Abstract
The importance of α-glucosidase in the endosperm starch metabolism of barley (Hordeum vulgare) seedlings is poorly understood. The enzyme converts maltose to glucose (Glc), but in vitro studies indicate that it can also attack starch granules. To discover its role in vivo, we took complementary chemical-genetic and reverse-genetic approaches. We identified iminosugar inhibitors of a recombinant form of an α-glucosidase previously discovered in barley endosperm (ALPHA-GLUCOSIDASE97 [HvAGL97]), and applied four of them to germinating grains. All four decreased the Glc-to-maltose ratio in the endosperm 10 d after imbibition, implying inhibition of maltase activity. Three of the four inhibitors also reduced starch degradation and seedling growth, but the fourth did not affect these parameters. Inhibition of starch degradation was apparently not due to inhibition of amylases. Inhibition of seedling growth was primarily a direct effect of the inhibitors on roots and coleoptiles rather than an indirect effect of the inhibition of endosperm metabolism. It may reflect inhibition of glycoprotein-processing glucosidases in these organs. In transgenic seedlings carrying an RNA interference silencing cassette for HvAgl97, α-glucosidase activity was reduced by up to 50%. There was a large decrease in the Glc-to-maltose ratio in these lines but no effect on starch degradation or seedling growth. Our results suggest that the α-glucosidase HvAGL97 is the major endosperm enzyme catalyzing the conversion of maltose to Glc but is not required for starch degradation. However, the effects of three glucosidase inhibitors on starch degradation in the endosperm indicate the existence of unidentified glucosidase(s) required for this process.
Following germination, starch degradation in the endosperm of cereal grains produces Glc, which is taken up into the scutellum and converted to Suc as the source of carbon for growth of the embryo (Nomura et al., 1969; Palmiano and Juliano, 1972; Aoki et al., 2006). Four enzymes are believed to be involved in the conversion of starch to Glc in the endosperm: α-amylase, β-amylase, the debranching enzyme limit dextrinase, and α-glucosidase (Fox et al., 2003; Bamforth, 2009). The aim of the work described below is to assess the importance of α-glucosidase (EC 3.2.1.10) in starch degradation following germination of barley (Hordeum vulgare) grains.
Starch degradation in barley has been the subject of intensive study in the context of brewing. In the initial, malting stage, barley grains are steeped in water for 1 d and then allowed to germinate and grow for about 5 d. Activities of all four enzymes increase, starch degradation is initiated, and the grain sprouts. Grains are then heated to arrest growth (kilned), milled, and heated in water (mashed) to promote rapid conversion of starch to Glc and oligosaccharides for fermentation. This conversion is catalyzed by the amylases and limit dextrinase present in the grains. Because of its heat lability, α-glucosidase activity is largely destroyed by kilning and is thought to be unimportant during mashing (Muslin et al., 2000). As a result, it is relatively poorly characterized (Bamforth, 2009). However, there are good indications that it may be crucial for normal postgerminative growth of the grain, as follows.
The major forms of α-glucosidase found in germinating cereal grains are exo-acting enzymes belonging to glycoside hydrolase family 31 (Frandsen and Svensson, 1998; Naested et al., 2006). The conventional view is that these enzymes act on maltose and other short maltooligosaccharides produced by amylases and limit dextrinase, converting them to Glc (Dunn, 1974; Manners, 1974; Osman, 2002). Consistent with this view, α-glucosidase from barley endosperm has a Km for maltose well below likely concentrations of maltose in the endosperm during the period of starch degradation (Km values from 1.7 to 2.4 mm reported by Jørgensen, 1963; Tibbot et al., 1998; Frandsen et al., 2000; Muslin et al., 2000; Naested et al., 2006).
Two lines of evidence suggest that α-glucosidase is also directly involved together with α-amylase in the degradation of starch granules. First, in in vitro experiments, purified α-glucosidase from barley seedlings can attack starch granules with the release of oligosaccharides and Glc. It also acts synergistically with α-amylase in solubilizing starch: in other words, the rate of starch solubilization in vitro is faster in the presence of both enzymes than predicted from the separate effects of the two enzymes (Sun and Henson, 1991; Sissons and MacGregor, 1994). Second, treatment of wheat (Triticum aestivum) seedlings with the α-glucosidase inhibitor miglitol (Bay m 1099) not only increased the ratio of maltose to Glc in the kernel fraction of seedlings but also inhibited the loss of starch from the endosperm and seedling growth (Konishi et al., 1994).
Neither of these approaches provides unambiguous information about the role of α-glucosidase in vivo. In vitro experiments may not accurately mimic the conditions in the endosperm. Different types of in vitro experiment have provided different views on the importance of α-glucosidase in granule solubilization (Sun and Henson, 1990, 1991; Sissons and MacGregor, 1994; Henson and Sun, 1996). Although miglitol was shown to inhibit α-glucosidase but not total amylase activity, its specificity was not fully explored (Konishi et al., 1994). It remains possible that some of its effects on wheat seedling growth and metabolism were due to the inhibition of enzymes other than endosperm α-glucosidase. Further ambiguity is created by reports of multiple isoforms of glucosidase with distinct specificities in germinating barley grains (Stark and Yin, 1987; Sun and Henson, 1990), of which only one (HvAGL197) has been identified at a molecular level (Tibbot and Skadsen, 1996; Frandsen et al., 2000; GenBank accession nos. AF118226 and AAF76254.1).
