The current policies for cadaver kidney distribution were recently discussed in The Journal.1 Questions about liver allocation are even more important, because there is not the option of artificial organ support.2 Two principles of liver deployment have been advocated: efficiency of organ use and urgency of need.
The Efficiency Principle
Single Disease Studies
Primary Biliary Cirrhosis
Patients with this disease have been stratified retrospectively into low-, medium-, and high-risk categories, and their actual survival after liver transplantation has been compared with the outcome expected without such intervention.3 This comparison depended on a Mayo hazard prediction model of the natural history of primary biliary cirrhosis (Table 1).4 Before the National Institutes of Health Consensus Development Conference of 1983,5 we reserved liver transplantation candidacy for patients with chronic disease whose life expectancy was a few months.6 The effect of this restrictive policy could be seen in liver recipients treated for primary biliary cirrhosis between March 1980 and June 1987. Even in the low-risk group, the bilirubin level averaged 205 μmol/L (12 mg/dL), and in the high-risk group, it averaged 480 μmol/L (28 mg/dL). All three cohorts had hypoalbuminemia (Table 1).
Table 1. Mayo Cox Proportional Hazard Model for Primary Biliary Cirrhosis*.
| Risk Grade | |||
|---|---|---|---|
| Risk Factor | Low | Middle | High |
| Age, y | 47 | 49 | 54 |
| Bilirubin, μmol/L (mg/dL) | 205 (12) | 410 (24) | 480 (28) |
| Albumin, g/L | 30 | 27 | 25 |
| Prothrombin time, s | 13.5 | 15 | 20 |
| Edema score | 0.4 | 0.8 | 0.9 |
From Markus et al.3
The 75% 1-year patient survival rate after transplantation was a 30 percentage point gain over the 45% rate predicted with medical treatment.3 Because 1-year survival rates were 83%, 75%, and 58% in the low-, medium-, and high-risk categories, respectively, the results have been used to demonstrate the inefficient use of organs when they are transplanted to high-risk recipients. Viewed from a different perspective, the gain of survival in the first postoperative year relative to projected outcome without transplantation was actually highest (58 percentage points) in the high-risk patients, next highest (55 percentage points) in the medium-risk group, and lowest (only 14 percentage points) in the low-risk group, whose 1-year survival rate without intervention would have been 69% (Fig 1). The life survival slope and degree of rehabilitation after 1 year were the same in all groups.5
Fig 1.
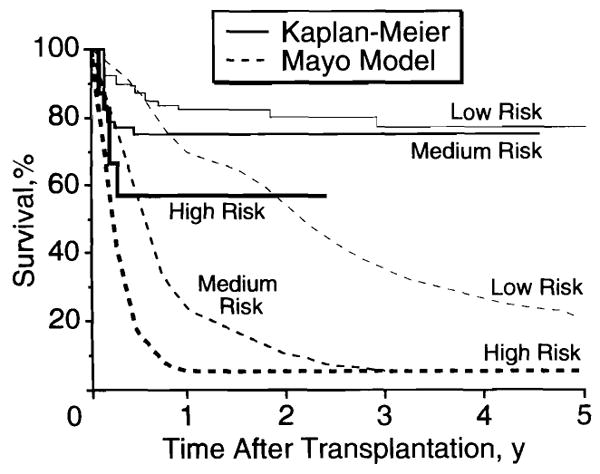
Actual (Kaplan-Meier) survival after transplantation and estimated survival without transplantation (Mayo model) in patients with primary biliary cirrhosis at low, medium, and high risk (from Markus et al3).
Sclerosing Cholangitis
Similar but more pronounced trends were seen with sclerosing cholangitis7 using a second Mayo hazard prediction model that factored in age, bilirubin level, splenomegaly, and histopathologic stage.8 The gain in 1-year survival rate with transplantation vs the predicted outcome without this procedure was only 7 percentage points in low-risk cases, a gain relative to the surrogate control that did not increase in the succeeding 7 years (Fig 2). In the medium-risk patients, the 1-year survival dividend from transplantation was zero, but this steadily increased thereafter. In contrast, the high-risk group achieved a stunning 40 percentage point life survival gain by 12 months, an improvement that had grown to nearly 80 percentage points at 7 years, by which time all patients without transplantation were long since projected dead. By 7 years, the best absolute survival rate belonged to the patients who originally had been the most ill (Fig 2).
