Abstract
In this study, the role of the recently identified class of phytohormones, strigolactones, in shaping root architecture was addressed. Primary root lengths of strigolactone-deficient and -insensitive Arabidopsis (Arabidopsis thaliana) plants were shorter than those of wild-type plants. This was accompanied by a reduction in meristem cell number, which could be rescued by application of the synthetic strigolactone analog GR24 in all genotypes except in the strigolactone-insensitive mutant. Upon GR24 treatment, cells in the transition zone showed a gradual increase in cell length, resulting in a vague transition point and an increase in transition zone size. PIN1/3/7-green fluorescent protein intensities in provascular tissue of the primary root tip were decreased, whereas PIN3-green fluorescent protein intensity in the columella was not affected. During phosphate-sufficient conditions, GR24 application to the roots suppressed lateral root primordial development and lateral root forming potential, leading to a reduction in lateral root density. Moreover, auxin levels in leaf tissue were reduced. When auxin levels were increased by exogenous application of naphthylacetic acid, GR24 application had a stimulatory effect on lateral root development instead. Similarly, under phosphate-limiting conditions, endogenous strigolactones present in wild-type plants stimulated a more rapid outgrowth of lateral root primordia when compared with strigolactone-deficient mutants. These results suggest that strigolactones are able to modulate local auxin levels and that the net result of strigolactone action is dependent on the auxin status of the plant. We postulate that the tightly balanced auxin-strigolactone interaction is the basis for the mechanism of the regulation of the plants’ root-to-shoot ratio.
Strigolactones, exuded from plants, have been known for a long time to act as germination stimulants for seeds of root parasitic plants such as Orobanche and Striga spp. (for review, see Bouwmeester et al., 2003, 2007). As root parasitic plants consume a large proportion of the host plants’ solutes, they cause wilting and early plant death. Initially, the discovery that strigolactones are also involved in the symbiotic interaction with arbuscular mycorrhizal fungi (Akiyama et al., 2005) was believed to provide an explanation for why the host plants’ capacity to produce strigolactones was not lost during evolution. Because arbuscular mycorrhizal fungi are potent providers of nutrients such as phosphate (Pi) and nitrogen to their host, the observation that Pi starvation induced strigolactone biosynthesis in host plants’ roots was not surprising (Yoneyama et al., 2007; López-Ráez et al., 2008). The recent discovery that strigolactones, or closely related compounds, also act as phytohormones inside the host plants and are involved in the inhibition of axillary bud outgrowth (Gomez-Roldan et al., 2008; Umehara et al., 2008) is an additional explanation why plants continue to produce these fatal germination stimulants: plants use the strigolactones to adjust their shoot architecture to the ever-changing environmental conditions. Indeed, Pi starvation was shown to reduce the number of shoot branches (Cline, 1991), which was recently proven to be related to increased strigolactone production observed under these conditions (Kohlen et al., 2011).
The discovery that strigolactones are the same as, or are at least closely related to this branching inhibiting signal (BIS), which is a major player in the process of apical dominance, unexpectedly merged two worlds of research and provides new mutual tools and insights. Early studies on BIS revealed that it concerns a mobile, long-distance signal, which moves acropetally (Turnbull et al., 2002; Sorefan et al., 2003; Booker et al., 2005). In Arabidopsis (Arabidopsis thaliana), the carotenoid dioxygenases MORE AXILLARY GROWTH3 (MAX3; AtCCD7), MAX4 (AtCCD8), and the cytochrome P450 MAX1 (AtCyp711A1) are involved in the biosynthesis of BIS (Turnbull et al., 2002; Sorefan et al., 2003; Booker et al., 2004, 2005; Auldridge et al., 2006), whereas the F-box and Leu-rich repeats containing MAX2 protein probably acts either in signal perception or transduction (Stirnberg et al., 2002, 2007; Booker et al., 2005). Plants mutated in any of the MAX genes all display increased numbers of shoot branches. This mutant phenotype can be rescued by the application of the synthetic strigolactone analog GR24 (Gomez-Roldan et al., 2008; Umehara et al., 2008) in a MAX2-dependent manner.
Besides strigolactones, auxin is another phytohormone that is essential during the process of shoot branching control in apical dominance. In contrast with strigolactones, auxin moves basipetally in the main stem and indirectly controls axillary bud outgrowth (Booker et al., 2003). In a recent study by Hayward et al. (2009), it was shown that auxin positively regulates the expression of the strigolactone biosynthetic genes MAX3 and MAX4. Reduced local endogenous auxin levels, in naphthylphthalamic acid (NPA)-treated or decapitated plants, resulted in a reduction of the expression levels of these genes. In addition, Bainbridge et al. (2005) showed that auxin can locally induce MAX4 expression in the root tip. Strigolactone-deficient max mutant plants carrying the auxin reporter gene DR5-GUS were shown to have relatively high GUS intensities in vascular tissue of the lower stem (Bennett et al., 2006), suggesting elevated auxin levels. Recently, Prusinkiewicz et al. (2009) indeed demonstrated the presence of increased auxin levels in the polar transport stream of max4. Furthermore, an increase in polar auxin transport capacity, elevated mRNA levels of the polar auxin efflux carrier PIN1, and higher pPIN1-GUS activity in max mutants have been reported by Bennett et al. (2006). Finally, Crawford et al. (2010) demonstrate that polar auxin transport can be reduced by exogenous application of GR24 and induced endogenous strigolactone production in a max1 mutant background. The combination of these results suggests that strigolactones and auxin tightly interact and modulate each other’s levels and distribution through a feedback mechanism (Hayward et al., 2009).
Also, root growth and root branching are tightly regulated processes coordinately controlled by several plant hormones, of which auxin is playing a key role. Shoot-derived auxin is delivered to the root tip through the polar transport stream that is facilitated by proteins of the PIN family. In the columella root cap, auxin is redistributed laterally toward the epidermal and cortical cell layers where acropetal auxin transport toward the elongation zone establishes a local auxin gradient regulating cell division and elongation. Finally, auxin is returned to the polar transport stream again (Blilou et al., 2005; for review and schematic representation, see Leyser, 2006). During this process of PIN activity–dependent auxin recirculation inside the root tip, lateral root (LR) initiation is triggered by the local accumulation of auxin in root pericycle cells adjacent to the xylem vessels (Casimiro et al., 2001; De Smet et al., 2006, 2007; Dubrovsky et al., 2008, Lucas et al., 2008). Subsequent tightly regulated cell division will then lead to LR primordial development and finally to emergence of a young LR from the parent root. In contrast with LR initiation, LR development is supported by auxin coming directly from the aerial part of the plant (Bhalerao et al., 2002). To allow and sustain lateral auxin influx from the polar auxin transport stream into the developing LR, PIN1 polarity is rearranged. This dynamic repolarization is mediated by endocytic recycling of the PIN1 protein (Jaillais et al., 2007). The subsequent establishment of a proper auxin gradient inside the developing LR primordia, which is also mediated by members of the PIN protein family, is crucial for correct LR development (Benková et al., 2003). Finally, at the stage when an autonomous meristem is formed, the LR primordium is able to produce its own auxin and becomes independent of auxin from the shoot (Casimiro et al., 2003).
Because auxin and auxin transport play such crucial roles in defining root system architecture (RSA) and strigolactones have been suggested to play a role in regulating auxin fluxes, we investigated the contribution of this new plant hormone to root developmental processes. In this study, we describe the effect of application of the synthetic strigolactone GR24 on primary root and LR development in relation to auxin in both strigolactone-deficient and -insensitive Arabidopsis mutants carrying the auxin reporter construct DR5-GUS. We report that GR24 application has a dual effect on primary root length, LR development, and LR initiation, of which the net result is dependent on the auxin status of the plant. Our results suggest that these effects are mediated through the modulation of local auxin levels in the root tip and developing lateral root primordia (LRP) and a reduction in free auxin levels in the aerial parts of the plant. Finally, we hypothesize that strigolactones are responsible for the changes in RSA of Arabidopsis plants growing under Pi-limiting conditions.
