Abstract
Reactivation of dormant meristems is of central importance for plant fitness and survival. Due to their large meristem size, potato (Solanum tuberosum) tubers serve as a model system to study the underlying molecular processes. The phytohormones cytokinins (CK) and gibberellins (GA) play important roles in releasing potato tuber dormancy and promoting sprouting, but their mode of action in these processes is still obscure. Here, we established an in vitro assay using excised tuber buds to study the dormancy-releasing capacity of GA and CK and show that application of gibberellic acid (GA3) is sufficient to induce sprouting. In contrast, treatment with 6-benzylaminopurine induced bud break but did not support further sprout growth unless GA3 was administered additionally. Transgenic potato plants expressing Arabidopsis (Arabidopsis thaliana) GA 20-oxidase or GA 2-oxidase to modify endogenous GA levels showed the expected phenotypical changes as well as slight effects on tuber sprouting. The isopentenyltransferase (IPT) from Agrobacterium tumefaciens and the Arabidopsis cytokinin oxidase/dehydrogenase1 (CKX) were exploited to modify the amounts of CK in transgenic potato plants. IPT expression promoted earlier sprouting in vitro. Strikingly, CKX-expressing tubers exhibited a prolonged dormancy period and did not respond to GA3. This supports an essential role of CK in terminating tuber dormancy and indicates that GA is not sufficient to break dormancy in the absence of CK. GA3-treated wild-type and CKX-expressing tuber buds were subjected to a transcriptome analysis that revealed transcriptional changes in several functional groups, including cell wall metabolism, cell cycle, and auxin and ethylene signaling, denoting events associated with the reactivation of dormant meristems.
Potato (Solanum tuberosum) is a staple food belonging to the three most important crops worldwide. Potato tubers are radially expanded stem axes formed by shortened internodes and nodes (“eyes”). They originate from underground stolons in a series of developmental processes comprising the cessation of growth at the apex, the swelling of the stolon by subapical radial growth, and the induction of longitudinal cell divisions and later randomly orientated divisions and enlargement of the body (Xu et al., 1998). The latter process is accompanied by the accumulation of starch and storage proteins and requires coordinated transcriptional and metabolic changes (Visser et al., 1994; Appeldoorn et al., 1999; Kloosterman et al., 2005, 2008). After formation, potato tubers undergo a period of dormancy that is characterized by the absence of visible bud growth. It is generally accepted that the onset of dormancy coincides with the cessation of meristematic activity during tuber initiation (Burton, 1989). The length of the dormancy period depends on the genetic background and is affected by preharvest and postharvest conditions (Sonnewald, 2001; Suttle, 2004a). With the onset of sprouting, the tuber turns into a source organ supporting growth of the developing sprout. This is associated with structural and metabolic changes (Viola et al., 2007) as well as with major changes in gene expression pattern (Ronning et al., 2003; Campbell et al., 2008). Additionally, endogenous plant hormones play a critical role in the regulation of dormancy and bud break (Hemberg, 1985; Suttle, 2004a).
GAs and cytokinins (CK) are thought to be involved in the release of dormancy (Smith and Rappaport, 1961; Turnbull and Hanke, 1985a), whereas abscisic acid (ABA) and ethylene have been associated with the onset and maintenance of tuber dormancy (Suttle, 1998b). While the role of ethylene in potato tuber dormancy is still obscure (Suttle, 2004a), the significance of ABA in regulating this process was first recognized by Hemberg (1949), while it was still known as β-inhibitor complex. Analyzing the ABA content of different potato varieties revealed a continuous decline in ABA during storage, but there was no correlation between the absolute ABA levels and the sprouting behavior of different potato varieties (Suttle and Hultstrand, 1994; Suttle, 1995; Biemelt et al., 2000). Therefore, a threshold level of ABA for the maintenance of dormancy could not be established. The observed decrease in ABA content during potato tuber storage is most likely due to the activation of catabolism while dormancy advances (Destefano-Beltrán et al., 2006).
To date, not much attention has been paid to the role of indole-3-acetic acid (IAA) in controlling tuber dormancy and sprouting. Recently, Sorce and colleagues (2009) performed a detailed study to investigate how auxin influences tuber dormancy. Although the concentration of free IAA in buds decreases during dormancy, additional immunolocalization experiments suggest that IAA might stimulate bud growth by enhancing early differentiation processes. Supporting this assumption, Faivre-Rampant et al. (2004) identified an auxin response factor gene (ARF6) as a marker for meristem reactivation in potato tubers.
Since their first isolation in 1935, GAs have been known to stimulate growth, and their dormancy-terminating capacity in potato was first reported in the mid 1950s (Brian et al., 1955; Rappaport, 1956). Subsequent studies showed an increase of endogenous GA-like substances prior to or together with the onset of sprouting (Smith and Rappaport, 1961; Bialek and Bielinska-Czarnecka, 1975), suggesting GA as a regulator of sprouting. Nevertheless, the role of GAs in dormancy regulation remains controversial, as quantitative analysis showed increased levels of GA1 and GA20, the predominant bioactive GA in potato and its immediate precursor, only in tubers that already exhibited actively elongating sprouts (Suttle, 2004b). Furthermore, manipulation of the endogenous GA levels by ectopic expression of the potato GA biosynthesis genes GA 20-oxidase (GA20ox1) and GA2ox1 did not significantly alter tuber dormancy (Carrera et al., 2000; Kloosterman et al., 2007), indicating a role for GA in sprout growth rather than in dormancy release.
CKs have been named for their ability to stimulate cell division and are involved in many growth and developmental processes. Early studies by Hemberg (1970) showed that both natural and synthetic CKs can rapidly induce sprouting in dormant tubers. Bioassays suggested an increase of endogenous CK levels before dormancy release, especially in the apical and lateral buds and the tissue surrounding them (Engelbrecht and Bielinska-Czarnecka, 1972; Van Staden and Dimalla, 1978). Evidence from immunological studies (Turnbull and Hanke, 1985b; Suttle, 1998a) confirmed an increase in bioactive CKs before dormancy release. Additionally, differences in sensitivity to applied CKs were observed in tuber tissues. At harvest and the beginning of the storage period, tubers were unresponsive to CK but exhibited increasing sensitivity as dormancy progressed (Turnbull and Hanke, 1985b; Suttle, 2001). Suttle (2001) also found that this was not associated with changes in CK metabolism and hypothesized that CK signal perception and/or transduction were influenced by the physiological status of the tuber.
At the cellular level, dormancy is most likely characterized by a G1-phase arrest of the meristematic cells, as indicated by microdensitometry (MacDonald and Osborne, 1988) and flow cytometry measurements (Campbell et al., 1996). Release from this arrest requires D-type cyclins (CycD), of which three groups have been isolated in Arabidopsis (Arabidopsis thaliana), CycD1, CycD2, and CycD3 (Soni et al., 1995; Renaudin et al., 1996). During the cell division cycle, D-type cyclins form complexes with cyclin-dependent kinases (CDKs), which then phosphorylate retinoblastoma-related proteins. This releases E2F transcription factors and triggers G1-to-S-phase transition (for review, see Horvath et al., 2003; Berckmans and De Veylder, 2009).
In this work, we aimed at improving our understanding of the regulation of potato tuber dormancy and sprouting by GAs and CKs. Therefore, we have established an in vitro assay for synchronous induction of sprouting that enabled us to monitor developmental changes caused by GA or CK. Furthermore, we examined transgenic plants with increased or decreased levels of either phytohormone to investigate their impact on sprouting behavior. Finally, we performed microarray studies by applying our in vitro assay to transgenic plants with decreased CK levels and wild-type potato tubers, which has allowed us to unravel the sequence of phytohormone action that leads to sprouting.
RESULTS
Treatment of Excised Potato Tuber Buds with GA3 Triggers Synchronous Sprouting
Although potato tuber dormancy has been extensively studied, the coordinated fashion in which phytohormones regulate this process is little understood. This may be due to the nonsynchronized sprouting of potato tubers, rendering kinetic studies during natural sprouting almost impossible. To circumvent this shortcoming and to synchronize sprouting, an in vitro sprout-release assay was developed. The assay is based on isolated tuber buds that are placed on wet filter paper in closed petri dishes. Upon the addition of different hormone solutions, bud growth can be followed over time. Since GA3 is known to support sprout growth (Suttle, 2004a), the initial assay was based on GA3 applications.
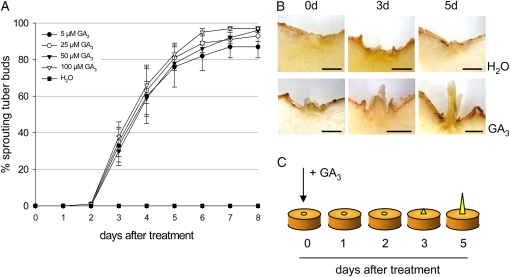
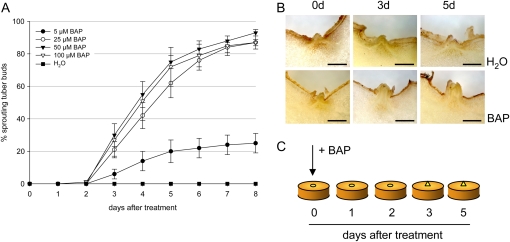
To determine the optimal concentration of GA3, different concentrations ranging from 5 to 100 μm were tested (Fig. 1A). Regardless of the concentration tested, first signs of sprouting became visible 3 d after GA3 treatment, and after 5 d, sprouts of 2 to 3 mm length could be clearly seen (Fig. 1B). After 7 d, at least 95% of the buds treated with 25, 50, or 100 μm GA3 had sprouted (Fig. 1A), the sprouts being thin and very elongated. A lower sprouting efficiency was observed for tuber buds treated with only 5 μm GA3 (Fig. 1A). Since there were essentially no differences in the ability to trigger sprouting between 25 and 100 μm GA3, 50 μm was chosen for further experiments. Control tuber discs treated with water showed no signs of sprouting during the period investigated (Fig. 1B).
Figure 1.
In vitro sprouting assay using different concentrations of GA3. Two weeks after storage, tuber discs containing one bud each were isolated and incubated for 5 min with 5, 25, 50, or 100 μm GA3 and water as a control. The tuber discs were kept in darkness and monitored daily for visible sprouting. A, The percentage of sprouted tuber buds after application of different GA3 concentrations during an 8-d period. The data represent means ± sd of two independent experiments (n = 25). B, Cross sections through tuber buds before and 3 and 5 d after treatment with either 50 μm GA3 or water. Bars = 1 mm. C, Schematic drawing of the in vitro tuber-sprouting experiment using GA3. [See online article for color version of this figure.]
In the experiment shown in Figure 1, tuber discs were excised from tubers 2 weeks after harvest. Since a time-dependent increase in GA sensitivity during the dormancy period has been published (Suttle, 2004b), we also wanted to test whether or not GA3 treatment can induce sprouting of discs taken from freshly harvested or deeply dormant tubers and whether GA3 responsiveness changes during storage. In all our experiments, incubation of tuber discs with 50 μm GA3 was sufficient to break dormancy with similar kinetics. However, the efficiency was reduced in discs from freshly harvested tubers (Supplemental Fig. S1A). Here, sprouting occurred in about 80% of tuber discs after 6 d, while almost all discs taken 3 or 6 weeks after harvest showed sprouting in the same time period. On the other hand, control tuber discs isolated from tubers stored for 6 weeks also started to sprout, although at a low rate (10%; Supplemental Fig. S1A), indicating that these tubers had probably lost their endodormancy.
Together these results show that GA3 can trigger tuber sprouting even of deeply dormant tubers within 3 d of treatment in a synchronous fashion, as is schematically depicted in Figure 1C. We anticipate that the in vitro sprout assay can be exploited cultivar independently since at least three other cultivars responded similarly (data not shown).
Overexpression of GA2ox and GA20ox from Arabidopsis Affects Plant Growth and Morphology But Has Only a Weak Impact on Potato Tuber Sprouting
To further investigate the role of GA in potato tuber dormancy, we generated transgenic plants with an altered endogenous GA content. The genomic Arabidopsis clones coding for GA2ox1 (GA2ox; accession no. AJ132435) and GA20ox1 (GA20ox; accession no. X83379) were inserted into the pBinAR binary vector between the cauliflower mosaic virus (CaMV) 35S promoter and the octopine synthase terminator as described by Biemelt et al. (2004). These constructs were used to transform potato plants via Agrobacterium tumefaciens-mediated gene transfer. About 60 transgenic lines were obtained per construct. Since the transgenic potato lines expressing GA20ox exhibited elongated shoots and potato plants expressing GA2ox showed a dwarf phenotype, as is typical for increased and reduced GA content, respectively, prescreening was based on phenotype. Expression of either transgene was confirmed by northern-blot analysis of the prescreened lines, and three highly expressing lines for each construct were selected for further characterization (Fig. 2A). Transformants overexpressing GA20ox exhibited elongated stems and light green leaves with elongated petioles (Fig. 2B). At harvest, the stem height was about twice that of wild-type controls (Table I) and correlated with the abundance of GA20ox transcript. These plants formed many long stolons, but both the number of tubers and tuber yield were reduced in the highest expressing line (Fig. 2C; Table I).