Fig 2.

Actual (Kaplan-Meier) survival after transplantation and estimated survival without transplantation (Mayo model) in patients with primary sclerosing cholangitis at low, medium, and high risk (from Abu-Elmagd et al7).
Heterogeneous Diseases: Risk Factors and Cost
At the New England Medical Center, Boston, Mass, 124 adults and children who had a full spectrum of diagnoses and medical urgency were given 142 livers between 1984 and 1992. These cases illustrated the relationship between the severity of pretransplant illness, patient survival, and cost of treatment.9 Urgency of need was determined with the 5-tier scale (called the United Network for Organ Sharing [UNOS] score) that was used nationally through 1990 (see legend of Fig 3). UNOS 1 and 2 candidates (the least ill) had the highest rates of posttransplant survival (Fig 3). The poorest results were with the UNOS 4 and 5 recipients. However, the rescue of the majority of these patients whose expected survival was essentially zero without transplantation was at least as noteworthy as the fact that the survival curve was degraded by their admission into candidacy. The cost of caring for the UNOS 4 and 5 recipients was high, reaching nearly $250000 and $190000 per case, respectively, including the expenditures before transplantation, which can exceed the expenses afterward.
Fig 3.

Survival rates and costs after liver transplantation by United Network for Organ Sharing (UNOS) severity score at the New England Medical Center, Boston, Mass. UNOS severity score: (1) working, (2) at home (many still working) but requiring close medical supervision and/or sporadic hospital care, (3) hospital-bound continuously or most of the time, (4) in the intensive care unit, usually with ventilator support, and (5) UNOStat, a life expectancy of only a few days without transplantation, often because of fulminant hepatic failure. The “zone of absurdity” (shaded area) has been added to illustrate the folly of going too far toward the use of liver transplantation for clinically well patients (data from Muto et al9).
Poorer and more expensive results also were evident when the high-risk patients were identified using the criteria of the Blue Cross/Blue Shield consortium or using the APACHE II (Acute Physiology and Chronic Health Evaluation) score, which expresses pretransplantation need for intensive care.9 No matter how sick the patients were before transplantation or how high the risk, however, those who lived (the majority in every subgroup) and were tested 1 year later had the same degree of rehabilitation, as determined by Karnofsky scores, which were satisfactory in all groups (Table 2).
Table 2. Karnofsky Scores by Risk Stratification, 1 Year After Transplantation*.
| Risk Stratification† | Karnofsky Score |
|---|---|
| Blue Cross/Blue Shield | |
| Elective patients | 82 |
| High-risk patients | 82 |
| APACHE II | |
| Elective patients | 82 |
| High-risk patients | 82 |
| UNOS score | |
| 1 | 85 |
| 2 | 83 |
| 3 | 79 |
| 4 | 83 |
| UNOStat | 84 |
Data obtained from Muto et al.9
APACHE II indicates Acute Physiology and Chronic Health Evaluation II; and UNOS, United Network for Organ Sharing.
Transplantation vs Options
The fact that extremely sick patients or those with ancillary risk factors have a reduced survival rate after liver transplantation has been used to decry their treatment. The extrapolation of this logic (Fig 3) could be the absurd recommendation that only asymptomatic (well) candidates be offered this service because they will yield predictably good life survival curves and generate small bills. However, for low-risk patients who still have adequate or restorable liver function, there often are other, safer treatment options short of transplantation.
For example, patients with autoimmune hepatitis frequently are referred as “liver transplant candidates” at a time when consideration of transplantation can be delayed for long periods by treatment with immunosuppression.10 Henderson et al11 recently emphasized the neglected use of distal splenorenal shunt (Warren procedure) for patients with cirrhosis whose principal complication was variceal hemorrhage. The 3- and 5-year survival rates in this series of patients who still had good (Child's A) or fair (Child's B) parenchymal function were superior to those of the generally sicker transplant recipients (predominantly Child's C) at the same institution, at less than one fourth the cost. The quality-of-life scores 1 year after both operations were essentially the same.
