Abstract
Plant-parasitic cyst nematodes penetrate plant roots and transform cells near the vasculature into specialized feeding sites called syncytia. Syncytia form by incorporating neighboring cells into a single fused cell by cell wall dissolution. This process is initiated via injection of esophageal gland cell effector proteins from the nematode stylet into the host cell. Once inside the cell, these proteins may interact with host proteins that regulate the phytohormone auxin, as cellular concentrations of auxin increase in developing syncytia. Soybean cyst nematode (Heterodera glycines) Hg19C07 is a novel effector protein expressed specifically in the dorsal gland cell during nematode parasitism. Here, we describe its ortholog in the beet cyst nematode (Heterodera schachtii), Hs19C07. We demonstrate that Hs19C07 interacts with the Arabidopsis (Arabidopsis thaliana) auxin influx transporter LAX3. LAX3 is expressed in cells overlying lateral root primordia, providing auxin signaling that triggers the expression of cell wall-modifying enzymes, allowing lateral roots to emerge. We found that LAX3 and polygalacturonase, a LAX3-induced cell wall-modifying enzyme, are expressed in the developing syncytium and in cells to be incorporated into the syncytium. We observed no decrease in H. schachtii infectivity in aux1 and lax3 single mutants. However, a decrease was observed in both the aux1lax3 double mutant and the aux1lax1lax2lax3 quadruple mutant. In addition, ectopic expression of 19C07 was found to speed up lateral root emergence. We propose that Hs19C07 most likely increases LAX3-mediated auxin influx and may provide a mechanism for cyst nematodes to modulate auxin flow into root cells, stimulating cell wall hydrolysis for syncytium development.
Cyst nematodes are sedentary endoparasites of plant roots that cause substantive damage and yield loss to many agricultural crops (Chitwood, 2003). These microscopic worms hatch from eggs in the soil, locate and penetrate a root, and then migrate through the root until they find and select a cell near the vasculature to initiate feeding (Hussey and Grundler, 1998). Using a hollow mouth spear, called a stylet, the nematode secretes esophageal gland-derived effector proteins into the host cell. These effector proteins developmentally reprogram the host cell into a specialized feeding site, called a syncytium (Davis et al., 2008). The nematode also induces an up-regulation of cell wall-modifying proteins (Goellner et al., 2001; Wieczorek et al., 2006, 2008; Karczmarek et al., 2008), causing the cell wall to dissolve. This allows the syncytium to expand in size by incorporating neighboring cells into the growing feeding site (Endo, 1964).
The phytohormone indole-3-acetic acid (IAA), or auxin, has been implicated in syncytium development (Goverse et al., 2000; Grunewald et al., 2009b). Auxin is involved in a myriad of plant cellular and developmental processes, including vascular development (Mattsson et al., 1999), embryonic axis development (Friml et al., 2003), phyllotaxis patterning (Reinhardt et al., 2003), gravitropism (Friml et al., 2002), phototropism (Stone et al., 2008), and lateral root development (Bhalerao et al., 2002; Swarup et al., 2008). The physiological responses associated with auxin are derived from a rapid transcriptional up-regulation of genes containing auxin-responsive elements (Dharmasiri et al., 2005; Kepinski and Leyser, 2005). Polar transport of auxin, both into and out of the cell, controls the amount of intracellular auxin available, thus providing regulation of the physiological processes affected by auxin. Several classes of plasma membrane auxin efflux transporters have been discovered, including those belonging to the PIN (Chen et al., 1998; Luschnig et al., 1998; Utsuno et al., 1998; Petrásek et al., 2006) and the MDR/PGP (Sidler et al., 1998) families. In Arabidopsis (Arabidopsis thaliana), the auxin influx transporter, AUX1 (Bennett et al., 1996), and three related family members, LAX1, LAX2, and LAX3, constitute the AUX/LAX family (Swarup et al., 2004), localize to the plasma membrane, and regulate auxin influx into the cell (Yang et al., 2006). LAX3 is required for lateral root emergence (Swarup et al., 2008) and is expressed specifically in cells overlaying lateral root primordia. By bringing auxin into these cells, LAX3 effectively up-regulates cell wall-modifying enzymes that loosen the cell wall and allow the newly formed lateral root to emerge through the existing root tissues (Swarup et al., 2008; Péret et al., 2009).
An increase in perceived auxin directly in the syncytium early during its development has been shown using auxin-responsive promoter-reporter constructs (Hutangura et al., 1999; Karczmarek et al., 2004; Wang et al., 2007; Grunewald et al., 2008). Cyst nematodes may manipulate auxin flow through the developing feeding site, as the Arabidopsis auxin influx transport protein, AUX1, is transcriptionally up-regulated in developing feeding sites (Mazarei et al., 2003) and the auxin efflux transporter, PIN3, relocalizes to the lateral plasma membrane of the developing syncytium (Grunewald et al., 2009a). Arabidopsis auxin-insensitive mutants with defects in auxin transport (pin) and signaling (axr2) support fewer cyst nematodes and produce smaller cysts compared with wild-type plants (Goverse et al., 2000; Grunewald et al., 2008). Furthermore, treatment with a polar auxin transport inhibitor, N-1-naphthylphthalamic acid, delays nematode development and inhibits nematode feeding site formation (Goverse et al., 2000). Taken together, these findings suggest a model in which an accumulation of auxin in the developing syncytium plays a substantive role in the formation of cyst nematode feeding sites from existing root cells.
The mechanisms that cyst nematodes use to alter auxin transport and signaling pathways to promote feeding site development are not well understood. A leading hypothesis is that cyst nematodes secrete effector proteins into the host cell and that these proteins function, among other things, to manipulate auxin flow through the feeding site by modulating auxin transport proteins or auxin signaling pathway components. Microaspiration of esophageal gland cell contents, construction of gland-enriched cDNA libraries, and analyses that predict secretion signal peptides (Gao et al., 2001; Wang et al., 2001) have led to the discovery of many putative effector proteins from the soybean cyst nematode (Heterodera glycines), 38 of which are novel (Gao et al., 2003). Subsequent in situ analyses have verified that these effector protein transcripts are present in esophageal gland cells (Gao et al., 2003). One effector protein transcript, Hg19C07 (accession no. AF490250), codes for a 207-amino acid protein that contains a 21-amino acid secretion signal. The Hg19C07 transcript is not detectable in preparasitic juveniles but is expressed specifically in the secretory dorsal gland cell of early parasitic life stages during feeding site development (Gao et al., 2003; Elling et al., 2009).
H. glycines and the beet cyst nematode (Heterodera schachtii) are closely related phylogenetically (Subbotin et al., 2001). Identification of putative effector proteins in H. schachtii has been accomplished, with many ortholog genes having a very high degree of sequence similarity and esophageal gland expression patterns (Patel et al., 2008). For example, H. schachtii ubiquitins (UBIs) have greater than 90% sequence identity with HgUBIs (Tytgat et al., 2004); Hs10A06 has 86% sequence identity with Hg10A06 (Hewezi et al., 2010); the H. schachtii cellulose-binding protein (CBP) has between 94% and 97% sequence homology to H. glycines CBP isoforms (Hewezi et al., 2008); the Hs4F01 annexin-like protein has 92% sequence identity to Hg4F01 (Patel et al., 2010); and H. schachtii CLAVATA3/ESR-like (CLE) proteins have greater than 80% homology with HgCLEs (Wang et al., 2010b), with the H. schachtii transcripts localizing to the same esophageal gland cell(s) as the H. glycines orthologs.
The H. schachtii-Arabidopsis pathosystem (Sijmons et al., 1991) is a useful tool for the study of cyst nematode parasitism on a molecular level. This infection system has led to the identification of several characterized protein-protein interactions between the H. schachtii effector proteins and Arabidopsis proteins expressed in tissues enriched in syncytial contents with yeast two-hybrid screens (Hewezi et al., 2008, 2010; Patel et al., 2010). In this paper, we report the results of a yeast two-hybrid approach that identified the Arabidopsis auxin influx transporter LAX3 as a potential interactor with the Hs19C07 effector protein. We demonstrate, with use of promoter-GUS lines, fluorescent protein fusions, and mutant lines, that the interaction is biologically relevant. Finally, we show that ectopic expression of Hs19C07 in Arabidopsis speeds up lateral root emergence, most likely by modulating the activity of LAX3.
RESULTS
Identification and Sequence Analysis of H. schachtii 19C07
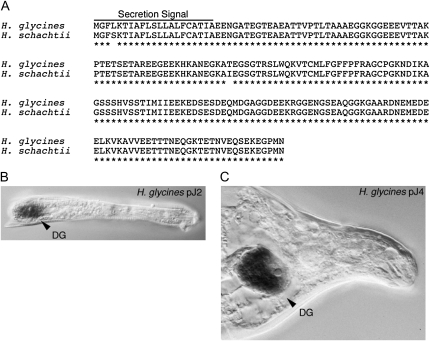
To identify the H. glycines putative effector protein 19C07 ortholog in H. schachtii, primers were designed from the previously reported sequence (Gao et al., 2003) and used in RACE-PCR on parasitic H. schachtii cDNA. The Hs19C07 gene codes for a 207-amino acid protein with a predicted molecular mass of 21.9 kD (Fig. 1A; accession no. HM142892). The Hs19C07 protein shares 99% sequence identity with Hg19C07. Both proteins contain the same number of amino acids as well as a 21-amino acid predicted secretion signal (Fig. 1A). No significant database matches were identified, classifying 19C07 as a novel protein. An in situ analysis demonstrated that Hg19C07 mRNA is induced only upon the onset of parasitism and is present exclusively inside of the single dorsal esophageal gland cell in both early-stage (Fig. 1B; parasitic second-stage juveniles [pJ2]) and late-stage (Fig. 1C; parasitic fourth-stage juveniles [pJ4]) nematodes, indicating that Hs19C07 is also a secreted protein from the dorsal esophageal gland cell expressed specifically during parasitism.
Figure 1.
The cyst nematode effector protein 19C07. A, Sequence comparison of 19C07 orthologs from H. glycines and H. schachtii. 19C07 is a novel 207-amino acid protein containing a putative 21-amino acid secretion signal. 19C07 is highly conserved between these two cyst nematode species containing 99% homologous amino acid sequence. B and C, In situ analysis of 19C07 in parasitic H. glycines cyst nematodes. B, pJ2 nematode. C, pJ4 nematode. DG, Dorsal gland.
