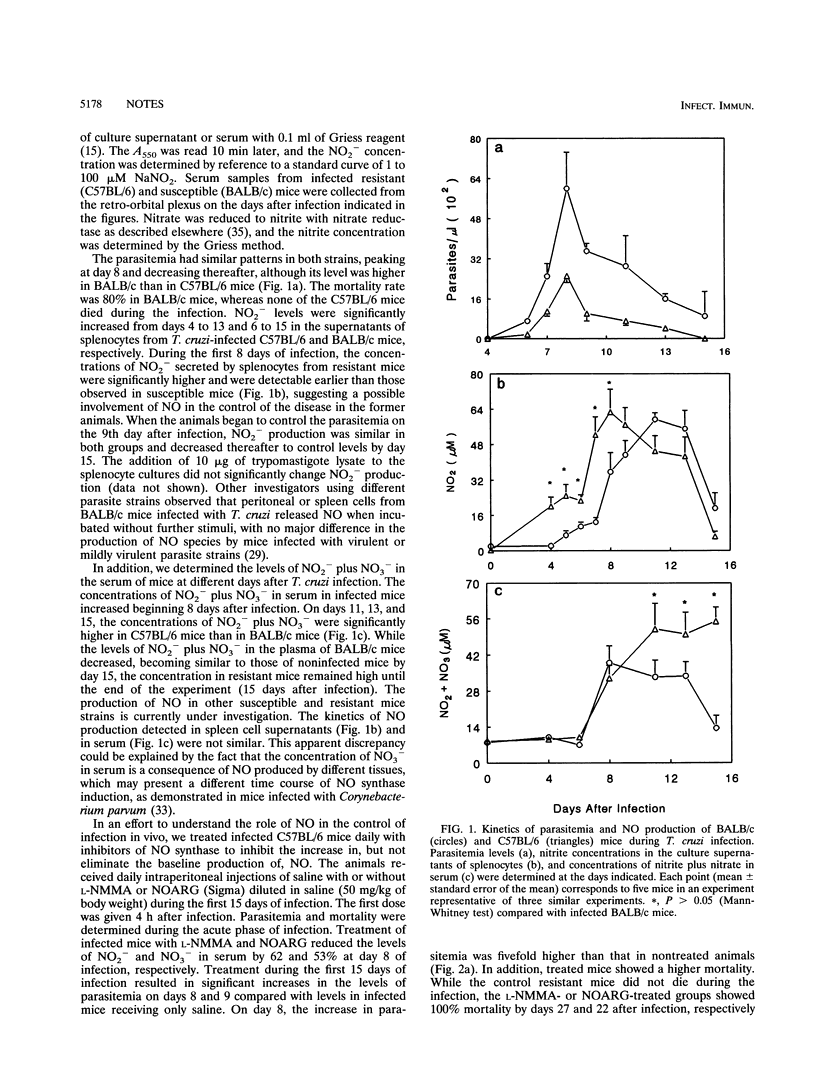
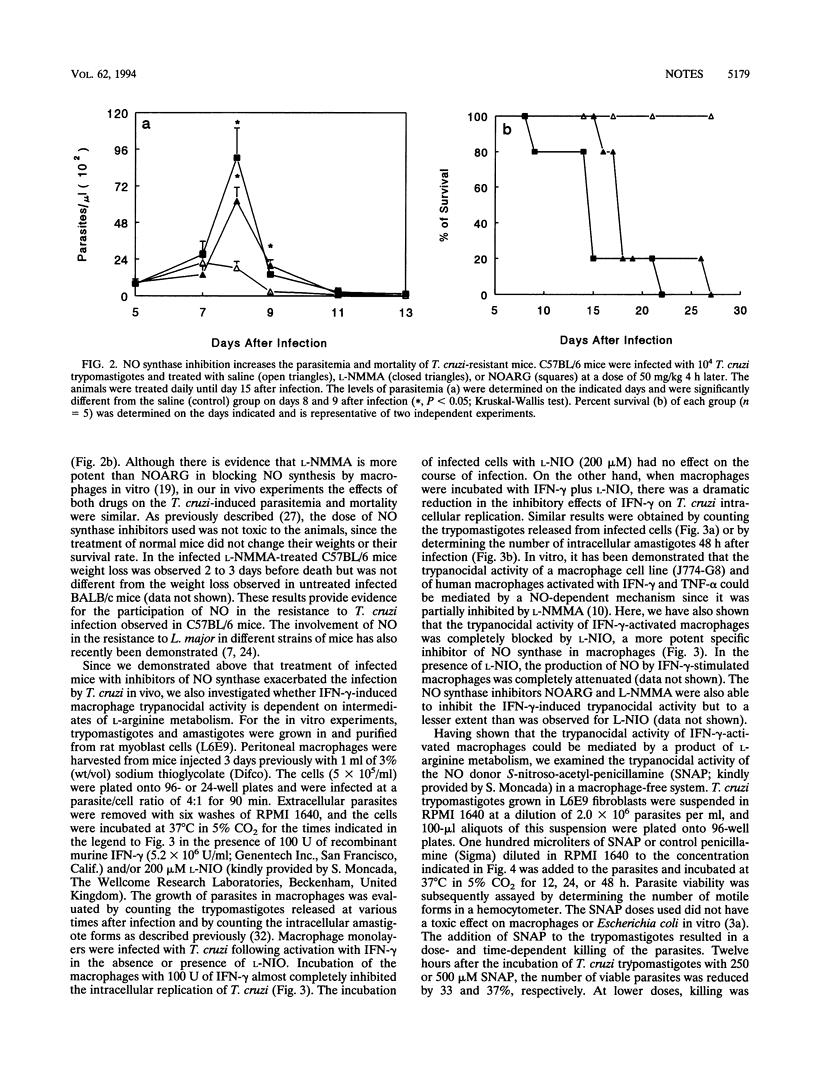
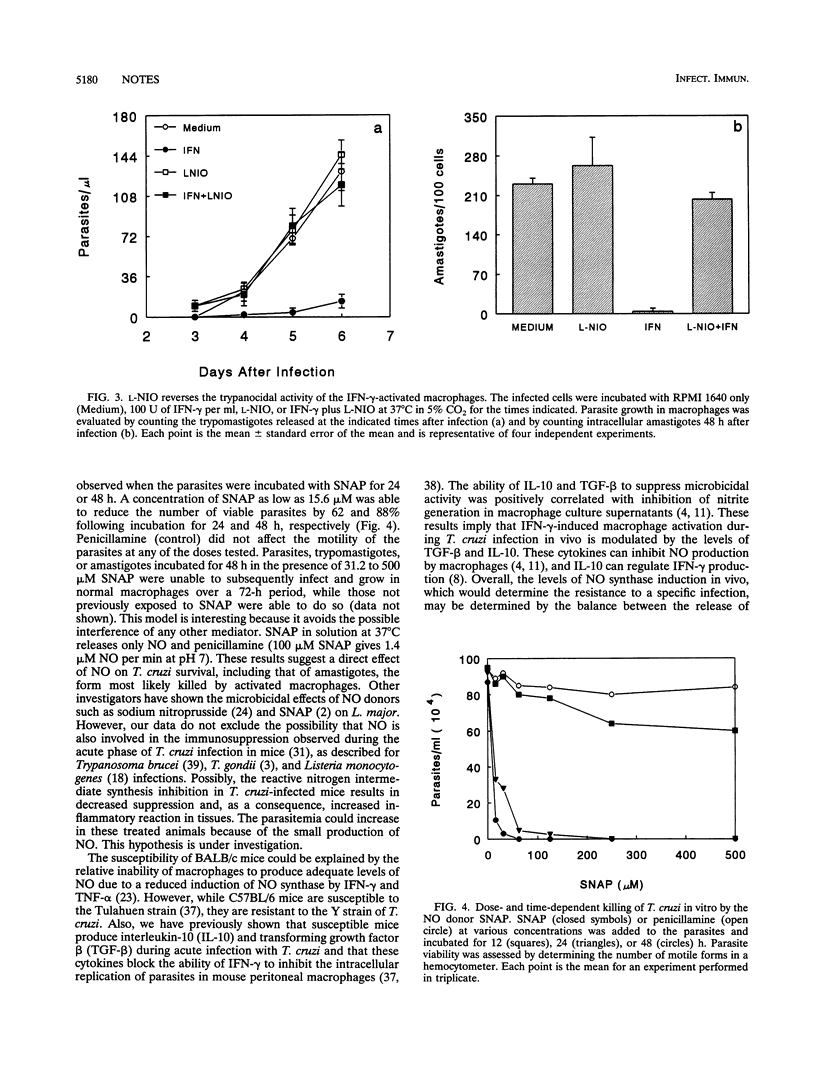
This study was carried out to determine the role of reactive nitrogen intermediates in Trypanosoma cruzi infection. In vitro, splenocytes obtained during the acute phase of infection produced elevated amounts of nitric oxide (NO) that were correlated with the resistance or susceptibility of the animals. In vivo, the levels of NO2- plus NO3- in plasma during the later phase of infection were higher in C57BL/6 mice than in BALBL/c mice. The treatment of infected C57BL/6 mice with inhibitors of NO synthase increased parasitemia and mortality. Finally, we found that the NO donor drug S-nitroso-acetyl-penicillamine is able to kill trypomastigotes in vitro in the absence of any other cells, suggesting a direct NO-mediated killing of T. cruzi.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Assreuy J., Cunha F. Q., Epperlein M., Noronha-Dutra A., O'Donnell C. A., Liew F. Y., Moncada S. Production of nitric oxide and superoxide by activated macrophages and killing of Leishmania major. Eur J Immunol. 1994 Mar;24(3):672–676. doi: 10.1002/eji.1830240328. [DOI] [PubMed] [Google Scholar]
- Candolfi E., Hunter C. A., Remington J. S. Mitogen- and antigen-specific proliferation of T cells in murine toxoplasmosis is inhibited by reactive nitrogen intermediates. Infect Immun. 1994 May;62(5):1995–2001. doi: 10.1128/iai.62.5.1995-2001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Ding A., Nathan C. F., Graycar J., Derynck R., Stuehr D. J., Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol. 1990 Aug 1;145(3):940–944. [PubMed] [Google Scholar]
- Drapier J. C., Wietzerbin J., Hibbs J. B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988 Oct;18(10):1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- Evans T. G., Thai L., Granger D. L., Hibbs J. B., Jr Effect of in vivo inhibition of nitric oxide production in murine leishmaniasis. J Immunol. 1993 Jul 15;151(2):907–915. [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991 Sep;59(9):3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOBLE F. C., BOYD J. L. Reticulo-endothelial blockade in experimental Chagas' disease. J Parasitol. 1962 Apr;48:223–228. [PubMed] [Google Scholar]