The Urgency Principle
A Field Trial (1987 Through 1990)
With the national liver distribution system that was in effect from November 1987 through 1990, livers obtained in a given area could be used locally without regard for urgency status. However, organs not used locally were freely shipped to wherever they were most badly needed (Fig 4). The distribution system used by UNOS was the same as one developed and tested in Pittsburgh, Pa, in the mid 1980s.12 A report of this field trial was used verbatim in writing the UNOS contract that became operational on October 1, 1987. The plan had two explicit objectives. One was to increase the incentive for organ donation by giving first priority to community recipients. The second was to allow new liver transplant teams to establish credibility by preferentially treating low-risk recipients while referring their gravely ill patients or those with complicated conditions to established programs.
Fig 4.

The United Network for Organ Sharing distribution policy in effect from November 1987 through 1990. This policy emphasized local primacy of organ use but with national distribution thereafter on the basis of urgency (see text). Beginning in 1991, the system was denationalized, with each of the 11 United Network for Organ Sharing regions (indicated by broken lines) having the first option to use livers before their release into a national organ pool.
Transplantation Results During Study
During the last 18 months of this allocation policy, 691 consecutive adult primary liver transplantations were carried out in Pittsburgh. The assessment of treatment efficacy was important in these cases, because two significant improvements had a potential impact on high-risk and all other patients: (1) use of University of Wisconsin (UW) solution beginning in 1988, which extended the safe time of liver preservation from 6 hours to 18 to 24 hours,13,14 and (2) use of the immunosuppressive drug, FK 506, which could be used for either primary or rescue treatment.15
As expected, the best results were in the low-risk recipients. Few in number (n=12 [1.7%]), those in UNOS class 1 survived for 1 year at a rate of 91%; those in combined classes 1 and 2 at 88%; those in class 5 (UNOStat [a life expectancy of only a few days without transplantation, often because of fulminant hepatic failure]) at 71%; and those in classes 3 and 4 at rates in between (Fig 5, left). The incidence of retransplantation was approximately the same in all cohorts, although it was less frequently successful in the sickest patients, as reflected in the graft survival curve (Fig 5, right). The crucial observation was that the gap in results with different urgency classes had narrowed to the point of nonsignificance except for the UNOStat group, which trailed the low-risk groups by 17%. Still, 71% of even this highest-risk cohort was alive at 1 year. After 1 year, the decline in survival rates was the same in all groups.
Fig 5.

Patient and graft survival following primary orthotopic liver transplantation in adults, stratified by pre-1991 United Network for Organ Sharing (UNOS) risk categories. The difference between patient and graft survival is accounted for by successful retransplantation.
Candidate Stability by UNOS Score
The case flow after evaluation is summarized in Fig 6. At the end of the first year, more than half (56%) of the patients who had entered as UNOS 1 candidates remained at this status, while 3% had died. As the risk level at entry increased from UNOS 1 to UNOS 3, the population of patients who had transplantations increased, frequently precipitated by worsening status and reclassification. The death rate while waiting was 10% for UNOS 3 patients and escalated to 17% for UNOS 4 patients and 28% for UNOS 5 patients (Fig 6). Of interest, 27 (3.4%) of the 796 patients in UNOS categories 3 through 5 improved enough with medical management to leave the hospital. These recoveries were dominated by patients with the entry diagnosis of fulminant hepatic failure.
Fig 6.