19C07 Interacts with the Auxin Influx Transporter AtLAX3
A yeast two-hybrid analysis was used to identify potential proteins in Arabidopsis that interact with Hs19C07. The Hs19C07 bait construct pGBKT7-Hs19C07 was used to screen approximately 8.67 × 107 yeast colonies from an Arabidopsis prey library constructed from H. schachtii-infected root tissues at 7 d post infection (Hewezi et al., 2008). Seventeen independent Arabidopsis proteins were identified from 37 clones selected on quadruple dropout synthetic defined (SD) medium that were 5-bromo-4-chloro-3-indolyl-α-d-galactopyranoside (x-α-gal) positive; seven were reconfirmed by cotransformation. One of these interactors (Y.C. 55) contained the complete 469-amino acid sequence coding for the auxin influx transporter, LAX3, as well as extra sequence upstream of LAX3 from another Arabidopsis gene, At3g18370, coding for 106 amino acids (amino acids 549–638).
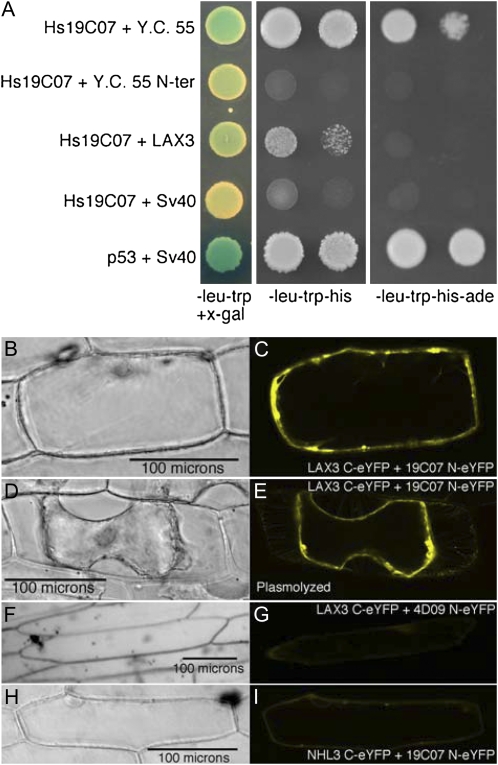
To further examine this interaction, both the full-length LAX3 (At1g77690) and the 318 nucleotides from At3g18370 upstream of LAX3 in Y.C. 55 were cloned separately into the prey pGADT7 activation domain vector and then cotransformed with pGBKT7-Hs19C07 into AH109 yeast cells along with appropriate controls (Fig. 2A). The full-length LAX3 cotransformed with Hs19C07 was x-α-gal positive, although the intensity was slightly weaker than that of the LAX3 Y.C. 55 and the positive control interaction between p53 and SV40 (Fig. 2A). A negative control cotransformation with pGBKT7-Hs19C07 and pGADT7 carrying SV40 did not turn as blue in the presence of x-α-gal, and neither did the cotransformation carrying the Y.C. 55 extra N-terminal (Y.C. 55 N-ter) sequence in pGADT7 cotransformed with pGBKT7-Hs19C07 when compared with full-length LAX3 coexpressed with Hs19C07. When plated on less stringent selective medium (SD-Leu-Trp-His), the full-length LAX3 did not grow as quickly as the original clone (Y.C. 55); however, no growth was observed with the Y.C. 55 N-ter or the negative control (Fig. 2A). Furthermore, no interaction was observed between the most closely related AUX/LAX family members, AUX1 or LAX2 and pGBKT7-Hs19C07 (Supplemental Fig. S1). On restrictive medium (SD-Leu-Trp-His-Ade), Y.C. 55 still interacted with Hs19C07, but the selection was too strong for the full-length LAX3 clone to overcome (Fig. 2A). On stringent selection plates (SD-/Leu-Trp-His-Ade), the full length LAX3 fusion may not drive enough of the activation domain expressed in the pGADT7 fusion to the nucleus, abolishing growth, while the N-terminal addition to LAX3 in Y.C. 55 may disrupt membrane localization, thus allowing the fusion to enter the nucleus, where it would be available for interaction. In contrast, overexpression of the full-length LAX3 on less stringent selection plates (SD-Leu-Trp-His) may have allowed enough of LAX3 to traffic to the nucleus for interaction with Hs19C07, allowing yeast growth (Fig. 2A, second column). Furthermore, the lack of interaction between Y.C. 55 N-ter and Hs19C07 on the less restrictive medium (SD-Leu-Trp-His) suggests that LAX3 is responsible for the interaction observed in Y.C. 55 (Fig. 2A). Because of the discrepancy of this yeast two-hybrid system to detect interactions with plasma membrane proteins, we used another approach to verify the interaction.
Figure 2.
H. schachtii 19C07 interacts with the Arabidopsis LAX3 auxin influx transporter. A, Yeast two-hybrid analysis. Left column, cotransformants grown on SD-Leu-Trp medium demonstrate that both bait and prey plasmids are present in yeast. Hs19C07 was cloned into the yeast two-hybrid vector pGBKT7, while the original recovered clone (Y.C. 55), the extra N-terminal sequence from Y.C. 55 (Y.C. 55 N-ter), and full-length LAX3 were cloned into pGADT7. pGBKT7 with Hs19C07 was cotransformed with pGADT7 carrying SV40 for a negative control. pGBKT7 with p53 and pGADT7 with SV40 provided a positive control. Blue colonies represent positive interactions in the presence of x-α-gal. Middle and left columns, positive selection of yeast two-hybrid interactions with minimal selection (SD-Leu-Trp-His) and restrictive (SD-Leu-Trp-His-Ade) media. B to I, BiFC confirmation of Hs19C07 interaction with AtLAX3. Bright-field images (B, D, F, and H) and fluorescent images (C, E, G, and I) of onion epidermal cells bombarded with BiFC constructs. B and C, Cobombardment of LAX3 C-eYFP and 19C07 N-eYFP. D and E, Plasmolyzed onion epidermal cell cobombarded with 19C07 N-eYFP and LAX3 C-eYFP. F and G, Cobombardment of LAX3 C-eYFP and 4D09 N-eYFP. H and I, Cobombardment of 19C07 N-eYFP and NHL3 C-eYFP.
To confirm a potential interaction between Hs19C07 and LAX3 in vivo, both proteins were transiently expressed using the pSAT4 bimolecular fluorescence complementation (BiFC) vector system (Citovsky et al., 2006) in onion (Allium cepa) peel epidermal cells. Hs19C07ΔSP fused to the N terminus of enhanced yellow fluorescent protein (eYFP), cobombarded into onion peels with LAX3 fused to the C terminus of eYFP, reconstituted the YFP signal specifically on the periphery of the cell (Fig. 2, B and C). After plasmolysis, the signal remained specifically with the plasma membrane (Fig. 2, D and E). To demonstrate the specificity of the interaction between Hs19C07 and LAX3, we coexpressed LAX3 C-eYFP with Hs4D09 N-eYFP, another H. schachtii esophageal gland protein (P. Howe and T.J. Baum, unpublished data). A slight YFP signal was detected, but at a much lower level than between LAX3 C-eYFP and Hs19C07 N-eYFP (Fig. 2, F and G). The YFP signal also was present within the cytoplasm of the cell surrounding the nucleus, unlike cells coexpressing LAX3 C-eYFP and Hs19C07 N-eYFP. We also tested Hs19C07 N-eYFP for interaction with another plasma membrane-localized protein, NHL3 (Varet et al., 2003), by fusing NHL3 to the C terminus of eYFP. Although a slight signal was detected, the signal was weak in comparison with the LAX3 and Hs19C07 interaction and was localized within the cytoplasm of the cell surrounding the nucleus (Fig. 2, H and I).
LAX3 and a LAX3-Inducible Gene Are Up-Regulated in Developing Syncytia
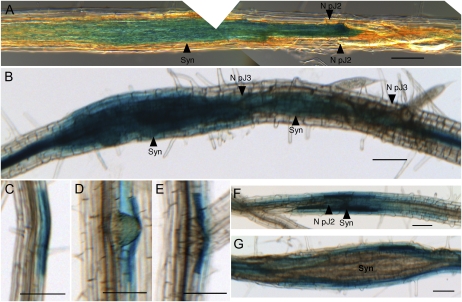
To examine the biological relevance of the interaction between Hs19C07 and LAX3, LAX3pro:GUS Arabidopsis seedlings were infected with H. schachtii juveniles and then stained at various stages of nematode development. Feeding cells stained blue as early as 2 d post inoculation (dpi) and before pJ2 nematodes had undergone their first in planta molt (Fig. 3A). LAX3 gene expression was constant in developing syncytia after the first in planta molt to pJ3 nematodes (Fig. 3B) and then subsided after feeding sites had finished developing.
Figure 3.
LAX3 and a LAX3-inducible gene are expressed in and surrounding developing syncytia. A and B, LAX3pro:GUS Arabidopsis roots infected with H. schachtii pJ2 (A) and pJ3 (B). C to E, PG expression demonstrated with PGpro:GUS Arabidopsis in uninfected roots. PG is expressed in cell layers overlaying developing lateral root primordia (C), emerging lateral roots (D), and in the cells shortly after the lateral root has emerged (E). F and G, PGpro:GUS Arabidopsis roots infected with H. schachtii. F, pJ2. G, Syncytium (Syn) at 8 dpi. Bars = 100 μm.
Syncytia development requires the dissolution of neighboring cell walls for fusion of adjacent cells into the growing feeding site (Endo, 1964). During lateral root emergence, normal expression of LAX3 in cells overlaying lateral root primordia results in an influx of auxin into these cells, inducing a plethora of cell wall-modifying enzymes (Swarup et al., 2008). Therefore, we examined the expression of a polygalacturonase (PG) gene that is regulated by LAX3 (Swarup et al., 2008) in response to nematode infection. PGpro:GUS transgenic plants infected with H. schachtii showed PG expression in cells overlaying the lateral root emergence site (Fig. 3C); expression was maintained after lateral root emergence (Fig. 3, D and E). PG was up-regulated in feeding sites several days after inoculation (Fig. 3F). As feeding sites developed and enlarged, PG expression shifted from the syncytium to surrounding cells (Fig. 3, F and G).
LAX3 Protein Is Expressed in the Syncytium and in Cells to Be Incorporated into the Syncytium
To examine LAX3 localization in roots following infection with H. schachtii, LAX3-YFP plants were grown on vertical plates and LAX3-YFP localization was imaged (Swarup et al., 2008). For comparative purposes, LAX3-YFP expression was also observed in noninfected plants. In noninfected plants, LAX3 protein expression was restricted to the vasculature and to the cells directly overlaying lateral root primordia, as described previously (Swarup et al., 2008; Fig. 4, A–D; Supplemental Fig. S2).
Figure 4.
