FK506, a potent immunosuppressive macrolide antibiotic, underwent initial clinical trails in liver recipients with intractable rejection or refractory drug toxicity (1, 2). The dramatic results achieved by this agent in “rescue” therapy were followed shortly by its introduction as a primary immunosuppressant in kidney and liver recipients beginning in March 1989 at the Presbyterian University and Children's Hospital of Pittsburgh (3, 4). In October 1989, a prospective clinical trial was begun using FK506 and low-dose steroids as the sole immunosuppression in patients undergoing orthotopic cardiac transplantation. The preliminary results using FK506 and low-dose steroids as primary immunosuppression in 23 patients following cardiac transplantation and in 4 patients as “rescue” therapy for refractory rejection or drug toxicity are reported here.
The 23 patients (19 males, 4 females) were prospectively entered into this study between 8 October 1989 and 1 June 1990. Informed consent was obtained from each patient prior to transplantation for the use of FK506. The age ranged from 22 days to 54 years, in 16 adult and 7 pediatric patients. The etiology of cardiomyopathy included ischemic (9), idiopathic (7), complex congenital (5) and valvular (2). Pretransplant mechanical circulatory support (MCS)* in the form of the Novacor left ventricular assist device (LVAD) (3), intraaortic balloon pump (IABP) (4) and extracorporeal membrane oxygenation (ECMO) (1), was required in 8 of the 23 patients (35%). Three children each had 5 previous thoracic operations, one child was on ECMO for 8 days prior to transplantation following extensive ventricular infarction subsequent to an arterial switch procedure, and one child was a neonate transplanted at 22 days of age for hypoplastic left heart syndrome (HLHS) (Table 1).
Table 1. FK506 immunosuppression in orthotopic cardiac transplantation, 10/89 to 6/90.
| Patients (n = 23) | Days | |
|---|---|---|
| Male/female | 19/4 | |
| Pediatric (<18) | 7 | |
| Pretransplant MCS | 8 (35%) | |
| Follow-up (days) | ||
| Range | 16–236 | |
| Median | 84 |
FK506 was administered intravenously for 24 to 72 hr following transplantation at a dose of 0.15 mg/kg/day in two divided doses. (Fig. 1) Oral administration was begun at 0.2 mg/kg/day in two divided doses within 36 to 72 hr posttransplant upon return of gastrointestinal function. Maintenance dose to attain an FK506 level of 1.5 to 3 ng/ml ranged from 0.2 to 0.4 mg/kg/day. Methylprednisolone 7 mg/kg was administered intravenously in the operating room and 5 mg/kg postoperatively for 24 hr in three divided doses. Thereafter patients received 0.15 mg/kg/day prednisone orally once a day. Prednisone was then selectively tapered or discontinued based upon rejection history.
Figure 1.
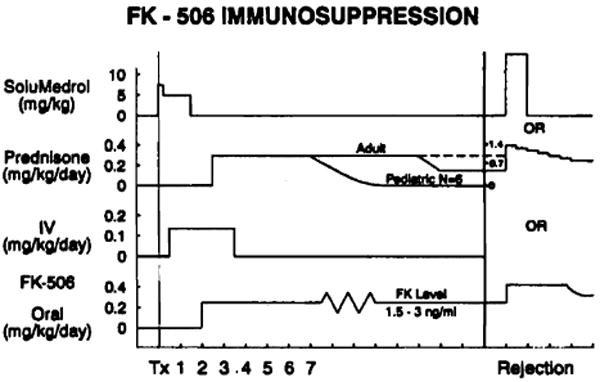
FK506 immunosuppression.
Rejection episodes of mild or moderate severity were preferentially treated by optimizing FK506 therapy, which consisted of increasing the oral dose of FK506 from 4 to 8 mg/day in the adult patients and from 1 to 4 mg/day in the pediatric patients. In some patients azotemia and oliguria limited the latitude with which to further increase FK506, particularly in the perioperative period; in these patients rejection episodes were treated either by intravenous methylprednisolone for 24 or 48 hr or with an oral prednisone taper, 100 mg to 20 mg over 5 days.
Twenty-two of the 23 patients (96%) entered into this trial are alive. Median follow-up was 84 days (16 to 236 days, as of 1 June 1990). One patient died in the immediate postoperative period from severe and unresponsive pulmonary hypertension and right heart failure. No mortality due to hyperacute, acute, or refractory cardiac rejection was seen during the use of FK506. The average left ventricular ejection fraction in the group measured by gated nuclear scan and/or echocardiography was 70% (range 58-75%) at the time of longest follow-up.
