Abstract
Isoprene emission from broadleaf trees is highly temperature dependent, accounts for much of the hydrocarbon emission from plants, and has a profound effect on atmospheric chemistry. We studied the temperature response of postillumination isoprene emission in oak (Quercus robur) and poplar (Populus deltoides) leaves in order to understand the regulation of isoprene emission. Upon darkening a leaf, isoprene emission fell nearly to zero but then increased for several minutes before falling back to nearly zero. Time of appearance of this burst of isoprene was highly temperature dependent, occurring sooner at higher temperatures. We hypothesize that this burst represents an intermediate pool of metabolites, probably early metabolites in the methylerythritol 4-phosphate pathway, accumulated upstream of dimethylallyl diphosphate (DMADP). The amount of this early metabolite(s) averaged 2.9 times the amount of plastidic DMADP. DMADP increased with temperature up to 35°C before starting to decrease; in contrast, the isoprene synthase rate constant increased up to 40°C, the highest temperature at which it could be assessed. During a rapid temperature switch from 30°C to 40°C, isoprene emission increased transiently. It was found that an increase in isoprene synthase activity is primarily responsible for this transient increase in emission levels, while DMADP level stayed constant during the switch. One hour after switching to 40°C, the amount of DMADP fell but the rate constant for isoprene synthase remained constant, indicating that the high temperature falloff in isoprene emission results from a reduction in the supply of DMADP rather than from changes in isoprene synthase activity.
Isoprene emission from plants is one of the largest hydrocarbon fluxes from the biosphere into the atmosphere (Guenther et al., 2006). This large flux has profound effects on atmospheric chemistry. Reactions of isoprene with hydroxyl radicals contribute to the formation of tropospheric ozone and affect aerosol formation (Went, 1960; Trainer et al., 1987; Chameides et al., 1988; Fehsenfeld et al., 1992; Kiendler-Scharr et al., 2009). At the same time, isoprene could potentially affect current global climate change by lengthening the lifetime of greenhouse gases, such as methane. Considering the large effects of isoprene emission on atmospheric chemistry and thus on future climate changes, it is important to study isoprene emission patterns under different environmental conditions.
It has long been known that isoprene emission is highly temperature dependent (Sanadze and Kalandaze, 1966; Tingey et al., 1979, 1987; Monson et al., 1992). Isoprene emission increases up to 35°C to 40°C even when carbon assimilation is declining. This uncoupling of emission from photosynthesis contributed to the hypothesis that isoprene may protect plants against heat stress (Sharkey and Singsaas, 1995; Singsaas et al., 1997). The rate of isoprene emission declines above its optimum, but the optimum temperature is significantly affected by the protocol of isoprene emission measurement (Singsaas et al., 1999; Singsaas, 2000). If measurements are made quickly, the optimum is much higher than if the measurements are made slowly. This occurs because isoprene emission above 35°C is unstable, increasing when the temperature is first raised but then falling back after 10 to 20 min at the higher temperature. A mechanistic understanding of the regulation of isoprene emission with changes in temperature is important to accurately model isoprene output in future environments where global mean temperature is predicted to rise.
Whether isoprene emission is primarily controlled by enzyme activity or substrate (dimethylallyl diphosphate [DMADP]) availability has been under debate. While it was originally proposed that changes in isoprene synthase (IspS) activity account for changes in emission levels (Fall and Wildermuth, 1998; Lehning et al., 1999), recent evidence suggests that, instead, variations in substrate levels may be responsible (Schnitzler et al., 2005; Rasulov et al., 2009b). In vitro Km values determined for IspS are in the millimolar range; these unusually high values indicate that changes in substrate levels could affect the reaction rate (Wildermuth and Fall, 1996; Schnitzler et al., 2005). A recently developed method using postillumination isoprene emission to measure DMADP levels, in particular, contributed to the understanding of the regulation of isoprene emission (Rasulov et al., 2009a). The principle of this method is similar to the in vivo measurement of ribulose 1,5-bisphosphate (RuBP) by postillumination CO2 assimilation (Laisk et al., 1984; Ruuska et al., 1998). Assuming that the methylerythritol 4-phosphate (MEP) pathway is rapidly shut off by darkness while isoprene synthase remains active, total isoprene released during darkness stoichiometrically approximates plastidic DMADP levels. Using this nonintrusive method, it has been reported that in poplar (Populus spp.) trees, dependence of isoprene emission on light, carbon dioxide, and oxygen is regulated by changes in substrate levels, and temperature response reflects mixed regulations on both enzyme activities and substrate levels (Rasulov et al., 2009b, 2010).
In this study, the effects of temperature on postillumination isoprene emission in oak (Quercus robur) and poplar (Populus deltoides) leaves were investigated. Following the initial decline in isoprene emission rate upon darkening of the leaf, we observed a burst of isoprene emission. The time of appearance of this burst is highly temperature dependent, and its size was markedly larger than the DMADP peak. Assuming that this postillumination burst represents a separate pool of MEP pathway metabolites, we measured isoprene emission and substrate levels under different temperatures in an effort to understand the temperature regulation of MEP pathway metabolites and to gain insight into the nature of the postillumination burst. Isoprene emission and metabolite levels as determined by postillumination isoprene emission during a rapid temperature switch from 30°C to 40°C were also measured, and the findings are discussed.
RESULTS
Characteristics of Postillumination Isoprene Emission
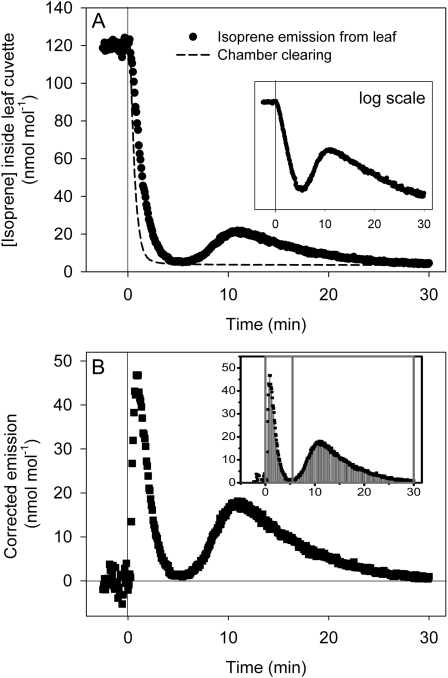
Postillumination isoprene emissions in oak leaves at different temperatures were measured following the procedures of Rasulov et al. (2009a). At a leaf temperature of 30°C, isoprene emission initially dropped to nearly zero after light was turned off (Fig. 1A). This initial decline was exponential, consistent with one homogenous pool being converted to isoprene (Fig. 1A, inset). The rate of isoprene emission rose again in darkness and then slowly dropped and leveled off in 30 min. When integrated and corrected for system clearing, this showed up as a second peak apart from the classic DMADP peak on the postillumination emission trace (Fig. 1B). It was hypothesized that this peak represents an intermediate pool of metabolites that accumulated upstream of DMADP, possibly in the MEP pathway. We refer to this pool as “early metabolites.” This phenomenon was also observed in poplar.