To elucidate the role and importance of α-glucosidase in the postgermination growth of barley seedlings, we have taken complementary chemical-genetic and reverse-genetic approaches. We describe the effects of a range of different α-glucosidase inhibitors on starch metabolism and growth in seedlings of two different barley cultivars. For one of these cultivars, we compare the effects of inhibitors with those of the expression of RNA interference (RNAi) for the α-glucosidase believed to be responsible for maltose hydrolysis in the endosperm (referred to as HvAGL97, encoded by HvAgl97). We conclude that the primary role of this enzyme is in the conversion of maltose to Glc in the endosperm. It is not required for starch degradation per se or for normal seedling growth.
RESULTS
Growth and Carbohydrate Content of Germinating Grains
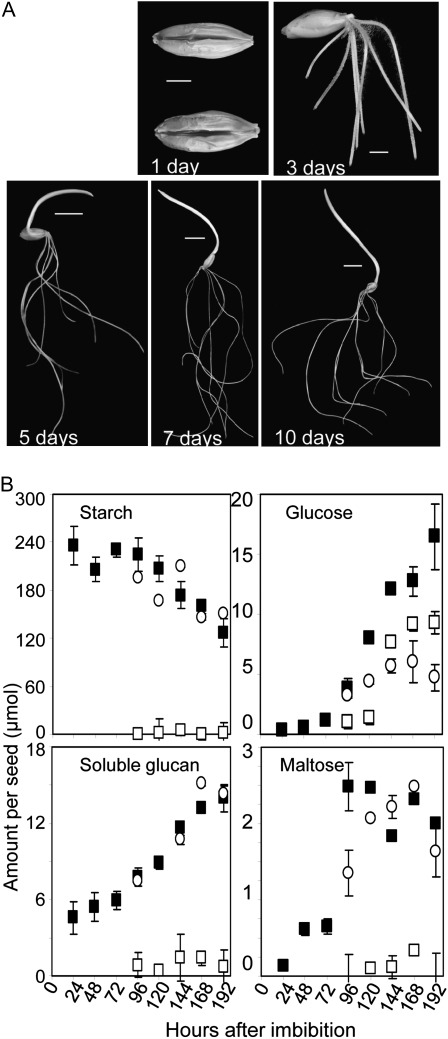
To establish suitable time points for studying the impact of inhibitors on seedling growth and metabolism, we measured starch and the starch breakdown products soluble glucan, maltose, and Glc at 24-h intervals after imbibition of grains of barley cv Optic. Under our conditions, roots emerged at 2 d and the coleoptile at 4 d, and by 10 d these had typically both reached lengths of 8 to 10 cm (Fig. 1A). Measurements were made on whole grains and from 4 d onward also on embryos (including roots and coleoptile) and the remainder of the grain after embryo removal. This latter fraction of the grain contains the testa and pericarp, most of the scutellum, the aleurone, and the endosperm, but its content of starch catabolites is dominated by the endosperm. Therefore, we refer to this fraction as the endosperm.
Figure 1.
Postimbibition development and metabolite levels in barley seedlings. A, Grains and seedlings at the number of days postimbibition indicated on the photographs. Bars = 0.3 cm for 1- and 3-d images and 1 cm for the remaining images. B, Amounts of starch and metabolites derived from starch in whole seedlings (black squares), endosperm (white circles), and embryos (white squares) during postimbibition growth. Values for starch and soluble glucans are given as μmol Glc equivalents. Values are means ± sd of measurements made on three individual seedlings, endosperms, or embryos. All grains were germinated at the same time in the same conditions.
The soluble glucan and Glc contents of whole seedlings rose from about 72 h after imbibition. Maltose content rose at around 72 h but showed no further increase and remained low in comparison with Glc (Fig. 1B). The starch content started to decline markedly at about 120 h after imbibition. After 192 h (8 d), 30% to 40% of the starch had been consumed, and after 10 d, about 75% of the starch had been consumed. Whereas most of the starch, soluble glucan, and maltose were contained in the endosperm, the rise in Glc content occurred in both endosperm and embryo. The molar ratio of Glc to maltose in the endosperm was between 3:1 and 2:1 from day 4 to day 8 after imbibition.
To allow the effects of inhibitors on starch degradation to be distinguished clearly, subsequent experiments were carried out between 8 and 10 d after imbibition, by which time a substantial proportion of the starch in control seedlings had been consumed.
Selection of Candidate Inhibitors
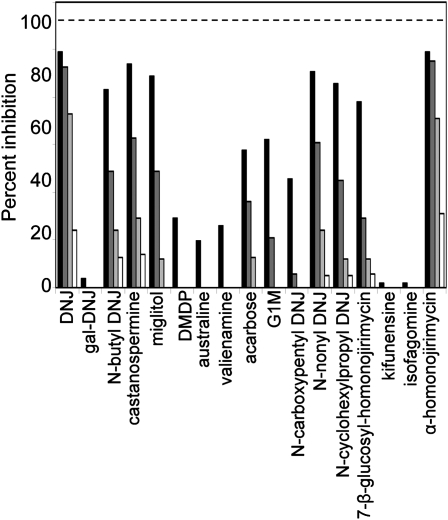
To select potential inhibitors of barley grain α-glucosidase, the hydrolysis of para-nitrophenyl α-d-glucopyranoside (pNPG) by the purified recombinant α-glucosidase (Naested et al., 2006) was assayed in the presence of a range of compounds available from MicroSource (GenPlus library; 960 compounds) and Phytoquest (Phytopure library; 1,197 compounds). The former library is composed of commercial drugs and known bioactives, while the latter is biased toward natural products and iminosugar-like compounds. The libraries were screened at 1 μm concentration using a stopped percentage inhibition assay. Four compounds out of 2,157 showed greater than 10% inhibitory activity (hit rate of 0.2%). One compound (α-homonojirimycin) was followed up in this study; the remaining three compounds are the subject of ongoing studies. α-Homonojirimycin and a range of other commercial inhibitors that are either known to inhibit α-glucosidases from other systems or closely resemble such inhibitors structurally were evaluated in duplicate at 10 μm, 1 μm, 100 nm, and 10 nm (Fig. 2). Structures of the selected compounds are given in Supplemental Figure S1. We observed a wide range of inhibitory effects (Fig. 2). Several of the more potent compounds inhibited recombinant enzyme activity by more than 80% at 10 μm. Two of these, 1-deoxynojirimycin (DNJ) and α-homonojirimycin, inhibited activity by 20% to 30% at 10 nm. Other structurally related compounds were ineffective. For subsequent experiments on germinating grains, we chose five iminosugar-based compounds spanning the range of inhibitory activities. DNJ, miglitol, and N-butyl DNJ are strong inhibitors, 4-O-α-glucosyl-moranoline (G1M) is a relatively weak inhibitor, and 1-deoxygalactonojirimycin (gal-DNJ) has almost no inhibitory activity.