Figure 2.
Expression of GA20ox and GA2ox from Arabidopsis in transgenic potato plants. A, Northern-blot analysis of GA20ox-expressing (lines 5, 15, and 58) and GA2ox-expressing (lines 27, 38, and 50) potato plants. Twenty micrograms of total RNA isolated from leaves was loaded per lane and probed with Arabidopsis GA20ox1 or GA2ox1. Hybridization with the small subunit of Rubisco (RbcS) was used as a loading control. B, Phenotypic alteration of transgenic potato plants approximately 8 weeks after transfer into the greenhouse. From left to right: GA20ox lines 58 and 5, the wild type (WT), and GA2ox lines 50 and 27. C to E, Effect on stolon and tuber development in GA20ox line 5 (C), the wild type (D), and GA2ox line 27 (E). [See online article for color version of this figure.]
Table I. Phenotypic characteristics of transgenic potato plants expressing either a GA20ox or a GA2ox gene from Arabidopsis.
Parameters were determined from soil-grown plants at harvest. Results are shown for three independent experiments and represent means ± se of 19 to 21 plants. Statistically significant differences from the wild type were determined using one-tailed t tests assuming unequal variance and are indicated by asterisks (P ≤ 0.05).
| Plant | Stem Height | Tuber Yield | No. of Tubers per Plant |
| cm | g plant−1 | ||
| Wild type (Solara) | 55.2 ± 3.0 | 166.3 ± 7.6 | 7.8 ± 0.5 |
| GA20ox | |||
| GA20ox-5 | 102.4 ± 3.5* | 166.6 ± 5.3 | 7.2 ± 0.5 |
| GA20ox-15 | 99.8 ± 3.0* | 135.0 ± 4.9* | 7.0 ± 0.5 |
| GA20ox-58 | 120.4 ± 5.9* | 116.3 ± 9.4* | 4.5 ± 0.3* |
| GA2ox | |||
| GA2ox-27 | 38.2 ± 4.3* | 94.9 ± 6.4* | 3.7 ± 0.3* |
| GA2ox-38 | 35.9 ± 3.5* | 102.0 ± 9.0* | 4.3 ± 4.3* |
| GA2ox-50 | 59.1 ± 4.4 | 80.5 ± 8.3* | 4.1 ± 0.4* |
In contrast, stem length of GA2ox-expressing potato plants was strongly reduced (Fig. 2B), and the leaves were small, thick, and dark green compared with the wild type. Stolons of these plants were shorter than those of the untransformed controls (Fig. 2E). On average, fewer tubers were formed, leading to a significantly decreased tuber yield (Table I).
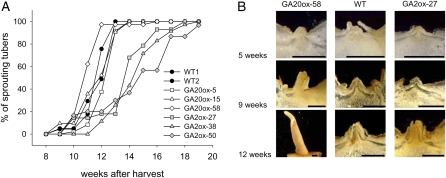
To study the effect of altered GA content on potato tuber dormancy, tubers of five plants per line were stored in darkness at room temperature directly after harvest and sprouting behavior was monitored. Control tubers started to sprout 9 weeks after harvest and reached 100% at 13 weeks after harvest. Onset of sprouting was quite similar in transgenic GA20ox-expressing tubers. Only in the highest expressing line, GA20ox-58, was sprouting slightly earlier as compared with the wild type (Fig. 3A). Conversely, sprouting onset was delayed in GA2ox-expressing tubers (Fig. 3A). Tubers of lines GA2ox-27 and GA2ox-50 started sprouting 10 weeks after harvest and reached a rate of about 60% sprouted tubers after 14 weeks. Line GA2ox-38 exhibited the strongest delay in sprouting, with the first sprouts appearing 3 weeks later than in the wild type. Similar to the observed changes in stem growth, sprouts of GA20ox-expressing lines were strongly elongated and thinner, whereas sprouts formed by GA2ox-expressing tubers were shorter and thicker (Fig. 3B).
Figure 3.
Impact of GA2ox and GA20ox expression on potato tuber sprouting. A, Sprouting behavior of the wild type (WT) and GA2ox- and GA20ox-expressing tubers was monitored over 20 weeks until more than 95% of tubers had started sprouting. Tubers were stored at room temperature in darkness (n = 25–48). B, Cross sections through tuber buds of the wild type and lines GA2ox-27 and GA20ox-58 after 5, 9, and 12 weeks of storage. Bars = 1 mm. [See online article for color version of this figure.]
Taken together, constitutive overexpression of GA20ox did not lead to a significantly altered dormancy period, whereas transgenic potato tubers expressing GA2ox showed a slightly prolonged rest period.
GA Measurements Confirm Changes in Endogenous GA Levels in the Transgenic Lines
Although the strong growth phenotype of the transgenic plants indicated changes in GA content, only an undetermined effect on tuber dormancy could be observed. Therefore, we aimed at confirming altered content of endogenous GAs in the transgenic potato lines.
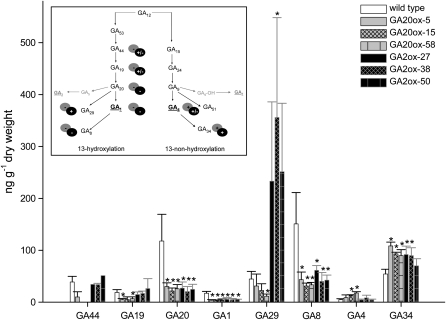
Initially, we sought to determine the content of different GAs in buds of dormant and sprouting tubers. However, as published previously, GA levels were below the detection limit in tuber tissues (Morris et al., 2006). Therefore, we determined endogenous GA levels in apical shoot tips of transgenic and wild-type plants. Consistent with previous reports (Van den Berg et al., 1995; Morris et al., 2006), the major bioactive GA detected in wild-type plants was GA1 (Fig. 4). In addition, high levels of its precursor GA20 and its inactivation product GA8 were measured as well as small amounts of the bioactive GA4. Strikingly, levels of all products of the early 13-hydroxylation pathway, the main path of GA biosynthesis in potato plants (Van den Berg et al., 1995), such as GA44, GA19, GA20, GA1, GA29, and GA8, were reduced in GA20ox-expressing lines. Nevertheless, expression of GA20ox led to an increase in the amount of the bioactive GA4 and its inactivation product GA34 (Fig. 4). This indicates that overexpression of the GA20ox in potato caused a shift to the 13-nonhydroxylation pathway, emulating the major path in Arabidopsis (Sponsel et al., 1997; Coles et al., 1999).
Figure 4.
Endogenous GA levels in potato wild-type and transgenic plants overexpressing either GA20ox or GA2ox. GAs were isolated from the shoot apex of growing plants (leaf nos. 1–5) and analyzed using gas chromatography-mass spectrometry. Values are shown as ng GA g−1 dry weight and represent means ± sd of three to five independent experiments. * Statistically significant differences from the wild type were determined in one-tailed t tests assuming unequal variance (P ≤ 0.05). The inset shows a schematic drawing of the changes in GA metabolism due to the expression of GA2ox (black circles) or GA20ox (gray circles) in potato plants. GA12 is the common precursor for all GAs. The 13-hydroxylation pathway leads to GA1, whereas the 13-nonhydroxylation pathway leads to GA4, as major bioactive forms. Bioactive GA species are underlined.
Analysis of GA content in GA2ox-expressing potato plants revealed a clear decrease in the amount of GA1 compared with the wild type and an increased content of GA29 and GA34, which are inactivation products of the 13-hydroxylation and 13-nonhydroxylation pathways, respectively (Fig. 4). Together with the observed phenotypic changes, this is consistent with a high GA2ox activity in these transgenic potato plants.
Since there was an obvious shift toward GA4 as the major bioactive product in the GA20ox plants, we wanted to investigate whether GA4 treatment is capable of triggering tuber sprouting in our sprout-release assay in a similar fashion to GA3 and GA1. Therefore, discs of wild-type tubers were treated with 50 μm GA1, GA3, or GA4. Water served as a negative control. As shown in Supplemental Figure S2, all GAs tested induced tuber sprouting within 3 d. Even though GA1 and GA4 appeared to have a slightly lower performance, more than 90% of tuber discs had sprouted after 6 or 7 d. Thus, the shift to the 13-nonhydroxylation pathway of GA biosynthesis in GA20ox-expressing lines would not account for the weak effect on tuber dormancy.
Expression of Arabidopsis GA20ox under Control of the Chimeric STLS1/CaMV35 Promoter Leads to Early Tuber Sprouting
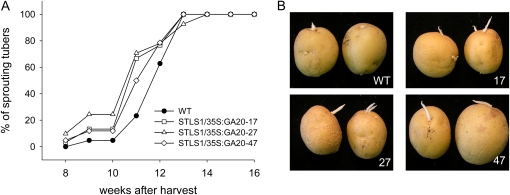
Another possible explanation for the small effect on sprouting in GA20ox tubers could be that expression of the transgene in buds was too low and the resulting production of bioactive GAs was not sufficient to induce sprouting. In another study, substantially higher expression of a target gene (Escherichia coli pyrophosphatase) was obtained in potato tubers by using a chimeric STLS1 enhancer-CaMV 35S promoter compared with the unmodified CaMV 35S promoter (Hajirezaei and Sonnewald, 1999). Thus, in order to achieve elevated GA20ox expression in potato tubers, a construct was designed containing the GA20ox clone under control of the chimeric STLS1/CaMV 35S promoter and transformed into potato plants by Agrobacterium-mediated gene transfer. More than 50 transgenic plants (named STLS1/35S:GA20ox) were regenerated, transferred into the greenhouse, and prescreened for elongated shoot growth compared with untransformed wild-type plants. Expression of the transgene was confirmed for five lines by northern blotting (Supplemental Fig. S3), and three highly expressing lines were selected for further analyses. After propagation in tissue culture, five plants per line were cultivated in the greenhouse. Tubers were harvested after about 12 weeks and stored in darkness at room temperature, and sprouting behavior was monitored. Five weeks after harvest, both wild-type and STLS1/35S:GA20ox tubers were still dormant. However, 8 weeks after harvest, sprouting had already commenced in up to 10% of the transgenic tubers, and 3 weeks later, almost 80% of these tubers had developed long sprouts (Fig. 5). In contrast, first signs of visible sprouting in wild-type tubers were apparent after 9 weeks, and a rate of 62% sprouting was observed after 12 weeks (Fig. 5A). However, almost all wild-type and transgenic tubers had started sprouting after 13 weeks.
Figure 5.
Impact of GA20ox expression under the control of the chimeric STLS1/CaMV 35S promoter on potato tuber sprouting and sprout development. A, Sprouting behavior of wild-type (WT) and GA20ox-expressing tubers was monitored over 14 weeks until all tubers had started sprouting. Tubers were stored at room temperature in darkness (n = 30–45). B, Phenotypic changes of tuber sprouts from STLS1/35S:GA20ox transgenic lines 17, 27, and 47 compared with the wild type. Photographs of two representative tubers per line were taken 14 weeks after harvest. [See online article for color version of this figure.]
In order to compare the expression level of the GA20ox in tubers expressing the gene under control of the chimeric and the unmodified CaMV 35S, respectively, northern-blot analysis was performed on samples taken during tuber storage. As shown in Supplemental Figure S4, there is a more constant expression of the GA20ox gene under control of the STLS1/CaMV 35S promoter during the rest period of potato tubers compared with the unchanged CaMV 35S promoter. This was especially marked after 2 and 3 months of storage when sprouting is initiated.
Together, these results show that higher expression of GA20ox was obtained in potato tubers by using the chimeric STLS1/CaMV 35S promoter, which led to an earlier onset of tuber sprouting. This supports the idea that GA is able to terminate tuber dormancy and promotes sprout outgrowth.
Exogenously Applied CK Breaks Tuber Dormancy in Sprout-Release Assays
Subsequently, we wanted to study the role of CKs in tuber sprouting. CKs were suggested to be the “primary factor” regulating the switch from a dormant to a nondormant state (Turnbull and Hanke, 1985a). In their experiments, the authors showed that CKs were able to trigger tuber sprouting, but this was dependent on “tissue sensitivity” (Turnbull and Hanke, 1985a).