- Gazzinelli R. T., Oswald I. P., Hieny S., James S. L., Sher A. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an L-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur J Immunol. 1992 Oct;22(10):2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R. T., Oswald I. P., James S. L., Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992 Mar 15;148(6):1792–1796. [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990 Jan;85(1):264–273. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Ruiz de Luzuriaga K., Wagner D. A., Rand W., Istfan N., Young V. R., Tannenbaum S. R. Nitrate biosynthesis in man. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7764–7768. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. J., Crawford R. M., Hockmeyer J. T., Meltzer M. S., Nacy C. A. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990 Dec 15;145(12):4290–4297. [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Gregory S. H., Wing E. J., Hoffman R. A., Simmons R. L. Reactive nitrogen intermediates suppress the primary immunologic response to Listeria. J Immunol. 1993 Apr 1;150(7):2901–2909. [PubMed] [Google Scholar]
- Gross S. S., Stuehr D. J., Aisaka K., Jaffe E. A., Levi R., Griffith O. W. Macrophage and endothelial cell nitric oxide synthesis: cell-type selective inhibition by NG-aminoarginine, NG-nitroarginine and NG-methylarginine. Biochem Biophys Res Commun. 1990 Jul 16;170(1):96–103. doi: 10.1016/0006-291x(90)91245-n. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. L., Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989 Dec 15;143(12):4208–4212. [PubMed] [Google Scholar]