Figure 2.
Effects of candidate inhibitors on the activity of recombinant barley grain α-glucosidase. The pure enzyme was assayed using pNPG as a substrate either without inhibitors or in the presence of inhibitors at 10 μm (black bars), 1 μm (dark gray bars), 100 nm (light gray bars), or 10 nm (white bars). The specific activity of the enzyme used in this assay was 25.6 units mg−1. One unit of activity is defined as the amount of enzyme that releases 2 μmol min−1 Glc from maltose when using 15 mm maltose as substrate at 37°C (Naested et al., 2006). The structures and full names of inhibitors are given in Supplemental Figure S1.
Effects of Inhibitors on Seedling Growth
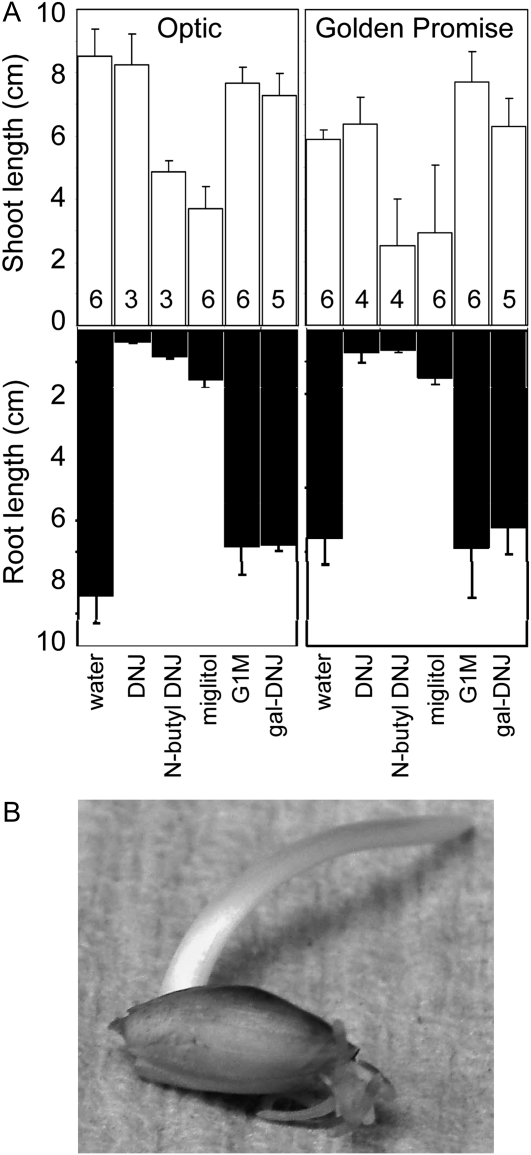
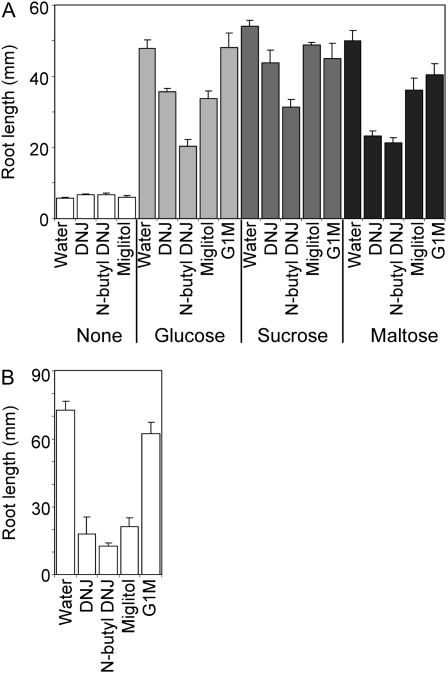
Batches of grains were allowed to germinate from the point of imbibition in the presence (at 500 μm) or absence (controls, water only) of the selected inhibitors. The three strongest inhibitors of the recombinant barley grain α-glucosidase, DNJ, miglitol, and N-butyl DNJ, very strongly inhibited root growth of seedlings of barley cv Optic (Fig. 3A). Complete inhibition of root growth occurred at about 3 d after imbibition. Roots became brown and extremely brittle (Fig. 3B). N-Butyl DNJ and miglitol also retarded coleoptile growth, but to a lesser degree, and DNJ had no effect on coleoptile growth. Gal-DNJ and G1M had little or no effect on root or coleoptile growth. Very similar results were obtained with a second cultivar of barley, Golden Promise (Fig. 3A).
Figure 3.
Effects of inhibitors on root and shoot growth of seedlings. A, Seedlings of cv Optic and Golden Promise were grown for 10 d postimbibition in the absence of inhibitor (water) or in the presence of 500 μm inhibitor. Values for root and coleoptile (shoot) length are means of measurements made on the numbers of seedlings indicated in the top part of the graph. Error bars represent se. B, A seedling of Optic after 7 d of growth in the presence of 500 μm DNJ. An equivalent seedling grown without inhibitor is shown in Figure 1A.