RESULTS
Application of the Strigolactone Analog GR24 Leads to a MAX2-Dependent Increase in Primary Root Length
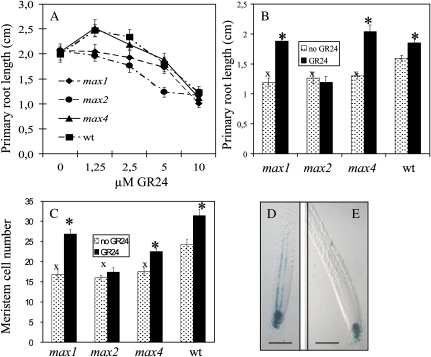
Growth and development of roots of 8, 11, and 14-d-old Arabidopsis seedlings of the strigolactone biosynthesis mutants max1-1 and max4-1, the strigolactone signaling mutant max2-1, and their corresponding wild type (Columbia-0) was examined. Plants were grown in the presence of different concentrations, ranging from 1.25 to 10 μm, of the synthetic strigolactone analog GR24. This concentration range was based on the level of GR24 required for a complete rescue of the branched phenotype of the max1, 3, and 4 mutant plants (data not shown). Application of 1.25 μm GR24 resulted in increased primary root lengths in 8-d-old wild-type plants and max4 but not in max1 and max2 (Fig. 1A). GR24 concentrations above 2.5 to 5 μm inhibited primary root elongation in a MAX2-independent manner. In 11- and 14-d-old plants, the increase in root length was not observed anymore (data not shown). To explore the possibility that under optimal growing conditions a general high rate of primary root growth could obscure the specific root growth stimulating effect of GR24, plants were subsequently grown under less favorable conditions. Because carbohydrate starvation leads to a decrease in primary root length that is not mediated through reduced meristem activity (Jain et al., 2007), Suc was omitted from the medium. Under these conditions, all genotypes, including max1, but not max2, showed a clear response to GR24 (2.5 μm) treatment, further confirming that the response to GR24 is mediated through MAX2 (Fig. 1B). Moreover, roots of untreated max mutant plants were significantly shorter than those of untreated wild-type plants. This correlated with a lower number of cortical cells in the primary root meristem (Fig. 1C) and a higher DR5-GUS intensity in primary root tips of max mutant plants containing the auxin reporter construct DR5-GUS (Fig. 1, D and E). Finally, GR24 application at 2.5 μm resulted in an increase in meristem cortical cell number in all genotypes except max2 (Fig. 1C).
Figure 1.
GR24 affects primary root length in a concentration-dependent way. A, Primary root lengths of 8-d-old max1-1, max2-1, max4-1, and wild-type (wt) Arabidopsis plants grown on vertical MS plates containing 0.5% Suc and different levels of GR24. B, Primary root length of 12-d-old plants grown on vertical MS plates containing no Suc and 0 or 2.5 μm GR24. Data are means ± se (n = 16–20). C, Cortical meristem cell number, expressed as the number of cells in one cell file extending from the quiescent center to the first elongated cell, of 7-d-old Arabidopsis plants grown on vertical MS plates containing 0.5% Suc and 0 or 2.5 μm GR24. Data are means ± se (n = 5). Marks indicate a statistically significant difference compared with untreated (asterisk) or wild-type plants (x) as determined by Student’s t test (P < 0.05). D and E, Nomarski images of GUS-stained primary roots of max4 (D) and wild-type (E) plants containing the DR5-GUS reporter construct. Bars = 0.1 mm. [See online article for color version of this figure.]
GR24-Mediated Increase in Primary Root Length Is Accompanied by an Increase in Meristem and Transition Zone Sizes
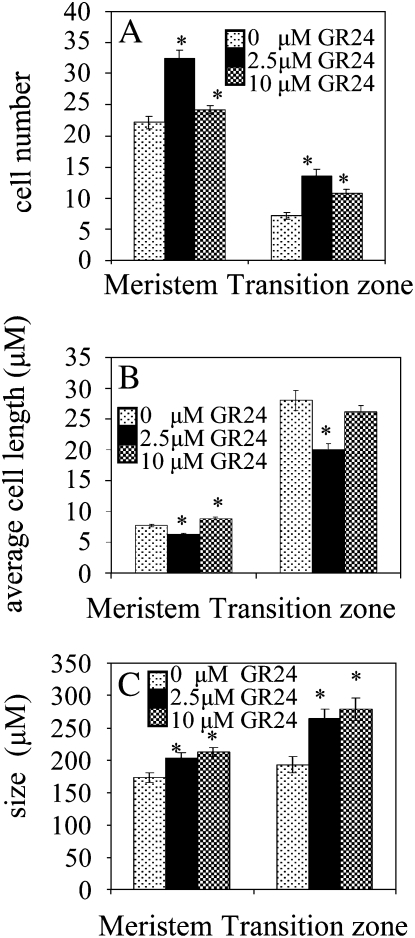
To further investigate the effect of GR24 on meristem cells as well as on cells present in the transition zone (the region between meristem and elongation zone), the number and length of all root cortical cells in one cell file extending from the 10th cell above the quiescent center until the elongation zone were determined in wild-type plants. By plotting the cell number of individual plants against their cumulative cell length, an impression of root cell dynamics in response to different concentrations of GR24 is obtained (Supplemental Fig. S1, A–C). Application of 2.5 μm GR24 resulted in a tremendous increase in the number of cells in this region (Fig. 2A). When 10 μm GR24 was applied, the meristem and transition zones only showed a minor increase in cell number when compared with untreated plants (Fig. 2A). For both GR24 concentrations, cells in the transition zone showed a strikingly slow and irregular increase in cell length before they reached their final stabilized elongated state (Supplemental Fig. S1, B–D). Cells in the meristem zone of plants treated with 2.5 μm GR24 were shorter than in untreated plants, whereas 10 μm GR24 treatment resulted in a small increase in meristem cell length (Fig. 2B). Both GR24 concentrations resulted in decreased cell lengths in the transition zone. Despite these reduced cell sizes, the increased cell numbers of the transition zone finally gave rise to an increase in transition zone size (Fig. 2C). Also, meristem size was increased by both GR24 treatments. With the higher doses of GR24 (5–10 μm), root curvature was induced in some plants (Supplemental Fig. S1E). When primary root tips of these plants were studied using confocal microscopy, a distortion of the columella and quiescent center was observed (Supplemental Fig. S1F).
Figure 2.
Application of GR24 affects cortical root cell number and length of the meristem and transition zone in a concentration-dependent way. A, Average cortical cell number present in a cell file starting from the quiescent center throughout the meristem and transition zone. B, Average length of cortical cells in meristem and transition zone. C, Meristem and transition zone size. Measurements were performed using 7-d-old wild-type plants grown in the presence of different concentrations of GR24. Data are means ± se (n = 12–16). Asterisks indicate a statistically significant difference between treated and untreated plants as determined by Student’s t test (P < 0.05).
The localization of the transition point, as well as the size of the meristem and transition zone, is largely controlled by the local establishment of an auxin gradient. High auxin levels stimulate cell proliferation, whereas low auxin levels favor cell elongation. The establishment of the auxin concentration gradient in the cortical meristem and transition zone is regulated by auxin efflux facilitating proteins of the PIN family. These proteins jointly control the recirculation of auxin in the root tip (Blilou et al., 2005).
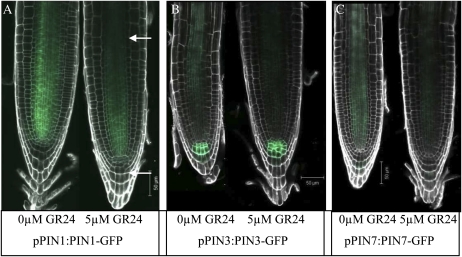
To explore a potential effect of GR24 on the levels and distribution of the PIN1, PIN2, PIN3, and PIN7 proteins, which are involved in this auxin circulation process, we examined 6-d-old wild-type plants carrying constructs encoding the respective PINpromoter-PINprotein-GFP fusion proteins. In the provascular region, application of 2.5, 5 (Fig. 3), and 10 μm GR24 resulted in a significant (P < 0.001) reduction in GFP intensity in the PIN1 (38% reduction), PIN3 (50% reduction), and PIN7 (73% reduction) reporter lines. There was no significant effect on PIN2-GFP levels (data not shown). In the columella, treatment with 2.5 μm GR24 resulted in a minor reduction in PIN7-GFP intensity, while the level of PIN3-GFP was not significantly affected. Interestingly, upon GR24 treatment, PIN3-GFP signal was observed in a larger number of columella cells that also displayed irregular shapes (Fig. 3B). Although the latter was also occasionally observed in GR24-treated nontransformed plants, the incidence was higher in transgenic plants carrying the pPIN3:PIN3-GFP construct. Finally, root curvature was found to be associated with this distortion of columella cells.