Figure 1.
A typical postillumination isoprene emission trace in oak leaf under standard conditions. A, Light was turned off at time 0. After emission dropped to almost zero in approximately 5 min, isoprene emission increased again and then fell slowly in 20 to 30 min. The decline in the first part of the trace was exponential (inset). B, In the chamber-clearing trace, preclearing isoprene levels were normalized to predarkness emission levels in the emission trace. Subtracting the clearing trace from the emission trace yielded the true postillumination isoprene emission. This difference showed up as two distinct peaks at temperatures less than 45°C. DMADP (first peak) and early metabolites (second peak) were then separated and integrated in Origin (inset).
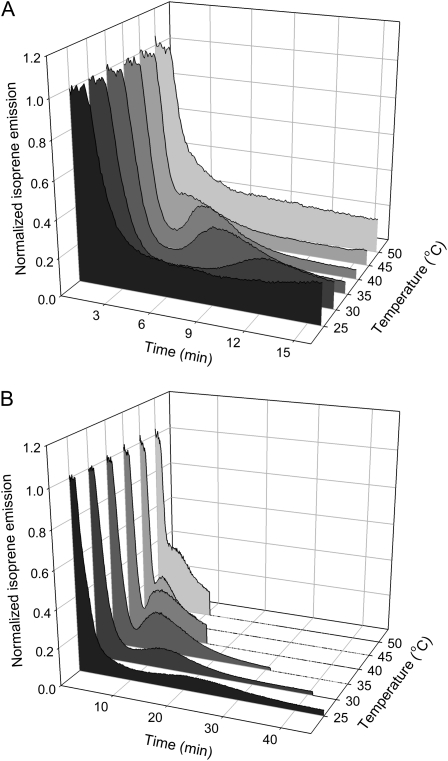
The time of appearance of this burst was highly temperature dependent in both oak and poplar (Fig. 2). The peak appeared sooner at higher temperatures, and at temperatures greater than 40°C, it appeared to merge with the initial decline in isoprene emission. This peak was observed even at the lowest temperature used, 25°C. At this temperature, the peak took more than 15 min before appearing and occurred over a much longer time (more than 30 min) than at higher temperatures.
Figure 2.
Time of appearance of the postillumination burst is temperature dependent in both oak (A) and poplar (B). In both cases, the peak in the postillumination isoprene emission trace appeared progressively sooner at higher temperatures and merged with the initial decline in isoprene emission at temperatures above 40°C. Light was turned off at 50 s. Isoprene emissions at different temperatures were normalized to predarkness levels. The postillumination period (darkness) in B was intentionally shortened at higher temperatures. This was intended to minimize thermodamage, since heat damage is exacerbated in darkness.
Temperature Response of Isoprene Emission, DMADP, and Early Metabolites
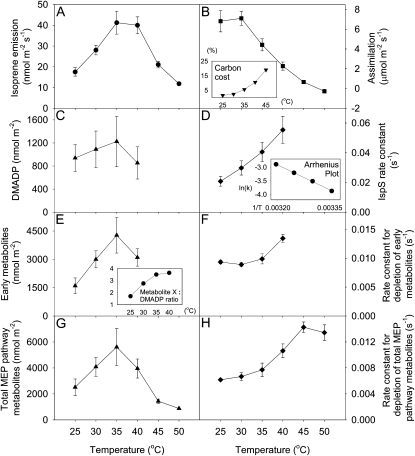
Isoprene emission in oak leaves increased with temperature until 35°C to 40°C, at which point it started to decrease (Fig. 3A). Carbon assimilation decreased with temperature at temperatures above 30°C and became negative at 50°C. The carbon cost of isoprene emission as a percentage of net assimilation increased exponentially with temperature, up to 20% at 45°C (Fig. 3B, inset). This percentage rose to more than 100% at 50°C, as net assimilation had become negative at this temperature.
Figure 3.
Temperature dependence of isoprene emission rate (A), assimilation rate (B), DMADP (C), IspS rate constant (D), early metabolites (E), the rate constant for depletion of early metabolites (F), total MEP pathway metabolites (G), and the rate constant for depletion of total MEP pathway metabolites (H) in oak leaf. The amounts of early metabolites and total MEP pathway metabolites are in isoprene units. The carbon cost of isoprene as a percentage of assimilation at a given temperature was calculated assuming that the synthesis of each isoprene requires six fixed carbons (B, inset). The rate constant for depletion of a certain metabolite was calculated by dividing isoprene emission rate by the amount of the metabolite. An Arrhenius plot of ln(k) versus 1/temperature (K) for isoprene synthase is shown in D, inset. Early metabolites to DAMDP ratio are shown in E, inset. DMADP and early metabolite data for temperatures over 40°C could not be obtained, since it was impossible to separate the two peaks at higher temperatures. Each point denotes the mean ± se (n = 4).
Plastidic DMADP levels increased with temperature between 25°C and 35°C before decreasing at 40°C (Fig. 3C). DMADP and early metabolite levels at temperatures above 40°C could not be obtained, since we could not separate the two peaks at higher temperatures (Fig. 2A). DMADP levels at 35°C were approximately 30% more than at 25°C. The variations, however, were not significant due to large se values (P = 0.137, by one-way repeated-measures ANOVA). The calculated rate constant for isoprene synthase (isoprene emission rate divided by the amount of DMADP) increased steadily from 25°C to 40°C, with an Arrhenius activation energy of 50.4 kJ (Fig. 3D). The early metabolites showed a similar temperature response curve as DMADP, increasing with temperature between 25°C and 35°C and decreasing at 40°C (Fig. 3E). Pools of early metabolites were 1.7 to 3.6 times the size of DMADP pools, and this ratio increased with temperature (Fig. 3E, inset). The early metabolite pool is on average 2.9 times the size of the DMADP pool. Variations in early metabolites were statistically significant (P = 0.007). The “rate constant” for the process of early metabolite depletion did not increase between 25°C and 35°C but increased when temperature was raised to 40°C (Fig. 3F).
Total MEP pathway metabolites were calculated by integrating the total area below the postillumination emission curve. The size of this pool increased with temperature up to 35°C before decreasing to very low levels at 45°C and 50°C (Fig. 3G). The rate constant for the process of total MEP pathway metabolite depletion increased up to 45°C with an Arrhenius activation energy of 33.6 kJ and decreased only slightly at 50°C (Fig. 3H).