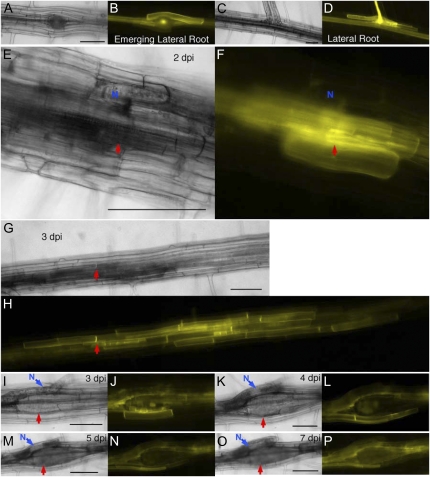
LAX3 is induced early in developing feeding sites and expressed in cells to be incorporated into syncytia. Arabidopsis lax3 and LAX3-YFP lines were observed before and after infection. A, C, E, G, I, K, M, and O, Bright-field images. B, D, F, H, J, L, N, and P, Fluorescent images. A and B, LAX3-YFP expression in the cells directly above an emerging lateral root primordium. C and D, LAX3-YFP expression persists in the cells above the emerged lateral root. E and F, H. schachtii-infected root 2 dpi with LAX3-YFP expression initiated in the feeding site as well as in several cells surrounding the developing feeding site. Blue N is on the infecting nematode. G and H, The same root infection site from E and F at 3 dpi. The LAX3-YFP signal has spread about 1 mm from the original infection site. Red arrows point to the nematode head. I to P, LAX3-YFP expression in the same syncytium at 3 dpi. Blue arrows point to the nematode (N), and red arrows point to the syncytium. Times are as follows: 3 dpi (I and J), 4 dpi (K and L), 5 dpi (M and N), and 7 dpi (O and P). Bars = 100 μm.
LAX3-YFP expression was monitored and imaged at the same infection site every 24 h for 7 dpi. LAX3-YFP was specifically up-regulated in root cells associated with nematode infection sites. LAX3-YFP expression was first observed in the feeding cell and then in the developing feeding site within 2 dpi (Fig. 4, E and F, red arrows). Nematode-induced LAX3-YFP expression was easy to distinguish from the normal lateral root-induced expression pattern (Supplemental Fig. S2, A, B, E, and F). LAX3-YFP expression expanded substantially between 2 and 3 dpi and was localized in the plasma membrane of cells to be incorporated into the developing syncytia (Fig. 4, G and H). The red arrows in Figure 4, E to H, point to the same location on the infected root at 2 and 3 dpi. At another feeding site at 3 dpi, the central structure of the syncytium was observable, and the remnants of cells merging into the feeding site through cell wall dissolution were also visible in the center of the developing feeding site (Fig. 4, I and J). LAX3-YFP expression was detectable in cells directly contacting the expanding feeding site (Fig. 4, I and J). At 4 dpi, the cell remnants observed at 3 dpi were completely incorporated into the growing feeding site, as were the perimeter syncytium cells that had LAX3-YFP in their plasma membrane. At 4 dpi, LAX3-YFP expression was detectable in the plasma membrane of the new layer of cells surrounding the developing syncytium (Fig. 4, K and L). Cells expressing LAX3-YFP at 4 dpi were incorporated into the syncytium by 7 dpi, with LAX3-YFP continually being expressed in the layer of cells proximal to the feeding site (Fig. 4, M–P). Both rapid expansion of the LAX3-YFP expression pattern and incorporation of cells into a developing feeding site are evident in a single root section between 5 and 6 dpi (Supplemental Fig. S2, A–D, N*).
LAX1 Is Up-Regulated in Developing Syncytia
Up-regulation of LAX3 and AUX1 (Mazarei et al., 2003) in developing syncytia led us to examine the expression of the other Arabidopsis AUX/LAX family members during cyst nematode infection. Both LAX1pro:GUS and LAX2pro:GUS Arabidopsis seedlings were infected with H. schachtii and stained at various stages of nematode development. Uninfected roots were examined from each line to determine basal expression patterns of LAX1 and LAX2 in Arabidopsis roots. LAX1 is expressed in vasculature cells surrounding the site of emergence of lateral roots (Supplemental Fig. S3, A–C). Expression starts coincident with the expansion of the emerging lateral root tip (Supplemental Fig. S3A) and increases in cells directly adjacent to the site of emergence shortly after lateral root tip emergence (Supplemental Fig. S3B). LAX1 expression continues for several days past the emergence of the lateral root (Supplemental Fig. S3C), then expression subsides. LAX1 is induced shortly after feeding site cell selection (Supplemental Fig. S3D) and stays up-regulated until after the J3 molt.
LAX2 is expressed in lateral root primordia and emerging lateral roots (Supplemental Fig. S3, E and F) and in the root tips of both lateral roots (Supplemental Fig. S3G) and the primary root (Supplemental Fig. S3H). LAX2 is not expressed in nematode feeding sites at any time during infection (Supplemental Fig. S3I).
Auxin Influx Transport Mutants Decrease Infectivity
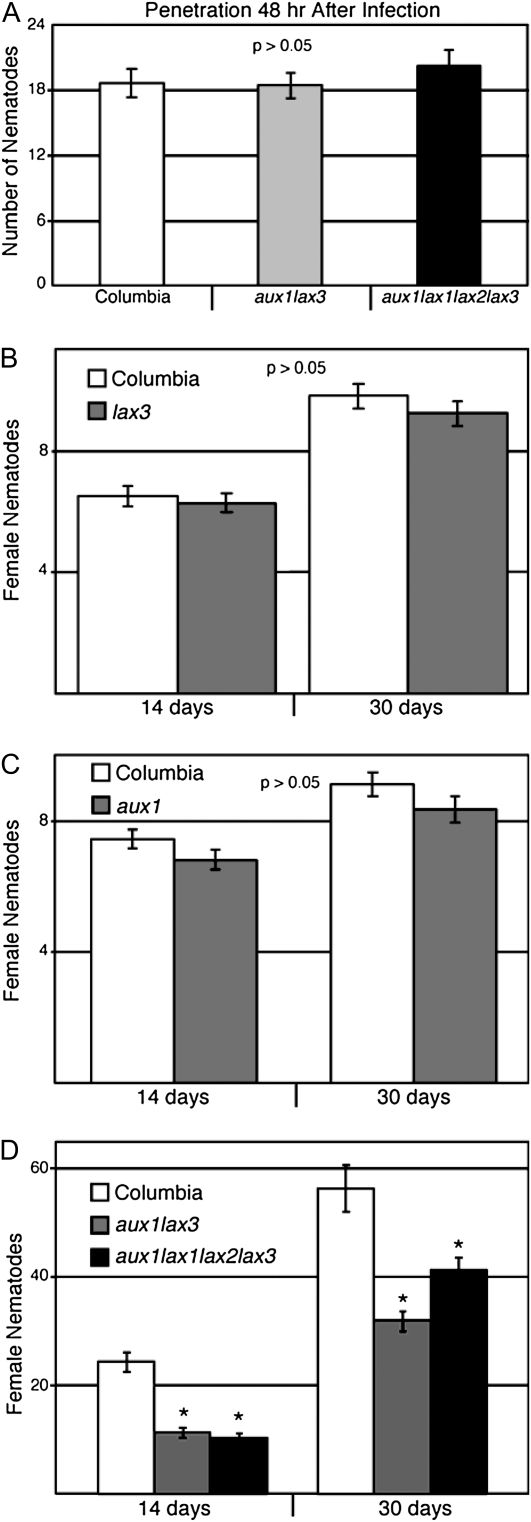
A role for auxin in developing syncytia is well documented (Hutangura et al., 1999; Goverse et al., 2000; Mazarei et al., 2003; Karczmarek et al., 2004; Grunewald et al., 2008, 2009a). However, infection assays on auxin influx transporter mutants are limited to a single allele of the aux1 mutant, aux1-7 (Goverse et al., 2000). Therefore, we examined H. schachtii infection on Arabidopsis auxin influx transporter single mutant lines, aux1-22 and lax3, as well as on the aux1lax3 double mutant and the aux1lax1lax2lax3 quadruple mutant to determine if the AUX/LAX family plays a critical role in syncytia development.
We first tested whether H. schachtii nematodes could perceive and penetrate into the roots of these mutant lines. We found no statistical difference between penetration in wild-type and lax3 lines (data not shown) or between the wild type and the aux1lax3 double mutant or the aux1lax1lax2lax3 quadruple mutant (Fig. 5A). Infection was assayed by measuring the development of parasitic J4 females at 14 dpi and adult females at 30 dpi. We found no significant reduction in nematode development in lax3 (Fig. 5B) or aux1 (Fig. 5C) single mutants. The aux1lax3 double mutant and the aux1lax1lax2lax3 quadruple mutant are agravitropic and fail to produce lateral roots up until 14 d post germination (Swarup et al., 2008; B. Peret and R. Swarup, unpublished data). Hence, an infection assay was performed on both the double and quadruple mutant seedlings at 7 d post germination prior to emergence of the first lateral root in order to start with the same amount of root tissue for infection; females were counted per plate rather than by individual plants, leading to larger infection numbers. Both the aux1lax3 double mutant and the aux1lax1lax2lax3 quadruple mutant had significant decreases in nematode numbers at both 14 and 30 dpi (Fig. 5D).
Figure 5.
Auxin influx transport mutants decrease nematode infectivity. A, At least 30 roots from Arabidopsis Col-0, aux1lax3, and aux1lax1lax2lax3 infected with H. schachtii were compared for penetration 48 h after infection. Means were not significantly different from the wild type using a two-tailed t test assuming unequal variances. Error bars indicate se. For aux1lax3, P = 0.901; for aux1lax1lax2lax3, P = 0.414 B, Three replicates of 36 individuals from Col-0 and lax3 lines were infected on 12-well plates with 250 infective J2 of H. schachtii. pJ4 female nematodes were counted per plant at 14 dpi, and adult female nematodes were counted at 30 dpi. Error bars indicate se. C, Three replicates of 36 individuals from Col-0 and aux1 lines were infected on 12-well plates with 250 infective J2 of H. schachtii. pJ4 female nematodes were counted per plant at 14 dpi, and adult female nematodes were counted at 30 dpi. Error bars indicate se. D, Three replicates of at least four vertical plates with eight individuals of Col-0, aux1lax3, and aux1lax1lax2lax3 genotypes were infected with H. schachtii. pJ4 female nematodes were counted per plate at 14 dpi, and adult female nematodes were counted at 30 dpi. Error bars indicate se. * P < 0.05 using a two-tailed t test.