Figure 3.
PIN-GFP protein levels and localization in roots of GR24-treated and untreated Arabidopsis plants. A to C, Confocal midplane sections of roots of untreated and 5 μm GR24-treated 6-d-old plants carrying the pPIN1:PIN1-GFP (A), pPIN3:PIN3-GFP (B), and pPIN7:PIN7-GFP (C) transgenes. The upper arrow indicates the provascular region, and the lower arrow indicates the localization of the columella. PI-stained cell walls are represented in white. The intensity of the GFP signal was quantified by converting RGB pixels to brightness values using the program ImageJ (Abramoff et al., 2004).
GR24 Decreases LR Density through a Suppression of LR Outgrowth and a Reduction in LR-Forming Potential
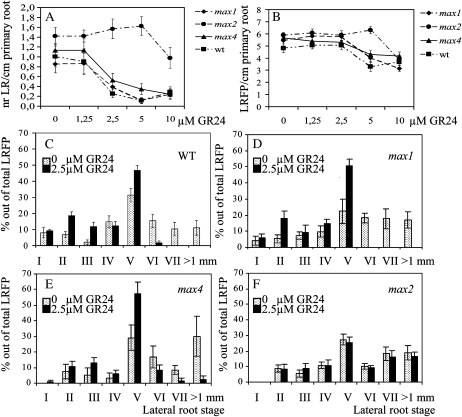
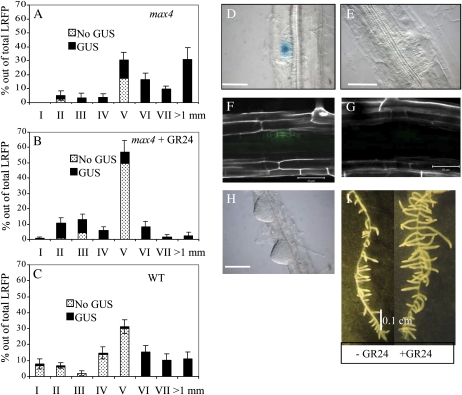
GR24 also affected lateral root density (LRD) and total lateral root-forming potential (LRFP). The latter is defined as the sum of emerged LRs plus LRP. At concentrations of 2.5 and 5 μM GR24, the biosynthetic max mutants (max1 and max4) and wild-type Arabidopsis showed a significant reduction in LRD but not max2 (Fig. 4A). A MAX2-independent decrease in LRD was observed at the highest concentration of 10 μm GR24. LRFP was not affected at the lower concentrations of 1.25 and 2.5 μm GR24. However, at 5 μm GR24, a clear MAX2-dependent decrease was observed (Fig. 4B). Finally, like LRD, LRFP also was negatively affected in the max2 mutant line when 10 μm GR24 was applied.
Figure 4.
GR24 treatment decreases LRD (A) and total LRFP (B) and delays LR development (C–F). LRFP is defined as the sum of all LRs plus LRP. max1-1, max2-1, max4-1, and wild-type (wt) plants were grown either on a range of GR24 levels (A and B) or on a fixed concentration of 2.5 μm GR24 (C–F) on vertical MS plates with (A and B) or without (C–F) 0.5% Suc and were evaluated at 14 (A and B) or 12 (C–F) d after germination. Data are means ± se (n = 20–25). LR developmental stages were characterized according to the scheme of Malamy and Benfey (1997).
Because at 2.5 μm GR24 LRD was decreased while LRFP was not affected, it seems likely that at this concentration the reduction in LRD is caused by a reduction in LRP outgrowth. To explore this assumption and assess whether LR outgrowth was affected randomly or if it concerned the suppression of a specific LRP developmental stage, all LRP in the primary roots of all genotypes under investigation were classified and counted according to the histological scale of Malamy and Benfey (1997). For each stage, the number of LRP was expressed as a percentage of the total LRFP. GR24 treatment resulted in a significantly higher accumulation of LRP stage V in all genotypes except max2 (Fig. 4, C–F). Also, 2.5 μm GR24 almost completely suppressed the developmental transition of LRP stage V into stage VI and abolished LR formation in both the wild type and max1, but much less so in max4, and it did not affect max2.
LR development is primarily determined by auxin signaling (for review, see Woodward and Bartel, 2005). To explore if the suppressive effect of GR24 on LRP outgrowth is mediated through the modulation of auxin levels and/or auxin distribution patterns in LRP, the percentage of LRP associated with GUS staining of the auxin reporter gene construct DR5-GUS present in GR24-treated and untreated max4 plants was determined. Although LRP with low (or absent) DR5-GUS levels were observed in untreated max4 plants (Fig. 5A), GR24 treatment resulted in a strong increase in the percentage of non-DR5-GUS-stained stage V LRP in max4 (Fig. 5B). Wild-type plants (producing endogenous strigolactones) also exhibited more non-GUS-stained stage V LRP than max4 (Fig. 5, C–E), suggesting that the effect of GR24/endogenous strigolactones on LR outgrowth is mediated through a reduction in free auxin levels, thereby leading to an arrest in LRP development. Arrested stage V LRP (Fig. 5E) were not able to form the polarized central cell files that are characteristic of stage VI LRP. Because PIN1 activity contributes to the formation of an auxin gradient that is directing cellular organization in developing LRP (Benková et al., 2003), we studied PIN1-GFP abundance in GR24-treated and untreated wild-type stage V LRP expressing pPIN1:PIN1-GFP. Figure 5, F and G, shows that GR24 treatment resulted in decreased PIN1-GFP intensities.
Figure 5.
The GR24-induced accumulation and developmental arrest of stage V LRP is mediated through an increase in the percentage of stage V LRP associated with low or no intensities of DR5-GUS. Untreated max4-1 (A) and wild type (WT; C) and treated (2.5 μm GR24) max4-1 plants (B) carrying the DR5-GUS transgene were grown on vertical MS plates and GUS stained when plants were 12 d old. LRP were characterized according to Malamy and Benfey (1997). For each developmental stage, DR5-GUS intensities were scored and the percentage out of the total LRFP was calculated. Data are means ± se (n = 15–20). D and E, Nomarski microscopy images of a GUS-stained stage V LRP showing DR5-GUS intensities and distribution patterns representative for the majority of nonarrested stage V LRP (D) and arrested stage V LRP (E). Scale bars represent 50 μm. F and G, Confocal microscopy images of untreated (F) and GR24-treated (2.5 μm; G) wild-type plants carrying the pPIN1:PIN1-GFP transgene. Scale bars represent 50 μm. When plants are grown in the presence of high exogenous auxin levels, the suppressive effect of GR24 on LRP development is lost while LR outgrowth is enhanced. H, Wild-type plants, pregrown for 5 d on standard vertical MS plates, were transferred to MS plates containing 10 μm IAA in combination with 10 μm GR24. The Nomarski image was taken 72 h after the start of the treatment. Scale bar represents 100 μm. I, Image taken 8 d after start of the treatment, showing LR elongation and increased LR density of the GR24-treated plants.