- Langermans J. A., Van der Hulst M. E., Nibbering P. H., Hiemstra P. S., Fransen L., Van Furth R. IFN-gamma-induced L-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. J Immunol. 1992 Jan 15;148(2):568–574. [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Moss D., Parkinson C., Rogers M. V., Moncada S. Resistance to Leishmania major infection correlates with the induction of nitric oxide synthase in murine macrophages. Eur J Immunol. 1991 Dec;21(12):3009–3014. doi: 10.1002/eji.1830211216. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- McCabe R. E., Mullins B. T. Failure of Trypanosoma cruzi to trigger the respiratory burst of activated macrophages. Mechanism for immune evasion and importance of oxygen-independent killing. J Immunol. 1990 Mar 15;144(6):2384–2388. [PubMed] [Google Scholar]
- McCall T. B., Feelisch M., Palmer R. M., Moncada S. Identification of N-iminoethyl-L-ornithine as an irreversible inhibitor of nitric oxide synthase in phagocytic cells. Br J Pharmacol. 1991 Jan;102(1):234–238. doi: 10.1111/j.1476-5381.1991.tb12159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. A. Trypanosoma cruzi: in vitro induction of macrophage microbicidal activity. J Exp Med. 1978 Jul 1;148(1):288–300. doi: 10.1084/jem.148.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petray P., Rottenberg M. E., Grinstein S., Orn A. Release of nitric oxide during the experimental infection with Trypanosoma cruzi. Parasite Immunol. 1994 Apr;16(4):193–199. doi: 10.1111/j.1365-3024.1994.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Plata F., Wietzerbin J., Pons F. G., Falcoff E., Eisen H. Synergistic protection by specific antibodies and interferon against infection by Trypanosoma cruzi in vitro. Eur J Immunol. 1984 Oct;14(10):930–935. doi: 10.1002/eji.1830141013. [DOI] [PubMed] [Google Scholar]
- Reed S. G. In vivo administration of recombinant IFN-gamma induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. J Immunol. 1988 Jun 15;140(12):4342–4347. [PubMed] [Google Scholar]