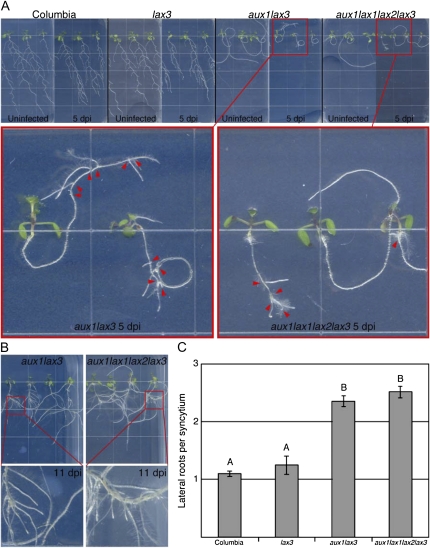
Nematode Infection Induces Lateral Root Formation in AUX/LAX Double and Quadruple Mutants
The severe lateral root phenotype of both the aux1lax3 double and aux1lax1lax2lax3 quadruple mutants, in which lateral root formation is blocked until at least 14 d after germination, has been documented (Swarup et al., 2008; B. Peret and R. Swarup, unpublished data). During infection assays with both the double and quadruple mutants, we observed hyper lateral root formation at the site of infection; this was examined further. Seven-day-old seedlings were infected with H. schachtii and then monitored for lateral root formation specifically from developing feeding sites. Infection of all lines resulted in decreased root growth as compared with uninfected roots (Fig. 6A). Although lateral root formation from syncytia occurred in all lines examined (Fig. 6), it was very apparent on the aux1lax3 double mutant and aux1lax1lax2lax3 quadruple mutant (Fig. 6A, insets, red arrowheads) because of the lack of lateral root formation in the uninfected plants at 5 dpi. By 11 dpi, lateral roots had grown substantially from feeding sites (Fig. 6B). Infection induced about one lateral root per infection site in both the wild type and the lax3 single mutant and around 2.5 lateral roots in both the aux1lax3 double and aux1lax1lax2lax3 quadruple mutants (Fig. 6C).
Figure 6.
Nematode infection induces lateral root formation in the aux1lax3 double and aux1lax1lax2lax3 quadruple mutants. A, Col-0, lax3, aux1lax3, and aux1lax1lax2lax3 seedlings either uninfected or infected (5 dpi) grown on vertical plates. Insets show infected aux1lax3 and aux1lax1lax2lax3 mutant lines. Arrowheads point to infection sites. Edge of squares = 1.3 cm. B, Lateral roots emerging from syncytia in infected aux1lax3 and aux1lax1lax2lax3 roots at 11 dpi. C, Lateral roots from all feeding sites were counted on three replicates of at least two vertical plates sown with six individuals from each line. Error bars indicate se. Letter grouping above bars indicates significance groups (P < 0.05). Plant lines that share a letter are not significantly different from each other using a two-tailed t test.
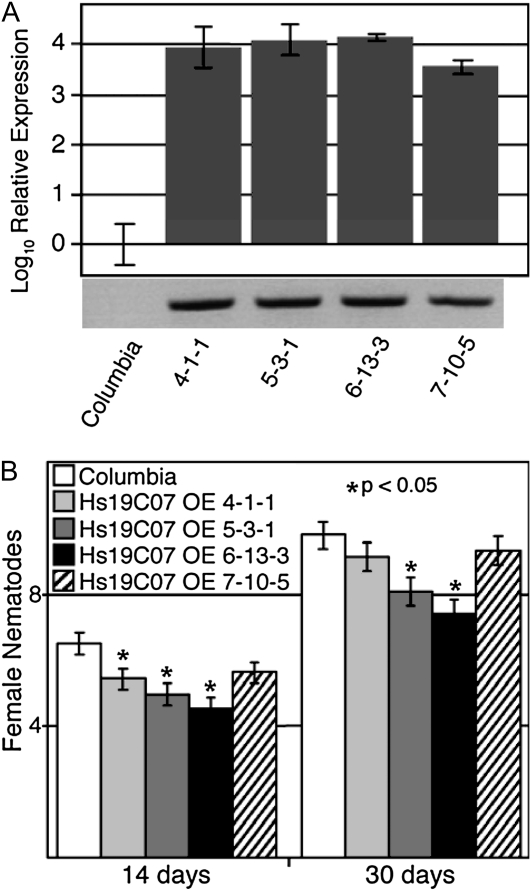
Ectopic Expression of 19C07 in Arabidopsis Reduces the Infectivity of H. schachtii
Because Hs19C07 interacted with AtLAX3 in the yeast two-hybrid screen, we generated Arabidopsis lines overexpressing Hs19C07 without the signal peptide to examine the effect of the nematode protein. Multiple independent transformants expressing Hs19C07ΔSP driven by the 35S promoter were selfed to homozygosity, and then expression levels were measured by reverse transcription (RT)-PCR and quantitative RT-PCR (qPCR). Four independent Hs19C07 overexpression lines were chosen with varying levels of transcript abundance (i.e. from relatively low expression [7-10-5] to high expression levels [6-13-3; Fig. 7A]). No obvious phenotypes were observed in the root systems of the Hs19C07 overexpression lines when grown on vertical plates. When examined for increased susceptibility to H. schachtii infection, we found a significant decrease in nematode number correlated to Hs19C07 expression level compared with the wild type at both 14 and 30 dpi, with greater amounts of Hs19C07 leading to fewer nematodes (Fig. 7B).
Figure 7.
Ectopic expression of 19C07 in Arabidopsis reduces infectivity of H. schachtii. A, Relative expression assayed by qRT-PCR of Hs19C07 in four independent homozygous Arabidopsis overexpression lines. Transcript levels were compared with Col-0 and are expressed in a logarithmic scale. Error bars indicate 95% confidence intervals. The bottom shows RT-PCR analysis of the coding region of the 19C07 transgene in the lines tested. B, Three replicates of 36 individuals from Col-0 and 19C07 overexpression lines were infected on 12-well plates with 250 infective J2 of H. schachtii. pJ4 female nematodes were counted on individuals at 14 dpi, and adult female nematodes were counted at 30 dpi. Error bars indicate se. * = P < 0.05.
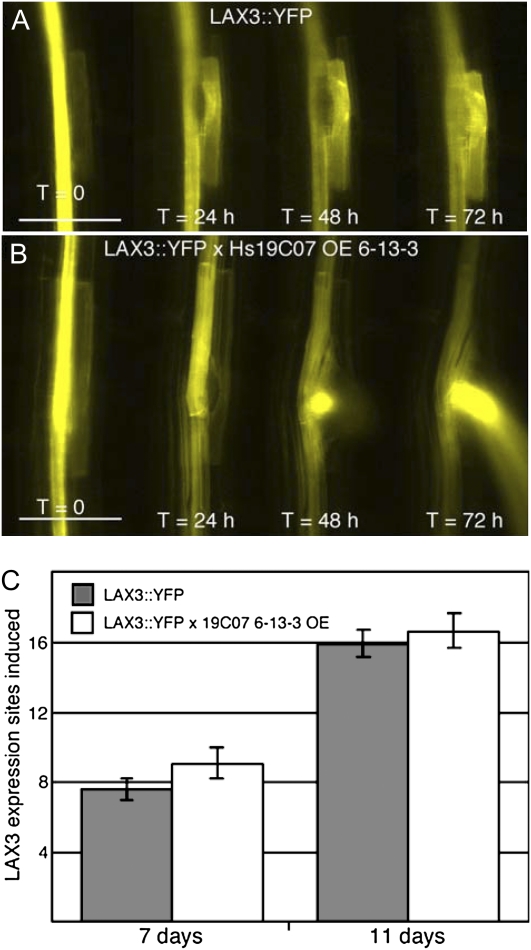
19C07 Expression Speeds up Lateral Root Emergence
To examine the effects of Hs19C07 on LAX3 directly, the LAX3-YFP line was crossed with the 6-13-3 Hs19C07ΔSP overexpression line (Fig. 8). F1 progeny from the cross were examined. The first lateral root emergence site was monitored daily as soon as LAX3-YFP was detectable. LAX3-YFP × Hs19C07 was compared with the LAX3-YFP line. Lateral root emergence was about 24 h sooner in the Hs19C07 background, with lateral roots emerging within 48 h of the onset of LAX3-YFP expression (Fig. 8B) compared with 72 h in the LAX3-YFP line (Fig. 8A). However, lateral roots were quantified at 7 and 11 d after planting, and no difference in the number of lateral roots was observed between the LAX3-YFP × 19C07 overexpression line and the LAX3-YFP line (Fig. 8C). From these data, we can conclude that 19C07 does not block LAX3 function, leading to a decrease in lateral root emergence, as reported in the lax3 mutant line (Swarup et al., 2008), but rather may stimulate its activity.
Figure 8.
Ectopic expression of 19C07 in Arabidopsis leads to earlier lateral root emergence. A and B, Initial lateral root emergence of lax3 and LAX3-YFP (A) and lax3 and LAX3-YFP × 19C07 6-13-3 overexpression line 5 d after seeding (T = 0) through 8 d after seeding (T = 72 h). Bars = 100 μm. C, The number of LAX3-YFP sites of expression on 7- and 11-d-old seedlings. Lateral root initiation sites were counted on two replicates of two vertical plates seeded with six individuals for each line. No significant difference was found using a two-tailed t test. Error bars indicate se.
DISCUSSION
Auxin is most likely involved in several aspects of syncytium development. Auxin-responsive genes, including the transcription factor AtWRKY23, are up-regulated in H. schachtii-derived syncytia (Grunewald et al., 2008; Szakasits et al., 2009). Thus, auxin most likely triggers a developmental cascade of events similar to the developmental cues triggered by auxin in developing organs, such as lateral roots (Swarup et al., 2008), or in the development of the root meristem early in embryogenesis (Friml et al., 2003). Although auxin has been shown to be required for cyst nematode infection, nothing is known about the role that nematode effector proteins may play in controlling the auxin flow into and out of developing feeding sites. Our results suggest that the cyst nematode effector protein 19C07 may modulate the activity of the auxin influx transporter LAX3 directly in the host cell destined to become the syncytium as well as in surrounding cells subsequently incorporated into the growing feeding site.