The Effect of GR24 on LRP Development and Outgrowth Is Dependent on the Auxin Status of the Plant
To investigate the auxin-mediated nature of strigolactone action, the effect of GR24 in the presence of high exogenous levels of auxin was investigated. Wild-type plants were grown for 5 d on vertical plates and subsequently transferred to plates containing 10 μm of the naturally occurring auxin indole-3-acetic acid (IAA) or 2.5 μm of the synthetic auxin naphthylacetic acid (NAA), either supplemented with or without 5 or 10 μm GR24. Roots were evaluated after 12, 24, and 72 h and after 8 d. NAA and IAA treatment strongly stimulated the initiation of LRP. GR24 treatment did not affect the timing of LRP initiation. All LRP in all treatments readily developed into LRs without showing any sign of deviating cellular organization (Fig. 5H), indicating that the previously observed inhibitory effect of GR24 on LR development is absent if enough auxin is supplied to the developing LRP. Treatment with 5 μm GR24 did not reduce PIN1-GFP intensities in developing LRP of NAA-treated (2.5 μm) plants (data not shown), suggesting that the previously observed reduction in PIN1-GFP intensity is a secondary effect caused by reduced auxin levels. Only when 10 μm GR24 was applied, a minor decrease in pPIN1:PIN1-GFP intensity was observed. Still, a physiological effect of 5 μm GR24 on LRP development was observed. In contrast with the previous observations showing an inhibitory effect of GR24 application on LRP development, simultaneous application of 2.5 μm NAA and 5 μm GR24 resulted in a stimulation of LRP development. Moreover, after 8 d of treatment, GR24 application resulted in significantly longer roots (0.5 instead of 0.4 mm; P < 0.02) in both NAA- and IAA-treated plants (Fig. 5I). Surprisingly, LRD also increased from 41 to 64 LR/cm (P < 0.0005) as a result of GR24 application.
These results suggest that the physiological response upon GR24 treatment is mediated through a modulation of local auxin levels and is therefore dependent on the auxin status and/or sensitivity of the plant. To test this hypothesis, the effect of GR24 on LR development was investigated in max mutant and wild-type plants grown under Pi starvation. Pérez-Torres et al. (2008) showed that the increase in LR formation in Pi-starved Arabidopsis seedlings is, at least in part, mediated by an increase in auxin sensitivity of root cells. Plants were grown for 5 d under Pi-sufficient conditions and then transferred to plates containing a limiting (20 μm) Pi concentration supplemented with or without 2.5 μm GR24. The distribution of the different LRP stages was determined 12 d after germination. In wild-type plants, GR24 application resulted in a reduction in the proportion of emerged LRs (Supplemental Fig. S2A). However, GR24 failed to reduce this proportion in the max mutants (Supplemental Fig. S2, B and C). This is also reflected by the absence of a clear effect of GR24 on the accumulation of stage V LRP in these latter genotypes, which did occur in the wild type. Apparently, the combination of higher initial DR5-GUS intensities as observed in max mutant roots, either suggesting elevated auxin levels or increased auxin sensitivity, and the increased auxin sensitivity induced by Pi deprivation (López-Bucio et al., 2002; Pérez-Torres et al., 2008) together lead to a loss of the inhibitory effect of GR24 on LR development.
GR24-Induced Suppression of LRP Outgrowth Is Partially Mediated through Decreased Shoot-Derived Auxin Levels
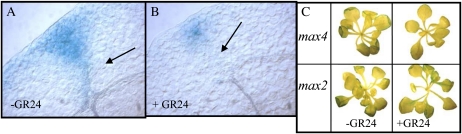
LRP development is stimulated by shoot-derived auxin. To explore whether the GR24-mediated reduction in DR5-GUS levels in LRP, leading to the suppression of LRP development, could have been mediated through reduced auxin levels in the aerial parts of the plants, DR5-GUS intensities in the rosette leaves and cotyledons of transgenic DR5-GUS max and wild-type plants were studied. Plants were grown for 12 d on Murashige and Skoog (MS) agar plates containing 0, 1.25, 2.5, 5, or 10 μm GR24. All GR24 concentrations tested decreased DR5-GUS intensities in the leaf margins and the tissue surrounding the hydathodes (Fig. 6, A and B), which are the primary sites of auxin production in young Arabidopsis plants (Aloni et al., 2003). The observed reduction was dependent on the presence of MAX2 (Fig. 6C). Besides decreased DR5-GUS levels around the auxin biosynthesis sites, vascularization between hydathodes and leaf veins was also negatively affected (Fig. 6, A and B). Quantification of auxin in leaf material from max2 and max4 mutant lines using liquid chromatography-tandem mass spectrometry showed a 79% reduction in auxin levels of max4 (14.44–2.98 pg/mg fresh weight, Student’s t test, P = 0.013) upon GR24 treatment, while no significant reduction was observed when max2 was treated with GR24. Finally, the leaf surface area was decreased by application of 2.5 μm GR24 in a MAX2-dependent way (Supplemental Fig. S3).
Figure 6.
GR24 treatment results in decreased intensities of the auxin reporter DR5-GUS in aerial parts of the plant. A and B, Close-up of a leaf of an untreated (B) and GR24-treated (C) max4-1 plant. Arrows point at the locations where developing vasculature is either present (A) or absent (B). C, GUS staining of the leaves of 12-d-old max4-1 and max2-1 plants carrying the DR5-GUS transgene grown in the presence or absence of 2.5 μm GR24 showing that the decrease in GUS intensities is dependent on MAX2.
Endogenous Strigolactones Stimulate LR Outgrowth during Pi-Limiting Conditions
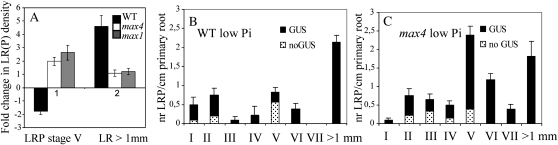
Because the high levels and potential ectopic localization of GR24 may obscure the true effects that endogenous strigolactones have on root system architecture, the relevance of endogenous strigolactones in determining RSA was investigated. Therefore, LR development in 12-d-old max1, max4, and wild-type plants grown under Pi-sufficient and Pi-limiting conditions was studied. Under Pi-sufficient conditions, LRFP was equal for all genotypes (data not shown). However, max4 plants showed a significantly (P < 0.05) higher LRD than wild-type plants, implying that during Pi-sufficient conditions, the endogenous strigolactones of wild-type plants had a suppressive effect on LR development (Supplemental Fig. S4A).
However, Pi limitation in the wild type resulted in an almost 2-fold decrease in LRP of stage V and a more than 4-fold increase in LRD (Fig. 7A; Supplemental Fig. S4B). In the max mutants, Pi limitation induced accumulation of stage V LRP instead. DR5-GUS-mediated visualization of the auxin status of all stages of LRP in wild-type and max4 plants grown under Pi-limiting conditions revealed that the majority of the accumulated stage V LRP of max4 plants consisted of intensely stained cells (Fig. 7, B and C).
Figure 7.
Endogenous strigolactones in wild-type (WT) plants allow a more rapid development of LRP into LRs during phosphate-limiting conditions. A, Graph showing fold change in LRP V and LRD of 12-d-old wild-type, max4-1, and max1-1 plants as a response to Pi-limiting conditions relative to sufficient Pi conditions. Plants were pregrown for 5 d on vertical MS plates containing sufficient Pi levels (1.5 mm), after which all plants were transferred to either Pi-deficient (20 μm Pi) or Pi-sufficient MS plates. B and C, DR5-GUS distribution in LRP of wild-type (B) and max4-1 (C) plants carrying the DR5-GUS transgene grown under Pi-limiting conditions showing that the majority of the highly accumulated max4-1 stage V LRP is associated with extremely high GUS intensities. Data are means ± se (n = 15–20).
DISCUSSION
In this study, a novel role for strigolactones in determining root architecture through an effect on primary root growth and LR development is described. Evidence for this role is provided by experiments studying the application of the synthetic strigolactone analog GR24 (discussed in the first half of the discussion). Differences in root architecture between wild-type and strigolactone-deficient plants provide evidence for the role of endogenous strigolactones in this trait. This, together with a broader view on strigolactone action at the whole-plant level, is discussed in the second half of the “Discussion.”
GR24 Affects Primary Root Growth in a Concentration-Dependent Manner
Arabidopsis plants, grown in the presence of different concentrations of GR24, showed an increase in primary root length at lower levels of GR24 (1.25 and 2.5 μm) and a decrease at higher levels of GR24 (Fig. 1A). This decrease was also observed in max2. Because a high dose of GR24 also affects the entire plant’s appearance and fitness, this is likely to be the result of general toxicity. Still, a high dose of GR24 did not disturb the GR24-specific elongating effect on meristem and transition zone size (Fig. 2) and could explain why the primary root length in max2 shows a stronger decrease at 5 μm GR24 when compared to the other genotypes.