CO2 Response of Isoprene Emission, DMADP, and Early Metabolites
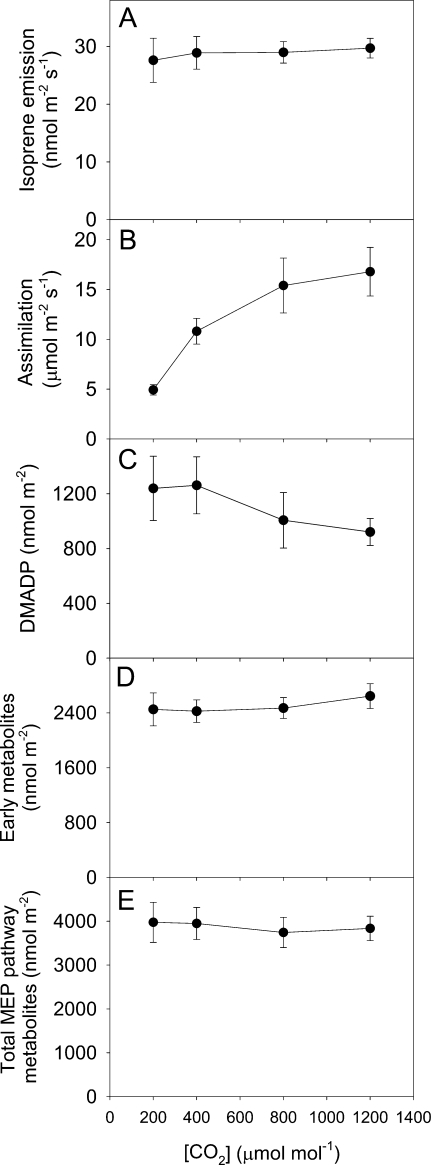
To determine if the early metabolite pool was related to Calvin-Benson cycle intermediates, isoprene emission was measured over a range of CO2 concentrations in oak leaves. Metabolite levels at 200, 400, 800, and 1,200 μmol mol−1 CO2 were then determined with postillumination isoprene emission. Isoprene emission stayed steady with increasing CO2 concentrations in this range, and carbon assimilation increased with CO2 concentrations (Fig. 4, A and B). DMADP levels decreased slightly at high CO2 concentrations (P = 0.157; Fig. 4C). Both early metabolites and total MEP pathway metabolites stayed constant with increasing CO2 concentrations (Fig. 4, D and E). The time at which the early metabolite peak appeared was not affected by CO2 concentration (data not shown).
Figure 4.
CO2 response of isoprene emission (A), assimilation rate (B), DMADP (C), early metabolites (D), and total MEP pathway metabolites (E) in oak leaf. The amounts of early metabolites and total MEP pathway metabolites are in isoprene units. Each point denotes the mean ± se (n = 3).
Changes in Isoprene Emission and Metabolites during a Rapid Temperature Switch from 30°C to 40°C
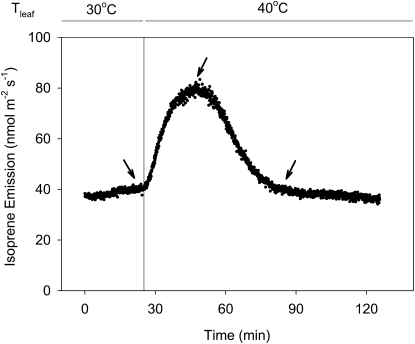
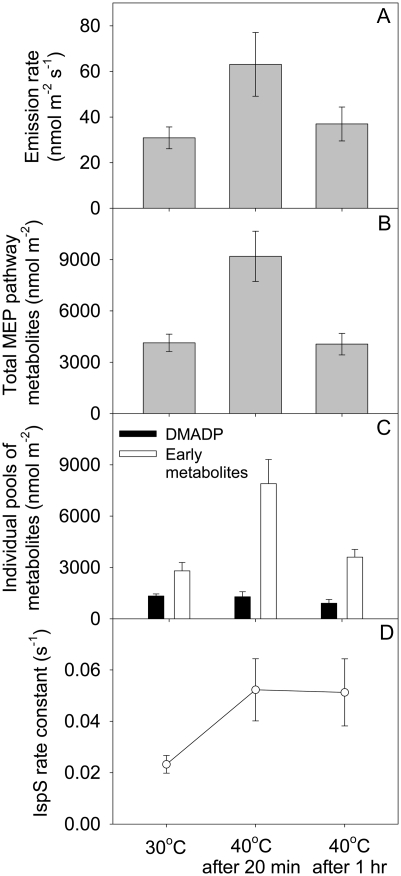
When the temperature of an oak leaf was rapidly raised from 30°C to 40°C, isoprene emission initially increased but then fell 20 min after the switch and eventually leveled off, often to preswitch levels (Fig. 5). Figure 6A shows emission levels before the temperature switch, 20 min after the switch (when emission was at a maximum), and 1 h after the switch (when emission level had dropped and leveled off). Total MEP pathway metabolites as measured by postillumination isoprene emission followed the same trend as emission rates and were highest at 20 min after the switch (Fig. 6B). However, contributions from DMADP and early metabolites to the total pool were disparate (Fig. 6C). DMADP levels did not increase upon the temperature increase. Early metabolites, being a bigger pool by itself, increased significantly and were primarily responsible for the increase in total MEP pathway metabolites. Both DMADP and the early metabolite pool decreased when the high temperature was maintained. Calculated rate constants for isoprene synthase more than doubled during the temperature switch and remained constant when high temperature was maintained (Fig. 6D).
Figure 5.
Isoprene emission of an oak leaf during a rapid switch from 30°C to 40°C. Isoprene emission increased initially upon the switch but then fell at approximately 20 min after the switch. Isoprene emission then leveled off, often to preswitch levels, within 1 h after the switch. Measurements in Figure 6 were made at the time points shown by the arrows.
Figure 6.
Isoprene emission rates (A), total MEP pathway metabolites (B), DMADP and early metabolites (C), and IspS rate constant (D) during a rapid switch from 30°C to 40°C in oak leaf. The amounts of total MEP pathway metabolites and early metabolites are in isoprene units. The rate constant for isoprene synthase was calculated by dividing emission rate by DMADP level. Each point denotes the mean ± se (n = 3).
DISCUSSION
Characteristics and Source of Postillumination Isoprene Emission
In addition to the postillumination isoprene emission that declined exponentially, we observed a later burst of isoprene emission in darkness (Fig. 1A). This peak could be measured at 25°C but became more prominent as temperature increased (Fig. 2). It also occurred earlier at higher temperature, and at 45°C it fused with the initial postillumination isoprene emission that is believed to come from DMADP present when the light is turned off.