- Reed S. G., Nathan C. F., Pihl D. L., Rodricks P., Shanebeck K., Conlon P. J., Grabstein K. H. Recombinant granulocyte/macrophage colony-stimulating factor activates macrophages to inhibit Trypanosoma cruzi and release hydrogen peroxide. Comparison with interferon gamma. J Exp Med. 1987 Dec 1;166(6):1734–1746. doi: 10.1084/jem.166.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M., Starobinas N., Ribeiro-Dos-Santos R., Minoprio P., Eisen H., Hontebeyrie-Joskowicz M. Susceptible mice present higher macrophage activation than resistant mice during infections with myotropic strains of Trypanosoma cruzi. Parasite Immunol. 1989 Jul;11(4):385–395. doi: 10.1111/j.1365-3024.1989.tb00675.x. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Wilke P., Evers B., Böhme E. Enzymatic formation of nitrogen oxides from L-arginine in bovine brain cytosol. Biochem Biophys Res Commun. 1989 Nov 30;165(1):284–291. doi: 10.1016/0006-291x(89)91067-x. [DOI] [PubMed] [Google Scholar]
- Schmuñis G. A. Chagas' disease and blood transfusion. Prog Clin Biol Res. 1985;182:127–145. [PubMed] [Google Scholar]
- Silva J. S., Morrissey P. J., Grabstein K. H., Mohler K. M., Anderson D., Reed S. G. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992 Jan 1;175(1):169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. S., Twardzik D. R., Reed S. G. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor beta (TGF-beta). J Exp Med. 1991 Sep 1;174(3):539–545. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg J., McGuigan F. Nitric oxide mediates suppression of T cell responses in murine Trypanosoma brucei infection. Eur J Immunol. 1992 Oct;22(10):2741–2744. doi: 10.1002/eji.1830221041. [DOI] [PubMed] [Google Scholar]
- TALIAFERRO W. H., PIZZI T. Connective tissue reactions in normal and immunized mice to a reticulotropic strain of Trypanosoma cruzi. J Infect Dis. 1955 May-Jun;96(3):199–226. doi: 10.1093/infdis/96.3.199. [DOI] [PubMed] [Google Scholar]
- Vincendeau P., Daulouède S. Macrophage cytostatic effect on Trypanosoma musculi involves an L-arginine-dependent mechanism. J Immunol. 1991 Jun 15;146(12):4338–4343. [PubMed] [Google Scholar]