Unexpectedly, max1 did not show the initial increase in primary root length upon 1.25 μm GR24 application as was observed in max4 and wild-type plants (Fig. 1A). This may be due to a so far unexplained reduced sensitivity to GR24, which was also observed when a concentration range of GR24 was used to rescue the branching phenotype of the max1, max3, and max4 mutants (data not shown). Because root length was significantly reduced in all genotypes at 5 and 10 μm GR24, indicating a general MAX2-independent response, a putative MAX2-dependent increase in max1 root lengths at higher GR24 levels could have been masked. Although Kohlen et al. (2011) show that the Arabidopsis max1 mutant is compromised in strigolactone levels, it could be that MAX1 is also involved in more downstream hydroxylation steps and is able to modify the synthetic strigolactone GR24 increasing its biological activity.
GR24-Mediated Changes in Root Meristem Patterning Are Indicative of Altered Local Auxin Concentrations
The effect of GR24 on primary root growth is accompanied by a GR24 concentration-dependent change in both cell number and cell length of cells located in the root meristem and transition zones (Fig. 2). Since high auxin levels are known to stimulate cell division whereas low auxin levels favor cell elongation, it can be concluded that lower levels (2.5 μm) of GR24 induce an increase in auxin levels in the primary root meristem, while higher doses tend to reduce these levels. These responses reflect the auxin-mediated nature of strigolactone action. Local auxin concentrations are established by the combined action of five auxin efflux proteins of the PIN family, jointly regulating auxin fluxes circulating in the primary root tip (Blilou et al. 2005). Therefore, auxin transport is a major contributor to root meristem patterning (Sabatini et al., 1999; Friml et al., 2003). Prusinkiewicz et al. (2009) suggested that strigolactones act by modulating PIN protein cycling between the plasma membrane and endomembrane system, hereby regulating the allocation of PINs to the plasma membrane. A GR24-mediated reduction in PIN protein cycling would then result in a decrease in auxin transport capacity in vascular tissue of both root and shoot, as these are the main sites of MAX2 expression (Stirnberg et al., 2007). Indeed, Crawford et al. (2010) recently demonstrated that both endogenous strigolactones and GR24 are able to reduce basipetal auxin transport. This could finally explain the changes in root meristem patterning we observed in this study. In an in silica study in which reduced auxin transport was enforced by simulating a reduction in vascular PIN expression, increased auxin levels were also observed in the border cells (defined as the layer of cells between the vascular and epidermal region) of the primary root tip (Grieneisen et al., 2007). Although the underlying cause for reduced auxin transport in that study may differ from this study, it illustrates that a GR24-mediated reduction in auxin transport is likely to be involved. Interestingly, in the same modeling work, Grieneisen et al. (2007) also show that the absence of lateral epidermal PINs, responsible for the auxin reflux to the main polar transport stream, results in a more spread out and uneven auxin distribution. This is provoking the loss of a clear transition point separating the meristem zone from the elongation zone, which was also observed in this study. The latter may thus be the consequence of a negative effect of GR24 on the efficiency of these lateral epidermal PINs.
Biological Relevance of the GR24-Mediated Changes in Root Meristem Patterning
The contribution of the GR24-mediated increase in transition zone size to the total increase in primary root length is relatively high (Fig. 2C). Expansion of the transition zone is also observed in radicle growth during seed germination (Sliwinska et al., 2009). Interestingly, in Arabidopsis, the strigolactone biosynthetic gene CCD8 is specifically expressed in the root cortical and epidermal cells of the transition-elongation zone upon auxin treatment (Bainbridge et al., 2005). Moreover, MAX2 expression also is elevated in this part of the root (Brady et al., 2007). Therefore, strigolactone-mediated modulation of the lateral auxin reflux, which occurs in this particular region, could be responsible for the increase in transition zone size during radicle growth. It is not unlikely that this process is at the basis of the germination of seeds of most plant species, including parasitic plants. Still, the underlying mechanism for exogenous strigolactone dependency of parasitic plant germination remains an intriguing issue.
Interesting is the GR24-induced lateral expansion of PIN3 protein localization to adjacent cells in the root cap. The disturbed cellular organization in this region furthermore suggests irregular cell divisions (Fig. 3B). If strigolactones are involved in PIN protein cycling as suggested by Prusinkiewicz et al. (2009), a GR24-mediated distortion of PIN3 polarization could have resulted in nonregular auxin fluxes and ectopic PIN3 distribution. Asymmetric lateral distribution of the PIN3 protein, leading to auxin asymmetry in the elongation zone, is also characteristic for the induction of root curvature during gravitropism (Friml et al., 2002; Ottenschläger et al., 2003) and may explain the observed induction of root curvature in this study when using higher levels of GR24 (Supplemental Fig. S1E). In tomato (Solanum lycopersicum), Koltai et al. (2009) also observed asymmetric root growth with high levels of GR24 (27 μm). In our study, root curvature was found to be associated with a distortion of the columella and quiescent center (Supplemental Fig. S1F). Interestingly, especially in the view of the effect of GR24 on auxin levels in the leaves, a similar collapse of this structure was observed in primary root tips that were depleted of auxin (Friml et al., 2004).
It would be of interest to explore whether strigolactones are involved in stimulating directional growth of the parasitic plants’ radicle toward the host root. Asymmetric perception of exuded strigolactones by the parasitic plants’ radicle might lead to a single-sided reduction in PIN cycling efficiency and subsequent auxin accumulation. As in gravitropism, this would result in the redirection of radicle growth, in this case toward the strigolactone source, the host root.
GR24 Application Leads to a Reduction of Auxin Levels in Leaf Tissue
GR24 reduces the intensity of the auxin reporter DR5-GUS and auxin levels in young expanding rosette leaves in a MAX2-dependent way. This is accompanied by a decrease in the number of vascular connections between the auxin production sites surrounding the hydathodes and the major leaf veins. Also, a reduction in leaf size was observed that is a known consequence of a reduction in auxin content (Ljung et al., 2001). Because MAX2 is expressed in vascular tissue throughout the entire plant, it is likely that the putative GR24-mediated reduction in PIN1 cycling as suggested by Prusinkiewicz et al. (2009) also occurs in vascular tissue of the leaf and stem resulting in a decrease in auxin transport capacity. Besides this effect, a GR24-induced reduction in PIN1 cycling could also be responsible for a reduction in the auxin-induced, PIN polarization-dependent canalization properties responsible for the formation of new vascular tissue (Sachs, 2000; Sauer et al., 2006; Prusinkiewicz et al., 2009). These effects would lead to a local and temporal accumulation of auxin, finally provoking a negative feedback on free auxin levels, explaining the observed reduction in DR5-GUS intensities and auxin levels in leaf tissue of GR24-treated plants. In a study applying the auxin transport inhibitor NPA to young Arabidopsis plants, Ljung et al. (2001) also observed a feedback inhibition of auxin biosynthesis in expanding leaves and cotyledons. In our study, a reduction in both auxin biosynthesis and auxin transport capacity would subsequently lead to a reduction in the auxin supply to the root system, influence primary root growth and meristem patterning, and reduce LRP initiation and development.
Combining the results of Brewer et al. (2009), showing that strigolactones act downstream of auxin, and the results of this study in which we show that strigolactones in their turn are able to modulate auxin levels, we postulate that strigolactones and auxin operate in a tightly regulated feedback circuit.
GR24 Application Influences LRP Development
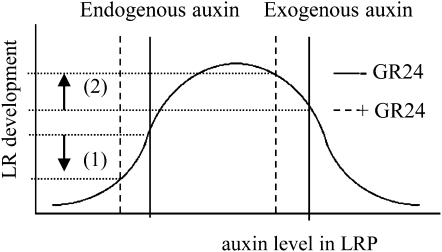
GR24 significantly reduced LRD, which is the combined result of suppressed LRP development and reduced total LRFP (Fig. 4). These processes are dependent on different auxin fluxes (Lucas et al., 2008). LR initiation is stimulated by auxin circulating in the root tip (Casimiro et al., 2003), being the net result of auxin influx from aerial parts of the plant and the dynamics of auxin (re)fluxes and production in the root tip itself. By contrast, LRP development is solely dependent on auxin sources directly derived from aerial parts of the plant. Auxin is delivered to the LRP through polar auxin transport (Bhalerao et al., 2002) and is subsequently imported into the developing LRP through repolarization of the PIN1 protein allowing lateral auxin influx. Interestingly, the results of this study indicate that GR24/strigolactones are able to modulate all these processes mentioned above. Since it was observed that GR24 reduces auxin levels in aerial parts of the plant, it is likely that the amount of auxin reaching the majority of the LRP is not sufficient to sustain subsequent LRP development beyond stage V (Fig. 8, left part of graph).