Four serious infections in 3 patients were present prior to cardiac transplantation. One patient had two pretransplant infections; during IABP support this patient was treated for an episode of staphylococcal line sepsis. This same patient had pulmonary emboli and infarctions and eventually required Novacor LVAD support. At the time of transplantation, 3 weeks following LVAD the wound culture was positive for candida. Amphotericin therapy was administered for 5 weeks after transplantation, and the sternal wound healed without incident. One neonatal patient had several episodes of line sepsis. This patient received no steroids during the postoperative period, FK506 being his only immunosuppressant. No episodes of cardiac rejection had been detected on routine weekly endomyocardial biopsies. The neonate with HLHS had several episodes of line sepsis. The other patient with a pretransplant infection was treated for a Klebsiella pneumonia during IABP support for 8 days when a donor heart became available. After transplantation, this patient required rehospitalization for hyperglycemia, dehydration, and diarrhea, the work-up of which revealed CMV gastroenteritis. Ganciclovir therapy resulted in complete resolution of symptoms and no recurrence has been noted. Two patients, both children, have had culture-negative episodes of sinusitis. One other child has developed a pneumonia and is recovering; the etiologic agent has not yet been identified. No other major or minor infections have occurred to date.
As would be expected with random matching, poor HLA compatibility was present in all but 2 recipients. One recipient received her donor heart from her sister, who had suffered brain death from a cerebrovascular accident. This rare scenario resulted in a six-antigen match. All patients had a panel reactive antibody of 0% except for 3 patients: 2 had a PRA of 3% and one patient had a PRA of 20%.
During this early follow-up period there have been ten episodes of moderate or severe rejection (Billingham grade 3 or greater) in 8 patients. (Fig. 2) Three episodes were treated with one or two doses of methylprednisolone (500 mg i.v.). Three episodes were treated with a 10-20% increase in the oral FK506 dosage. Three patients were treated with a prednisone taper. All nine of these episodes resolved with these respective treatments. One patient was treated with OKT3 and is the only patient in whom azathioprine was added.
Figure 2.
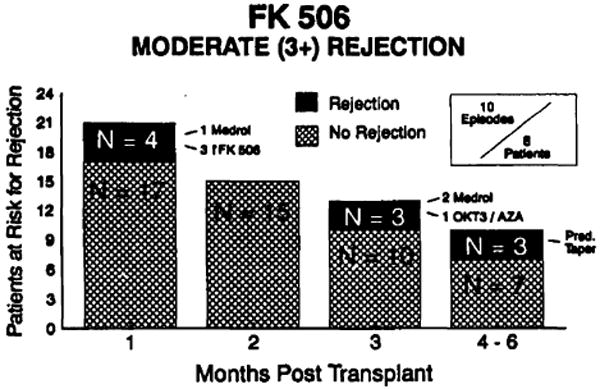
FK506: moderate (3+) rejection.
The incidence of rejection treated with augmented immunosuppression within the first 90 days posttransplant in patients on FK506 immunotherapy was 0.22 episodes per patient. (Fig. 3) This compared favorably to our most recent prospective randomized trial using conventional cyclosporine-based triple-drug immunotherapy comparing rabbit antithymocyte globulin (RATG) vs. OKT3 prophylaxis. (5) The incidence of treated rejection in the first 90 days following transplant in the RATG group (n = 39) was 0.42 episodes per patient and in the OKT3 (n = 43) group was 1.2 episodes per patient, while 9 patients receiving no immunoprophylaxis had 1.55 episodes of treated rejection.
Figure 3.
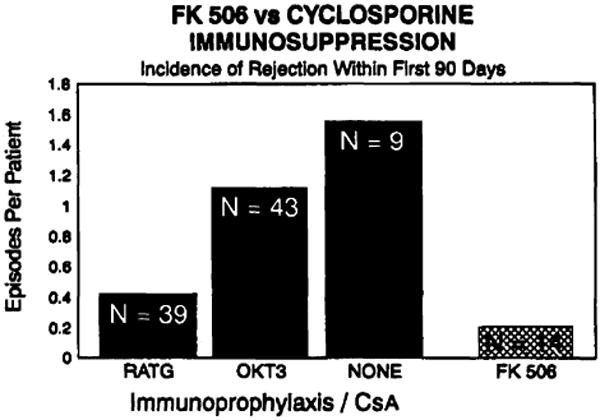
FK506 vs. cyclosporine immunosuppression.
The high FK506 levels attained in the perioperative period by administering intravenous drug overlapped with the institution of oral therapy uniformly exacts a price to be paid in terms of renal function in cardiac transplant recipients.
Although 3 patients had no significant alteration in urine output or renal function, the remainder suffered significant oliguria and azotemia, which peaked at an average on the 6th postoperative day (mean event ± 1). Renal function returned toward, but not back to, preoperative levels in all patients, around the 14th postoperative day (mean event ± 1).