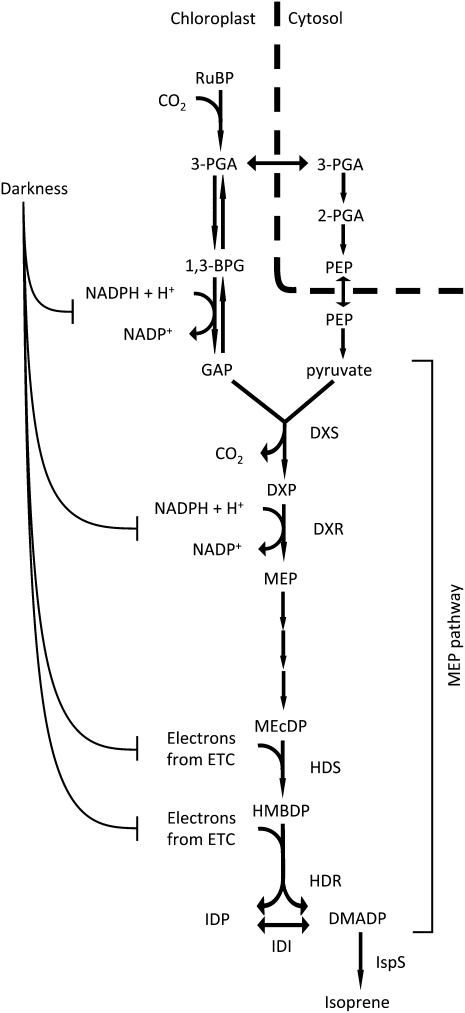
The use of postillumination isoprene emission as a measure of DMADP was first suggested by Rasulov et al. (2009a). This method depends on darkness stopping DMADP production at a point in metabolism where DMADP is the only significant metabolite pool between the block caused by darkness and isoprene. Potential points at which DMADP production could be stopped by imposing darkness are shown in Figure 7. These steps are most susceptible because energetic cofactors produced from the electron transport chain are quickly depleted in darkness (Sharkey et al., 1986; Rasulov et al., 2009a). In this view, the observation of a postillumination isoprene burst is interesting. This phenomenon was first documented by Monson et al. (1991), where it was suggested that isoprene emission may be controlled by ATP levels. A “light-independent isoprene emission process” was also observed at higher temperatures by Rasulov et al. (2010). Assuming that there is only one homogeneous DMADP pool in the chloroplasts, this burst of isoprene in darkness has to represent DMADP that is either transported into chloroplasts or synthesized de novo from some unknown metabolite, “metabolite X.” The possible sources are explored and discussed below.
Figure 7.
Proposed inhibition by darkness of steps leading to isoprene production in the short term. Steps in the MEP pathway expected to be most susceptible to depletion of reducing power upon darkening are indicated here. 1,3-BPG, 1,3-Bisphosphoglycerate; DXR, deoxyxylulose phosphate reductoisomerase; DXS, deoxyxylulose phosphate synthase; ETC, electron transport chain; HDS, hydroxymethylbutenyl diphosphate synthase; MEcDP, methylerythritol 2,4-cyclodiphosphate; PEP, phosphoenolpyruvate. ATPs are omitted here for simplicity. Steps between 3-PGA and GAP are reversible. Coupling of NADPH is only shown in the forward reaction for simplicity.
(1) Cytosolic DMADP. DMADP can also be produced by the mevalonic acid pathway in the cytosol, and this pool can be large. However, reports that examined DMADP transport into chloroplasts showed that this activity is very low (Bick and Lange, 2003). Cross talk between the MEP pathway and the mevalonic acid pathway is limited and unidirectional out of chloroplasts (Bick and Lange, 2003; Laule et al., 2003; Dudareva et al., 2005; Hampel et al., 2005). Rasulov et al. (2009a) measured total leaf DMADP and found a significant pool of DMADP in darkened leaves, which they hypothesized to be cytosolic DMADP, and that cytosolic DMADP cannot be used for isoprene production. We agree.
(2) Plastidic isopentenyl diphosphate (IDP). This is unlikely, since the plastidic DMADP pool is considerably larger than the IDP pool in the presence of IDP isomerase (IDI). IDI is needed in isoprene-emitting plants to increase the DMADP pool for isoprene production, since 5 times more IDP than DMADP is produced in the last step of the MEP pathway (Adam et al., 2002; Rohdich et al., 2002, 2003; Tritsch et al., 2010). Different groups have reported equilibrium DMADP:IDP ratios ranging from 3:1 to 13:1 in the presence of IDI (Agranoff et al., 1960; Koyama et al., 1983; Lützow and Beyer, 1988; Ramos-Valdivia et al., 1997). The metabolite X in this study is much larger than the pool size of DMADP, so it is unlikely to be IDP (Fig. 3E, inset). Besides, assuming that IDI is active in darkness, IDP should isomerize to DMADP and convert to isoprene during the first phase of postillumination isoprene emission (first peak in Fig. 1B). It is likely that the first phase of postillumination isoprene emission represents both DMADP and IDP (DMADP being the majority) and that metabolite X is further upstream in the metabolic pathway leading to isoprene.
(3) Metabolites upstream of the MEP pathway, for example, sugar phosphates in the Calvin-Benson cycle. Sugars and sugar phosphates, such as RuBP, 3-phosphoglycerate (3-PGA), and triose phosphates, are large pools and ultimately the source of plastidic DMADP. Nonetheless, the amount of metabolite X was unchanged over a large range of CO2 concentrations (Fig. 4D). Changes in CO2 would affect the amount of 3-PGA and RuBP present in leaves when the light is turned off, although the effect is less on the amount of glyceraldehyde 3-phosphate (GAP; Badger et al., 1984; Loreto and Sharkey, 1993). However, GAP levels fall to very low levels within 5 min of darkness (Sharkey et al., 1986; Loreto and Sharkey, 1993). In addition, a previous study showed that carbon isotope labeling is unchanged during the postillumination period (Kreuzwieser et al., 2002), supporting the view that metabolite X and DMADP are located in the same pathway. It thus appears unlikely that this postillumination burst represents a metabolite in the Calvin-Benson cycle upstream of the MEP pathway.
(4) MEP pathway metabolites (upstream of DMADP). Metabolites in the MEP pathway upstream of IDP and DMADP seem to be the most likely source. While the regulation of the MEP pathway is still not well understood, several enzymes such as deoxyxylulose phosphate synthase, deoxyxylulose phosphate reductoisomerase, hydroxymethylbutenyl diphosphate synthase, and hydroxymethylbutenyl diphosphate reductase (HDR) have all been proposed as highly regulated steps that could potentially let intermediate metabolites build up to significant levels (Sharkey et al., 2008; Rivasseau et al., 2009). For instance, a recent report suggested that a cyclic intermediate in the MEP pathway, methylerythritol cyclodiphosphate, accumulates to measurable levels in spinach (Spinacia oleracea) leaves (Rivasseau et al., 2009). Maybe early metabolites upstream of DMADP, such as methylerythritol cyclodiphosphate, are converted to DMADP in darkness. But some tricky questions remain to be answered. Why should these early metabolites convert to DMADP, if reducing power is depleted in darkness? Why is the conversion of early metabolites to DMADP delayed in darkness?
This phenomenon can be explained if the MEP pathway is limited by energetic cofactors in the short term and by carbon supply in the long term. When light is turned off, electron transport stops essentially immediately. This effectively shuts off the MEP pathway, as the second and the last two steps of the MEP pathway all require reducing power (Fig. 7). In addition, steps requiring ATP are also turned off within a few seconds. HDR may be the most susceptible enzyme, as it accepts electrons directly from ferredoxin in light (Seemann et al., 2005, 2006; Seemann and Rohmer, 2007). If the ATP supply limits more than reducing power during the first few seconds, then postillumination isoprene emission will measure several metabolites in addition to DMADP. Given that ATP can be made from a stored proton motive force across the thylakoid membranes after the light is turned off, it seems much less likely that ATP supply is more limiting than reduced ferredoxin supply in the first moments of darkness.