Figure 8.
Schematic representation of the putative mechanism of GR24 action in root system architecture. Bell-shaped auxin response model for the auxin-mediated effect of the synthetic strigolactone GR24 on LR development. (1) Under sufficient Pi conditions, GR24 application has an inhibitory effect on LR development mediated through a reduction in auxin levels in the polar auxin transport stream coming from the shoot. (2) In the presence of exogenous auxin, LR development is increased instead. Under these conditions, a GR24-mediated reduction of auxin levels reaching LRP, through reduced auxin import into the developing LRP, results in an auxin concentration closer to the auxin optimum, thereby increasing the LR developmental rate.
The GR24-Mediated Reduction of PIN-GFP Levels in LRP Is a Secondary Effect
When similar levels of GR24, which were previously found to reduce PIN1-GFP intensities in the LRP, were applied in the presence of exogenous auxin, PIN1-GFP intensities were not affected. This suggests that GR24 is not directly affecting PIN expression but that the decrease in PIN-GFP levels is a consequence of reduced auxin levels. Indeed, PIN gene expression is known to be auxin inducible (Vieten et al., 2005).
Although no changes in PIN1-GFP levels were observed, simultaneous application of GR24 and auxin still affected LRP development. Interestingly, however, under these conditions, GR24 had a stimulatory effect on LRP development instead. Because it is hypothesized that a reduction in PIN cycling is the direct effect resulting from strigolactone action (Prusinkiewicz et al., 2009), it is likely that the lateral auxin influx into the LRP, which is facilitated by repolarization of PIN1 proteins located in vascular tissue of the root, is disturbed by GR24 treatment. This would lead to a reduction in the supra-optimal auxin levels inside the NAA-treated LRP, provoking a shift toward the auxin optimum that is stimulating maximum LRP development (Fig. 8, right part of graph). Apparently, this auxin reduction was too low to be reflected by decreased PIN1-GFP intensities. Another observation that is in line with this one is the reduction (in the wild type) or loss (in max mutants) of the suppressive effect of GR24 on LR development when plants were grown under Pi deficiency, a condition known to enhance auxin sensitivity (López-Bucio et al., 2002; Pérez-Torres et al., 2008; Supplemental Fig. S2). These results once more demonstrate that strigolactones act through the modulation of auxin levels and that the net result of strigolactone action is dependent on the auxin status of the plant.
GR24 Has a Dual Effect on LR Initiation Depending on the Auxin Status of the Plant
LR initiation starts with the auxin-induced division of pericycle founder cells. Auxin reaches these cells through the PIN protein-mediated lateral auxin reflux at the level of the transition zone in the root tip (Casimiro et al., 2001; De Smet et al., 2007). Within the founder cells, a fraction of the auxin reflux accumulates until a LR initiation threshold is reached (Lucas et al., 2008). GR24 has a dual effect on LR initiation depending on the auxin status of the plant. During normal physiological conditions, low levels of GR24 application do not affect LR initiation. As already described, under these conditions the GR24-mediated decrease in meristem cell size suggests increased auxin levels in the epidermal/cortical meristem zone. When these locally increased auxin levels are combined with a slight reduction in the rate of lateral auxin reflux, which is transporting auxin from the epidermal/cortical cell layers back to the polar transport stream in provascular tissue, net levels of accumulated auxin in founder cells will remain unchanged. Only higher doses of GR24, further reducing the apical auxin supply, hereby reducing the auxin levels in epidermal/cortical cell layers, will lead to a reduction in LR initiation. These results agree with the root branching model, as proposed by Lucas et al. (2008), which explains differences in root branching by local changes in auxin transport.
When plants are grown in the presence of NAA, LR initiation is significantly enhanced by GR24 application. A constant high exogenous auxin supply, combined with a GR24-induced reduction in lateral cellular auxin efflux, will result in an increase in auxin accumulation in pericycle founder cells. Interestingly, increased LR initiation was also observed for the pin2 pin3 pin7 triple mutant (Laskowski et al., 2008), which may partially mimic the effect of GR24 application under exogenous NAA administration.
Implications for the Role of Endogenous Strigolactones under Natural Conditions at the Whole-Plant Level
Our results show that exogenous application of the strigolactone analog GR24 affects both primary root and LR growth as well as LRP initiation and development. GR24 is a synthetic strigolactone and, as used in our experiments, a mixture of two stereoisomers (Mangnus et al., 1992) that may have a different effect on root development. Experiments with pure, natural strigolactones are therefore needed to confirm our findings. However, in our study, we also used strigolactone-deficient mutants, which form the best material to study the true action of strigolactones on root architecture. max mutants have shorter primary roots containing fewer meristem cells (Fig. 1, B and C), while they have a higher DR5-GUS intensity in the provascular region (Fig. 1D). Interestingly, max mutant plants also have smaller leaves (Supplemental Fig. S3), which probably reflect higher auxin levels (Ljung et al., 2001). Because LRP development is dependent on auxin derived from the shoot (Bhalerao et al., 2002), this could explain why max mutants tend to have a higher LRD. Although this was only significant for max4 and not max1 and max2 (Fig. 4, C–F), the majority of stage V LRP in max mutant plants were associated with a clear DR5-GUS signal, showing the presence of a properly formed auxin maximum. It is likely that most of these stage V LRP will develop into LRs. Therefore, the relatively small difference in LRD observed between young wild-type and max mutant plants is likely to become larger during later stages of plant development. The difference in LR development between wild-type and strigolactone-deficient plants could be analogous to GR24 application during Pi-sufficient conditions (Fig. 8, left part of graph). Interestingly, under Pi-limiting conditions, the opposite is observed (Fig. 7A). In this situation, LR development in wild-type plants is enhanced when compared to the max mutants. The mechanism leading to this result is likely to be similar to the situation in which GR24 application in the presence of exogenous NAA was shown to enhance LR outgrowth (Figs. 5I and 8, right part of graph).
Strigolactones are also known to suppress bud outgrowth (Gomez-Roldan et al., 2008; Umehara et al., 2008). Because strigolactone production is enhanced under Pi deficiency in tomato (López-Ráez et al., 2008), red clover (Trifolium pratense; Yoneyama et al., 2007), and Arabidopsis (Kohlen et al., 2011), the desirable response of reduced shoot branching under low Pi conditions would be achieved. Recent results in our lab using the Arabidopsis max mutants demonstrate that low Pi-induced strigolactone biosynthesis is indeed responsible for the reduction in shoot branching under low Pi conditions (Kohlen et al., 2011). This results in enhanced carbon allocation to the roots sustaining an increase in root branching to expand the exploratory capacity of the root system (Bates and Lynch, 1996; López-Bucio et al., 2002; Sánchez-Calderón et al., 2005). In this study, we demonstrate that, in addition to controlling shoot architecture, endogenous strigolactones also play an important role in stimulating LR development under Pi-limiting conditions. This is in contrast with Pi-sufficient conditions, during which endogenous strigolactones limit the outgrowth of LRP. Therefore, we postulate that the major role of strigolactones in plant development lies in the coordinated, balanced control of the root-to-shoot branching ratio under continuously changing environmental conditions.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of the max1-1, max2-1 (Stirnberg et al., 2002), max4-1 (Sorefan et al., 2003), and their parental Columbia-0 wild-type lines either carrying (Bennett et al., 2006) or not carrying the DR5-GUS transgene were kindly provided by Prof. O. Leyser (University of York, UK). Seeds of the pPIN1/2/3/7::PIN1/2/3/7:GFP lines (Benková et al., 2003; Friml et al., 2003) were kindly provided by Prof. J. Friml (Ghent University, Belgium). Before sowing on MS plates, seeds were surface sterilized in 10% (w/v) chlorine bleach and then washed with 70% (w/v) ethanol and sterile distilled water. Seeds were imbibed on wet filter paper at 4°C for 2 to 4 d and plated on MS/agar plates (0.5× MS salts supplemented with 1× Gamborg’s B5 vitamin mix, 0.8% [w/v] agar [Daichin], without [unless stated otherwise] Suc at pH 5.8). Plants were grown either on horizontal (for leaf surface measurements) or on near vertical plates (for root system architecture analysis) in a climate chamber under a 22°C/18°C 16-h-light/8-h-dark regime (80 μmol m−2 s−1).