To investigate the ability of CK to break tuber dormancy, we applied 6-benzylaminopurine (BAP) to excised tuber buds in the sprout-release assay. Again, discs containing a single bud were excised from tubers 2 weeks after harvest, and different concentrations ranging from 5 to 100 μm BAP were tested. Similar to GA3-treated tuber discs, first signs of sprout induction became visible 3 d after BAP application (Fig. 6). But in contrast to GA3, BAP did not support further sprout outgrowth even after longer observation times. Soon after bud break, sprout growth was arrested at a length of approximately 0.5 to 1.5 mm (Fig. 6B). Generally, treatment with 5 μm BAP results in the lowest frequency of induced bud break, with 25, 50, and 100 μm BAP inducing increasingly higher rates of bud break, although by 7 d these three dosages gave similar proportions of open buds (more than 80%; Fig. 6A). The water controls remained dormant during the period investigated.
Figure 6.
In vitro tuber-sprouting assay using different concentrations of BAP. Two weeks after storage, tuber discs were isolated and incubated for 5 min with 5, 25, 50, or 100 μm BAP and water as a control. Tuber discs were kept in darkness under tissue culture conditions and scored daily for visible sprouting. A, The percentage of sprouted tuber buds after incubation with different BAP concentrations or water over an 8-d period. The data represent means ± sd of three independent experiments (n = 25). B, Cross sections through tuber buds before and 3 and 5 d after treatment with either 50 μm BAP or water. Bars = 1 mm. C, Schematic drawing of the in vitro tuber-sprouting experiment using BAP. [See online article for color version of this figure.]
In order to investigate whether the sensitivity to BAP changes with tuber age, tuber discs from freshly harvested tubers and tubers stored for 3 or 6 weeks were sampled and incubated with 50 μm BAP (Supplemental Fig. S1B). The response to application of BAP was comparable in tubers that had been stored for 3 or 6 weeks, resulting in bud break that was first detectable after 3 d. In contrast, sprouting efficiency was clearly decreased in tuber samples taken directly after harvest, where bud break occurred 1 d later. Five days after treatment, about 35%, 80%, or 90% of discs showed open buds in samples taken from freshly harvested, 3-week-stored, or 6-week-stored tubers, respectively. However, at the end of the experiment, more than 80% of tubers showed broken dormancy regardless of the previous storage time (Supplemental Fig. S1B).
Although differences in the kinetics of the BAP response were found, these results reveal that CK induces bud break but does not support further sprout growth during the endodormancy period. Notable sprout elongation was achieved only by an additional application of one droplet of 50 μm GA3 onto the bud (Supplemental Fig. S5).
Overexpression of IPT Severely Affects Plant Growth and Morphology
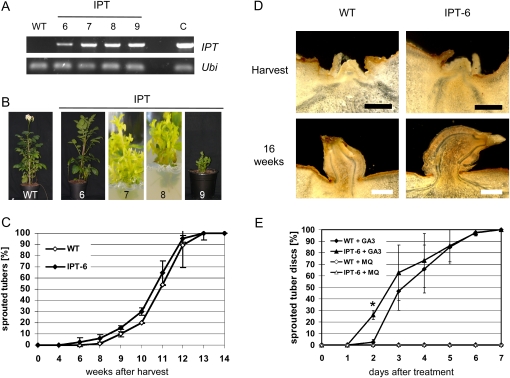
To further investigate the role of CK in the regulation of potato tuber dormancy, we aimed at generating transgenic potatoes with modified levels of active CKs. In order to increase endogenous CK content, plants were transformed with the isopentenyltransferase (IPT) gene (accession no. AF242881) from the Agrobacterium T-DNA, which encodes a rate-limiting enzyme, under control of the CaMV 35S promoter. Out of two rounds of transformation, 15 transgenic plants could be regenerated. Three lines already displayed a typical CK-responsive phenotype with bushy growth, small leaflets, and inhibition of root growth (Fig. 7B) in tissue culture. Two of the lines did not survive the transfer into soil. A third one (line 9) was characterized by strongly retarded growth, a loss of apical dominance, small leaves, and a severe reduction in tuber yield under greenhouse conditions (Fig. 7B; Table II). Since only very few and small tubers were formed, these could not be further analyzed. However, another line (IPT-6) was obtained that resembled wild-type plants with respect to growth and tuber yield. PCR analysis confirmed lower expression of the IPT gene compared with the other lines (Fig. 7A). Tubers were stored at room temperature in darkness, and sprouting behavior was monitored. Sprout induction of IPT-expressing tubers was not significantly earlier than in wild-type tubers (Fig. 7C), but intriguingly, they formed thicker and longer sprouts, as shown by cross sections through spouting tuber buds (Fig. 7D). To further investigate whether IPT expression leads to an earlier onset of sprouting, discs of dormant tubers were excised and tested for their sprouting behavior using the in vitro sprout-release assay. As described before, tuber buds derived from wild-type controls started to sprout 3 d after GA3 treatment. In contrast, transgenic tubers responded 1 d earlier to the GA3 treatment, as shown in Figure 7E. About 25% of tuber discs had already started sprouting after 2 d, indicating that CKs may be important for the initiation of tuber sprouting.
Figure 7.
Characterization of IPT-expressing potato plants. A, PCR analysis of IPT-expressing potato plants (lines 6, 7, 8, and 9). Five micrograms of total RNA isolated from leaves was reverse transcribed using oligo(dT) primers and RevertAid H− reverse transcriptase. An aliquot of first-strand cDNA was amplified with gene-specific primers for IPT or ubi3. B, Phenotypic alteration of transgenic potato plants. From left to right: the wild type (WT) and IPT lines 6, 7, 8, and 9. Photographs were taken 8 weeks after transfer to the greenhouse (the wild type and IPT lines 6 and 9) and from plants propagated in tissue culture (IPT lines 7 and 8) 6 weeks after cutting. C, Sprouting behavior of IPT-expressing line 6 (IPT-6) compared with the wild type. Tubers were stored at room temperature in darkness and monitored for 14 weeks after harvest until 100% sprouting was reached. The data show means ± sd of two independent harvests (n = 25–36). Lines 7, 8, and 9 did not produce any or not sufficient numbers of tubers. D, Tuber cross sections at harvest and after 16 weeks of storage. Bud complexes of freshly harvested tubers and sprouts of 16-week-old tubers stored at room temperature were sectioned by hand and imaged on a stereomicroscope. From top left to bottom right: the wild type at harvest, IPT-6 at harvest, the wild type at 16 weeks, and IPT-6 at 16 weeks. Black bars = 500 μm, and white bars = 1 mm. E, In vitro sprouting assay of wild-type and IPT-6 tubers. Tuber discs containing one bud complex were incubated with 50 μm GA3 or sterile water and scored daily for visible sprouting. The data show means ± se of two independent experiments (n = 20–30). * Statistically significant differences from the wild type (P ≤ 0.05). [See online article for color version of this figure.]
Table II. Phenotypic characteristics of transgenic potato plants expressing either a CKX gene from Arabidopsis or an IPT gene from Agrobacterium.
Parameters were determined from soil-grown plants at harvest. Results are shown for three independent experiments and represent means ± se of 25 to 40 plants. Statistically significant differences from the wild type were determined using one-tailed t tests assuming unequal variance and are indicated by asterisks (P ≤ 0.05).
| Plant | Stem Height | Tuber Yield | No. of Tubers per Plant |
| cm | g plant−1 | ||
| Wild type (Solara) | 56.7 ± 2.0 | 176.7 ± 10.4 | 7.6 ± 0.4 |
| CKX | |||
| CKX-4 | 39.2 ± 0.8* | 20.0 ± 0.7* | 6.5 ± 0.6 |
| CKX-10 | 46.5 ± 1.2* | 136.1 ± 5.1* | 3.9 ± 0.4* |
| CKX-11 | 55.3 ± 1.3 | 168.0 ± 2.7 | 5.8 ± 0.3* |
| IPT | |||
| IPT-6 | 54.2 ± 1.1 | 170.2 ± 3.9 | 7.2 ± 0.2 |
| IPT-9 | 11.1 ± 0.9* | 10.7 ± 3.4* | 2.8 ± 0.6* |
Transgenic Potato Plants Expressing a CK Oxidase Show an Altered Phenotype and a Prolonged Dormancy Period
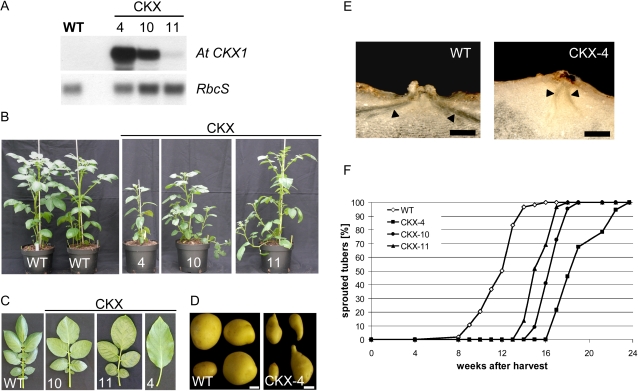
The catabolic enzyme cytokinin oxidase/dehydrogenase1 (CKX) was shown to play a critical role in controlling CK levels in plants and was successfully exploited to reduce the endogenous content of CKs by means of overexpression in transgenic tobacco (Nicotiana tabacum) or Arabidopsis plants (Werner et al., 2001, 2003). We have chosen the Arabidopsis CKX1 (CKX) gene (At2g41510) for expression in potato plants to analyze the impact of decreased CK content on tuber dormancy. To this end, the gene was amplified by PCR from Arabidopsis cDNA and cloned into the BinAR vector (Höfgen and Willmitzer, 1990). The construct was transformed into potato plants by Agrobacterium-mediated gene transfer, and a total of 12 transgenic plants were obtained. Based on northern-blot analysis, three lines with different expression levels of the transgene were selected for more detailed analysis, with CKX-4 exhibiting the strongest and CKX-11 the lowest expression of the CKX gene (Fig. 8A). Plants were propagated in tissue culture and transferred into the greenhouse. Compared with the wild type, transgenic lines showed reduced shoot growth, and the degree of retardation in final shoot size corresponded with the expression of the transgene (Fig. 8, A and B; Table II). Moreover, the plants produced a high number of fragile side shoots and had a lower number of leaflets per leaf. The strongest line, CKX-4, formed only single lanceolate leaves instead of the typical compound leaves (Fig. 8C). With increasing CKX expression, tuber yield was reduced severely and only a few, small, drop-shaped tubers were formed (Table II; Fig. 8D). Cross section through the tuber buds revealed that the size of the meristem is much smaller than that of wild-type tubers and that the two main vascular bundles were in close proximity to each other (Fig. 8E). Interestingly, onset of sprouting of stored tubers was delayed 5 to 8 weeks in CKX-expressing tubers compared with the wild type, and the delay correlated with the CKX transcript abundance (Fig. 8, A and F). Moreover, while sprouts of lines CKX-10 and CKX-11 developed normally after bud breakage, sprouts formed by the strong expressing line CKX-4 remained diminutive and failed to achieve proper growth (data not shown), further supporting the assumption that CKs are important players for the reactivation and maintenance of meristematic activity during the onset of tuber sprouting.
Figure 8.
Characterization of CKX-expressing potato plants. A, Northern-blot analysis of CKX-expressing (lines 4, 10, and 11) potato plants. Thirty micrograms of total RNA isolated from leaves was loaded per lane and probed with AtCKX1. Hybridization with the small subunit of Rubisco (RbcS) was used as a loading control. B, Phenotypic alteration of transgenic potato plants approximately 8 weeks after transfer into the greenhouse. From left to right: two wild-type plants (WT) and CKX lines 4, 10, and 11. C and D, Effect on leaf morphology (C) and on tuber formation (D). E, Cross sections of wild-type and CKX-expressing (line 4; CKX-4) tubers at harvest. Freshly harvested tubers were hand-sectioned and imaged on a stereomicroscope. Arrowheads indicate vascular strands. Bars = 1 mm. F, Sprouting behavior of CKX-expressing lines (lines 4, 10, and 11) in comparison with the wild type monitored for 24 weeks after harvest until 100% sprouting was reached. Tubers were stored at room temperature in darkness (n = 25–48). [See online article for color version of this figure.]