Two of the 23 patients in this trial required dialysis. The first was the patient transplanted at 22 days of age for HLHS. This small patient suffered multiple bouts of line sepsis, nearly constant antibiotic therapy including vancomycin, and vena caval and renal vein thrombosis. Peritoneal dialysis was instituted during the 3rd postoperative week and the child and his renal function are recovering. This patient is excluded from the analysis of renal function in the group as a whole because of his multiple complicating variables. The other patient who has required dialysis suffered a cardiac arrest on induction of anesthesia, which required emergency placement of an IABP. FK506 levels attained in the early postoperative period were also very high. This patient's renal function also is returning toward normal. In Figures 4 and 5 the serum BUN and creatinine levels in patients on FK506 immunotherapy were compared with those of the 40 patients on cyclosporine immunotherapy transplanted at our institution prior to October 1989.
Figure 4.
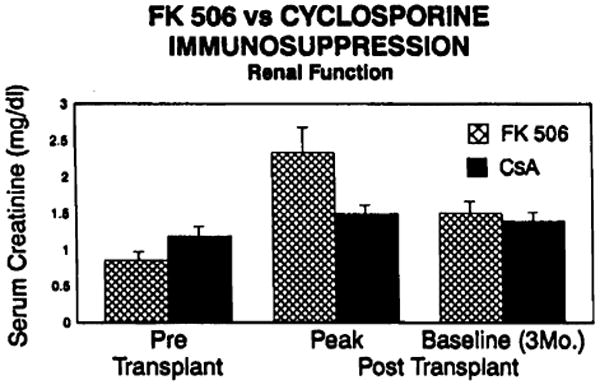
FK506 vs. cyclosporine immunosuppression–renal function; serum creatinine levels.
Figure 5.
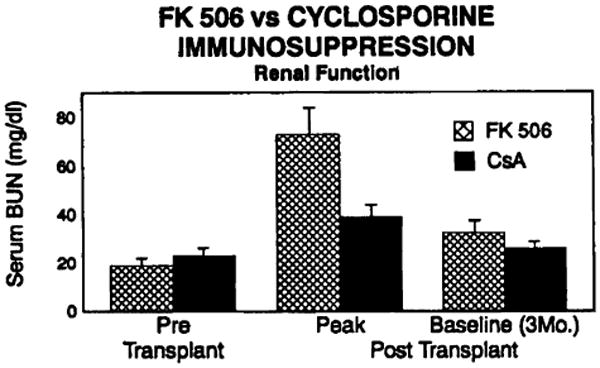
FK506 vs. cyclosporine immunosuppression–renal function; serum BUN levels.
Hypertension is notably rate in patients following cardiac transplantation treated with FK506 and low-dose steroids. Only 3 of 15 patients (20%) on FK506, followed for greater than 3 months, have required antihypertensive treatment, and all are well controlled on a single agent. In contrast, of the 40 previous cardiac transplant recipients treated with cyclosporine-based triple-drug immunotherapy, 28 (70%) required at least single-drug antihypertensive therapy (Fig. 6). This difference is statistically significant (P<0.001).
Figure 6.
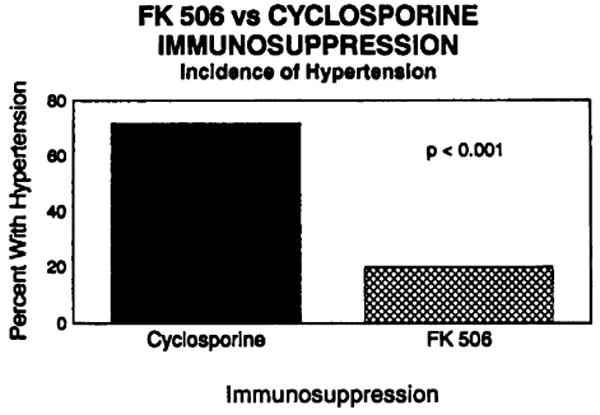
FK506 vs. cyclosporine immunosuppression–incidence of hypertension.
Two of the 23 patients developed insulin-dependent diabetes following cardiac transplantation. One patient, mentioned above, returned with a CMV enteritis and required steroid treatment for rejection; hyperglycemia was first evident on this readmission and has required insulin for control. Of note is this patient's positive DR3 locus. One other patient, also still on steroids, has required insulin following transplant.
Continuous monitoring of hematologic and coagulation indices, as well as liver function, cholesterol, triglycerides, LDL, HDL, and VLDL have failed to reveal any abnormalities in our patients at this interval. Only 2 of 22 patients have abnormal uric acid levels, one of whom had gout pretransplantation.