The subsequent increase in isoprene emission could occur when HDR switches to NADPH as a source of reducing power during darkness (Zepeck et al., 2005; Seemann et al., 2006). NADPH (or NADH; Adam et al., 2002) produced elsewhere in the chloroplast could supply the MEP pathway, leading to a rise in isoprene emission, but this would take time. There is likely also to be NADH in the plastid, although generally NADH-NAD ratios are lower than NADPH-NADP ratios. Because plastidic GAP dehydrogenase can use either NADH or NADPH (McGowan and Gibbs, 1974), it is not clear whether these redox pairs would be in different ratios. Thus, we cannot speculate on how NADH may affect the results we observed. Another way that a delay could be introduced comes from the redox regulation of the reductive phase of the pentose phosphate pathway. The first two steps of this pathway produce NADPH but are inhibited in the light by regulatory dithiols. These enzymes could become activated over a period of minutes after darkening the leaf.
In the long term, carbon supply to the MEP pathway may become the limiting factor causing emission to fall eventually. The amount of GAP is rapidly reduced in darkness (Sharkey et al., 1986; Loreto and Sharkey, 1993). When light is turned off, 3-PGA levels increase initially, which could lead to an excess of pyruvate (Fig. 7), but then it falls quickly to low levels at 10 min into darkness (Loreto and Sharkey, 1993). We suspect that this is because low levels of NADPH and ATP during the first few minutes of darkness drive the reactions between 3-PGA and GAP in the reverse direction, depleting GAP and pushing up 3-PGA levels. On a longer time frame, redox-sensitive enzymes in the Calvin-Benson cycle such as phosphoribulokinase and GAP dehydrogenase are turned off, causing a drop in PGA levels (Wara-Aswapati et al., 1980; Wedel and Soll, 1998; Scheibe et al., 2002). Thus, the first decline in isoprene emission following darkness may result from a lack of reduced ferredoxin, while the second decline may result from a lack of carbon intermediates.
The amount of plastidic DMADP found in our study (approximately 1 μmol m−2 under standard conditions) is in good agreement with previous reports (Rasulov et al., 2009a, 2009b, 2010). The rate constant of isoprene synthase derived from this number predicts that the entire DMADP pool turns over in 39 s under standard conditions. On the other hand, the early metabolites constitute a much larger pool that turns over in 146 s, or approximately 2.5 min, under the same conditions.
Temperature and CO2 Response of DMADP and Early Metabolites in Oak
Our results support the view that isoprene emission under different temperature regimes is regulated both by enzyme activity and substrate levels. DMADP levels increase with temperature until 35°C, where it starts to drop (Fig. 3C). This trend was also observed in aspen (Populus spp.; Rasulov et al., 2010). The rate constant of isoprene synthase increased with temperature at 25°C to 40°C, and the rate constant for depletion of total MEP pathway metabolites increased up to 45°C (Fig. 3, D and H). Again, this is in agreement with IspS activity measured in vitro and in vivo (Monson et al., 1992; Lehning et al., 1999; Rasulov et al., 2009b). The early metabolites showed a similar temperature response to DMADP, increasing up to 35°C before it dropped at 40°C. The changes in early metabolites, however, were much sharper: early metabolites at 35°C more than doubled the amount at 25°C, whereas DMADP increased by just 30% (Fig. 3, C and E). This is reflected in the rate constant calculated for each of these two metabolites: while IspS activity rose sharply with temperature, the activities that converted early metabolites to isoprene (via DMADP) remained relatively unchanged from 25°C to 35°C before increasing at higher temperatures (Fig. 3, D and F). This shows that variation of early metabolites with temperature is nonenzymatic but instead regulatory, implying a complex regulatory role that early metabolites play in the temperature response of isoprene emission.
Isoprene emission and substrate levels did not respond to increased CO2 concentrations in this study (Fig. 4). Reduced isoprene emissions at elevated CO2 concentrations are often reported in CO2 response studies (Jones and Rasmussen, 1975; Monson and Fall, 1989; Loreto and Sharkey, 1990; Rosenstiel et al., 2003; Scholefield et al., 2004; Possell et al., 2005; Calfapietra et al., 2008; Rasulov et al., 2009b; Wilkinson et al., 2009). However, in one study, the CO2 response of isoprene emission in red oak (Quercus rubra) was variable (Loreto and Sharkey, 1990). In another, isoprene emission from leaves of red oak was significantly higher under high-CO2 growth conditions and did not change with increasing experimental CO2 concentrations (Sharkey et al., 1991). Under field conditions, an increase (in oak) and no change (in poplar) in isoprene emission in trees grown under elevated CO2 conditions have been reported (Calfapietra et al., 2007; Loreto et al., 2007). It was also reported that the reduction in emission at high CO2 concentrations, when observed, is temperature sensitive (Loreto and Sharkey, 1990; Rasulov et al., 2010). Our results here further demonstrate that the CO2 response of isoprene emission between experiments can be different.
Metabolite Levels during a Rapid Switch from 30°C to 40°C
It was observed in this laboratory some years ago that upon a rapid increase in temperature, isoprene emission in oak increased, but only transiently when the high temperature was maintained (Singsaas et al., 1999; Singsaas and Sharkey, 2000). We were able to repeat this result (Fig. 5) and used postillumination isoprene emission to measure changes in metabolite levels in this experiment.
Our results show that a change in emission levels during the fast temperature switch is attributable to an increase in IspS activity rather than substrate level (Fig. 6, C and D). DMADP levels at 30°C and 40°C were similar. This is consistent with the temperature response curve of DMADP (Fig. 3). Early metabolites were high just after the temperature switch (Fig. 6, B and C) but declined after 1 h. The early metabolites may serve as a buffer allowing for short-term high rates of isoprene emission. However, IspS appears to be able to drain the early metabolite pool faster than it can be refilled at 40°C, causing the control of the rate of isoprene emission to shift from DMADP concentration plus IspS kinetics to upstream reactions needed to make the early metabolites. In the field, temperatures of red oak leaves could rise up to 39°C and could vary by more than 10°C on a sunny day (Singsaas et al., 1999). The rapid temperature shift used in this experiment in a sense mimics a real-world scenario where leaf temperature rises rapidly in a sun fleck. It appears that metabolic control can shift at these physiologically relevant temperatures.
When high temperature is maintained for a period of time, metabolite levels drop and enzyme activity is maintained (Fig. 6, B–D). This could be explained by a limitation on energetic cofactors. Rates of photosynthetic electron transport decrease (Niinemets et al., 1999) and thylakoid membrane conductance increases at higher temperatures (Zhang et al., 2009; Zhang and Sharkey, 2009), causing a drop in proton motive force. This leads to a decrease in ATP synthesis. On the other hand, ATP and NADPH usage increases as photorespiration increases. This drawdown of energetic cofactors could affect the MEP pathway, where three NADPH and three ATP equivalents are needed for the synthesis of one isoprene molecule.