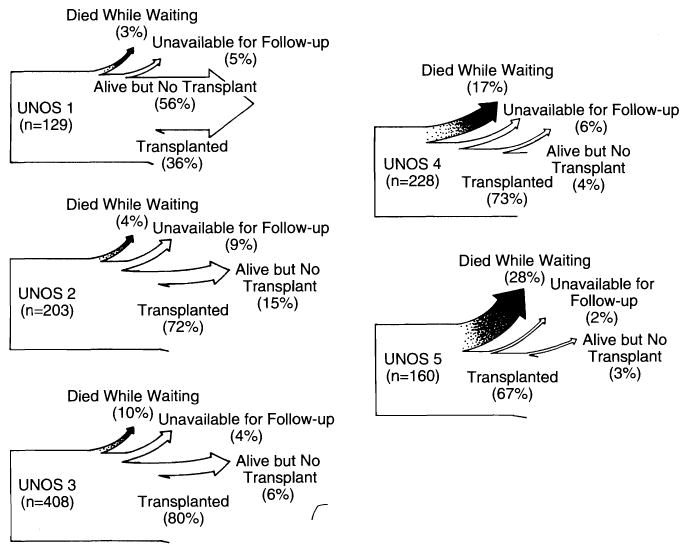
Increasing incidence of death while waiting with each increase of the United Network for Organ Sharing (UNOS) entry score of liver transplant candidates. The percentage of patients alive who had not received transplants decreased with increasing UNOS score.
Termination of Trial
These recipients, including those who were gravely ill at entry or whose condition deteriorated while waiting, could be treated efficiently because of the emphasis on urgency of patient need and the national donor reservoir designed to meet this need. This was changed when a directive from UNOS, effective January 1, 1991, created a functional confederacy in the United States of 11 regions from which the free national movement of organs was discouraged in favor of elective regional use. Urgency of need at a national level was removed as the most pervasive internal principle of the American organ allocation framework.
Consequences of the UNOS Rules Change
Loss of Patient Choice
Factors that preclude candidacy at any given center, such as age, technical or medical complexity, or advanced illness, frequently are not contraindications in other centers to which rejected candidates can go for a second opinion. Under the new system, such patients no longer have easy access to a national reservoir of organs after leaving their region. Even livers donated in their home community encounter an administrative barrier at the regional boundary. This significantly impairs patient choice of center.
A particularly troubling example of organ flow restriction developed in the Veterans Affairs hospitals, among which two liver transplantation centers were designated to which referrals from all 50 states were directed. Most of the organs available to the veterans, however, were expected to come from the local procurement areas in which the national veteran centers were placed.
Loss of Dispersal Autoregulation
Because urgency of need was a magnet for organs not used electively in their local procurement areas, allocation until 1991 tended to be autoregulatory. Urgent cases surfaced most frequently in centers where large numbers of patients waited and drifted toward greater illness, as described above. It was a patient-driven system. The new system is center-driven, in that the administrative control of a liver has become as much of a factor as the medical indication in recipient selection.
Proliferation of Centers
The development of new centers, which had proceeded in an orderly manner before 1991, turned into a stampede, particularly in organ-rich regions. The concern of existing programs that had been focused on preventing organs from leaving their region was superseded by the threat to their organ supply posed by new teams nearby. In this competitive interface, a syndrome of entry triage was encouraged when government agencies established minimum life survival curves as a measure of medical competence, without an attempt to stratify disease severity using the criteria of UNOS, Blue Cross/Blue Shield, APACHE II, or some other system.
The consequence of denying candidacy to sick patients or those with complicated conditions by the argument of “inefficient organ use” cannot be assessed from the data accrued in this recent period and used in 1993 congressional hearings to defend the probity of current allocation practices. Because many such patients die without being listed, the numbers reported as “died while waiting” are understated. Omitted from the record are patients who were not permitted to wait so that organs could be used for elective recipients. Except for those who are medically sophisticated or wealthy, such disenfranchised potential candidates can neither find treatment within nor escape from their regions. The result has been uneven quality of and access to liver transplantation throughout the United States. This heterogeneity also applies to the standards used for donor acceptance in different regions.
Effect on Donor Procurement
A predictable sobering consequence will be shrinkage of the donor pool. Livers with minor functional or anatomic imperfections or from older donors will be systematically discarded and never offered from regions with an excess of donor organs where recipient candidacy is limited to elective patients. Even if they were procured, organs pronounced not good enough to be used locally would be accepted reluctantly elsewhere.
Conclusions
Liver transplantation services should be offered at hepatology centers where the requisite medical support and hepatobiliary surgical procedures are also available. In individual cases, it is necessary first to determine whether a meaningful quality of life can be restored using a full armamentarium of therapeutic options, second to decide in what setting health care can best be provided, and third to establish the degree of urgency.