Auxin Influx Transporters Play a Role in Syncytium Development
LAX3 plays a role in lateral root emergence. Its up-regulation in cells overlaying lateral root primordia results in an influx of auxin to promote the emergence of lateral roots. This auxin influx is followed by the up-regulation of cell wall-modifying enzymes, such as PG (Wen et al., 2006; Swarup et al., 2008), that loosen the cell wall and allow the lateral root to emerge. Likewise, auxin influx into the developing feeding site from LAX3 and, presumably, AUX1 (Mazarei et al., 2003) and LAX1 (Supplemental Fig. S3), induces PG expression directly in the feeding site early (Fig. 3F) and most likely the other cell wall-modifying enzymes that are induced by LAX3-associated auxin influx in the roots (Swarup et al., 2008). Furthermore, in early syncytium development, PIN1, an auxin efflux protein, is down-regulated, presumably increasing the influx transporter-mediated auxin concentration in the developing feeding site that triggers the syncytium-specific auxin-induced physiological changes (Grunewald et al., 2009a). Within several days of syncytium initiation, LAX3 (Figs. 3B and 4, G to P; Supplemental Fig. S2, A–D) and PG (Fig. 3G) expression is shifted to cells neighboring the developing feeding site. This may be accomplished by a similar mechanism to LAX3 expression in cells overlaying emerging lateral roots (i.e. auxin flow from the lateral root primordia to the overlaying cells leads to the degradation of SLR/IAA14 repressor protein). Degradation of the repressor allows the auxin-inducible LAX3 to influx auxin into the cells, leading to a loosening of the cell wall by up-regulating a suite of cell wall-modifying proteins (Swarup et al., 2008; Péret et al., 2009). Thus, cyst nematodes may use existing auxin transport mechanisms to accomplish the same auxin-induced LAX3 expression in surrounding cells as the auxin efflux transporter PIN3 localizes to the periphery of developing syncytium (Grunewald et al., 2009a). LAX3 induction in cells surrounding the syncytium leads to an auxin influx into these cells, amplifying the auxin signal and, most likely, priming them for incorporation into the feeding site (Grunewald et al., 2009b). After several weeks, when adult females are present and feeding, LAX3-YFP expression is relegated to the locale farthest from the nematode or dissipated altogether (data not shown), suggesting that auxin is necessary for early events in syncytium setup and development. When the syncytium is large enough to maintain feeding, it stops expanding and incorporating cells, thus relieving the necessity for auxin-induced cell wall-modifying enzymes.
Prior experimental evidence and the work described herein now demonstrate that AUX1 (Mazarei et al., 2003), LAX1 (Supplemental Fig. S3), and LAX3 (Figs. 3, A and B, and 4, E–P) may be involved in auxin influx in the developing syncytium. Nevertheless, nematode infection and development observed on the aux1lax1lax2lax3 quadruple mutant (Fig. 5D) suggest that auxin may not be required for, but may enhance, syncytium development. In our studies, H. schachtii juveniles were able to sense, locate, and penetrate auxin transporter mutants (Fig. 5A), which suggests that auxin transporter mutants only affect syncytia initiation and development. The lack of a significant decrease in nematode infection on both aux1 and lax3 single mutants compared with the wild type (Fig. 5, B and C) suggests that removing one of four potential transporters does not affect infectivity, likely due to functional redundancy among these transporter proteins, as is observed in phyllotactic patterning (Bainbridge et al., 2008) and embryonic root cell organization (Ugartechea-Chirino et al., 2010). When more than one of the four potential AUX/LAX influx transporters were removed, overall auxin flow into the developing syncytia most likely decreased, leading to a delay in nematode development (Fig. 5D; 14 d). Although feeding sites matured and nematode development proceeded, a significant decrease in female numbers was observed 30 d after infection (Fig. 5D). A similar delay in nematode development was also observed previously in plants treated with an auxin transport inhibitor (Goverse et al., 2000).
Decreased auxin transport in the double and quadruple mutants results in a severe lateral root defect (i.e. no lateral roots form for 14 d) and a complete loss of gravitropism (Swarup et al., 2008). Interestingly, however, we observed a hyperinduction of lateral roots from aux1lax3 and aux1lax1lax2lax3 mutant feeding sites (Fig. 6). The fact that auxin promotes lateral root emergence (Péret et al., 2009) suggests that other classes of auxin influx transporters may be induced during syncytium development to fulfill the auxin requirement for developing feeding sites in the double and quadruple mutants. For example, the NRT1.1 nitrate transporter has also been shown to influx auxin in Xenopus oocytes, yeast, and in planta (Krouk et al., 2010). While auxin may presumably still accumulate in the feeding sites from other influx transporters on the aux1lax3 and aux1lax1lax2lax3 lines, the auxin accumulation that promotes lateral root growth may trigger the signaling events that precede lateral root formation, or alternatively, the nematodes may provide these lateral root induction signals. Following infection, the nematodes may potentially bypass the auxin requirement and up-regulate auxin-inducible genes with an unidentified parasitism factor (Grunewald et al., 2008). This might be the case during infection of the aux1lax3 double mutant and the aux1lax1lax2lax3 quadruple mutant that still support nematode growth and development, although the numbers are substantively decreased from wild-type plants (Fig. 5D). Potential nematode-induced auxin-independent up-regulation of auxin-inducible genes (Grunewald et al., 2008) could come from either uncharacterized nematode effector proteins or other nematode-derived signals, such as nematode-synthesized auxin (De Meutter et al., 2005), as other classes of plant pathogens have been shown to produce auxin or related compounds during parasitism (Glickmann et al., 1998; Chung et al., 2003). The up-regulation of auxin-inducible genes might also be explained by auxin biosynthesis occurring directly within developing syncytia, as auxin synthesis genes have been shown to be up-regulated in rapidly dividing root cells (Petersson et al., 2009), and from meristematic cells originating in both the primary and lateral roots (Ljung et al., 2005). The regulation of auxin biosynthesis by cytokinin has also been demonstrated (Jones et al., 2010). Therefore, alterations to cytokinin metabolism within developing syncytia might also explain the up-regulation of auxin-inducible genes in response to nematode infection.
Hs19C07 May Modulate AtLAX3 Activity in Developing Syncytia
Here, we describe the identification of the H. schachtii effector protein ortholog of H. glycines 19C07. As with Hg19C07 and Hs19C07, many H. glycines effector proteins have high sequence identity (80%–97%) to orthologs in H. schachtii (Tytgat et al., 2004; Hewezi et al., 2008, 2010; Patel et al., 2008, 2010; Wang et al., 2010b). The first transcript synthesis can be detected specifically within the dorsal gland cell after the nematode has penetrated the root (Gao et al., 2003). The increased expression of 19C07 at the onset of parasitism is similar to other known effector proteins (Ithal et al., 2007; Elling et al., 2009). Furthermore, the presence of an N-terminal secretion signal implies that this protein can enter the secretory pathway to the gland’s ampulla, where it is released by exocytosis and delivered through the feeding stylet into the host plant either intracellularly or extracellularly, as has been demonstrated with the cyst nematode HgCLEs (Wang et al., 2010a), the cyst nematode β,1-4-endoglucanases (Wang et al., 1999; Goellner et al., 2001), and the root-knot nematode Meloidogyne incognita calreticulin (Jaubert et al., 2005).
We demonstrate that Hs19C07 interacts directly with the syncytia-expressed auxin influx transporter AtLAX3 in situ. Using BiFC, a robust reconstitution of the YFP signal occurred specifically on the plasma membrane of onion peel epidermal cells when both Hs19C07 and AtLAX3 were coexpressed (Fig. 2, B–E) compared with the negative controls. When AtNHL3, an unrelated plasma membrane protein (Varet et al., 2003), and Hs19C07 were coexpressed, a faint signal was produced in the cytoplasm and surrounding the nucleus (Fig. 2, H and I), as is often observed in cytoplasmically expressed proteins, which may suggest that this signal is not associated with real interaction at the plasma membrane. Furthermore, substituting Hs19C07 with Hs4D09 (P. Howe and T.J. Baum, unpublished data), another effector protein expressed in the dorsal esophageal gland cell with a similar expression profile (Elling et al., 2009), and coexpressing with LAX3 produced a weak YFP signal in the cytoplasm surrounding the nucleus (Fig. 2, F and G), suggesting that the observed fluorescence was not comparable to the specific interaction observed between 19C07 and LAX3 at the plasma membrane.
We observed that overexpression of Hs19C07 in a LAX3-YFP background led to the faster emergence of lateral roots (24 h earlier; Fig. 8, A and B) compared with the LAX3-YFP line, suggesting that Hs19C07 increased the auxin influx rate from LAX3 to increase the rate of lateral root emergence. Thus, we propose that the nematode-secreted Hs19C07 most likely functions to increase the activity of LAX3 in such a way that the increased auxin accumulation in the developing feeding site stimulates cell wall hydrolysis to accelerate syncytia expansion and development. However, the results from our infection assays of Hs19C07 overexpression lines are counterintuitive to this idea. In previous studies, expression of H. schachtii effector proteins in Arabidopsis led to greater susceptibility to nematode infection (Hewezi et al., 2008, 2010; Patel et al., 2010). In contrast, we found that overexpression of Hs19C07 using the constitutive 35S promoter led to a slightly decreased cyst number inversely correlated to the amount of transcript expression. We speculate that this may have been an effect of Hs19C07 activity on normal auxin flux throughout the root, thereby dissipating the auxin concentrations in the root and decreasing the amount of auxin available for import into the developing feeding sites. To more carefully examine the effects of 19C07 overexpression on nematode infectivity, future studies will need to be performed using promoters more specific to developing feeding sites.
In summary, we present data that suggest that a nematode effector protein, Hs19C07, most likely modulates the activity of a plant auxin influx transporter protein, LAX3, during feeding site formation. This work adds to the growing body of evidence that auxin is an integral part of syncytia formation (for review, see Grunewald et al., 2009b). Like other symbiotic and parasitic interactions with plants (Mathesius et al., 1998; Boot et al., 1999; Huo et al., 2006; Chen et al., 2007; van Noorden et al., 2007; Felten et al., 2009), cyst nematodes also appear to use and manipulate auxin transport and sensing mechanisms in their host in order to dedifferentiate and up-regulate many genes leading to the development of transcriptionally unique syncytia (Szakasits et al., 2009). Further studies will be performed to examine the effects of Hs19C07 coexpression with LAX3 on auxin influx in cultured cells.
MATERIALS AND METHODS
Plant Materials
The LAX3pro::GUS, PGpro::GUS, and aux1-22lax3 lines were as described (Swarup et al., 2008). The LAX3pro::LAX3-YFP line was as described (Swarup et al., 2004, 2008). The aux1-22 and lax3 lines were as described (Tissier et al., 1999). The LAX1pro::GUS, LAX2pro::GUS, and aux1lax1lax2lax3 quadruple mutant were as described (Bainbridge et al., 2008). Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 (Col-0) was used for wild-type controls. The 19C07 overexpression lines were generated by the floral dip method (Clough and Bent, 1998) of Arabidopsis Col-0 using Agrobacterium tumefaciens strain GV3101 carrying pMHs19C07ΔSP. Lines were self-pollinated and carried to the T3 generation; homozygous lines were used in this study. To create the LAX3-YFP × 19C07 6-13-3 line, the LAX3pro::LAX3-YFP line was crossed to the T3 19C07 6-13-3 overexpression line as a female. Heterozygous F1 individuals were analyzed from this cross.