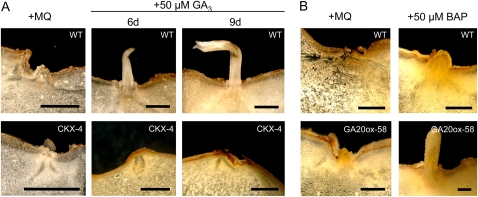
To further investigate the observed delay in sprouting of CKX-expressing tubers, we isolated buds from wild-type tubers and from the strongest expressing line, CKX-4, and performed a sprout-release assay using 50 μm GA3. While sprouting could be induced in wild-type tuber buds as observed before, no signs of sprouting could be seen in transgenic tuber buds even when kept for a longer time (9 d; Fig. 9A). To confirm that this phenotype is due to a reduced level of CKs, CKX-expressing tuber discs were also incubated with 50 μm BAP. As shown in Supplemental Figure S6, BAP treatment of the transgenic tubers restored the wild-type phenotype.
Figure 9.
In vitro tuber sprouting of transgenic lines expressing CKX or GA20ox treated with GA3 or BAP. A, Cross sections through buds of wild-type (WT) and CKX-4 tubers treated with water (MQ) or 50 μm GA3 after 6 and 9 d. B, Cross sections through buds of wild-type and GA20ox-58 tubers treated with water or 50 μm BAP 6 d after treatment. Bars = 1 mm. [See online article for color version of this figure.]
In addition, tuber discs taken from the strongest GA20ox-expressing line, line 58, were also incubated with 50 μm BAP. While in wild-type tubers BAP treatment induced only bud break, it also promoted outgrowth of the sprout in tubers with increased GA levels (Fig. 9B). Together, these data led us to conclude that GA requires CKs to release tuber dormancy.
Microarray Analysis of GA3-Treated Wild-Type and CKX-Expressing Tubers
In order to explore the cause for the lack of sprouting of CKX-expressing tubers after GA3 application, a transcript analysis was conducted using microarrays. To this end, dormant wild-type and CKX-4 tuber buds were subjected to a sprout-release assay and transcript profiles were prepared from samples taken immediately (0 d) and after 3 d of GA3 treatment using the Agilent 4 × 44K array (Kloosterman et al., 2008). As before, wild-type tubers started sprouting 3 d after GA3 treatment, and in about 30% of the samples, little sprouts were already visible, indicating meristem reactivation and the beginning of sprout outgrowth (Fig. 1). In contrast, no sign of sprouting could be seen in CKX-4 tubers.
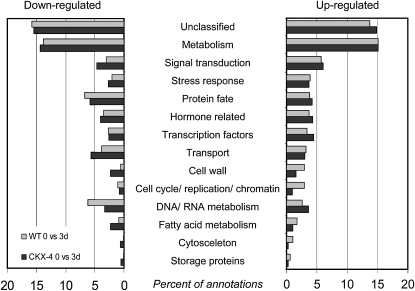
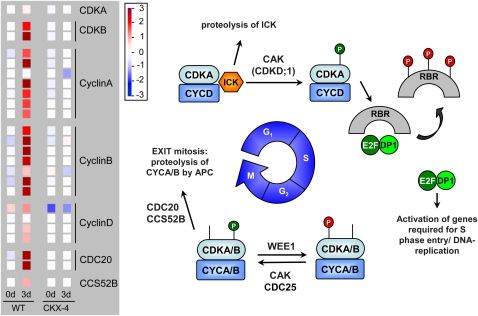
Data analysis was performed as described in “Materials and Methods” using the GeneSpring GX7.3.1 software. Comparing expression profiles at 0 and 3 d after GA3 application, 4,410 and 5,629 features were found to be differentially expressed in wild-type and CKX-4 tuber buds, respectively (Table III; Supplemental Tables S1 and S2). Among them, 2,792 and 3,218 features were up-regulated while 1,617 and 2,411 were down-regulated in wild-type and CKX-4 tuber buds, respectively. The differentially regulated genes were grouped into 15 functional categories (Fig. 10), with two groups, transcripts of unknown function and transcripts that could not be assigned to any other category, accounting for about 50% of the transcripts. With induction of sprouting in wild-type tubers, there was a clear increase in the percentage of transcripts falling into the functional groups “cell cycle activity and replication,” “cytoskeleton,” and “cell wall,” which indicates increased cellular metabolism and cell proliferation during the reactivation of meristematic activity (Fig. 10; Supplemental Table S1). In contrast, there was no increase or even a lower number of transcripts up-regulated in these categories in CKX-4 tuber buds after 3 d, reflecting the observation that no sprout growth has been initiated (Fig. 10; Supplemental Table S2). Closer inspection of the data revealed that transcript quantity of most cell cycle regulators, such as cyclins A, B, and D as well as CDKB, was strongly increased in wild-type tubers, correlating with the resumption of growth as illustrated using the MapMan tool in Figure 11. This was accompanied by an activated replication, as indicated by a clearly increased expression of marker genes such as histone H4, histone H3, and deoxyuridine triphosphatase (dUTPase; Brandstädter et al., 1994; Senning et al., 2010; Supplemental Table S1). Strikingly, transcripts coding for components of the cytoskeleton that are necessary for cell division and expansion are predominantly increased in wild-type tuber buds 3 d after GA3 treatment. In addition, a number of genes controlling the biosynthesis of cellulose (cellulose synthase) and genes involved in cell wall modification and loosening, such as polygalacturonases, pectin-(methyl)esterases, and xyloglucan endotransglucosylase-hydrolases, were found to be induced exclusively in wild-type tubers (Supplemental Tables S1 and S2). This is in line with the fact that cell proliferation and expansion require cell wall biosynthesis and assembly as well as extensive modifications.
Table III. Number of transcripts significantly (P ≤ 0.05) regulated in wild-type and CKX-4 tuber buds during the GA3-mediated sprout-release assay.
| Comparison | Greater Than 2-Fold Regulated Transcripts | Overlapping Transcriptsa | 3-d GA3 UPb | 3-d GA3 DOWNb |
| Wild type 0 d versus 3-d GA3 | 4,410 | 1,867 | 2,793 | 1,617 |
| CKX-4 0 d versus 3-d GA3 | 5,629 | 3,218 | 2,411 |
Transcripts regulated in both wild-type and CKX-4 tuber buds, identified by Venn diagram.
Number of up- and down-regulated transcripts, identified by K means clustering.
Figure 10.
Functional assignment of transcripts differentially expressed in wild-type (WT) and CKX-expressing tuber buds during the sprout-release assay. Transcripts differentially expressed 3 d after GA3 treatment in buds of wild-type or CKX-4 tubers were classified into functional groups. Bars illustrate the percentage of transcripts within various categories based on total numbers. The category “unknown,” containing about 35% of differentially expressed transcripts, is not shown to increase clarity (for more detailed information, see Supplemental Tables S1 and S2).
Figure 11.
Expression of cell cycle genes in wild-type (WT) and CKX-4 tubers after GA3-mediated tuber sprouting. Expression data of differentially expressed ESTs representing genes of the cell cycle are shown in the left-hand panel and a schematic overview of the mitotic cell cycle, modified after Inzé and De Veylder (2006), is shown on the right. Color bars as displayed by the MapMan tool were copied to and aligned in Microsoft PowerPoint. The color scale next to the panel indicates transcript levels, with red representing an increase and blue representing a decrease in transcript levels. The colors saturate at 8-fold change. Details on the assignment of individual POCI identifiers to genes shown are listed in Supplemental Table S3. At the G1/S-phase transition, CDK inhibitory protein ICK dissociates from CDKA-CYCD complexes and is degraded via the proteasome pathway. Phosphorylation of CDKA then activates the CDKA-CYCD complex, which initiates phosphorylation of the retinoblastoma-related protein (RBR), releasing the E2F-DP1 complex. This complex promotes the transcription of genes required for progression into and through S-phase, where DNA replication takes place. The G2/M-phase transition is accompanied by a marked increase in transcription of both A- and B-type CDKs and A- and B-type cyclins. CDKA/B-CYCA/B complexes are activated by removal of the inhibitory phosphate by CDC25 and phosphorylation by CDK-activating kinase (CAK), both enzymes acting on the CDK part of the complex. The reverse reaction, inhibitory phosphorylation of CDK, is catalyzed by kinase WEE1 and promotes endoreduplication. During mitosis, CDC20- and CCS25-activated anaphase-promoting complex (APC) degrades cyclins A and B, leading to the transition from mitosis into G1-phase. CDC, Cell division cycle; DP, docking protein.
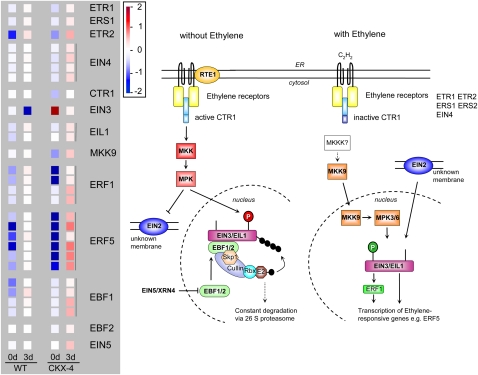
In relation to hormone signaling, the most obvious differences between wild-type and CKX-expressing tubers were apparent for ethylene and auxin signaling pathways, which were also visualized using the MapMan tool (Figs. 12 and 13; Supplemental Table S3). The entire ethylene signaling pathway appeared to be activated more strongly in CKX-4 tuber buds compared with the wild type, with the exception of the key transcription factor ETHYLENE-INSENSITIVE3 (EIN3), expression of which is about 6-fold down-regulated 3 d after GA3 treatment, probably due to the transcriptional activation of the F-box proteins EIN3-BINDING F-BOX1 (EBF1) and EBF2, which in turn are controlled by the activity of EIN5, a 5′-3′ exoribonuclease (for review, see Stepanova and Alonso, 2009; Yoo et al., 2009). Expression of transcripts with homology to EBF1, EBF2, and EIN5 was found to be 2- to 4-fold induced 3 d after GA3 treatment of CKX-4 tubers, while there was a weaker induction in wild-type tubers with the exception of a gene coding for an ortholog of AtEBF1 (Micro.1924.C1; Fig. 12; Supplemental Tables S1 and S2). Besides the Ethylene Response Factors (ERFs), EBF2 is the only gene directly activated by EIN3 and EIN3-like (EIL) transcription factors, most likely as part of a rapid feedback regulatory loop. Among the ERFs, various ESTs with homology to ERF5 from Arabidopsis or tomato (Solanum lycopersicum) showed strongly increased transcript abundance in the CKX-4 line in the sprout-release assay when compared with the wild type (Fig. 12; Supplemental Tables S1 and S2).
Figure 12.
Expression of ethylene signaling genes in wild-type (WT) and CKX-4 tubers after GA3-induced tuber sprouting. Expression data of differentially expressed ESTs representing genes of the ethylene signaling pathway are shown in the left-hand panel and a schematic overview of this pathway, as assumed from knowledge in Arabidopsis (Kendrick and Chang, 2008; Yoo et al., 2009), is shown on the right. Color bars as displayed by the MapMan tool were copied to and aligned in Microsoft PowerPoint. The color scale next to the panel indicates transcript levels, with red representing an increase and blue representing a decrease in transcript levels. The colors saturate at 4-fold change. Details on the assignment of individual POCI identifiers to genes shown are listed in Supplemental Table S3. Without ethylene binding, the ethylene receptor complexes in the endoplasmic reticulum membrane activate CTR1. RTE1 acts as a negative regulator of ethylene response by regulating the ethylene receptor ETR1. CTR1 is proposed to be a MKKK that activates a cytosolic kinase signaling cascade, leading to phosphorylation of residues Thr-592 and Thr-546 of the transcription factors EIN3 and EIL1 in the nucleus as well as the inhibition of membrane-bound protein EIN2. Phosphorylated EIN3 and EIL1 interact with F-box proteins EBF1 and EBF2, resulting in constant degradation of the transcription factors and thus suppression of ethylene signaling. EIN5/XRN4 indirectly influences transcript levels of EBF1 and EBF2. Upon binding of ethylene, CTR1 is inactivated, which leads to inactivation of the CTR1-MAPK signaling and activation of another kinase signaling cascade involving MKK9, MPK3, and MPK6 and probably an as yet unknown MKKK. This cascade leads to the phosphorylation of residues Thr-176 and Thr-176 of EIN3 and EIL1, presumably resulting in increased stability because of a reduced interaction with EBF1 and EBF2. Membrane-bound protein EIN2 also positively influences EIN3 accumulation through a mechanism that has not been elucidated yet. EIN3/EIL1 activates ethylene responses both directly and indirectly through expression of the transcriptional activator ERF1. CTR1, Constitutive triple response 1; ERS, ethylene response sensor; MKK, mitogen-activated protein (MAP) kinase kinase; MKKK, MAP kinase kinase kinase; MPK, MAP protein kinase; RBX, RING box protein; RTE1, reversion to ethylene sensitivity 1; SCF complex, SKP1-cullin-F-box complex; SKP1, S-phase kinase-associated protein 1; XRN4, exoribonuclease 4.