Effects of Inhibitors on Starch Metabolism in Seedlings
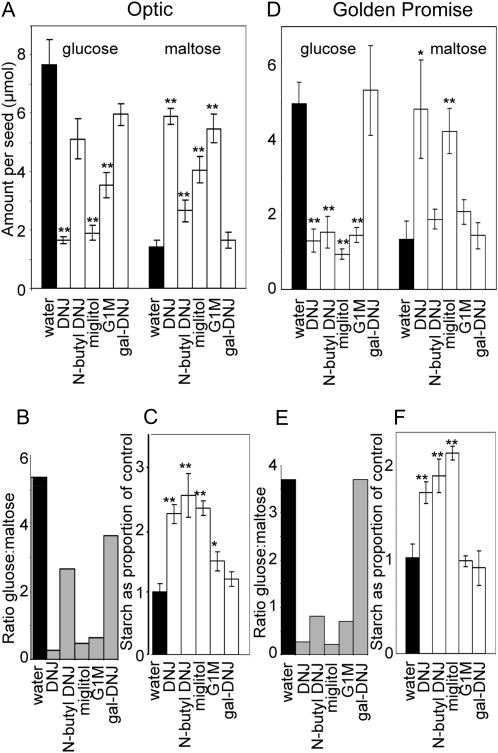
The presence of inhibitors of α-glucosidase had marked effects on levels of Glc, maltose, and starch in the endosperm after 10 d of germination (Fig. 4). For both Optic and Golden Promise, the presence of either DNJ or miglitol resulted in higher levels of maltose and lower levels of Glc than in controls. In Optic, the presence of G1M also affected both maltose and Glc levels, and the presence of N-butyl DNJ affected maltose but not Glc levels (Fig. 4A). In Golden Promise, the presence of N-butyl DNJ or G1M strongly reduced Glc levels relative to controls, but neither inhibitor caused a statistically significant elevation of maltose (Fig. 4D). Thus, in Optic, the presence of DNJ, miglitol, and G1M reduced the ratio of Glc to maltose from the control value of 5.4 to less than 0.7 (Fig. 4B), and the presence of N-butyl DNJ reduced the ratio to 2.7. In Golden Promise, the presence of any of these four inhibitors reduced the ratio of Glc to maltose from the control value of about 3.7 to 0.8 or less (Fig. 4E). The presence of gal-DNJ had no statistically significant effect on maltose or Glc levels in the endosperm of either Optic or Golden Promise.
Figure 4.
Effects of inhibitors on starch, Glc, and maltose levels in seedlings. Seedlings were grown for 10 d postimbibition in the absence of inhibitor (water) or in the presence of 500 μm inhibitor, and amounts of metabolites in the endosperm fraction were assayed. Identical experiments were carried out on grains of cv Optic (A–C) and Golden Promise (D–F). Values marked with asterisks are statistically significantly different from control (water) values (t test; ** P < 0.01, * 0.01 > P < 0.05). A and D, Values for Glc and maltose are means of measurements on the following numbers of individual seedlings. For Optic: water 10, G1M 9, DNJ 8, other inhibitors each 7; for Golden Promise: N-butyl DNJ 4, other inhibitors each 6. Error bars represent se. Values are shown as μmol Glc equivalents. B and E, Values for the ratio of Glc to maltose are calculated from the absolute amounts of these metabolites shown in A and D. C and F, Amounts of starch in seedlings grown in the presence of inhibitors are shown as a proportion of the amounts in seedlings grown in the absence of inhibitor (water). Values are means of measurements on the following numbers of individual seedlings. For Optic: G1M 11, water 10, miglitol 9, DNJ and gal-DNJ 8, N-butyl DNJ 7; for Golden Promise: N-butyl DNJ 3, DNJ 4, water and other inhibitors each 6. Error bars represent se.
Starch degradation in seedlings of both Optic and Golden Promise was strongly inhibited in the presence of DNJ, miglitol, or N-butyl DNJ (Fig. 4, C and F). Whereas Optic controls contained an average of 51 μmol Glc equivalents of starch per endosperm after 10 d of growth, the endosperm of seedlings grown with DNJ, miglitol, or N-butyl DNJ contained 2.2 to 2.5 times this amount. Thus, whereas control seedlings had degraded approximately 75% of their original starch content after 10 d of growth, only 40% of the starch was degraded in the presence of these inhibitors. For Golden Promise, controls contained 48 μmol Glc equivalents of starch per endosperm, and seedlings grown with DNJ, miglitol, or N-butyl DNJ contained between 1.7 and 2.1 times this amount. The presence of G1M inhibited starch degradation in the endosperm fraction to a small extent in Optic and not at all in Golden Promise, and the presence of gal-DNJ had no effect in either cultivar.
Effects of Inhibitors on Starch-Degrading Enzymes and Growth of Isolated Embryos
The results presented above revealed marked differences between the inhibitors in the extent to which they affected maltose metabolism, seedling growth, and starch degradation. DNJ, N-butyl DNJ, and miglitol all inhibited root growth and starch degradation as well as maltose metabolism. In contrast, G1M appeared to be a very effective inhibitor of maltose metabolism but had no effect on root growth and very little effect on starch degradation.
The broad effects of DNJ, N-butyl DNJ, and miglitol might be attributable to inhibition of enzymes on the pathway of starch degradation between the starch granule and maltose. Inhibition in this part of the pathway could be responsible for the reduction in starch degradation, and this in turn might reduce root growth by restricting the supply of sugars from the endosperm to the embryo. To test whether the inhibitors may affect enzymes of starch degradation other than α-glucosidase, we examined their effects on purified α-amylase and β-amylase from barley and on activities in seedling extracts.