CONCLUSION
In this study, we examined the effect of temperature on the postillumination isoprene burst and discussed possible sources of this phenomenon. Timing of this burst is highly temperature dependent. This burst likely represents a pool of metabolites that has accumulated in the MEP pathway, but the identity of this metabolite and the mechanism by which it is converted to DMADP remain unclear. It will be helpful to perform more studies to extend our understanding of chloroplast energetic status during the postillumination period. Studies of the MEP pathway metabolome will also go a long way toward solving this puzzle.
Our results also confirm the finding that the response of isoprene emission to temperature is regulated both by enzyme activity and substrate availability. Isoprene emission and metabolite levels did not respond to high CO2 concentrations in this study. During a rapid temperature switch from 30°C to 40°C, isoprene emission increased initially due to an increase in enzyme activity and then decreased over time as substrate became limiting.
MATERIALS AND METHODS
Plant Material
Leaves were harvested from oak (Quercus robur) trees on the Michigan State University campus (42°43′N, 84°28′W) between April and September 2010. Whenever possible, leaves from the periphery (sun exposed) of the canopy were harvested. Small twigs with desired leaves were cut from the trees with razor blades or a pole pruner and immediately recut underwater with a sharp razor blade. Leaves with a small portion of branch were then transferred underwater to a floral tube and carried back to the laboratory to be analyzed. We have found in the past that leaves harvested in this way were physiologically active for at least 6 h. To maintain consistency, leaves used in the same experiment were harvested from the same tree.
Poplar trees (Populus deltoides) were propagated by stem cuttings in soil (Baccto planting mix; Michigan Peat Co.) and grown in a 25°C/23°C, 14-h photoperiod growth chamber. Photosynthetic photon flux density at leaf level was set at 500 μmol m−2 s−1. Mature leaves from 2-month-old plants were used in this study. Poplar leaves remained attached during the experiment.
Gas Exchange
During an experiment, leaves were enclosed in a custom-built aluminum cuvette with a glass window. Air in the cuvette was mixed with three small Micronel D241L fans (Micronel US). Synthetic air used for gas exchange was mixed from cylinders of N2, O2, and 5% CO2 in air and humidified as described by Tennessen et al. (1994). CO2 concentration was controlled to 400 μmol mol−1 unless otherwise noted. Total flow rate through the chamber was measured with a flow meter before the air entered the leaf chamber. CO2 levels in reference and sample air were measured with a LI-6262 CO2/water analyzer (LI-COR Biosciences). Air coming out of the analyzer was then connected to a fast isoprene sensor (Hills Scientific), and isoprene was measured at 5-s intervals. The temperature of the leaf chamber was controlled by two water baths linked to a six-way valve that allowed ultrafast switching between different temperatures. Both leaf and air temperatures were monitored with thermocouples; only leaf temperatures were used and reported.
Experiments were carried out under standard conditions (30°C, 1,000 μmol m−2 s−1 photosynthetic photon flux density at leaf level) unless otherwise noted. Light was provided with an SL3500 white light-emitting diode array (Photon Systems Instruments). Each leaf was acclimatized for at least 45 min before starting the experiment. Assimilation rate was calculated according to methods used by von Caemmerer and Farquhar (1981).
Measurement of Postillumination Isoprene Emission
Postillumination isoprene emission and chamber clearing were measured according to procedures described by Rasulov et al. (2009a). Light in the chamber was turned off by quickly switching off the light-emitting diode and at the same time covering the chamber window with a thick stack of paper. This procedure drops light level in the chamber to essentially zero (0.00 μmol m−2 s−1 as measured by a quantum sensor in the same condition). In practice, it was found that a first dark period in the experiment caused the postillumination burst to appear earlier than succeeding dark periods, while subsequent darkenings caused consistent responses in following dark periods. This problem was overcome by giving the leaf one dark period for which the data were not used before the actual experiment began.
To generate the chamber-clearing trace, a small flow of external isoprene source (Airgas Great Lakes) was first fed into the chamber until isoprene levels in the chamber were steady. The isoprene source was then cut off at the chamber while isoprene level was monitored at the fast isoprene sensor (Fig. 1A).
The true postillumination isoprene emission was obtained by subtracting chamber clearing from the observed emission. This usually generated two distinct peaks: the first assumed to be DMADP and the second referred to as early metabolites (Fig. 1B). Since we do not know the kinetics of reactions that convert the early metabolites to isoprene, the two-peaked postillumination trace cannot be precisely deconvoluted. A realistic estimate is obtained by simply drawing a vertical line at the minimum between the two peaks and separating the two peaks by this line (Fig. 1B, inset). This data analysis process is automated using the Peak Analyzer module in Origin (Originlab).
Data Analysis
One-way ANOVA and t tests (P < 0.05) were used to detect significant differences between groups. Mann-Whitney and Kruskal-Wallis tests were used in cases where a normality test failed. Statistical tests were carried out in SigmaPlot (Systat Software).
References
- Adam P, Hecht S, Eisenreich WG, Kaiser J, Grawert T, Arigoni D, Bacher A, Rohdich F. (2002) Biosynthesis of terpenes: studies on 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase. Proc Natl Acad Sci USA 99: 12108–12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agranoff BW, Eggerer H, Henning U, Lynen F. (1960) Biosynthesis of terpenes. VII. Isopentenyl pyrophosphate isomerase. J Biol Chem 235: 326–332 [PubMed] [Google Scholar]
- Badger MR, Sharkey TD, von Caemmerer S. (1984) The relationship between steady-state gas exchange of bean leaves and the levels of carbon-reduction-cycle intermediates. Planta 160: 305–313 [DOI] [PubMed] [Google Scholar]
- Bick JA, Lange BM. (2003) Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membrane. Arch Biochem Biophys 415: 146–154 [DOI] [PubMed] [Google Scholar]
- Calfapietra C, Mugnozza GS, Karnosky DF, Loreto F, Sharkey TD. (2008) Isoprene emission rates under elevated CO2 and O3 in two field-grown aspen clones differing in their sensitivity to O3. New Phytol 179: 55–61 [DOI] [PubMed] [Google Scholar]