Pi starvation experiments were conducted by transferring plants pregrown (5 d) on Pi-sufficient (1.25 mm) MS plates to low Pi (20 μm) MS plates. LR induction by high levels of NAA (2.5 and 10 μm) or IAA (10 μm) was performed according to Himanen et al. (2002) with the exception that during the 5-d period of pregrowing NPA was omitted from the medium. All experiments were repeated at least three times.
GUS Staining
Histochemical GUS staining was performed according to Stomp (1992). The GUS activities were visualized by incubating the seedlings with the GUS substrate 5-bromo-4-chloro-3-indolyl-β-glucuronic acid for 13 h at 37°C. After clearing overnight in 70% ethanol, the plants were stored in 4°C prior to imaging.
Root System Architecture Measurements
Pictures of the root systems grown on MS plates were taken with a digital camera (Canon EOS 350 d) and were proportionally enlarged and printed to measure primary root lengths using a curvimeter. Images of NAA/IAA-treated plants were taken with a digital camera connected to a stereomicroscope at 5× magnification. LRP developmental stages were counted and evaluated using a Nikon Optiphot microscope equipped with Nomarski optics at 10× magnification. Roots were cleared for 2 to 16 h in a drop of Hoyer’s solution (7.5 g gum arabic, 100 g chloral hydrate, and 5 mL glycerol in 30 mL water) on a microscope slide. LRP developmental stages were classified according to the system of Malamy and Benfey (1997). The number of root meristem cells was determined by counting cortical cells in one cell file, starting from the quiescent center until the first cell showing signs of rapid elongation using confocal microscopy.
Confocal Microscopy
Roots of seedlings expressing GFP were incubated for 10 min in 1 μm propidium iodide (PI) in growth medium prior to imaging, washed, and coverslip mounted for imaging on an Axiovert 200M with a Zeiss 510 META confocal laser scanning microscope (Carl Zeiss). Representative root tips closest to the coverslip were selected for imaging with a 10× (numerical aperture of 0.3) or 20× (numerical aperture of 0.4) Fluar objective. Imaging was done in a reproducible manner starting with similar sample preparation, to image acquisition settings and data processing, for all experiments. Samples were excited with 5% of a 488-nm laser (emission from a 30-mW argon tube) for GFP excitation and 80% of a 543-nm laser (emission from a 1-mW helium-neon tube) for PI excitation. Optical sections of roots and subsequent z series were made using DM 488/543, EM 505 to 530 (GFP in green), and EM LP 615 (PI in white). Transmission images were simultaneously collected.
Single midplane optical sections were selected and compared, while LRP could most accurately be counted from z series, flat projected for maximal pixel value. Image analysis was done using Zeiss LSM Image Examiner (version 3.5), ImageJ version 1-32, and Adobe Photoshop CS2 (Adobe Systems).
Stereomicroscopy
Roots were imaged on a Zeiss stereo Discovery (A12) with a Plan S 1.0× FWD 81 mm (1–100×) objective. Images were taken with an AxioCam MRc5 (5 MPix camera; Zeiss) and analyzed using AxioVision 4.6 software.
IAA Extraction of Arabidopsis Leaf Material
For IAA analysis, 200 mg of root or shoot tissue was ground in a mortar with liquid nitrogen. The samples were extracted with 1 mL of cold methanol containing [phenyl 13C6]-IAA (0.1 nmol/mL) as internal standard in a 2-mL Eppendorf tube. The tubes were vortexed and sonicated for 10 min in a Branson 3510 ultrasonic bath (Branson Ultrasonics) and placed overnight in orbital shaker at 4°C. The samples were centrifuged for 10 min at 11,500 rpm in a Heraeus Fresco 17 centrifuge (Thermo Scientific) at 4°C, after which the organic phase was transferred to a 4-mL glass vial. The pellets were reextracted with another 1 mL of methanol. The combined methanol fractions were further purified by anion-exchange column (Grace Pure Amino 500 mg/3 mL SPE) as previously described (Chen et al., 1988), dried in a SpeedVacuum Savant SPD121P (Thermo Scientific), and the residue dissolved in 200 μL of acetonitrile:water:formic acid (25:75:0.1, v/v/v). Before liquid chromatography-tandem mass spectrometry analysis, samples were filtered through Minisart SRP4 0.45-μm filters (Sartorius).
IAA Detection and Quantification by Liquid Chromatography-Tandem Mass Spectrometry
Analysis of IAA in Arabidopsis (Arabidopsis thaliana) leaf extracts was performed by comparing retention times and mass transitions with those of IAA standard using a Waters Xevo tandem quadruple mass spectrometer using the settings previously described by Kohlen et al. (2011) and the gradient described for ABA by López-Ráez et al. (2010). Multiple reaction monitoring (MRM) was used for IAA quantification. Parent-daughter transitions were set according to the tandem mass spectrometry spectra obtained for the standards IAA and [phenyl 13C6]-IAA. Transitions were selected based on the most abundant and specific fragment ions for which the collision energy was optimized. For identification, the MRM transitions mass-to-charge ratio 176 > 103 at a collision energy of 30 eV and 176 > 130 at 16 eV; and for [phenyl 13C6]-IAA, the transitions mass-to-charge ratio 182 > 109 at 28 eV and 182 > 136 at 16 eV were selected. Cone voltage was set to 18 eV. IAA was quantified using a calibration curve with known amount of standards and based on the ratio of the area of the MRM transition 176 > 130 for IAA to the MRM transition 182 > 136 for [phenyl 13C6]-IAA. Data acquisition and analysis were performed using MassLynx 4.1 software (Waters). The summed area of all the corresponding MRM transitions was used for statistical analysis.
Leaf Surface Quantification
Twenty-five seedlings were grown horizontally for 12 d on 9-cm-wide petri dishes in triplicates. Images were taken using a digital camera (Nikon D80 with Nikkor AF-S 60 mm f/2.8 G Micro ED) connected to a computer using Nikon camera control pro software version 2.0. Image analysis was performed using ImageJ based on segmentation by color-thresholding using visual scripting for ImageJ according to Joosen et al. (2010).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Application of GR24 affects cortical root cell dynamics of the proliferation and transition zone in a concentration-dependent way.
Supplemental Figure S2. The inhibitory effect of GR24 application on LRP development is decreased in plants grown under phosphate-limiting conditions.
Supplemental Figure S3. GR24 treatment results in a decrease in leaf surface.
Supplemental Figure S4. The effect of endogenous strigolactones on lateral root development in Pi-sufficient and Pi-limiting conditions.