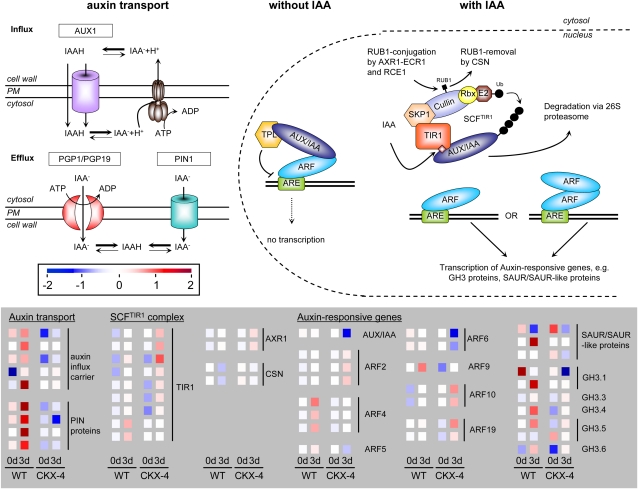
Figure 13.
Expression of auxin transport and signaling genes in wild-type (WT) and CKX-4 tubers after GA3-mediated tuber sprouting. An overview of auxin transport proteins is shown on the left, and the auxin signaling pathway is shown on the right as derived from Arabidopsis (Mockaitis and Estelle, 2008; Weijers and Friml, 2009). Expression data of differentially expressed ESTs representing genes of auxin transport and signaling are shown in the gray panel. Color bars as displayed by the MapMan tool were copied to and aligned in Microsoft PowerPoint. The color scale above the panel indicates transcript levels, with red representing an increase and blue representing a decrease in transcript levels. The colors saturate at 4-fold change. Details on the assignment of individual POCI identifiers to genes shown are listed in Supplemental Table S3. Auxin transport: IAA is the most abundantly occurring auxin in nature. It can enter the cell in its protonated form either by diffusion through the plasma membrane or facilitated by AUX1 permeases. Inside the cell, IAAH is deprotonated and trapped due to the change in pH. Auxin efflux is performed by two types of transporters, PGP1/PGP19 ATP-binding cassette transporters and PIN proteins. PGP1/PGP19 usually has a nonpolar localization, whereas PIN proteins show a polar localization that determines the orientation of the auxin flow within the cell. Auxin signaling, without IAA: ARF transcription factors bind to auxin-responsive elements (AREs) in the promoters of auxin-responsive genes and interact with Aux/IAA proteins, which in turn recruit the transcriptional corepressor TPL to prevent gene expression. When accumulating in the nucleus, IAA binds to Aux/IAA proteins and the TIR1 subunit of the SCFTIR1 complex, targeting Aux/IAA for degradation via the 26S proteasome and releasing ARF transcription factors from inhibition. The activity of the SCRTIR1 complex can be regulated by the addition and removal of ubiquitin-like protein RUB1 through the action of conjugating (AXR1, RCE1, and ECR1) and deconjugating (CSN) enzymes. ARFs released from inhibition act as monomers or dimers to promote the expression of auxin-responsive genes (e.g. those coding for SAUR and GH3 proteins). AUX1, Auxin 1; AXR1, auxin-resistant 1; CSN, COP9 signalosome; ECR1, E1 C-terminal-related 1; PGP, P-glycoprotein; PIN, PIN-formed; PM, plasma membrane; RBX, RING box protein; RCE1, RUB1-conjugating enzyme; RUB1, related to ubiquitin; SCF complex, SKP1-cullin-F-box complex; SKP1, S-phase kinase-associated protein 1; TIR1, transport inhibitor response 1; TPL, topless; Ub, ubiquitin.
In the wild type, we found a clear increase in transcripts coding for two different types of auxin biosynthetic enzymes, namely aldehyde oxidase(s) and flavin monooxygenase-like protein (Yucca-like in Arabidopsis) 3 d after GA3 incubation, while either their total expression level was lower or their induction was less pronounced in CKX-4 (Supplemental Tables S1 and S2). In accordance with a stimulated auxin biosynthesis, there was a substantial increase in the expression of several of the primary auxin-responsive genes SAUR (for small auxin up-regulated RNAs) and GH3-type (for review, see Hagen and Guilfoyle, 2002) as well as of auxin influx and PIN1-like auxin efflux carrier(s) in wild-type as compared with CKX-4 tubers (Fig. 13). Interestingly, ARF4 was induced up to 3.5-fold with the onset of sprout outgrowth in wild-type tubers, while in CKX-4, ARF2, -10, and -19 were strongly transcriptionally activated by GA3.
Fewer differences between the genotypes were found in the expression of GA and CK biosynthetic and signaling genes. The amount of transcripts for several GA biosynthetic genes such as ent-kaurene oxidase, GA2ox, and GA3ox as well as of the GA receptor declined in both genotypes 3 d after GA3 application. These changes could be directly or indirectly caused by the GA3 treatment. However, expression of Gip1-like genes was exclusively induced in wild-type tubers (Supplemental Tables S1 and S2). As expected from the genetic modification, expression of purine transport proteins as well as those of some response regulators (e.g. ARR4 homolog) was lower in the CKX-4 line than in the wild type both before and after GA3 treatment. In contrast, increased transcript amounts were found for zeatin O-xylosyltransferase, encoding an enzyme thought to be involved in CK homeostasis (Martin et al., 1993).
A higher percentage of transcripts coding for metabolic enzymes were up-regulated than down-regulated following GA3 treatment of both wild-type and CKX-4 tuber buds, indicating an accelerated metabolic rate (Fig. 10). Among them were a high number of ESTs that showed similar changes of expression in both genotypes and therefore can be found within the population of 1,867 overlapping transcripts as determined by Venn diagrams (Table III). However, ESTs coding for enzymes of fatty acid biosynthesis were clearly induced in wild-type tubers, suggesting an increased demand for building blocks of plasma membranes, whereas a high fraction of them were down-regulated in CKX-4 tuber buds (Fig. 10).
Interestingly, a number of transcripts coding for transcription factors involved in maintenance of the shoot apical meristem (Carraro et al., 2006) were among the group of transcripts with similar changes in expression level in the wild type and CKX-4 (e.g. the homeobox transcription factors knotted1-like and knotted2 or those of the class III HD-ZIP family). In contrast, expression of putative transcription factors with homology to AINTEGUMENTA (ANT), PHAN2TASTICA (PHAN), GROWTH-REGULATING FACTOR3 (GRF3), or OVATE, which have been associated with organ differentiation and (out)growth (Kim et al., 2003; Hackbusch et al., 2005; Carraro et al., 2006), was more strongly or exclusively induced in GA3-treated wild-type tuber discs. These data also reflect that in wild-type tubers, meristematic activity is regained upon GA3 application followed by outgrowth of the sprout, while in CKX-4 tubers, initiation and outgrowth of the sprout cannot be induced, probably due to the loss of activation of cell cycle activity and downstream differentiation processes.
DISCUSSION
GA Is Sufficient to Break Tuber Dormancy and to Stimulate Sprouting
Although the dormancy-terminating capacity of GA was shown a long time ago (Brian et al., 1955), there is still a debate about its mode of action (Suttle, 2004a). Early studies showed an increase of endogenous GA-like substances prior to or together with the onset of sprouting, as summarized by Suttle (2004a), while recent analyses using gas chromatography-mass spectrometry showed increased levels of GA1, GA20, and GA19, the predominant bioactive GA in potato and its direct precursors, only in tubers with actively elongating sprouts (Suttle, 2004b). Furthermore, both antisense expression of the endogenous GA biosynthesis gene GA20ox1 and overexpression of GA2ox1 resulted in reduced GA levels and dwarf phenotypes but did not affect tuber dormancy (Carrera et al., 2000; Kloosterman et al., 2007). In contrast potato plants with higher expression of potato GA20ox1 had elongated shoots and their tubers exhibited a decreased dormancy period (Carrera et al., 2000). Together, these results suggested a role for GA in sprout growth rather than in dormancy release (Suttle, 2004a).
In this study, we used an in vitro assay to stimulate sprouting of excised tuber buds using GA3. This triggered sprouting independent of tuber age, although efficiency was reduced in freshly harvested, deeply dormant tubers. However, 6 d after treatment, more than 80% of these tuber discs had started sprouting. A slightly different result was obtained by Suttle (2004b), who injected different GA species of the 13-hydroxylation pathway into tubers. Only the bioactive GA1 was sufficient to terminate the dormancy of tubers stored for a short time, while its precursors GA19 and GA20 also promoted sprouting of aged tubers, indicating that GA20ox activity is rate limiting. Our experiments differed from that of Suttle (2004b), since we applied GA3, which is less rapidly metabolized, to isolated tuber discs containing one bud. This may result in more stable effects. Nevertheless, in all studies, treatment with bioactive GA species was sufficient to terminate tuber dormancy and to stimulate sprout outgrowth.
To further substantiate our observation, transgenic potato plants with modified GA biosynthesis were generated by heterologous expression of the Arabidopsis GA20ox1 or GA2ox1 gene under the control of the CaMV 35S promoter. The observed slender or dwarf phenotypes of the plants were consistent with those of previous reports for overexpression of GA20ox or GA2ox genes in Arabidopsis, tobacco, or potato plants (Coles et al., 1999; Carrera et al., 2000; Biemelt et al., 2004; Kloosterman et al., 2007) and supported the important role of GA for plant growth (Hedden and Phillips, 2000). Similar to transgenic potato plants with increased expression of the endogenous GA20ox1 or GA2ox1 gene (Carrera et al., 2000; Kloosterman et al., 2007), tuber yield was reduced in both groups of transgenic plants in our study. However, in our experiments, length of dormancy was extended in GA2ox tubers, while it remained almost unchanged in GA20ox tubers, opposite to previous observations (Carrera et al., 2000; Kloosterman et al., 2007). As in the other studies, we could confirm changes in the levels of different GA species in the transgenic plants. Interestingly, overexpression of the Arabidopsis GA20ox1 in potato plants caused a shift toward the 13-nonhydroxylation pathway, which is the prevailing pathway in Arabidopsis (Coles et al., 1999), indicated by increased amounts of GA4 and GA34. A similar observation was made by Vidal et al. (2001) by ectopic expression of the citrus GA20ox1 in tobacco, which caused an accumulation of GA4 at the expense of GA1. However, the shift to GA4 in the transgenic potatoes would not account for the failure to induce tuber sprouting in these lines, since the dormancy-releasing ability of GA4 was found to be quite similar to that of GA1 and GA3. One possible explanation for the different and weak effects in the transgenic plants might be that the amount of bioactive GA(s) must exceed a critical level in certain cell populations to exert its function. Hence, the constitutive CaMV 35S promoter originally used in this study might not be sufficiently active in stored tubers to achieve the required expression level. In fact, higher and more stable expression of GA20ox was obtained in aged tubers when driven by a chimeric STLS1 enhancer/CaMV 35S promoter (Hajirezaei and Sonnewald, 1999) compared with the unmodified CaMV 35S promoter. Interestingly, these transgenic tubers exhibited earlier induction of sprouting compared with wild-type tubers, indicating that high expression of the transgene was necessary to cause an effect. However, this also demonstrates the requirement of specific promoters conferring strong expression in stored tubers.
CK Is Essential for Bud Break
Early studies have demonstrated that application of CKs results in the termination of tuber dormancy. These studies also revealed an increasing sensitivity to the phytohormone during postharvest storage, with tubers being insensitive immediately after harvest and for a period thereafter (Hemberg, 1970; Turnbull and Hanke, 1985a; Suttle, 1998a; Suttle and Banowetz, 2000). In a more recent study, Suttle (2008) showed that synthetic CKs such as N-2-(chloro-4-pyridyl)-N′-phenylurea and 1-(α-ethylbenzyl)-3-nitroguanidine are more effective in dormancy termination than the natural zeatin, probably because they escape degradation by CKX, but they did not eliminate the initial resistance period. Applying BAP to excised tuber buds in our sprout-release assay confirmed the ability of CKs to remove tuber dormancy. Moreover, we also observed a time-dependent increase in sensitivity to BAP treatment, but it could also release the dormancy of freshly harvested tubers. Thus, the difference in the response to CK may be due to the different experimental systems, substances, and/or potato cultivars used; however, potato tubers may have also attained a certain metabolic competence before sprouting occurred (Sonnewald, 2001). In contrast to the previous studies, BAP induced only bud break but did not support further sprout outgrowth. Sprout growth could be stimulated only after an additional dosage of GA3.