To date, 4 patients who have had refractory cardiac rejection on augmented conventional immunosuppression have undergone “rescue” therapy with FK506. Two of these patients are from our center. One patient with an elevated PRA was retransplanted at 6 years due to chronic rejection. Subsequent to retransplantation, on maintenance cyclosporine she suffered continued rejection despite OKT3 prophylaxis and treatment with steroids and RATG. She was switched to FK506 after 4 months and her prednisone has been tapered to 5 mg. She has had two episodes of 3+ rejection since her switch; however, these have cleared with increased oral dose FK506. Her cushingoid habitus has melted away with her loss of 27 pounds, and she is very strikingly pleased with her “new” self. The second of our patients to undergo a switch to FK506 is a pediatric patient with hypertension, hypercholesterolemia, severe steroid morbidity, and persistent grade 2 rejection. Steroids have been tapered to 5 mg and at this early interval cardiac rejection is resolving. Resolution of his other drug-related toxicities are anticipated.
Two other patients have undergone cardiac rescue with FK506, after repeated attempts to reverse cardiac rejection with conventional rescue therapy. Persistent rejection has been reversed in both patients with subsequent normalization of their endomyocardial biopsies.
Two patients with cystic fibrosis have undergone double-lung transplantation and one patient with Eisenmenger's physiology has had a heart-lung transplant with FK506 as the sole immunosuppressant. No steroids have been used with the exception of a prednisone taper given to one patient several months after transplant. All 3 patients are surviving with a median follow-up of 213 days.
Serious or even mild side effects from FK506 have been exceedingly rare with the exception of renal dysfunction detailed above. During the intravenous administration, flushing, nausea, and anorexia have been encountered occasionally. The oral form of FK506 is very well tolerated, and the only reported side effects, even after extensive and routine patient questioning, have been tingling and temperature malsensation in the hands and feet. These latter symptoms are most often associated with toxicity and high blood levels. Only one patient has been removed from FK506 therapy due to an allergic-type pneumonitis; however, it remains unclear if this is related to FK therapy, and the child is to be placed back on FK506 in the near future and pulmonary functions will be followed with great interest.
Notably absent in these patients have been complaints of gingival hyperplasia or hirsutism.
Our preliminary results with FK506 and low-dose steroids for cardiac transplantation are extremely promising. During this very early follow-up period FK506 and low-dose steroids attain immunosuppression at least comparable, if not superior, to the very best immunosuppressive therapy of the past decade employed at the University of Pittsburgh, using RATG immunoprophylaxis and cyclosporine-based triple-drug immunotherapy. Not immediately apparent in these results is a significant flexibility in the clinical use of FK506. In the primary group and in the “rescue” group mild and moderate episodes of rejection have been reversed with small increases in oral FK506 dosage.
The most obvious toxicity of FK506 was renal impairment. Certainly some of the azotemia and oliguria had occurred in our attempts to attain high FK506 levels early on following transplantation. In the future, as was experienced with cyclosporine, some modification of the perioperative use of FK506 may diminish early renal dysfunction. The dramatic absence of hypertension in this group of patients and the very well tolerated form of oral FK506 are encouraging for better preservation of long-term renal function. Initial evaluation of the histology from renal biopsies of kidney transplant recipients showed no specific pathology attributable to FK506 (Demetris AJ, verbal communication). The powerful immunosuppressant effects of FK506 are underscored in four rather dramatic areas. First FK506 appeared to be able to reverse cardiac rejection that had been refractory to all other known forms of therapy. Second, the pediatric group of patients, particularly troublesome compared to their adult counterparts in controlling rejection, have fared extremely well, and 6 of the 7 children are on no steroids. Third, FK506 introduces a new degree of flexibility in immunosuppressant management in the ability to merely increase oral dosage and reverse moderate episodes of rejection. Finally, the potential benefits of FK506-based immunotherapy, with little need for large steroid doses and nonspecific bone marrow suppressants, may be realized in decreased infection rates.
Footnotes
Abbreviations: ECMO, extracorporeal membrane oxygenation; HLHS, hypoplastic left heart syndrome; IABP, intraaortic balloon pump; LVAD, left ventricular assist device; MCS, mechanical circulatory support.
References
- 1.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramanan R, Jain A. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fung JJ, Todo S, Jain A, et al. Conversion of liver allograft recipients with cyclosporine related complications from cyclosporine to FK 506. Transplant Proc. 1990;22:6. [PMC free article] [PubMed] [Google Scholar]
- 3.Todo S, Fung JJ, Demetris AJ, Jain A, Venkataramanan R, Starzl TE. Early trials with FK 506 as primary treatment in liver transplantation. Transplant Proc. 1990;22:13. [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Fung JJ, Jordan M, et al. Kidney transplantation under FK 506. JAMA. 1990;264:63. [PMC free article] [PubMed] [Google Scholar]
- 5.Kormos RL, Armitage JM, Dummer JS, Miyamoto Y, Griffith BP, Hardesty RL. Optimal perioperative immunosuppression in cardiac transplantation using rabbit antithymocyte globulin. Transplantation. 1990;49:306. doi: 10.1097/00007890-199002000-00016. [DOI] [PubMed] [Google Scholar]