Nematode Materials
The beet cyst nematode (Heterodera schachtii) was propagated on greenhouse-grown Beta vulgaris ‘Monohi’. The soybean cyst nematode (Heterodera glycines) was propagated on Glycine max roots. Preparation and hatching of nematode eggs were performed as described (de Boer et al., 1999; Goellner et al., 2001; Mitchum et al., 2004). After 48 h of hatching, second-stage preparasitic juveniles were collected by low-speed centrifugation (8,000 rpm) in a tabletop centrifuge and surface sterilized for 10 min in a 0.004% mercuric chloride, 0.002% sodium azide, and 0.001% Triton X-100 solution, followed by five washes in sterile distilled water. Sterilized nematodes were resuspended in sterile 0.1% agarose. Nematodes were resuspended at a concentration of 200 nematodes 25 μL−1 with 25 μL applied to each root for GUS staining experiments. Nematodes were resuspended at a concentration of 50 nematodes 25 μL−1 with 25 μL applied to each root for LAX3-YFP line infections. Nematodes were resuspended at a concentration of 250 nematodes 25 μL−1 with 25 μL added to each individual plant for the 12-well plate, vertical plate, and penetration infection assays as well as the lateral root emergence from the feeding site assay.
In Situ Analysis
Parasitic H. glycines was isolated from G. max, fixed with paraformaldehyde, prepared, and labeled with a Hg19C07 digoxigenin probe as described (de Boer et al., 1998; Gao et al., 2001).
H. schachtii 19C07 Sequence Identification
Hs19C07 sequence was obtained using RACE with the BD SMART cDNA amplification kit (Clontech) according to the manufacturer’s directions. To obtain Hs19C07 flanking sequences, both 5′ and 3′ RACE were performed utilizing primers (5′ RACE primer, 5′-GTTCTCTCCGCCCCGTTTCTCTTCGT-3′; 3′ RACE primer, 5′-ACGAAGAGAAACGGGGCGGAGAGAAC-3′) designed from the H. glycines 19C07 sequence (Gao et al., 2003). The full-length Hs19C07 clone was obtained by using the primers Hs19C07 long 5′UTR (5′-CTCTCTCCCATTGGAATTATTCATTG-3′) and Hs19C07 long 3′UΤΡ (5′-CCGATCAGTCGTTCCCTTTCG-3′) and then cloned into the PCR4TOPO vector to create PCR4TOPO-easy-Hs19C07MC1. Hs19C07 was independently cloned multiple times and sequence verified. The Hs19C07 GenBank accession number is HM142892.
Constructs
Overexpression Construct
Hs19C07 without the signal peptide was PCR amplified using the primers Hs19C07BamHIDSPF (5′-CCAAGGATCCATGGAAGAAAATGGGGCGACAGA-3′) and Hs19C07XhoIR (5′-GGTTCTCGAGTCAGTTCATCGGCCCCTCC-3′). The resulting PCR product was cloned into pGEM-T-Easy (Promega) to make pGHs19C07ΔSPw. Hs19C07ΔSP was cut from pGHs19C07ΔSPw as a BamHI and SalI fragment and ligated into pMD1, a derivative of pBI121 (Clontech), under the control of the 35S promoter to make pMHs19C07ΔSP.
Yeast Two-Hybrid Constructs
Hs19C07 without the signal peptide was PCR amplified using the primers Hs19C07NdeIY2HDSPF (5′-CCAACATATGGAAGAAAATGGGGCGACAGA-3′) and Hs19C07Y2HBamHIR (5′-GGTTGGATCCTCAGTTCATCGGCCCCTCCTTTTC-3′) and then cloned into pGEM-T-Easy (Promega) to make pGHs19C07NB. Hs19C07ΔSP was digested from pGHs19C07NB as an NdeI-BamHI fragment and cloned into the GAL4 DNA-binding domain of pGBKT7 (Clontech) to generate the bait construct pGBKT7-Hs19C07. Full-length LAX3 was amplified from Arabidopsis root cDNA using the primer set LAX3EcoRIY2HF (5′-CCAAGAATTCATGGCGGCAGAGAAAATAGA-3′) and LAX3Y2HBamHIR (5′-GGTTGGATCCTCATGGCTTGTGAGGAGGGC-3′) and then cloned into pGADT7 as an EcoRI-BamHI fragment to make pGADT7-Lax3. The extra N-terminal sequence recovered in Y.C. 55 was amplified from the recovered pGADT7-Rec2 Y.C. 55 plasmid using the primers Y.C. 55 extra F (EcoRI) (5′-GCAAGAATTCCACCCAAGCAGTGGTATCAACG-3′) and Y.C. 55 extra R (XhoI) (5′-GCAACTCGAGCTATTTTCTCTTCTTCTCTCCATATTG-3′) and cloned into pGADT7 as an EcoRI-XhoI fragment to make pGADT7-Y.C. 55 N-ter.
BiFC Constructs
Hs19C07ΔSP was amplified using the primers 19C07 nC (cC) Sense (XhoI) (5′-GCAACTCGAGCTGAAGAAAATGGGGCGACAGA-3′) and 19C07 nC (cC) AS (BamHI) (5′-GCAAGGATCCTCATCAGTTCATCGGCCCCTCC-3′) and then cloned into pSAT4-nEYFP-C1 (Citovsky et al., 2006) to make pSAT4-nEYFP-Hs19C07ΔSP. AtLAX3 was amplified from root cDNA using the primers Lax3 nC (cC) Sense (XhoI) (5′-GCAACTCGAGCTATGGCGGCAGAGAAAATAGAGAC-3′) and Lax3 nC (cC) AS (BamHI) (5′-GCAAGGATCCTCATCATGGCTTGTGAGGAGGGC-3′) and then cloned into pSAT4-cEYFP-C1-B (Citovsky et al., 2006) to make pSAT4-cEYFP-Lax3. AtNHL3 (Varet et al., 2002) was amplified from seedling cDNA using the primers NHL3 cC Sense (XhoI) (5′-GCAACTCGAGCTATGGCGGACTTAAACGGTGCGTATTAC-3′) and NHL3 cC AS (BamHI) (5′-GCAAGGATCCTCATCAAAAGTCAACGTCACACTTGGTC-3′) and then cloned into pSAT4-cEYFP-C1-B to make pSAT4-cEYFP-NHL3.
Yeast Two-Hybrid Screening
Yeast two-hybrid screening was carried out as described in the BD Matchmaker Library Construction and Screening Kits according to the manufacturer’s instructions (Clontech). Three Arabidopsis cDNA libraries from roots of ecotype C24 at 3 d (pJ2), 7 d (pJ3), and 10 d (pJ4) post H. schachtii infection were generated in Saccharomyces cerevisiae strain AH109 as a fusion to the GAL4 activation domain of pGADT7-Rec2 (Hewezi et al., 2008). Screens were conducted using the mating method according to the manufacturer’s instructions. pGBKT7-Hs19C07 was used to screen approximately 8.67 × 107 yeast colonies from the pJ2, pJ3, and pJ4 prey libraries.
BiFC
Onion (Allium cepa) peel epidermal cells were bombarded with 0.5 μg of each pSAT4 plasmid (nEYFP or cEYFP) coated onto 1.6-μm-diameter gold particles (Bio-Rad) at 1,100 p.s.i. and 9-cm distance with a Biolistic Particle Delivery System (PDS-1000/He; Bio-Rad). Onion peels were incubated at room temperature in the dark for 24 h on half-strength Murashige and Skoog medium, pH 5.7, prior to imaging for YFP signal. At least 20 onion peel cells were examined from each of three replicates for each cobombardment.
GUS Staining
Seedlings were sown on modified Knops medium plates (Sijmons et al., 1991), grown vertically for 8 d, and then subjected to infection with H. schachtii. Infected seedlings were collected at various time points and vacuum infiltrated with a GUS substrate buffer (1 mm 5-bromo-4-chloro-3-indolyl glucuronide, 100 mm Tris, pH 7.0, 50 mm NaCl, 0.06% Triton X-100, and 0.5 mm potassium ferrocyanide) and then incubated in GUS substrate buffer overnight (Jefferson et al., 1987; Wang et al., 2007) at 37°C.
RNA Isolation, Quantitative Real-Time PCR, and RT-PCR
Total RNA was extracted from fresh root tissue isolated from the Hs19C07 overexpression Arabidopsis lines using the RNeasy Plant Mini Kit (Qiagen) and treated with RNase-free DNaseI (Qiagen). cDNA was synthesized from 750 ng of RNA using the First Strand cDNA Synthesis Kit for RT-PCR (Roche Diagnostics) according to the manufacturer’s instructions. qPCR was performed using the Applied Biosystems 7500 Real-Time PCR system. qPCR primers were designed from the Hs19C07 coding sequence and an endogenous control gene (GAPDH; accession no. AAG51517.1) by using Primer Express (Applied Biosystems). The following primers were designed to analyze Hs19C07: 19C07 qRT F (5′-CTGTATGTTGTTCGGCTTTTTCC-3′) and 19C07 qRT R (5′-CCCGCTTTTATATCGTTTTTGC-3′). The following primers were used for the GAPDH internal control: ATGAPDHF (5′-TCCCGTGTGGTCGACTTGA-3′) and ATGAPDHR (5′-CCCTATCATTCGAGATCTGCTTCT-3′). Triplicate qPCRs were set up and analyzed as described (Wang et al., 2007), except that the primer dimer amplification of the Hs19C07 primers in the wild-type background enabled us to set the expression level of 19C07 in the ecotype Col-0 background to zero for a relative comparison with the overexpression lines. Confidence intervals were calculated as described (Wang et al., 2007).
RT-PCR was performed on root cDNA from the Arabidopsis Hs19C07 overexpression lines to visually examine the expression of the Hs19C07 transcript. PCR was performed using the primer set 19C07 SSdel (HindIII) (5′-GCAAAAGCTTATGGAAGAAAATGGGGCGACAGA-3′) and 19C07 AS (SalI) (5′-GCAAGTCGACGGGTTCATCGGCCCCTCCTTTTC-3′) with ExTaq DNA polymerase (Takara) with the following conditions: 95°C for 90 s followed by 30 cycles of 95°C for 30 s, 63°C for 45 s, and 72°C for 45 s.
Microscopy
Images for the onion peels for BiFC cobombardment were acquired with an LSM 510 META confocal system mounted on an Axiovert 200M inverted microscope (Carl Zeiss) equipped with a 20× Plan Apochromat objective lens. YFP was excited with a 514-nm laser, and emitted fluorescence was detected through a 535- to 590-nm long-pass filter. Several onion peels were plasmolyzed with a plasmolysis buffer (50 mm Tris, pH 7.1, 2 m NaCl) before imaging.