The importance of CK for initiating the release of tuber dormancy also became evident from experiments with transgenic plants. In tubers harvested from transgenic potato plants expressing a bacterial 1-deoxy-d-xylose 5-phosphate synthase, sprout growth had already occurred at harvest to a length of about 1 to 2 mm (Morris et al., 2006). This was accompanied by an increased level of trans-zeatin riboside and isopentenyl adenosine produced in these transgenic tubers. Bud break was followed by a phase of growth arrest before further sprout growth could be detected, probably due to a hormonal and/or metabolic imbalance. Furthermore, transgenic potato plants transformed with T-DNA from Agrobacterium to increase CK biosynthesis displayed a variety of phenotypic changes, including premature sprouting (Ooms and Lenton, 1985). We exploited the IPT gene from Agrobacterium to enhance endogenous CK levels. As reported before (Ooms and Lenton, 1985; Macháčková et al., 1997), our transgenic potato plants hardly formed roots and exhibited a stunted, bushy phenotype with small leaflets. Hence, only one weakly IPT-expressing line formed enough tubers of similar size as the wild type to be suitable for further experiments. Strikingly, these tubers began to sprout 1 d earlier when used in a sprout-release assay with 50 μm GA3, even though there was no significant effect on sprouting behavior when stored under normal conditions. Besides the transgenic IPT plants, we generated potato plants overexpressing the Arabidopsis CKX1 gene to reduce the endogenous CK content. These plants also displayed considerable morphological and developmental changes. As reported for transgenic Arabidopsis and tobacco plants expressing the same gene (Werner et al., 2001, 2003), shoots of transgenic potato plants were retarded and leaf size and shape were changed. Remarkably, the numbers of leaflets per leaf went down with increasing expression of the transgene, to only single lanceolate leaves formed in the strongest line instead of the typical composite wild-type leaves. In this line, the tuber shape was changed and only a few, droplet-like tubers were formed. These tubers exhibited a clearly prolonged dormancy period, and most interestingly for our study, tuber discs did not respond to exogenously applied GA3. This result led us to conclude that GA requires the presence of CK to trigger tuber sprouting and that CK is essential for the initiation of bud break. The assumption is confirmed by the observations that BAP application to excised wild-type tuber buds stimulated bud break but not further sprout growth; however, it was sufficient to induce sprouting of GA20ox-expressing tubers.
CKs are characterized by their ability to stimulate cell division of plants in vivo and in vitro (Francis and Sorrell, 2001; Werner et al., 2001, and refs. therein). In the cell cycle, they regulate the G1/S-phase transition, the stage in which most cells of dormant buds are arrested, at least partially, by inducing CycD-type cyclins (Campbell et al., 1996; Francis and Sorrell, 2001; Horvath et al., 2003; Francis, 2007). Meanwhile, there are numerous studies providing evidence for a CK-CycD3 connection. For instance, Soni et al. (1995) showed an induction of CycD3 by CK and Riou-Khamlichi et al. (1999) found elevated CycD3 transcript levels in Arabidopsis mutants with high CK content, while cycD3 mutants described by Dewitte et al. (2007) showed reduced CK response. Moreover, the onset of tuber sprouting was found to be accompanied by a large increase in cell division (Campbell et al., 1996). Consistent with these results, expression of almost all cell cycle regulators including cyclin D3 was found to be induced in wild-type tubers following GA3-mediated sprouting but not in transgenic tubers expressing CKX. Expression of genes controlling DNA replication such as histone H4 and dUTPase was also only activated in wild-type tubers when sprout growth commenced. Both genes were recently described as molecular markers defining the transition from a dormant to a sprouting tuber meristem (Senning et al., 2010). In addition, we found clear differences in the expression of genes involved in cell wall biosynthesis and modification, as well as of fatty acid biosynthetic enzymes, between wild-type and CKX-expressing tubers; these genes are involved in processes necessary to initiate cell division and growth. More of these genes were activated by GA3 treatment of wild-type tubers than of CKX-4 tubers, reflecting that sprout growth had commenced in wild type tubers but not in the transgenic tubers.
Interestingly, cross sections through sprouting IPT-expressing tubers indicated an increased meristem size, whereas the tuber meristem of CKX-expressing plants was smaller and had less developed vasculature. Similarly, Werner et al. (2001, 2003) observed smaller shoot apical meristems in CKX-expressing tobacco and Arabidopsis plants and suggested that CKs are not only required to maintain cell division but might also be involved in cell differentiation.
In accordance with this assumption, a strongly increased expression of putative transcription factors controlling organ differentiation and (out)growth, such as ANT, PHAN, GRF3, or OVATE (Kim et al., 2003; Hackbusch et al., 2005; Carraro et al., 2006), was seen in wild-type tuber discs as opposed to CKX discs, indicating that organ differentiation is not promoted in CK-deficient tubers. Moreover, clear differences in the response to GA3 were found between both genotypes with respect to auxin biosynthesis and signaling. Recent work elucidated an essential role for auxin in primordium initiation and outgrowth of lateral organs (for review, see Carraro et al., 2006; Leyser, 2009). Thus, expression of auxin-responsive SAUR and GH3 genes was dampened in CKX-4 tubers after GA3 treatment when compared with the wild type. Interestingly, expression of ESTs with homology to ARF2 was 2- to 4- fold induced by GA3 in CKX-expressing tubers. In Arabidopsis, ARF2 was described as a general repressor of cell division (Schruff et al., 2006). In contrast, transcript amounts of ARF4 homologs and of auxin transport proteins accumulated in sprouting wild-type buds. The latter proteins are important for the establishment of an active auxin transport, and in particular PIN proteins generate high local auxin maxima necessary to initiate organ outgrowth (Reinhardt et al., 2000; Friml et al., 2003; Carraro et al., 2006; Leyser, 2006). Recently, strigolactones have been discovered as the long-missing signal involved in auxin-mediated suppression of lateral bud outgrowth (Gomez-Roldan et al., 2008; Umehara et al., 2008; Dun et al., 2009). Interestingly, expression of four individual ESTs with homology to the F-box gene RAMOSUS4 from pea (Pisum sativum), which seems to be required for strigolactone signaling, was more than 2-fold higher in CKX-4 tuber buds when compared with the wild type (data not shown). However, its expression was not changed due to GA3 treatment. This may suggest that strigolactones are involved in the suppression of bud outgrowth in CKX-expressing tubers, but further experiments are required to figure this out.
In addition to auxin signaling components, at least two auxin biosynthesis genes appeared to be transcriptionally activated in wild-type tubers. This is in line with results of Sorce et al. (2000), who documented a positive correlation between the concentration of IAA and the end of tuber dormancy. In a recent, more detailed study, the same authors reported a progressive decrease in free IAA content during the dormancy period (Sorce et al., 2009). In addition, they performed immunolocalization studies that showed an accumulation of the hormone in the apical meristem and the vascular tissue beneath the dormant tubers, whereas in sprouting tubers, IAA was localized mainly in the primordia. From these results, Sorce et al. (2009) postulated that auxin supports early developmental processes that underlie dormancy break. This assumption is also strengthened by Faivre- Rampant et al. (2004), who found that expression of ARF6 was strongly up-regulated at dormancy release, especially in the developing vasculature, as shown by in situ hybridization. Hence, ARF6 was discussed as a marker for meristem activation (Faivre-Rampant et al., 2004). In our transcript profiling experiment, expression of ARF6 was not changed in the wild type, which might be due to the time points investigated (after 3 d, sprouting had already commenced), but it was clearly decreased in CKX-expressing tubers. However, our data support an important role for auxin in the onset of tuber sprouting, possibly by stimulation of vascular tissue differentiation and determination of leaf primordia formation of the newly developing sprout via directed auxin transport and distribution. This activation seems to be mediated by GA3 and dependent on a functional CK metabolism and signaling pathway.
Besides auxin, transcripts coding for components of the ethylene signaling pathway were found to be differentially expressed in GA3-treated wild-type and CKX-4 tubers. Thus, expression of transcripts with homology to ERF1 and ERF5 from tomato was more strongly activated in CKX-4 tubers than in the wild type, which could also be seen for EBF1 and EBF2 homologs. EBF1 and EBF2 are F-box proteins that target the central transcription factors EIN3 and EIL1 to proteasome-dependent degradation (for summary, see Stepanova and Alonso, 2009; Yoo et al., 2009). EIN3 and EIL1 activate the expression of ERF1 and other primary response genes, which induce the expression of secondary response genes by binding to the GCC box in the promoter (Solano et al., 1998). Interestingly, the transcript of the potato EIN3 homolog accumulated in untreated CKX-4 tubers and its expression decreased following GA3 treatment. This might be caused by the activation of EBF1 and EBF2 seen in CKX-4 tubers. Expression of both genes was found to be induced by ethylene and might provide an additional layer of regulation (Stepanova and Alonso, 2009; Yoo et al., 2009). On the other hand, a MKK9-like (for mitogen-activated protein kinase kinase 9) EST was transcriptionally induced in CKX-4 lines but not in wild-type tubers in response to GA3. MKK9 is part of a positively acting signaling cascade and may stabilize EIN3. However, it needs to be considered that almost nothing is known about ethylene signaling in potato, and even in Arabidopsis many fundamental questions still remain to be addressed (Stepanova and Alonso, 2009). Nevertheless, ethylene has been shown to play a pivotal role in the initiation and maintenance of tuber dormancy, but conflicting results have been published concerning its impact on terminating the rest period (Suttle, 1998b, 2004a, 2009). Hence, transient treatment with ethylene could hasten dormancy release, while continuous treatment resulted in sprout growth inhibition (Rylski et al., 1974). Application of ethylene inhibitors to developing microtubers resulted in a dose-dependent increase in premature sprouting (Suttle, 1998b). Conversely, several publications described an increased rate of ethylene production as sprouting commenced (Suttle, 2004a, and refs. therein). Inspection of our array data also revealed an increased expression of several ESTs coding for the biosynthetic genes 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase (Supplemental Tables S1 and S2) upon GA3 treatment, with different ESTs being induced in the wild type and CKX-4. This induction may indicate increased ethylene formation. However, its perception and signal transduction are clearly different in both genotypes, and our data suggest that ethylene signaling negatively influences sprout outgrowth. Together with stimulated auxin biosynthesis, transport, and signaling pathways, a dampened ethylene response might trigger cell differentiation and finally sprout outgrowth upon GA3 application. Examination of transcript profiles at earlier time points after GA3 treatment might contribute to elucidation of initial steps in hormonal signaling in the future. However, reactivation of meristematic activity is clearly dependent on the availability of CKs, as is evident from transgenic lines with increased expression of the CK-degrading enzyme CKX, in which sprouting cannot be induced by GA3 treatment. This was also reflected by the suppressed cell proliferation and differentiation in these transgenic tubers.
In summary, our data point to CK as an essential component controlling dormancy release. GA requires CK to stimulate the resumption of meristematic activity but is sufficient to support sprout growth once bud break has occurred.
MATERIALS AND METHODS
Plant Material and Growing Conditions
Potato (Solanum tuberosum ‘Solara’) plants were obtained from Bioplant. Plants were propagated in tissue culture under a 16-h-light/8-h-dark period on Murashige and Skoog medium (Murashige and Skoog, 1962) containing 2% Suc. Plants used for the analyses were cultivated in the greenhouse in individual pots (diameter of 20 cm, depth of 15.5 cm) at 50% humidity with 16 h of supplemental light (150 μmol quanta m−2 s−1) and 8 h of darkness. The temperature regime followed the light/dark cycle with 21°C and 18°C. After harvest, tubers were stored at room temperature in darkness.
Plasmid Construction and Plant Transformation
Standard procedures were performed as described by Sambrook et al. (1989). The cloning of Arabidopsis (Arabidopsis thaliana) GA20ox (accession no. X83379; AtGA20ox1) and GA2ox (accession no. AJ132435; AtGA2ox1) into the BinAR vector (Höfgen and Willmitzer, 1990) containing the CaMV 35S promoter and the octopine synthase polyadenylation signal was described by Biemelt et al. (2004). To express GA20ox under the control of the chimeric STLS1/CaMV 35 promoter, an approximately 1,300-bp fragment of the STLS1 promoter was amplified by PCR using the primers L700-5′ (5′-AGAATTCGCGGCCGCCCATTCCTTAAAAATTCCC-3′) and L700-3′ (5′-GAATTCCTGCTCTCACTACTTAGTATG-3′). The fragment was subcloned into pCR 2.1 vector (Invitrogen) and subsequently inserted into the binary construct 5′ upstream of the CaMV 35S promoter using EcoRI restriction sites.