Supplementary Material
Acknowledgments
We thank Jiři Friml for providing the Arabidopsis lines carrying the pPIN1/2/3/7:PIN1/2/3/7-GFP transgenes. We are grateful to Ottoline Leyser who supplied us with seeds of the max1-1, max2-1, and max4-1 Arabidopsis lines carrying the DR5-GUS transgene. We thank Binne Zwanenburg for supplying GR24.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. (2004) Image processing with ImageJ. Biophotonics International 7: 36–42 [Google Scholar]
- Akiyama K, Matsuzaki K, Hayashi H. (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827 [DOI] [PubMed] [Google Scholar]
- Aloni R, Schwalm K, Langhans M, Ullrich CI. (2003) Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta 216: 841–853 [DOI] [PubMed] [Google Scholar]
- Auldridge ME, Block A, Vogel JT, Dabney-Smith C, Mila I, Bouzayen M, Magallanes-Lundback M, DellaPenna D, McCarty DR, Klee HJ. (2006) Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J 45: 982–993 [DOI] [PubMed] [Google Scholar]
- Bainbridge K, Sorefan K, Ward S, Leyser O. (2005) Hormonally controlled expression of the Arabidopsis MAX4 shoot branching regulatory gene. Plant J 44: 569–580 [DOI] [PubMed] [Google Scholar]
- Bates TR, Lynch JP. (1996) Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ 19: 529–538 [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16: 553–563 [DOI] [PubMed] [Google Scholar]
- Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G. (2002) Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29: 325–332 [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 [DOI] [PubMed] [Google Scholar]
- Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser O. (2004) MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr Biol 14: 1232–1238 [DOI] [PubMed] [Google Scholar]
- Booker J, Chatfield S, Leyser O. (2003) Auxin acts in xylem-associated or medullary cells to mediate apical dominance. Plant Cell 15: 495–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, Turnbull C, Srinivasan M, Goddard P, Leyser O. (2005) MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell 8: 443–449 [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Matusova R, Zhongkui S, Beale MH. (2003) Secondary metabolite signalling in host-parasitic plant interactions. Curr Opin Plant Biol 6: 358–364 [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Roux C, López-Raez JA, Bécard G. (2007) Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci 12: 224–230 [DOI] [PubMed] [Google Scholar]
- Brady SM, Orlando DA, Lee JY, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, Benfey PN. (2007) A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318: 801–806 [DOI] [PubMed] [Google Scholar]
- Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA. (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 150: 482–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ. (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8: 165–171 [DOI] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, et al. (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KH, Miller AN, Patterson GW, Cohen JD. (1988) A rapid and simple procedure for purification of indole-3-acetic acid prior to GC-SIM-MS analysis. Plant Physiol 86: 822–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline MG. (1991) Apical dominance. Bot Rev 57: 318–358 [Google Scholar]
- Crawford S, Shinohara N, Sieberer T, Williamson L, George G, Hepworth J, Müller D, Domagalska MA, Leyser O. (2010) Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137: 2905–2913 [DOI] [PubMed] [Google Scholar]
- De Smet I, Tetsumura T, De Rybel B, Frey NF, Laplaze L, Casimiro I, Swarup R, Naudts M, Vanneste S, Audenaert D, et al. (2007) Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134: 681–690 [DOI] [PubMed] [Google Scholar]
- De Smet I, Vanneste S, Inzé D, Beeckman T. (2006) Lateral root initiation or the birth of a new meristem. Plant Mol Biol 60: 871–887 [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benková E. (2008) Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci USA 105: 8790–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G. (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PBF, Ljung K, Sandberg G, et al. (2004) A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306: 862–865 [DOI] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194 [DOI] [PubMed] [Google Scholar]
- Grieneisen VA, Xu J, Marée AFM, Hogeweg P, Scheres B. (2007) Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449: 1008–1013 [DOI] [PubMed] [Google Scholar]
- Hayward A, Stirnberg P, Beveridge C, Leyser O. (2009) Interactions between auxin and strigolactone in shoot branching control. Plant Physiol 151: 400–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inzé D, Beeckman T. (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14: 2339–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Santambrogio M, Rozier F, Fobis-Loisy I, Miège C, Gaude T. (2007) The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell 130: 1057–1070 [DOI] [PubMed] [Google Scholar]
- Jain A, Poling MD, Karthikeyan AS, Blakeslee JJ, Peer WA, Titapiwatanakun B, Murphy AS, Raghothama KG. (2007) Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiol 144: 232–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosen RV, Kodde J, Willems LA, Ligterink W, van der Plas LH, Hilhorst HW. (2010) GERMINATOR: a software package for high-throughput scoring and curve fitting of Arabidopsis seed germination. Plant J 62: 148–159 [DOI] [PubMed] [Google Scholar]
- Kohlen W, Charnikhova T, Liu Q, Bours R, Domagalska MA, Beguerie S, Verstappen F, Leyser O, Bouwmeester H, Ruyter-Spira C. (2011) Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol 155: 974–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltai H, Dor E, Hershenhorn J, Joel DM, Weininger S, Lekalla S, Shealtiel H, Bhattacharya C, Eliahu E, Resnick N, et al. (2009) Strigolactones’ effect on root growth and root-hair elongation may be mediated by auxin-efflux carriers. J Plant Growth Regul 29: 129–136 [Google Scholar]
- Laskowski M, Grieneisen VA, Hofhuis H, Hove CA, Hogeweg P, Marée AF, Scheres B. (2008) Root system architecture from coupling cell shape to auxin transport. PLoS Biol 6: e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser O. (2006) Dynamic interaction of auxin transport and signaling. Curr Biol 16: 424–433 [DOI] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G. (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28: 465–474 [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L. (2002) Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol 129: 244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Ráez JA, Charnikhova T, Gómez-Roldán V, Matusova R, Kohlen W, De Vos R, Verstappen F, Puech-Pages V, Bécard G, Mulder P, et al. (2008) Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol 178: 863–874 [DOI] [PubMed] [Google Scholar]
- López-Ráez JA, Kohlen W, Charnikhova T, Mulder P, Undas AK, Sergeant MJ, Verstappen F, Bugg TDH, Thompson AJ, Ruyter-Spira C, et al. (2010) Does abscisic acid affect strigolactone biosynthesis? New Phytol 187: 343–354 [DOI] [PubMed] [Google Scholar]
- Lucas M, Guédon Y, Jay-Allemand C, Godin C, Laplaze L. (2008) An auxin transport-based model of root branching in Arabidopsis thaliana. PLoS ONE 3: e3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Mangnus EM, Dommerholt FJ, de Jong RLP, Zwanenburg B. (1992) Improved synthesis of strigol analogue GR24 and evaluation of the biological activity of its diastereomers. J Agric Food Chem 40: 1230–1235 [Google Scholar]
- Ottenschläger I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K. (2003) Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 100: 2987–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Torres CA, López-Bucio J, Cruz-Ramírez A, Ibarra-Laclette E, Dharmasiri S, Estelle M, Herrera-Estrella L. (2008) Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 20: 3258–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O. (2009) Control of bud activation by an auxin transport switch. Proc Natl Acad Sci USA 106: 17431–17436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, et al. (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472 [DOI] [PubMed] [Google Scholar]
- Sachs T. (2000) Integrating cellular and organismic aspects of vascular differentiation. Plant Cell Physiol 41: 649–656 [DOI] [PubMed] [Google Scholar]
- Sánchez-Calderón L, López-Bucio J, Chacón-López A, Cruz-Ramírez A, Nieto-Jacobo F, Dubrovsky JG, Herrera-Estrella L. (2005) Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana. Plant Cell Physiol 46: 174–184 [DOI] [PubMed] [Google Scholar]
- Sauer M, Balla J, Luschnig C, Wiśniewska J, Reinöhl V, Friml J, Benková E. (2006) Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev 20: 2902–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinska E, Bassel GW, Bewley JD. (2009) Germination of Arabidopsis thaliana seeds is not completed as a result of elongation of the radicle but of the adjacent transition zone and lower hypocotyl. J Exp Bot 60: 3587–3594 [DOI] [PubMed] [Google Scholar]
- Sorefan K, Booker J, Haurogné K, Goussot M, Bainbridge K, Foo E, Chatfield SP, Ward S, Beveridge CA, Rameau C, et al. (2003) MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev 17: 1469–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P, Furner IJ, Ottoline Leyser HM. (2007) MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J 50: 80–94 [DOI] [PubMed] [Google Scholar]
- Stirnberg P, van De Sande K, Leyser HMO. (2002) MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129: 1131–1141 [DOI] [PubMed] [Google Scholar]
- Stomp AM. (1992) Histochemical localization of β-glucuronidase activity. Gallagher SR, , GUS Protocols: Using the GUS Gene as a Reporter of Gene Expression. Academic Press, San Diego, pp 103–113 [Google Scholar]
- Turnbull CG, Booker JP, Leyser HM. (2002) Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J 32: 255–262 [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200 [DOI] [PubMed] [Google Scholar]
- Vieten A, Vanneste S, Wiśniewska J, Benková E, Benjamins R, Beeckman T, Luschnig C, Friml J. (2005) Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132: 4521–4531 [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95: 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H. (2007) Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 225: 1031–1038 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.