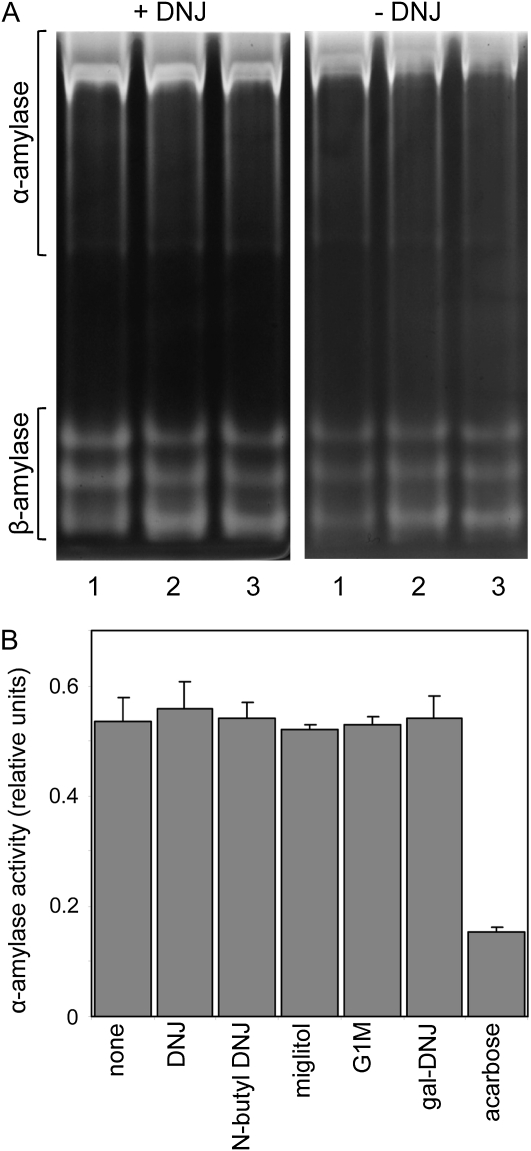
Purified α-amylase1 and β-amylase were insensitive to inhibition by DNJ. A concentration of 100 μm DNJ had no effect on the activity of either enzyme. At 1 mm, DNJ inhibited β-amylase by 9% and miglitol inhibited it by 12% (data not shown). None of the inhibitors affected α-amylase activity in extracts of seedlings (Fig. 5). In contrast, α-amylase activity was reduced by 70% in the presence of 500 μm acarbose, which is known to inhibit both isoforms of α-amylase in barley endosperm (Søgaard et al., 1993; Oudjeriouat et al., 2003). DNJ had no effect on the intensity of bands attributable to either α-amylase or β-amylase on amylopectin-containing native gels of seedling extracts (Fig. 5). This was true when grains were germinated with DNJ and DNJ was included in the extract, the gel itself, and the postelectrophoresis incubation.
Figure 5.
Effects of inhibitors on starch-hydrolyzing activities in endosperm extracts. A, Extracts of seedlings 3 d after imbibition were subjected to electrophoresis on native, 7.5% acrylamide gels containing 0.1% (w/v) amylopectin. After incubation for 15 min at room temperature, gels were stained with iodine solution and washed to reveal bands attributable to starch-hydrolyzing enzymes. The left gel and the incubation medium contained 500 μm DNJ; the right gel and incubation medium contained no DNJ. Lane 1, No DNJ present during seedling growth or extraction; lane 2, 500 μm DNJ present during seedling growth but not during extraction; lane 3, 500 μm DNJ present during seedling growth and extraction. The two gels were run at the same time and photographed identically. Positions of bands attributable to α- and β-amylases are indicated. Attributions were confirmed by transfer of proteins after electrophoresis to gels containing β-limit dextrins. Bands attributed to α-amylase were visible after incubation, but bands attributed to β-amylase activity were not (data not shown). B, An extract of seedlings 4 d after germination was assayed for α-amylase activity in the presence or absence (none) of 500 μm inhibitor. Values are means of measurements on three technical replicates. Error bars represent se.
To discover whether the inhibition of root growth by DNJ, N-butyl DNJ, and miglitol might be an indirect starvation effect brought about by inhibition of starch degradation in the endosperm, we tested the effects of the inhibitors on the growth of isolated embryos in a liquid medium (Fig. 6). In the absence of both sugars and inhibitors, growth of isolated embryos was severely retarded relative to that of whole seedlings grown in the same medium. The effect was greater for roots than for coleoptiles. Coleoptile growth was 65% less in isolated embryos than in intact seedlings (20 ± 1 mm [10] as opposed to 55 ± 2 mm [8]; values are means ± se of measurements on the number of embryos in brackets), but root growth was reduced by over 90% (5.7 ± 0.4 mm as opposed to 73 ± 4 mm; Fig. 6). Addition of Glc, Suc, or maltose to the growth medium restored the growth of both roots (Fig. 6) and coleoptiles to values much closer to those of intact seedlings (coleoptiles of isolated embryos were 47 ± 1 mm [14], 45 ± 1 mm [10], and 46 ± 1 mm [14] on Glc, Suc, and maltose respectively; on intact seedlings, they were 55 ± 2 mm [8]).
Figure 6.