GUS-stained roots were mounted on glass microscope slides, and images were acquired with an Olympus Vanox AHBT3 AH-3 equipped with 10× and 4× objectives fitted with a Leica DFC295 digital camera.
LAX3-YFP plant lines were grown on vertical plates with a thin layer of medium, keeping the root system in the dark until imaging. Images were acquired through the plate and medium with an Olympus IX 70 inverted microscope equipped with either a 20×/0.7 Plan Apochromat or a 10×/0.3 UPlanFL objective lens and a Hamamatsu Orca AG cooled CCD camera. For fluorescent images, a filter set with 490- to 510-nm excitation, a 515-nm long-pass dichroic mirror, and 520- to 550-nm emission was used (Chroma).
Infected root systems for the lateral root counts were imaged on an Epson Perfection V200 photo scanner and processed with Photoshop.
Infection Assays
All Arabidopsis seeds used in infection assays were surface sterilized with Cl2 gas for 6 h, incubated at 4°C for 48 h, and then seeded on modified Knops medium (Sijmons et al., 1991).
Penetration Assay
Two replicates of at least 30 Arabidopsis seeds from each genotype were grown on vertical plates for 8 d prior to infection with H. schachtii. Seedlings were collected 48 h after infection, and nematodes were stained with acid fuchsin fixation/staining solution (Hewezi et al., 2008) and counted on an inverted microscope. Nematodes penetrated into each root were scored.
Twelve-Well Plate Infection Assays
Three replicates of 36 seeds from each Arabidopsis genotype under investigation were individually and randomly distributed on 12-well plates and grown for 14 d prior to infection. At 14 and 30 d post infection, pJ4 females or adult females were counted on each individual.
Vertical Plate Infection Assay
Three replicates of at least four vertical plates with eight seeds from each genotype tested were grown vertically for 8 d prior to infection. pJ4 females or adult females were counted at 14 and 30 d post infection, respectively. Nematode counts were scored for each plate.
Lateral Root Scoring from Feeding Sites of Auxin Transport Mutant Lines
Three replicates of at least two vertical plates with six seeds from each genotype tested were grown vertically for 7 d in 16-h-light/8-h-dark conditions such that there was similar root length with almost no lateral root development when infection occurred. Lateral roots that emerged from all feeding sites at 11 dpi were counted and scored for each syncytium.
Analysis of Hs19C07 in the LAX3-YFP Background
LAX3-YFP × 19C07 6-13-3 seeds were surface sterilized for 6 h with Cl2 gas and then planted on vertical plates. Seedlings were grown on vertical plates on half-strength Murashige and Skoog medium, pH 5.7, with 2% Suc for 5 d. Seedlings were monitored and visualized for the next 4 d at 24-h intervals at the site of the first lateral root emergence on all seedlings. The total number of lateral roots per individual was counted from two replicates of two vertical plates for each genotype sown with six seeds each at 7 and 11 d after planting.
Statistical Analysis
Data analysis was performed with SAS version 9.2. Penetration assays, all infection assays, and lateral root counts were all compared with the wild type using unpaired two-tailed t test and assuming equal variance.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AF490250 (H. glycines 19C07), HM142892 (H. schachtii 19C07), AF469061 (H. schachtii 4D09), At5g01240 (LAX1), At2g21050 (LAX2), At1g77690 (LAX3), At5g06320 (NHL3), At5g14650 (PG), and AAG51517.1 (GAPDH).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Hs19C07 yeast two-hybrid screen of AUX/LAX family members.
Supplemental Figure S2. LAX3 induction in feeding sites and in cells immediately overlaying emerging lateral roots.
Supplemental Figure S3. LAX1 and LAX2 expression in response to nematode infection.
Supplementary Material
Acknowledgments
We thank Amy Replogle and Dr. Jianying Wang for critical discussions of the work; Melody Kroll for editing the manuscript; Robert Heinz for maintenance of the nematode lines; Peter Howe for the Hs4D09 BiFC control; and Esteban Fernandez and Stephanie Boyle for help with imaging.
References
- Bainbridge K, Guyomarc’h S, Bayer E, Swarup R, Bennett M, Mandel T, Kuhlemeier C. (2008) Auxin influx carriers stabilize phyllotactic patterning. Genes Dev 22: 810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA. (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273: 948–950 [DOI] [PubMed] [Google Scholar]
- Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G. (2002) Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29: 325–332 [DOI] [PubMed] [Google Scholar]
- Boot KJM, van Brussel AAN, Tak T, Spaink HP, Kijne JW. (1999) Lipochitin oligosaccharides from Rhizobium leguminosarum bv. viciae reduce auxin transport capacity in Vicia sativa subsp nigra roots. Mol Plant Microbe Interact 12: 839–844 [Google Scholar]
- Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH. (1998) The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci USA 95: 15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Agnew JL, Cohen JD, He P, Shan L, Sheen J, Kunkel BN. (2007) Pseudomonas syringae type III effector AvrRpt2 alters Arabidopsis thaliana auxin physiology. Proc Natl Acad Sci USA 104: 20131–20136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood DJ. (2003) Research on plant-parasitic nematode biology conducted by the United States Department of Agriculture-Agricultural Research Service. Pest Manag Sci 59: 748–753 [DOI] [PubMed] [Google Scholar]
- Chung KR, Shilts T, Ertürk U, Timmer LW, Ueng PP. (2003) Indole derivatives produced by the fungus Colletotrichum acutatum causing lime anthracnose and postbloom fruit drop of citrus. FEMS Microbiol Lett 226: 23–30 [DOI] [PubMed] [Google Scholar]
- Citovsky V, Lee LY, Vyas S, Glick E, Chen MH, Vainstein A, Gafni Y, Gelvin SB, Tzfira T. (2006) Subcellular localization of interacting proteins by bimolecular fluorescence complementation in planta. J Mol Biol 362: 1120–1131 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Davis EL, Hussey RS, Mitchum MG, Baum TJ. (2008) Parasitism proteins in nematode-plant interactions. Curr Opin Plant Biol 11: 360–366 [DOI] [PubMed] [Google Scholar]
- de Boer JM, Yan Y, Smant G, Davis EL, Baum TJ. (1998) In-situ hybridization to messenger RNA in Heterodera glycines. J Nematol 30: 309–312 [PMC free article] [PubMed] [Google Scholar]
- de Boer JM, Yan Y, Wang X, Smant G, Hussey RS, Davis EL, Baum TJ. (1999) Developmental expression of secretory β-1,4-endoglucanases in the subventral esophageal glands of Heterodera glycines. Mol Plant Microbe Interact 12: 663–669 [DOI] [PubMed] [Google Scholar]
- De Meutter J, Tytgat T, Prinsen E, Gheysen G, Van Onckelen H, Gheysen G. (2005) Production of auxin and related compounds by the plant parasitic nematodes Heterodera schachtii and Meloidogyne incognita. Commun Agric Appl Biol Sci 70: 51–60 [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. (2005) The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445 [DOI] [PubMed] [Google Scholar]
- Elling AA, Mitreva M, Gai X, Martin J, Recknor J, Davis EL, Hussey RS, Nettleton D, McCarter JP, Baum TJ. (2009) Sequence mining and transcript profiling to explore cyst nematode parasitism. BMC Genomics 10: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo BY. (1964) Penetration and development of Heterodera glycines in soybean roots and related anatomical changes. Phytopathology 54: 79–88 [Google Scholar]
- Felten J, Kohler A, Morin E, Bhalerao RP, Palme K, Martin F, Ditengou FA, Legué V. (2009) The ectomycorrhizal fungus Laccaria bicolor stimulates lateral root formation in poplar and Arabidopsis through auxin transport and signaling. Plant Physiol 151: 1991–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G. (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Gao B, Allen R, Maier T, Davis EL, Baum TJ, Hussey RS. (2001) Identification of putative parasitism genes expressed in the esophageal gland cells of the soybean cyst nematode Heterodera glycines. Mol Plant Microbe Interact 14: 1247–1254 [DOI] [PubMed] [Google Scholar]
- Gao B, Allen R, Maier T, Davis EL, Baum TJ, Hussey RS. (2003) The parasitome of the phytonematode Heterodera glycines. Mol Plant Microbe Interact 16: 720–726 [DOI] [PubMed] [Google Scholar]
- Glickmann E, Gardan L, Jacquet S, Hussain S, Elasri M, Petit A, Dessaux Y. (1998) Auxin production is a common feature of most pathovars of Pseudomonas syringae. Mol Plant Microbe Interact 11: 156–162 [DOI] [PubMed] [Google Scholar]
- Goellner M, Wang X, Davis EL. (2001) Endo-β-1,4-glucanase expression in compatible plant-nematode interactions. Plant Cell 13: 2241–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverse A, Overmars H, Engelbertink J, Schots A, Bakker J, Helder J. (2000) Both induction and morphogenesis of cyst nematode feeding cells are mediated by auxin. Mol Plant Microbe Interact 13: 1121–1129 [DOI] [PubMed] [Google Scholar]
- Grunewald W, Cannoot B, Friml J, Gheysen G. (2009a) Parasitic nematodes modulate PIN-mediated auxin transport to facilitate infection. PLoS Pathog 5: e1000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald W, Karimi M, Wieczorek K, Van de Cappelle E, Wischnitzki E, Grundler F, Inzé D, Beeckman T, Gheysen G. (2008) A role for AtWRKY23 in feeding site establishment of plant-parasitic nematodes. Plant Physiol 148: 358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald W, van Noorden G, Van Isterdael G, Beeckman T, Gheysen G, Mathesius U. (2009b) Manipulation of auxin transport in plant roots during Rhizobium symbiosis and nematode parasitism. Plant Cell 21: 2553–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewezi T, Howe P, Maier TR, Hussey RS, Mitchum MG, Davis EL, Baum TJ. (2008) Cellulose binding protein from the parasitic nematode Heterodera schachtii interacts with Arabidopsis pectin methylesterase: cooperative cell wall modification during parasitism. Plant Cell 20: 3080–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewezi T, Howe PJ, Maier TR, Hussey RS, Mitchum MG, Davis EL, Baum TJ. (2010) Arabidopsis spermidine synthase is targeted by an effector protein of the cyst nematode Heterodera schachtii. Plant Physiol 152: 968–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo X, Schnabel E, Hughes K, Frugoli J. (2006) RNAi phenotypes and the localization of a protein:GUS fusion imply a role for Medicago truncatula PIN genes in nodulation. J Plant Growth Regul 25: 156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey RS, Grundler FM. (1998) Nematode parasitism of plants. Perry RN, Wright J, , Physiology and Biochemistry of Free-Living and Plant Parasitic Nematodes. CAB International Press, Oxford, pp 213–243 [Google Scholar]