With nonhepatic or hepatic disease, we do not ordinarily pronounce the condition of a patient to be hopeless when it is merely grave and then withhold or impede treatment under the pretense that it would be better invested in patients who are less ill. If patient need dictates liver allograft flow from a national reservoir rather than allowing this to be center-driven, with piecemeal policies from region to region, the national liver graft shortage may not be as great as we repeatedly tell one another. Even if it is, our activities will be self-regulated in a genuinely ethical order.
Part of the self-regulation will involve wise decisions about what constitutes a hopeless condition. Such judgments presumably would be most discriminating in centers of excellence where they are made and audited frequently with direct knowledge of the needs of other candidates. Otherwise, livers could be wasted by being transplanted into moribund recipients who have no chance of recovery or, alternatively, into candidates who do not need this drastic therapy.
Acknowledgments
Work on this report was supported in part by project grant DK 29961 from the National Institutes of Health, Bethesda, Md.
Footnotes
References
- 1.Gaston RS, Ayres I, Dooley LG, Diethelm AG. Racial equity in renal transplantation: the disparate impact of HLA-based allocation. JAMA. 1993;270:1352–1356. [PubMed] [Google Scholar]
- 2.Delmonico FL, Jenkins RL, Freeman R, et al. The high-risk liver allograft recipient: should allocation policy consider outcome? Arch Surg. 1992;127:579–584. doi: 10.1001/archsurg.1992.01420050103013. [DOI] [PubMed] [Google Scholar]
- 3.Markus B, Dickson ER, Grambsch P, et al. Efficacy of liver transplantation in patients with primary biliary cirrhosis. N Engl J Med. 1989;320:1709–1713. doi: 10.1056/NEJM198906293202602. [DOI] [PubMed] [Google Scholar]
- 4.Dickson ER, Grambsch PM, Flening TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology. 1989;10:1–7. doi: 10.1002/hep.1840100102. [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health Consensus Development Conference on Liver Transplantation. Hepatology. 1984;4(suppl):1S–110S. [PubMed] [Google Scholar]
- 6.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu-Elmagd K, Malinchoc M, Dickson ER, et al. Efficacy of liver transplantation in patients with primary sclerosing cholangitis. Surg Gynecol Obstet. 1993;177:335–344. [PMC free article] [PubMed] [Google Scholar]
- 8.Dickson ER, Murtaugh PA, Grambsch PM, et al. Primary sclerosing cholangitis: refinement and validation of survival model. Gastroenterology. 1992;103:1893–1901. doi: 10.1016/0016-5085(92)91449-e. [DOI] [PubMed] [Google Scholar]
- 9.Muto P, Freeman RB, Haug CE, Lu A, Rohrer RJ. Liver transplant candidate stratification systems: implications for third-party payors and organ allocation. Transplantation. In press. [PubMed] [Google Scholar]
- 10.Van Thiel D, Wright H, Carroll P, et al. FK 506: a treatment for autoimmune chronic active hepatitis: results of an open label preliminary trial. Am J Gastroenterol. In press. [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson JM, Gilmore GT, Hooks MA, et al. Selective shunt in the management of variceal bleeding in the era of liver transplantation. Ann Surg. 1992;216:248–255. doi: 10.1097/00000658-199209000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Gordon RD, Tzakis A, et al. Equitable allocation of extrarenal organs: with special reference to the liver. Transplant Proc. 1988;20(suppl 1):5131–5138. [PMC free article] [PubMed] [Google Scholar]
- 13.Kalayoglu M, Sollinger HW, Stratta RJ, et al. Extended preservation of the liver for clinical transplantation. Lancet. 1988;1:617–619. doi: 10.1016/s0140-6736(88)91416-x. [DOI] [PubMed] [Google Scholar]
- 14.Todo S, Nery J, Yanaga K, Podesta L, Gordon RD, Starzl TE. Extended preservation of human liver grafts with UW solution. JAMA. 1989;261:711–714. [PMC free article] [PubMed] [Google Scholar]
- 15.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramanan R, Jain A. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