Effect of inhibitors on root growth of detached embryos supplied with sugars. A, Root length was measured on embryos that had been excised from dry grains and grown in liquid culture with MS medium for 6 d with 500 μm inhibitor or without inhibitor (water) and in the presence or absence (none) of 3% (w/v) sugar. Values are means ± se of measurements on the following numbers of seedlings (left to right for each sugar treatment). None: 10, 6, 4, 4; Glc: 14, 6, 4, 3, 4; Suc: 10, 6, 4, 8, 4; maltose: 14, 6, 3, 4, 4. B, Root lengths for whole seedlings, grown under the same conditions as embryos, in the absence of sugars and in the presence or absence (water) of 500 μm inhibitor. Values are means of measurements on the following numbers of seedlings (left to right): 8, 3, 2, 4, 2. Error bars represent se.
Growth of isolated embryos was affected by the inhibitors, but to a lesser extent than growth of intact seedlings. In the absence of added sugars, the inhibitors had no effect on root growth of isolated embryos (Fig. 6). In the presence of sugars, the maximum retardation of root growth of isolated embryos by DNJ, N-butyl DNJ, and miglitol was 58%, 54%, and 29%, respectively, whereas these inhibitors retarded root growth of intact seedlings by 75%, 82%, and 71%, respectively (Fig. 6). None of the inhibitors reduced coleoptile growth of isolated embryos by more than 30%, with or without sugars (data not shown). As with intact seedlings, G1M had little or no effect on the growth of either roots or coleoptiles of isolated embryos.
Starch Metabolism in Transgenic Seedlings with Reduced α-Glucosidase Activity
To provide independent information about the importance of the α-glucosidase HvAGL97 in germinating barley grains, we generated transgenic plants with reduced activities of this enzyme. Immature embryos of barley cv Golden Promise were transformed with an RNAi silencing cassette for HvAgl97 using an Agrobacterium tumefaciens-mediated protocol (Bartlett et al., 2008). Plants that regenerated in the presence of the selectable marker hygromycin were transferred to soil in a greenhouse. The transformed status, and the presence of the silencing cassette, were confirmed by PCR. Between 17 and 23 grains were germinated for each of four selected transgenic lines and found to segregate 3:1 for the presence:absence of the transgene in the T1 generation. All four lines contained a single copy of the transgene (Supplemental Table S1). Initially, we measured root and coleoptile length after 10 d of growth on T1 seedlings from each of 19 original T0 plants from 18 independent transgenic events (Supplemental Fig. S2). There was variation between T1 seedlings from different original T0 plants in the ratio of root length to coleoptile length (about 1.1–1.8), but there was no reduction in root length in relation to the Golden Promise parent.
To analyze the effects of the expression of the RNAi silencing cassette on α-glucosidase activity and products of starch metabolism, we studied individual T1 grains from the four selected transgenic lines. As controls, we used a fifth line (line 66) lacking the RNAi silencing cassette (Supplemental Table S1) and untransformed Golden Promise. Eight grains of each transgenic line were germinated for 10 d, then the embryo was removed and tested by PCR for the presence of the hygromycin resistance gene. Five to seven of the eight seedlings carried the hygromycin resistance gene. Analyses of enzyme activity and metabolites were carried out on the endosperm fraction of the transgenic and control seedlings.
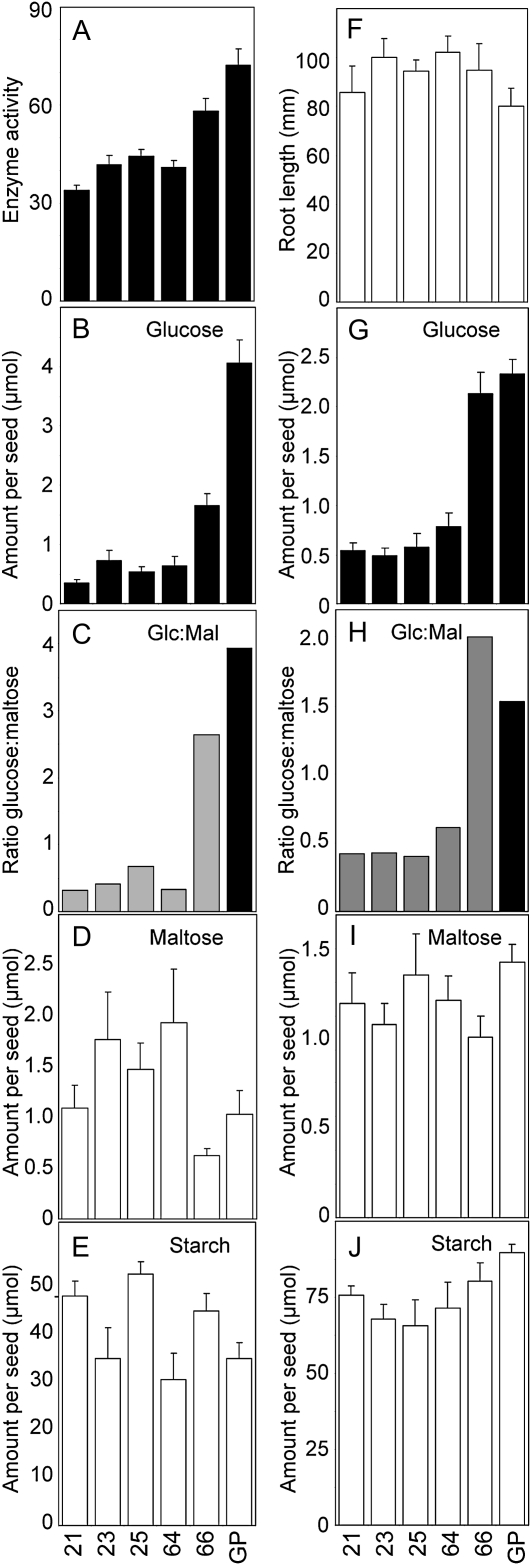
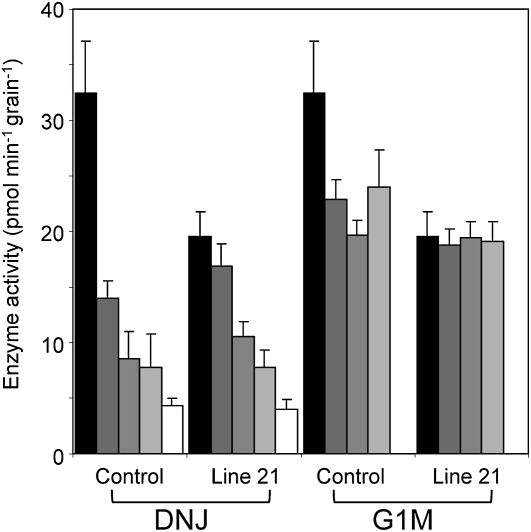