- Hutangura P, Mathesius U, Jones MGK, Rolfe BG. (1999) Auxin induction is a trigger for root gall formation caused by root-knot nematodes in white clover and is associated with the activation of the flavonoid pathway. Aust J Plant Physiol 26: 221–231 [Google Scholar]
- Ithal N, Recknor J, Nettleton D, Hearne L, Maier T, Baum TJ, Mitchum MG. (2007) Parallel genome-wide expression profiling of host and pathogen during soybean cyst nematode infection of soybean. Mol Plant Microbe Interact 20: 293–305 [DOI] [PubMed] [Google Scholar]
- Jaubert S, Milac AL, Petrescu AJ, de Almeida-Engler J, Abad P, Rosso MN. (2005) In planta secretion of a calreticulin by migratory and sedentary stages of root-knot nematode. Mol Plant Microbe Interact 12: 1277–1284 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Gunneras SA, Petersson SV, Tarkowski P, Graham N, May S, Dolezal K, Sandberg G, Ljung K. (2010) Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell 22: 2956–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczmarek A, Fudali S, Lichocka M, Sobczak M, Kurek W, Janakowski S, Roosien J, Golinowski W, Bakker J, Goverse A, et al. (2008) Expression of two functionally distinct plant endo-β-1,4-glucanases is essential for the compatible interaction between potato cyst nematode and its hosts. Mol Plant Microbe Interact 21: 791–798 [DOI] [PubMed] [Google Scholar]
- Karczmarek A, Overmars H, Helder J, Goverse A. (2004) Feeding cell development by cyst and root-knot nematodes involves a similar early, local and transient activation of a specific auxin-inducible promoter element. Mol Plant Pathol 5: 343–346 [DOI] [PubMed] [Google Scholar]
- Kepinski S, Leyser O. (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435: 446–451 [DOI] [PubMed] [Google Scholar]
- Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K, et al. (2010) Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell 18: 927–937 [DOI] [PubMed] [Google Scholar]
- Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Normanly J, Sandberg G. (2005) Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell 17: 1090–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U, Schlaman HR, Spaink HP, Of Sautter C, Rolfe BG, Djordjevic MA. (1998) Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J 14: 23–34 [DOI] [PubMed] [Google Scholar]
- Mattsson J, Sung ZR, Berleth T. (1999) Responses of plant vascular systems to auxin transport inhibition. Development 126: 2979–2991 [DOI] [PubMed] [Google Scholar]
- Mazarei M, Lennon KA, Puthoff DP, Rodermel SR, Baum TJ. (2003) Expression of an Arabidopsis phosphoglycerate mutase homologue is localized to apical meristems, regulated by hormones, and induced by sedentary plant-parasitic nematodes. Plant Mol Biol 53: 513–530 [DOI] [PubMed] [Google Scholar]
- Mitchum MG, Sukno S, Wang X, Shani Z, Tsabary G, Shoseyov O, Davis EL. (2004) The promoter of the Arabidopsis thaliana Cel1 endo-1,4-β glucanase gene is differentially expressed in plant feeding cells induced by root-knot and cyst nematodes. Mol Plant Pathol 5: 175–181 [DOI] [PubMed] [Google Scholar]
- Patel N, Hamamouch N, Li C, Hewezi T, Hussey RS, Baum TJ, Mitchum MG, Davis EL. (2010) A nematode effector protein similar to annexins in host plants. J Exp Bot 61: 235–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N, Hamamouch N, Li C, Hussey R, Mitchum M, Baum T, Wang X, Davis EL. (2008) Similarity and functional analyses of expressed parasitism genes in Heterodera schachtii and Heterodera glycines. J Nematol 40: 299–310 [Google Scholar]
- Péret B, Larrieu A, Bennett MJ. (2009) Lateral root emergence: a difficult birth. J Exp Bot 60: 3637–3643 [DOI] [PubMed] [Google Scholar]
- Petersson SV, Johansson AI, Kowalczyk M, Makoveychuk A, Wang JY, Moritz T, Grebe M, Benfey PN, Sandberg G, Ljung K. (2009) An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. Plant Cell 21: 1659–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrásek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertová D, Wisniewska J, Tadele Z, Kubes M, Covanová M, et al. (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312: 914–918 [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C. (2003) Regulation of phyllotaxis by polar auxin transport. Nature 426: 255–260 [DOI] [PubMed] [Google Scholar]
- Sidler M, Hassa P, Hasan S, Ringli C, Dudler R. (1998) Involvement of an ABC transporter in a developmental pathway regulating hypocotyl cell elongation in the light. Plant Cell 10: 1623–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons PC, Grundler FMW, von Mende N, Burrows PR, Wyss U. (1991) Arabidopsis thaliana as a new model host for plant-parasitic nematodes. Plant J 1: 245–254 [Google Scholar]
- Stone BB, Stowe-Evans EL, Harper RM, Celaya RB, Ljung K, Sandberg G, Liscum E. (2008) Disruptions in AUX1-dependent auxin influx alter hypocotyl phototropism in Arabidopsis. Mol Plant 1: 129–144 [DOI] [PubMed] [Google Scholar]
- Subbotin SA, Vierstraete A, De Ley P, Rowe J, Waeyenberge L, Moens M, Vanfleteren JR. (2001) Phylogenetic relationships within the cyst-forming nematodes (Nematoda, Heteroderidae) based on analysis of sequences from the ITS regions of ribosomal DNA. Mol Phylogenet Evol 21: 1–16 [DOI] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, Casimiro I, Péret B, Yang Y, Parry G, Nielsen E, De Smet I, Vanneste S, et al. (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol 10: 946–954 [DOI] [PubMed] [Google Scholar]
- Swarup R, Kargul J, Marchant A, Zadik D, Rahman A, Mills R, Yemm A, May S, Williams L, Millner P, et al. (2004) Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell 16: 3069–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakasits D, Heinen P, Wieczorek K, Hofmann J, Wagner F, Kreil DP, Sykacek P, Grundler FMW, Bohlmann H. (2009) The transcriptome of syncytia induced by the cyst nematode Heterodera schachtii in Arabidopsis roots. Plant J 57: 771–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissier AF, Marillonnet S, Klimyuk V, Patel K, Torres MA, Murphy G, Jones JDG. (1999) Multiple independent defective suppressor-mutator transposon insertions in Arabidopsis: a tool for functional genomics. Plant Cell 11: 1841–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat T, Vanholme B, De Meutter J, Claeys M, Couvreur M, Vanhoutte I, Gheysen G, Van Criekinge W, Borgonie G, Coomans A, et al. (2004) A new class of ubiquitin extension proteins secreted by the dorsal pharyngeal gland in plant parasitic cyst nematodes. Mol Plant Microbe Interact 17: 846–852 [DOI] [PubMed] [Google Scholar]
- Ugartechea-Chirino Y, Swarup R, Swarup K, Péret B, Whitworth M, Bennett M, Bougourd S. (2010) The AUX1 LAX family of auxin influx carriers is required for the establishment of embryonic root cell organization in Arabidopsis thaliana. Ann Bot (Lond) 105: 277–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsuno K, Shikanai T, Yamada Y, Hashimoto T. (1998) Agr, an Agravitropic locus of Arabidopsis thaliana, encodes a novel membrane-protein family member. Plant Cell Physiol 39: 1111–1118 [DOI] [PubMed] [Google Scholar]
- van Noorden GE, Kerim T, Goffard N, Wiblin R, Pellerone FI, Rolfe BG, Mathesius U. (2007) Overlap of proteome changes in Medicago truncatula in response to auxin and Sinorhizobium meliloti. Plant Physiol 144: 1115–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varet A, Hause B, Hause G, Scheel D, Lee J. (2003) The Arabidopsis NHL3 gene encodes a plasma membrane protein and its overexpression correlates with increased resistance to Pseudomonas syringae pv. tomato DC3000. Plant Physiol 132: 2023–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varet A, Parker J, Tornero P, Nass N, Nürnberger T, Dangl JL, Scheel D, Lee J. (2002) NHL25 and NHL3, two NDR1/HIN1-1ike genes in Arabidopsis thaliana with potential role(s) in plant defense. Mol Plant Microbe Interact 15: 608–616 [DOI] [PubMed] [Google Scholar]
- Wang J, Lee C, Replogle A, Joshi S, Korkin D, Hussey R, Baum TJ, Davis EL, Wang X, Mitchum MG. (2010a) Dual roles for the variable domain in protein trafficking and host-specific recognition of Heterodera glycines CLE effector proteins. New Phytol 187: 1003–1017 [DOI] [PubMed] [Google Scholar]
- Wang J, Replogle A, Hussey R, Baum T, Wang X, Davis EL, Mitchum MG. (2010b) Identification of potential host plant mimics of CLAVATA3/ESR (CLE)-like peptides from the plant-parasitic nematode Heterodera schachtii. Mol Plant Pathol (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Allen R, Ding X, Goellner M, Maier T, de Boer JM, Baum TJ, Hussey RS, Davis EL. (2001) Signal peptide-selection of cDNA cloned directly from the esophageal gland cells of the soybean cyst nematode Heterodera glycines. Mol Plant Microbe Interact 14: 536–544 [DOI] [PubMed] [Google Scholar]
- Wang X, Meyers D, Yan YT, Baum T, Smant G, Hussey R, Davis EL. (1999) In planta localization of a beta-1,4-endoglucanase secreted by Heterodera glycines. Mol Plant Microbe Interact 12: 64–67 [DOI] [PubMed] [Google Scholar]
- Wang X, Replogle A, Davis EL, Mitchum MG. (2007) The tobacco Cel7 gene promoter is auxin-responsive and locally induced in nematode feeding sites of heterologous plants. Mol Plant Pathol 8: 423–436 [DOI] [PubMed] [Google Scholar]
- Wen F, Laskowski M, Hawes M. (2006) Cell separation in roots. Annu Plant Rev 25: 91–105 [Google Scholar]
- Wieczorek K, Golecki B, Gerdes L, Heinen P, Szakasits D, Durachko DM, Cosgrove DJ, Kreil DP, Puzio PS, Bohlmann H, et al. (2006) Expansins are involved in the formation of nematode-induced syncytia in roots of Arabidopsis thaliana. Plant J 48: 98–112 [DOI] [PubMed] [Google Scholar]
- Wieczorek K, Hofmann J, Blöchl A, Szakasits D, Bohlmann H, Grundler FMW. (2008) Arabidopsis endo-1,4-β-glucanases are involved in the formation of root syncytia induced by Heterodera schachtii. Plant J 53: 336–351 [DOI] [PubMed] [Google Scholar]
- Yang Y, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E. (2006) High-affinity auxin transport by the AUX1 influx carrier protein. Curr Biol 16: 1123–1127 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.