- Calfapietra C, Wiberley AE, Falbel TG, Linskey AR, Mugnozza GS, Karnosky DF, Loreto F, Sharkey TD. (2007) Isoprene synthase expression and protein levels are reduced under elevated O3 but not under elevated CO2 (FACE) in field-grown aspen trees. Plant Cell Environ 30: 654–661 [DOI] [PubMed] [Google Scholar]
- Chameides WL, Lindsay RW, Richardson J, Kiang CS. (1988) The role of biogenic hydrocarbons in urban photochemical smog: Atlanta as a case study. Science 241: 1473–1475 [DOI] [PubMed] [Google Scholar]
- Dudareva N, Andersson S, Orlova I, Gatto N, Reichelt M, Rhodes D, Boland W, Gershenzon J. (2005) The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc Natl Acad Sci USA 102: 933–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall R, Wildermuth MC. (1998) Isoprene synthase: from biochemical mechanism to emission algorithm. J Geophys Res 103: 25599–25609 [Google Scholar]
- Fehsenfeld F, Calvert J, Fall R, Goldan P, Guenther AB, Hewitt CN, Lamb B, Liu S, Trainer M, Westberg H. (1992) Emissions of volatile organic compounds from vegetation and the implications for atmospheric chemistry: natural sources of acid precursors, neutralizing compounds, and oxidants. Global Biogeochem Cycles 6: 389–430 [Google Scholar]
- Guenther A, Karl T, Harley P, Wiedinmyer C, Palmer PI, Geron C. (2006) Estimates of global terrestrial isoprene emissions using MEGAN (model of emissions of gases and aerosols from nature). Atmos Chem Phys Discuss 6: 107–173 [Google Scholar]
- Hampel D, Mosandl A, Wüst M. (2005) Biosynthesis of mono- and sesquiterpenes in carrot roots and leaves (Daucus carota L.): metabolic cross talk of cytosolic mevalonate and plastidial methylerythritol phosphate pathways. Phytochemistry 66: 305–311 [DOI] [PubMed] [Google Scholar]
- Jones CA, Rasmussen RA. (1975) Production of isoprene by leaf tissue. Plant Physiol 55: 982–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiendler-Scharr A, Wildt J, Dal Maso M, Hohaus T, Kleist E, Mentel TF, Tillmann R, Uerlings R, Schurr U, Wahner A. (2009) New particle formation in forests inhibited by isoprene emissions. Nature 461: 381–384 [DOI] [PubMed] [Google Scholar]
- Koyama T, Katsuki Y, Ogura K. (1983) Studies on isopentenyl pyrophosphate isomerase with artificial substrates: Z-E isomerization of Z-3-methyl-3-pentenyl pyrophosphate. Bioorg Chem 12: 58–70 [Google Scholar]
- Kreuzwieser J, Graus M, Wisthaler A, Hanse A, Rennenberg H, Schnitzler JP. (2002) Xylem-transported glucose as an additional carbon source for leaf isoprene formation in Quercus robur. New Phytol 156: 171–178 [DOI] [PubMed] [Google Scholar]
- Laisk A, Kiirats O, Oja V. (1984) Assimilatory power (postillumination CO2 uptake) in leaves: measurement, environmental dependencies, and kinetic properties. Plant Physiol 76: 723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laule O, Fürholz A, Chang HS, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange M. (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 6866–6871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehning A, Zimmer I, Steinbrecher R, Bruggemann N, Schnitzler JP. (1999) Isoprene synthase activity and its relation to isoprene emission in Quercus robur L. leaves. Plant Cell Environ 22: 495–504 [Google Scholar]
- Loreto F, Centritto M, Barta C, Calfapietra C, Fares S, Monson RK. (2007) The relationship between isoprene emission rate and dark respiration rate in white poplar (Populus alba L.) leaves. Plant Cell Environ 30: 662–669 [DOI] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD. (1990) A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta 182: 523–531 [DOI] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD. (1993) On the relationship between isoprene emission and photosynthetic metabolites under different environmental conditions. Planta 189: 420–424 [DOI] [PubMed] [Google Scholar]
- Lützow M, Beyer P. (1988) The isopentenyl-diphosphate Δ-isomerase and its relation to the phytoene synthase complex in daffodil chromoplasts. Biochim Biophys Acta 959: 118–126 [Google Scholar]
- McGowan RE, Gibbs M. (1974) Comparative enzymology of the glyceraldehyde 3-phosphate dehydrogenases from Pisum sativum. Plant Physiol 54: 312–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson RK, Fall R. (1989) Isoprene emission from aspen leaves : influence of environment and relation to photosynthesis and photorespiration. Plant Physiol 90: 267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson RK, Hills AJ, Zimmerman PR, Fall RR. (1991) Studies of the relationship between isoprene emission rate and CO2 or photon-flux density using a real-time isoprene analyser. Plant Cell Environ 14: 517–523 [Google Scholar]
- Monson RK, Jaeger CH, Adams WW, Driggers EM, Silver GM, Fall R. (1992) Relationships among isoprene emission rate, photosynthesis, and isoprene synthase activity as influenced by temperature. Plant Physiol 98: 1175–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Tenhunen JD, Harley PC, Steinbrecher R. (1999) A model of isoprene emission based on energetic requirements for isoprene synthesis and leaf photosynthetic properties for Liquidambar and Quercus. Plant Cell Environ 22: 1319–1335 [Google Scholar]
- Possell M, Hewitt CN, Beerling DJ. (2005) The effects of glacial atmospheric CO2 concentrations and climate on isoprene emissions by vascular plants. Glob Change Biol 11: 60–69 [Google Scholar]
- Ramos-Valdivia AC, van der Heijden R, Verpoorte R. (1997) Isopentenyl diphosphate isomerase: a core enzyme in isoprenoid biosynthesis. A review of its biochemistry and function. Nat Prod Rep 14: 591–603 [DOI] [PubMed] [Google Scholar]
- Rasulov B, Copolovici L, Laisk A, Niinemets Ü. (2009a) Postillumination isoprene emission: in vivo measurements of dimethylallyldiphosphate pool size and isoprene synthase kinetics in aspen leaves. Plant Physiol 149: 1609–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Bichele I, Laisk A, Niinemets Ü. (2010) Temperature response of isoprene emission in vivo reflects a combined effect of substrate limitations and isoprene synthase activity: a kinetic analysis. Plant Physiol 154: 1558–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Välbe M, Laisk A, Niinemets Ü. (2009b) Evidence that light, carbon dioxide, and oxygen dependencies of leaf isoprene emission are driven by energy status in hybrid aspen. Plant Physiol 151: 448–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivasseau C, Seemann M, Boisson AM, Streb P, Gout E, Douce R, Rohmer M, Bligny R. (2009) Accumulation of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate in illuminated plant leaves at supraoptimal temperatures reveals a bottleneck of the prokaryotic methylerythritol 4-phosphate pathway of isoprenoid biosynthesis. Plant Cell Environ 32: 82–92 [DOI] [PubMed] [Google Scholar]