In the four selected transgenic lines, activity of α-glucosidase in the endosperm was between 40% and 53% lower than in Golden Promise. Activity in line 66 was not statistically significantly different from that in Golden Promise (Fig. 7A). There was a very strong correlation between the activity of α-glucosidase and the Glc content of the endosperm after 10 d of germination. In the four selected lines, the Glc content was only 9% to 12% of that in Golden Promise (Fig. 7B), and the ratio of Glc to maltose was 25% or less that in the controls (Fig. 7C). There was no correlation between α-glucosidase activity and the contents of maltose and starch in the endosperm, or between α-glucosidase activity and root length (Fig. 7, D–F). A further, identical experiment on a separate batch of grains gave essentially the same results (Fig. 7, G–I). The degree of reduction in Glc content in the endosperm of transgenic seedlings was greater than might be expected from the degree of reduction in α-glucosidase activity. Although activity was reduced by only about 50%, Glc content was reduced by 80% or more. We considered the possibility that the α-glucosidase assay measured both HvAGL97, the target of the RNAi construct, and another unknown activity capable of hydrolyzing the pNPG substrate. To test this idea, we investigated the inhibition by DNJ and G1M of the activity in endosperm extracts of Golden Promise and transgenic line 21. As found previously, activity without inhibitors was markedly lower in line 21 than in Golden Promise (reduction of 42%; Fig. 8). Activity in both sorts of seedlings was progressively inhibited by increasing the concentrations of DNJ. At 5 mm DNJ, 85% of Golden Promise activity and 75% of line 21 activity was inhibited. In contrast, G1M inhibited only about 40% of the Golden Promise activity and had no effect on activity in line 21. Increasing concentrations of G1M had no further effect. Notably, the activity in Golden Promise in the presence of G1M was the same as that in line 21 with or without G1M. This result is consistent with the idea that the activity measured with pNPG in Golden Promise endosperm includes both HvAGL97, which is inhibited by both G1M and DNJ, and one or more other activities that are inhibited by DNJ but not G1M.
Figure 7.
Metabolite content and α-glucosidase activity in seedlings from lines of barley transformed with an RNAi construct for α-glucosidase. Ten days after imbibition, seedlings from T1 plants of each of four independent transgenic lines carrying both the silencing and the hygromycin resistance cassettes (lines 21, 23, 25, and 64), one line carrying only the hygromycin resistance cassette (line 66), and the parental cv Golden Promise (GP) were separated into endosperm and embryo fractions. After measurement of root length, the endosperm fraction was frozen and the embryo fraction was used to test for the presence of the hygromycin resistance gene used as the selectable marker. For seedlings carrying the hygromycin gene, activity and metabolite analyses were carried out on the endosperm fraction. Data in A to F were obtained from a single experiment, and data in G to J were obtained in an independent identical experiment. A, α-Glucosidase activity, assayed with pNPG as the substrate. Values are pmol min−1 seedling−1. Values for lines 21, 23, 25, and 64 are statistically significantly different from that for Golden Promise (P = 0.0012, 0.0017, 0.0070, and 0.0004, respectively, by Student’s t test). The value for line 66 is not statistically significantly different from that for Golden Promise (P = 0.0608). B and G, Glc content. C and H, Ratio of Glc to maltose calculated from the absolute amounts of these metabolites shown in B and D and in G and I, respectively. D and I, Maltose content. E and J, Starch content. F, Root length. Starch, Glc, and maltose values are shown as μmol Glc equivalents. Except for E, values are means of measurements on the following number of seedlings (left to right within panels). A, 3, 4, 3, 5, 5, 6; B, D, and E, 4, 5, 5, 3, 5, 6; F, 6, 6, 5, 5, 6, 6; G, I, and J, 5, 5, 5, 6, 7, 8. Error bars represent se.
Figure 8.
Inhibition of α-glucosidase activity in extracts of transgenic barley seedlings by DNJ and G1M. Seedlings of transgenic line 21 carrying the hygromycin resistance cassette were selected as described for Figure 7. Extracts of the endosperm fraction of individual seedlings of line 21 and Golden Promise, 10 d after imbibition, were assayed for α-glucosidase activity in the absence of inhibitor (black bars) or in the presence of 50 μm, 500 μm, 1 mm, or 5 mm inhibitor (gray and white bars from left to right for each line). Values are means of measurements on six individual seedlings for each line. Error bars represent se.