The IPT clone (accession no. AF242881) originating from the Agrobacterium tumefaciens Ti plasmid pTi15955 (accession no. AF242881) was kindly provided by T. Schmülling as a T-DNA subclone in the pUC9 vector. This vector was used as a template to amplify the IPT gene by PCR using the primers IPT-5′ (5′-GGTACCATGGACCTGCATCTAATTTTC-3′) and IPT-3′ (5′-GTCGACCTAATACATTCCGAACGG-3′). The Arabidopsis CKX gene (accession no. NM_129714; At2g41510, AtCKX1) was amplified from Arabidopsis leaf cDNA using the following primers: CKX1-5′ (5′-GGATCCATGGGATTGACCTCATCC-3′) and CKX1-3′ (5′-GTCGACTTATACAGTTCTAGGTTTCG-3′). The resulting PCR fragments were subcloned into the pCR blunt vector (Invitrogen). The respective fragments were excised using Asp718 and SalI (IPT) or BamHI and SalI (CKX) restrictions sites and inserted into the binary vector BinAR (Höfgen and Willmitzer, 1990).
The binary constructs were transformed into Agrobacterium strain C58C1 carrying the virulence plasmid pGV2260 (Deblaere et al., 1985). Transformation of potato plants was performed as described (Rocha-Sosa et al., 1989).
Sprout-Release Assay (In Vitro Tuber-Sprouting Assay)
Discs of 5 mm height containing one bud each were excised from potato tubers using a Korkbohrer size 4 (8 mm). Discs were washed three times for 15 min in sterile filtered buffer containing 20 mm MES, 300 mm d-mannitol, and 5 mm ascorbic acid, pH 6.5. Discs were incubated with 5 to 100 μm GA3, 5 to 100 μm BAP, or sterile water for 5 min and subsequently placed in petri dishes lined with moist filter paper. Petri dishes were sealed with tape and stored in darkness under tissue culture conditions. The filter paper was regularly moistened by adding sterile water. Sprouting behavior of tuber discs was scored daily.
RNA Isolation, Reverse Transcription-PCR, and Northern-Blot Analysis
Isolation of total RNA was essentially performed as described by Logemann et al. (1987). For reverse transcription-PCR, cDNA was synthesized from 10 μg of total RNA as described (Biemelt et al., 2004). An aliquot was applied to PCR using the gene-specific primers IPT-5′ and IPT-3′. Ubiquitin was used as a loading control using primers described by Kloosterman et al. (2005).
For northern-blot analysis, 20 to 30 μg of total RNA was separated on 1.5% formaldehyde-containing agarose gels and blotted onto nylon membranes (GeneScreen; New England Nuclear) by capillary blotting overnight. The membranes were prehybridized and hybridized at 65°C. cDNA fragments of AtGA2ox1, AtGA20ox1, and AtCKX1 were used as probes and radioactively labeled with [32P]dCTP by means of the High Prime Kit (Roche). After stringent washing, radioactive membranes were exposed to x-ray films (Kodak) overnight at −70°C. Hybridization with a cDNA fragment of the small subunit of ribulose 1,5-bisphosphate carboxylase (accession no. X02353) served as a loading control.
Sample Preparation and Microarray Hybridization
For transcript profiling, an in vitro sprout experiment with tubers from wild-type and CKX-4 plants prestored for approximately 1 week was performed using 50 μm GA3. Samples were taken directly and 3 d after treatment by pooling eight tuber discs per sample. To reduce the portion of parenchyma in the samples, the size of the tuber discs was further reduced by cutting the eyes with Korkbohrer size 2 (4 mm). Total RNA from three replicates each was isolated as described above and purified using RNeasy Mini Spin Columns (Qiagen) following the manufacturer’s protocol. RNA quantity was measured with the ND-100 Spectrophotometer v3.3.0 (NanoDrop Technologies). RNA integrity was verified using an Agilent RNA 6000 Nano Chip on an Agilent 2100 BioAnalyzer (version B.02.03 BSI307) as recommended by the manufacturer’s protocol (Agilent RNA 6000 Nano Assay Protocol2).
Sample labeling and preparation for microarray hybridization were performed as described in the one-color microarray-based gene expression analysis protocol provided by Agilent including the one-color RNA spike-in kit (version 5.0.1; Agilent Technologies). Slides were scanned on the Agilent Microarray Scanner with extended dynamic range at high resolution (5 μm). Data sets were extracted by the feature extraction software package (version 9.5.3.1; Agilent Technologies) using a standard protocol.
Microarray Data Analysis
For data analysis, text files generated by the feature extraction software were imported into GeneSpring GX 7.3.1 (Silicon Genetics). Three normalization steps were applied: (1) data transformation: measurements less than 5.0 were set to 5.0; (2) per chip: normalization to the 50th percentile; (3) per gene: the signal of each feature was normalized to the median of its value across the data set. Features passing the quality check (flags present) and showing changes in expression levels equal or more than 2-fold were selected for further analysis. A volcano plot was applied to identify statistically significant (P ≤ 0.05; equal variances assumed), more than 2-fold differentially expressed genes between two conditions including the Benjamini-Hochberg multiple test correction. Annotations were taken from the POCI online tool (http://pgrc.ipk-gatersleben.de/poci). Functional assignment was done based on annotations, Gene Ontology terms, or by homology search against the Arabidopsis genome. Heat maps for selected pathways were generated using the MapMan tool (https://www.gabipd.org/projects/MapMan/).
Measurement of GA Contents
To analyze GA contents, apical shoot tips of potato plants comprising the first five leaves were harvested, frozen in liquid nitrogen, freeze dried, and stored at −80°C until analyzed. Approximately 500 mg (dry weight) of milled leaf material was extracted in the presence of 2H-labeled internal standards (5–25 ng μL−1) and 3H-labeled GA1, GA4, 16,17-dihydroGA19, and GA20 (50,000 dpm each) as markers at 4°C overnight in 100 mL of 80% (v/v) aqueous methanol. The extracts were purified, fractionated, and subjected to quantitative analysis by combined gas chromatography-mass spectrometry with selected ion monitoring as described by Griffiths et al. (2006).
Imaging
Images of tuber disc cross sections were captured with a Leica DFC480 digital camera on a Leica MZ16F stereomicroscope. Images were processed with Adobe Photoshop software.
Sequence data from this article can be found in GenBank/EMBL data libraries with the following accession numbers: AtGA20ox1 (X83379; At4g25420.1); AtGA2ox1 (AJ132435; At1g78440); IPT (AF242881); AtCKX 1 (NM_129714; At2g41510); STLS1 (X04753). The transcriptome data discussed here have been deposited at ArrayExpress (E-MEXP-2921).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. In vitro sprouting assay with potato tuber discs excised at different time points after harvest.
Supplemental Figure S2. In vitro tuber sprouting of wild-type tubers using different GA species.
Supplemental Figure S3. Expression of Arabidopsis GA20ox in transgenic potato plants under the control of the chimeric STLS1/CaMV 35S promoter.
Supplemental Figure S4. Expression level of the GA20ox gene caused by different plant promoters during tuber storage.
Supplemental Figure S5. Induction of growth in BAP-treated tuber discs by application of GA3.
Supplemental Figure S6. In vitro tuber-sprouting assay of CKX-4 tuber buds treated with BAP.
Supplemental Table S1. Differentially expressed transcripts found in the comparison of wild-type tuber buds immediately and 3 d after GA3 treatment.
Supplemental Table S2. Differentially expressed transcripts found in the comparison of AtCKX-4 tuber buds immediately and 3 d after GA3 treatment.
Supplemental Table S3. Assignment of individual POCI identifiers to MapMan terms corresponding to genes involved in cell cycle regulation, or auxin or ethylene signaling pathways.
Supplementary Material
Acknowledgments
We are grateful to Stephen Reid, Melanie Ruff, and Heike Nierig for excellent technical assistance and to Andrea Knospe, Christiane Börnke, and Anja Rust for plant transformation and maintenance. We thank Christine Hösl for taking care of plants in the greenhouse. We are indebted to Prof. Thomas Schmülling for providing the pUC9-iptΔp plasmid carrying the Agrobacterium IPT gene. We are thankful to Christian Prasch for providing cross sections through potato buds.
References
- Appeldoorn NJG, De Bruijn SM, Koot-Gronsveld EAM, Visser RGF, Vreugdenhil D, Van der Plas LHW. (1999) Developmental changes in enzymes involved in the conversion of hexose phosphate and its subsequent metabolites during early tuberization of potato. Plant Cell Environ 22: 1085–1096 [Google Scholar]
- Berckmans B, De Veylder L. (2009) Transcriptional control of the cell cycle. Curr Opin Plant Biol 12: 599–605 [DOI] [PubMed] [Google Scholar]
- Bialek K, Bielinska-Czarnecka M. (1975) Gibberellin-like substances in potato tubers during their growth and dormancy. Bull Acad Pol Sci Ser Sci Biol 23: 213–218 [Google Scholar]
- Biemelt S, Hajirezaei M, Hentschel E, Sonnewald U. (2000) Comparative analysis of abscisic acid content and starch degradation during storage of tubers harvested from different potato varieties. Potato Res 43: 371–382 [Google Scholar]
- Biemelt S, Tschiersch H, Sonnewald U. (2004) Impact of altered gibberellin metabolism on biomass accumulation, lignin biosynthesis, and photosynthesis in transgenic tobacco plants. Plant Physiol 135: 254–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstädter J, Rossbach C, Theres K. (1994) The pattern of histone H4 expression in the tomato shoot apex changes during development. Planta 192: 69–74 [DOI] [PubMed] [Google Scholar]
- Brian PW, Hemming HG, Radley M. (1955) A physiological comparison of gibberellic acid with some auxins. Physiol Plant 8: 899–912 [Google Scholar]
- Burton WB. (1989) The Potato, Ed 3 Longman Scientific and Technical, Essex, UK [Google Scholar]
- Campbell M, Segear E, Beers L, Knauber D, Suttle J. (2008) Dormancy in potato tuber meristems: chemically induced cessation in dormancy matches the natural process based on transcript profiles. Funct Integr Genomics 8: 317–328 [DOI] [PubMed] [Google Scholar]
- Campbell MA, Suttle JC, Sell TW. (1996) Changes in cell cycle status and expression of p34 (cdc2) kinase during potato tuber meristem dormancy. Physiol Plant 98: 743–752 [Google Scholar]
- Carraro N, Peaucelle A, Laufs P, Traas J. (2006) Cell differentiation and organ initiation at the shoot apical meristem. Plant Mol Biol 60: 811–826 [DOI] [PubMed] [Google Scholar]
- Carrera E, Bou J, García-Martínez JL, Prat S. (2000) Changes in GA 20-oxidase gene expression strongly affect stem length, tuber induction and tuber yield of potato plants. Plant J 22: 247–256 [DOI] [PubMed] [Google Scholar]
- Coles JP, Phillips AL, Croker SJ, García-Lepe R, Lewis MJ, Hedden P. (1999) Modification of gibberellin production and plant development in Arabidopsis by sense and antisense expression of gibberellin 20-oxidase genes. Plant J 17: 547–556 [DOI] [PubMed] [Google Scholar]
- Deblaere R, Bytebier B, De Greve H, Deboeck F, Schell J, Van Montagu M, Leemans J. (1985) Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res 13: 4777–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destefano-Beltrán L, Knauber D, Huckle L, Suttle JC. (2006) Effects of postharvest storage and dormancy status on ABA content, metabolism, and expression of genes involved in ABA biosynthesis and metabolism in potato tuber tissues. Plant Mol Biol 61: 687–697 [DOI] [PubMed] [Google Scholar]
- Dewitte W, Scofield S, Alcasabas AA, Maughan SC, Menges M, Braun N, Collins C, Nieuwland J, Prinsen E, Sundaresan V, et al. (2007) Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc Natl Acad Sci USA 104: 14537–14542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun EA, Brewer PB, Beveridge CA. (2009) Strigolactones: discovery of the elusive shoot branching hormone. Trends Plant Sci 14: 364–372 [DOI] [PubMed] [Google Scholar]
- Engelbrecht L, Bielinska-Czarnecka M. (1972) Increase of cytokinin activity in potato tubers near the end of dormancy. Biochem Physiol Pflanz 163: 499–504 [Google Scholar]
- Faivre-Rampant O, Cardle L, Marshall D, Viola R, Taylor MA. (2004) Changes in gene expression during meristem activation processes in Solanum tuberosum with a focus on the regulation of an auxin response factor gene. J Exp Bot 55: 613–622 [DOI] [PubMed] [Google Scholar]
- Francis D. (2007) The plant cell cycle: 15 years on. New Phytol 174: 261–278 [DOI] [PubMed] [Google Scholar]