- Rohdich F, Hecht S, Gärtner K, Adam P, Krieger C, Amslinger S, Arigoni D, Bacher A, Eisenreich W. (2002) Studies on the nonmevalonate terpene biosynthetic pathway: metabolic role of IspH (LytB) protein. Proc Natl Acad Sci USA 99: 1158–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohdich F, Zepeck F, Adam P, Hecht S, Kaiser J, Laupitz R, Gräwert T, Amslinger S, Eisenreich W, Bacher A, et al. (2003) The deoxyxylulose phosphate pathway of isoprenoid biosynthesis: studies on the mechanisms of the reactions catalyzed by IspG and IspH protein. Proc Natl Acad Sci USA 100: 1586–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstiel TN, Potosnak MJ, Griffin KL, Fall R, Monson RK. (2003) Increased CO2 uncouples growth from isoprene emission in an agriforest ecosystem. Nature 421: 256–259 [DOI] [PubMed] [Google Scholar]
- Ruuska S, Andrews TJ, Badger MR, Hudson GS, Laisk A, Price GD, von Caemmerer S. (1998) The interplay between limiting processes in C-3 photosynthesis studied by rapid-response gas exchange using transgenic tobacco impaired in photosynthesis. Aust J Plant Physiol 25: 859–870 [Google Scholar]
- Sanadze GA, Kalandaze AN. (1966) Light and temperature curves of the evolution of C5H8. Sov Plant Physiol 13: 458–461 [Google Scholar]
- Scheibe R, Wedel N, Vetter S, Emmerlich V, Sauermann SM. (2002) Co-existence of two regulatory NADP-glyceraldehyde 3-P dehydrogenase complexes in higher plant chloroplasts. Eur J Biochem 269: 5617–5624 [DOI] [PubMed] [Google Scholar]
- Schnitzler JP, Zimmer I, Bachl A, Arend M, Fromm J, Fischbach RJ. (2005) Biochemical properties of isoprene synthase in poplar (Populus × canescens). Planta 222: 777–786 [DOI] [PubMed] [Google Scholar]
- Scholefield PA, Doick KJ, Herbert BMJ, Hewitt CNS, Schnitzler JP, Pinelli P, Loreto F. (2004) Impact of rising CO2 on emissions of volatile organic compounds: isoprene emission from Phragmites australis growing at elevated CO2 in a natural carbon dioxide spring. Plant Cell Environ 27: 393–401 [Google Scholar]
- Seemann M, Rohmer M. (2007) Isoprenoid biosynthesis via the methylerythritol phosphate pathway: GcpE and LytB, two novel iron-sulphur proteins. C R Chim 10: 748–755 [Google Scholar]
- Seemann M, Tse Sum Bui B, Wolff M, Miginiac-Maslow M, Rohmer M. (2006) Isoprenoid biosynthesis in plant chloroplasts via the MEP pathway: direct thylakoid/ferredoxin-dependent photoreduction of GcpE/IspG. FEBS Lett 580: 1547–1552 [DOI] [PubMed] [Google Scholar]
- Seemann M, Wegner P, Schünemann V, Bui BTS, Wolff M, Marquet A, Trautwein AX, Rohmer M. (2005) Isoprenoid biosynthesis in chloroplasts via the methylerythritol phosphate pathway: the (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE) from Arabidopsis thaliana is a [4Fe-4S] protein. J Biol Inorg Chem 10: 131–137 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Loreto F, Delwiche CF. (1991) High carbon dioxide and sun/shade effects on isoprene emission from oak and aspen tree leaves. Plant Cell Environ 14: 333–338 [Google Scholar]
- Sharkey TD, Seemann JR, Pearcy RW. (1986) Contribution of metabolites of photosynthesis to postillumination CO2 assimilation in response to lightflecks. Plant Physiol 82: 1063–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Singsaas EL. (1995) Why plants emit isoprene. Nature 374: 769 [Google Scholar]
- Sharkey TD, Wiberley AE, Donohue AR. (2008) Isoprene emission from plants: why and how. Ann Bot (Lond) 101: 5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singsaas EL. (2000) Terpenes and the thermotolerance of photosynthesis. New Phytol 146: 1–2 [Google Scholar]
- Singsaas EL, Laporte MM, Shi JZ, Monson RK, Bowling DR, Johnson K, Lerdau M, Jasentuliytana A, Sharkey TD. (1999) Kinetics of leaf temperature fluctuation affect isoprene emission from red oak (Quercus rubra) leaves. Tree Physiol 19: 917–924 [DOI] [PubMed] [Google Scholar]
- Singsaas EL, Lerdau M, Winter K, Sharkey TD. (1997) Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol 115: 1413–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singsaas EL, Sharkey TD. (2000) The effects of high temperature on isoprene synthesis in oak leaves. Plant Cell Environ 23: 751–757 [Google Scholar]
- Tennessen DJ, Singsaas EL, Sharkey TD. (1994) Light emitting diodes as a light source for photosynthesis research. Photosynth Res 39: 85–92 [DOI] [PubMed] [Google Scholar]
- Tingey DT, Evans RC, Bates EH, Gumpertz ML. (1987) Isoprene emissions and photosynthesis in three ferns: the influence of light and temperature. Physiol Plant 69: 609–616 [Google Scholar]
- Tingey DT, Manning M, Grothaus LC, Burns WF. (1979) The influence of light and temperature on isoprene emission rates from live oak. Physiol Plant 47: 112–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainer M, Williams EJ, Parrish DD, Buhr MP, Allwine EJ, Westberg HH, Fehsenfeld FC, Liu SC. (1987) Models and observations of the impact of natural hydrocarbons on rural ozone. Nature 329: 705–707 [Google Scholar]
- Tritsch D, Hemmerlin A, Bach TJ, Rohmer M. (2010) Plant isoprenoid biosynthesis via the MEP pathway: in vivo IPP/DMAPP ratio produced by (E)-4-hydroxy-3-methylbut-2-enyl diphosphate reductase in tobacco BY-2 cell cultures. FEBS Lett 584: 129–134 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387 [DOI] [PubMed] [Google Scholar]
- Wara-Aswapati O, Kemble RJ, Bradbeer JW. (1980) Activation of glyceraldehyde-phosphate dehydrogenase (NADP) and phosphoribulokinase in Phaseolus vulgaris leaf extracts involves the dissociation of oligomers. Plant Physiol 66: 34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedel N, Soll J. (1998) Evolutionary conserved light regulation of Calvin cycle activity by NADPH-mediated reversible phosphoribulokinase/CP12/glyceraldehyde-3-phosphate dehydrogenase complex dissociation. Proc Natl Acad Sci USA 95: 9699–9704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Went FW. (1960) Blue hazes in the atmosphere. Nature 187: 641–643 [Google Scholar]
- Wildermuth MC, Fall R. (1996) Light-dependent isoprene emission: characterization of a thylakoid-bound isoprene synthase in Salix discolor chloroplasts. Plant Physiol 112: 171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MJ, Monson RK, Trahan N, Lee S, Brown E, Jackson RB, Polley HW, Fay PA, Fall R. (2009) Leaf isoprene emission rate as a function of atmospheric CO2 concentration. Glob Change Biol 15: 1189–1200 [Google Scholar]
- Zepeck F, Gräwert T, Kaiser J, Schramek N, Eisenreich W, Bacher A, Rohdich F. (2005) Biosynthesis of isoprenoids: purification and properties of IspG protein from Escherichia coli. J Org Chem 70: 9168–9174 [DOI] [PubMed] [Google Scholar]
- Zhang R, Cruz JA, Kramer DM, Magallanes-Lundback ME, Dellapenna D, Sharkey TD. (2009) Moderate heat stress reduces the pH component of the transthylakoid proton motive force in light-adapted, intact tobacco leaves. Plant Cell Environ 32: 1538–1547 [DOI] [PubMed] [Google Scholar]
- Zhang R, Sharkey TD. (2009) Photosynthetic electron transport and proton flux under moderate heat stress. Photosynth Res 100: 29–43 [DOI] [PubMed] [Google Scholar]