- Francis D, Sorrell DA. (2001) The interface between the cell cycle and plant growth regulators: a mini review. Plant Growth Regul 33: 1–12 [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G. (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194 [DOI] [PubMed] [Google Scholar]
- Griffiths J, Murase K, Rieu I, Zentella R, Zhang ZL, Powers SJ, Gong F, Phillips AL, Hedden P, Sun TP, et al. (2006) Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18: 3399–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackbusch J, Richter K, Müller J, Salamini F, Uhrig JF. (2005) A central role of Arabidopsis thaliana ovate family proteins in networking and subcellular localization of 3-aa loop extension homeodomain proteins. Proc Natl Acad Sci USA 102: 4908–4912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G, Guilfoyle T. (2002) Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol Biol 49: 373–385 [PubMed] [Google Scholar]
- Hajirezaei M, Sonnewald U. (1999) Inhibition of potato tuber sprouting: low levels of cytosolic pyrophosphate lead to non-sprouting tubers harvested from transgenic potato plants. Potato Res 42: 353–372 [Google Scholar]
- Hedden P, Phillips AL. (2000) Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci 5: 523–530 [DOI] [PubMed] [Google Scholar]
- Hemberg T. (1949) Significance of growth-inhibiting substances and auxin for the rest period of potato tuber. Physiol Plant 2: 24–36 [Google Scholar]
- Hemberg T. (1970) The action of some cytokinins on the rest-period and the content of acid growth-inhibiting substances in potato. Physiol Plant 23: 850–858 [Google Scholar]
- Hemberg T. (1985) Potato Rest. Academic Press, Orlando, FL [Google Scholar]
- Höfgen R, Willmitzer L. (1990) Biochemical and genetic analysis of different patattin isoforms expressed in various organs of potato (Solanum tuberosum). Plant Sci 66: 221–230 [Google Scholar]
- Horvath DP, Anderson JV, Chao WS, Foley ME. (2003) Knowing when to grow: signals regulating bud dormancy. Trends Plant Sci 8: 534–540 [DOI] [PubMed] [Google Scholar]
- Inzé D, De Veylder L. (2006) Cell cycle regulation in plant development. Annu Rev Genet 40: 77–105 [DOI] [PubMed] [Google Scholar]
- Kendrick MD, Chang C. (2008) Ethylene signaling: new levels of complexity and regulation. Curr Opin Plant Biol 11: 479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Choi D, Kende H. (2003) The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth in Arabidopsis. Plant J 36: 94–104 [DOI] [PubMed] [Google Scholar]
- Kloosterman B, De Koeyer D, Griffiths R, Flinn B, Steuernagel B, Scholz U, Sonnewald S, Sonnewald U, Bryan GJ, Prat S, et al. (2008) Genes driving potato tuber initiation and growth: identification based on transcriptional changes using the POCI array. Funct Integr Genomics 8: 329–340 [DOI] [PubMed] [Google Scholar]
- Kloosterman B, Navarro C, Bijsterbosch G, Lange T, Prat S, Visser RG, Bachem CW. (2007) StGA2ox1 is induced prior to stolon swelling and controls GA levels during potato tuber development. Plant J 52: 362–373 [DOI] [PubMed] [Google Scholar]
- Kloosterman B, Vorst O, Hall RD, Visser RGF, Bachem CW. (2005) Tuber on a chip: differential gene expression during potato tuber development. Plant Biotechnol J 3: 505–519 [DOI] [PubMed] [Google Scholar]
- Leyser O. (2006) Dynamic integration of auxin transport and signalling. Curr Biol 16: R424–R433 [DOI] [PubMed] [Google Scholar]
- Leyser O. (2009) The control of shoot branching: an example of plant information processing. Plant Cell Environ 32: 694–703 [DOI] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L. (1987) Improved method for the isolation of RNA from plant tissues. Anal Biochem 163: 16–20 [DOI] [PubMed] [Google Scholar]
- MacDonald MM, Osborne DJ. (1988) Synthesis of nucleic acids and protein in tuber buds of Solanum tuberosum during dormancy and early sprouting. Physiol Plant 73: 392–400 [Google Scholar]
- Macháčková I, Sergeeva L, Ondřej M, Zaltsman O, Konstantinova T, Eder J, Ovesná J, Golyanovskaya S, Rakitin Y, Aksenova N. (1997) Growth pattern, tuber formation and hormonal balance in in vitro potato plants carrying ipt gene. Plant Growth Regul 21: 27–36 [Google Scholar]
- Martin RC, Mok MC, Mok DW. (1993) Cytolocalization of zeatin O-xylosyltransferase in Phaseolus. Proc Natl Acad Sci USA 90: 953–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockaitis K, Estelle M. (2008) Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol 24: 55–80 [DOI] [PubMed] [Google Scholar]
- Morris WL, Ducreux LJM, Hedden P, Millam S, Taylor MA. (2006) Overexpression of a bacterial 1-deoxy-D-xylulose 5-phosphate synthase gene in potato tubers perturbs the isoprenoid metabolic network: implications for the control of the tuber life cycle. J Exp Bot 57: 3007–3018 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Ooms G, Lenton JR. (1985) T-DNA genes to study plant development: precocious tuberisation and enhanced cytokinins in A. tumefaciens transformed potato. Plant Mol Biol 5: 205–212 [DOI] [PubMed] [Google Scholar]
- Rappaport L. (1956) Growth regulating metabolites: gibberellin compounds derived from rice disease-producing fungus exhibit powerful plant growth regulating properties. Calif Agric 10: 4–11 [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C. (2000) Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12: 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudin J-P, Doonan JH, Freeman D, Hashimoto J, Hirt H, Inzé D, Jacobs T, Kouchi H, Rouzé P, Sauter M, et al. (1996) Plant cyclins: a unified nomenclature for plant A-, B- and D-type cyclins based on sequence organization. Plant Mol Biol 32: 1003–1018 [DOI] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JAH. (1999) Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283: 1541–1544 [DOI] [PubMed] [Google Scholar]
- Rocha-Sosa M, Sonnewald U, Frommer W, Stratmann M, Schell J, Willmitzer L. (1989) Both developmental and metabolic signals activate the promoter of a class I patatin gene. EMBO J 8: 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronning CM, Stegalkina SS, Ascenzi RA, Bougri O, Hart AL, Utterbach TR, Vanaken SE, Riedmuller SB, White JA, Cho J, et al. (2003) Comparative analyses of potato expressed sequence tag libraries. Plant Physiol 131: 419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylski I, Rappaport L, Pratt HK. (1974) Dual effects of ethylene on potato dormancy and sprout growth. Plant Physiol 53: 658–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, Ed 2 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Schruff MC, Spielman M, Tiwari S, Adams S, Fenby N, Scott RJ. (2006) The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Development 133: 251–261 [DOI] [PubMed] [Google Scholar]
- Senning M, Sonnewald U, Sonnewald S. (2010) Deoxyuridine triphosphatase expression defines the transition from dormant to sprouting potato tuber buds. Mol Breed 27: 525–531 [Google Scholar]
- Smith OE, Rappaport L. (1961) Endogenous gibberellins in resting and sprouting potato tubers. Adv Chem Ser 28: 42–48 [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni R, Carmichael JP, Shah ZH, Murray JAH. (1995) A family of cyclin D homologs from plants differentially controlled by growth regulators and containing the conserved retinoblastoma protein interaction motif. Plant Cell 7: 85–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald U. (2001) Control of potato tuber sprouting. Trends Plant Sci 6: 333–335 [DOI] [PubMed] [Google Scholar]
- Sorce C, Lombardi L, Giorgetti L, Parisi B, Ranalli P, Lorenzi R. (2009) Indoleacetic acid concentration and metabolism changes during bud development in tubers of two potato (Solanum tuberosum) cultivars. J Plant Physiol 166: 1023–1033 [DOI] [PubMed] [Google Scholar]
- Sorce C, Lorenzi R, Ceccarelli N, Ranalli P. (2000) Changes in free and conjugated IAA during dormancy and sprouting of potato tubers. Aust J Plant Physiol 27: 371–377 [Google Scholar]
- Sponsel VM, Schmidt FW, Porter SG, Nakayama M, Kohlstruk S, Estelle M. (1997) Characterization of new gibberellin-responsive semidwarf mutants of Arabidopsis. Plant Physiol 115: 1009–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Alonso JM. (2009) Ethylene signaling and response: where different regulatory modules meet. Curr Opin Plant Biol 12: 548–555 [DOI] [PubMed] [Google Scholar]
- Suttle JC, Hultstrand JF. (1994) Role of endogenous abscisic acid in potato microtuber dormancy. Plant Physiol 105: 891–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle J. (2001) Dormancy-related changes in cytokinin efficacy and metabolism in potato tubers during postharvest storage. Plant Growth Regul 35: 199–206 [Google Scholar]
- Suttle J. (2008) Effects of synthetic phenylurea and nitroguanidine cytokinins on dormancy break and sprout growth in Russet Burbank minitubers. Am J Potato Res 85: 121–128 [Google Scholar]
- Suttle J. (2009) Ethylene is not involved in hormone- and bromoethane-induced dormancy break in Russet Burbank minitubers. Am J Potato Res 86: 278–285 [Google Scholar]
- Suttle JC. (1995) Postharvest changes in endogenous ABA levels and ABA metabolism in relation to dormancy in potato tubers. Physiol Plant 95: 233–240 [Google Scholar]
- Suttle JC. (1998a) Postharvest changes in endogenous cytokinins and cytokinin efficacy in potato tubers in relation to bud endodormancy. Physiol Plant 103: 59–69 [Google Scholar]
- Suttle JC. (1998b) Involvement of ethylene in potato microtuber dormancy. Plant Physiol 118: 843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle JC. (2004a) Physiological regulation of potato tuber dormancy. Am J Plant Physiol 81: 253–262 [Google Scholar]
- Suttle JC. (2004b) Involvement of endogenous gibberellins in potato tuber dormancy and early sprout growth: a critical assessment. J Plant Physiol 161: 157–164 [DOI] [PubMed] [Google Scholar]
- Suttle JC, Banowetz GM. (2000) Changes in cis-zeatin and cis-zeatin riboside levels and biological activity during potato tuber dormancy. Physiol Plant 109: 68–74 [Google Scholar]
- Turnbull CGN, Hanke DE. (1985b) The control of bud dormancy in potato tubers: measurement of the seasonal pattern of changing concentrations of zeatin-cytokinins. Planta 165: 366–376 [DOI] [PubMed] [Google Scholar]
- Turnbull CGN, Hanke DE. (1985a) The control of bud dormancy in potato tubers: evidence for the primary role of cytokinins and a seasonal pattern of changing sensitivity to cytokinin. Planta 165: 359–365 [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200 [DOI] [PubMed] [Google Scholar]
- Van den Berg JH, Davies PJ, Ewing EE, Halinska A. (1995) Metabolism of gibberellin A12 and A12 aldehyde and the identification of endogenous gibberellins in potato (Solanum tuberosum ssp. andigena) shoots. J Plant Physiol 146: 459–466 [Google Scholar]
- Van Staden J, Dimalla GG. (1978) Endogenous cytokinins and the breaking of dormancy and apical dominance in potato tubers. J Exp Bot 29: 1077–1084 [Google Scholar]
- Vidal AM, Gisbert C, Talón M, Primo-Millo E, López-Díaz I, García-Martínez JL. (2001) The ectopic overexpression of a citrus gibberellin 20-oxidase enhances the non-13-hydroxylation pathway of gibberellin biosynthesis and induces an extremely elongated phenotype in tobacco. Physiol Plant 112: 251–260 [DOI] [PubMed] [Google Scholar]
- Viola R, Pelloux J, van der Ploeg A, Gillespie T, Marquis N, Roberts AG, Hancock RD. (2007) Symplastic connection is required for bud outgrowth following dormancy in potato (Solanum tuberosum L.) tubers. Plant Cell Environ 30: 973–983 [DOI] [PubMed] [Google Scholar]
- Visser RGF, Vreugdenhil D, Hendriks T, Jacobsen E. (1994) Gene expression and carbohydrate content during tuber to stolon transition in potatoes (Solanum tuberosum). Physiol Plant 75: 525–531 [Google Scholar]
- Weijers D, Friml J. (2009) SnapShot: auxin signaling and transport. Cell 136: 1172. [DOI] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15: 2532–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T. (2001) Regulation of plant growth by cytokinin. Proc Natl Acad Sci USA 98: 10487–10492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Vreugdenhil D, van Lammeren AAM. (1998) Cell division and cell enlargement during potato tuber formation. J Exp Bot 49: 573–582 [Google Scholar]
- Yoo SD, Cho Y, Sheen J. (2009) Emerging connections in the ethylene signaling network. Trends Plant Sci 14: 270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.