DISCUSSION
Iminosugar α-Glucosidase Inhibitors Can Inhibit Maltase Activity in Intact Barley Seedlings
Several iminosugars proved to be powerful inhibitors in vitro of recombinant α-glucosidase HvAGL97 from barley endosperm. The extent of inhibition varied greatly, with six-membered ring, gluco-configured compounds showing potent inhibitory activity, while five-membered ring and fused bicyclic inhibitors with similar relative stereochemistry (Supplemental Fig. S1) were very much less effective. Some of the inhibitory compounds are known inhibitors of a broad spectrum of α-glucosidases from other sources. For example, DNJ, which is a potent competitive inhibitor of recombinant barley α-glucosidase HvAGL97 (Ki = 39 nm; H. Naested and B. Svensson, unpublished data), is also an inhibitor of mammalian trehalase (Asano et al., 1996) and inhibits the glucosidases responsible for posttranslational processing of Asn-linked glycans in the endoplasmic reticulum (α-glucosidases I and II; Peyrieras et al., 1983; Winchester and Fleet, 1992). While some of the inhibitors are relatively specific for α-glucosidases (e.g. miglitol), others inhibit other classes of enzyme. For example, G1M inhibits purified cyclodextrin glucanotransferases (Arai et al., 1986; Maruo et al., 1993) and DNJ inhibits bacterial glucoamylase (Arai et al., 1986). We chose five compounds for application to barley grains, to include both strong and weak inhibitors, a compound with essentially no inhibitory effects (gal-DNJ), and inhibitors with different specificity profiles. One of the compounds, miglitol, had previously been shown to affect carbohydrate metabolism and growth in wheat seedlings, putatively through the inhibition of α-glucosidase (Konishi et al., 1994). The effects of the five selected compounds on seedling growth and levels of carbohydrate in the endosperm were very similar in two different cultivars of barley, giving confidence that these effects are of general importance. Results from the two cultivars are considered together for the purposes of discussion.
The four compounds that inhibited recombinant α-glucosidase HvAGL97 in vitro also strongly reduced the ratio of Glc to maltose in the endosperm. Levels of Glc were particularly strongly reduced. Levels of maltose were elevated in some but not all cases. These data imply that all four compounds inhibited the α-glucosidase responsible for the conversion of maltose to Glc in the endosperm (referred to below as maltase). The fact that the four inhibitors had approximately comparable effects on the Glc-to-maltose ratio, despite their differing potencies as inhibitors of recombinant α-glucosidase, suggests that the inhibitor concentration in the endosperm in our experiments may be sufficient to completely inhibit maltase. Consistent with its lack of effect on the activity of recombinant α-glucosidase, gal-DNJ had no effect on the Glc-to-maltose ratio in the endosperm.
Some Maltase Inhibitors Also Affect Other, Unknown Proteins Necessary for Starch Degradation and Root Growth
Despite the similarities of their effects on the Glc-to-maltose ratio in the endosperm, the four inhibitors had different effects on starch degradation and on seedling growth. DNJ, N-butyl DNJ, and miglitol strongly inhibited seedling growth and caused elevated starch content (i.e. reduced starch degradation) in the endosperm. Their effects on starch degradation were of comparable magnitude, but they differed in their effects on seedling growth. DNJ very strongly inhibited root growth but not coleoptile (shoot) growth, whereas N-butyl DNJ and miglitol reduced both root and coleoptile growth. These effects of miglitol are similar to those reported previously for wheat seedlings (Konishi et al., 1994). In contrast, G1M had no effect on seedling growth, no effect on starch content in Optic, and only a marginal effect on starch content in Golden Promise.
The broad effects of DNJ, N-butyl DNJ, and miglitol suggested that, in addition to inhibiting maltase, they also inhibit one or more additional enzymes crucial for starch degradation and seedling growth. To provide more information about their possible targets, we investigated first whether they inhibit endosperm amylase activity, and second whether their effects on seedling growth are secondary consequences of the reduction in starch degradation in the endosperm.
The degradation of starch granules in the endosperm is catalyzed primarily by α-amylases; thus, the reduced rate of starch degradation in seedlings treated with DNJ, N-butyl DNJ, or miglitol could reflect the inhibition of α-amylase by these compounds. This seemed unlikely a priori, because these molecules are too small to act as substrate analogs of α-amylase. DNJ does not inhibit amylases from a wide range of microbial and animal sources (Arai et al., 1986). Treatment of wheat seedlings with miglitol actually increased the extractable α-amylase activity (Konishi et al., 1994). We confirmed that DNJ does not inhibit either purified α-amylase or α-amylase activity in endosperm extracts. None of the other α-glucosidase inhibitors affected α-amylase activity in endosperm extracts. Purified β-amylase was not inhibited by DNJ, and native gels indicated that this was also true in endosperm extracts. Thus, the inhibition of starch degradation by these compounds is not due to direct inhibition of amylases in the endosperm. It is also highly unlikely that α-amylase was inhibited by a buildup of maltooligosaccharides as a result of the inhibition of maltase. Maltose and maltotriose can inhibit some α-amylases (porcine pancreatic α-amylase; Al Kazaz et al., 1998), and maltose inhibits the degradation of starch granules by barley α-amylase at very high concentrations (50% inhibition at approximately 250 mm; Hill et al., 1997). However, G1M inhibited maltase activity and elevated the maltose content of the endosperm with little or no effect on starch degradation. This argues against maltooligosaccharide inhibition of α-amylase as the cause of reduced starch degradation in the presence of the other inhibitors. Further proposals about the effects of the inhibitors on starch degradation are presented below.
The inhibition of starch degradation by DNJ, N-butyl DNJ, and miglitol does not account for their effects on root growth. If root growth were inhibited because of reduced starch degradation and hence sugar starvation, provision of sugars other than maltose to isolated embryos should permit growth that is not affected by the inhibitors. This was not the case. DNJ, N-butyl DNJ, and miglitol were able to inhibit root elongation in the presence of exogenous sugars. This result suggests that the inhibitors have direct effects on proteins in the roots that are necessary for growth.
The most likely targets for the inhibitors in the roots are the glucosidases that trim N-linked glycans during the maturation of glycosylated proteins in the endoplasmic reticulum (Peyrieras et al., 1983; Gross et al., 1986; Winchester and Fleet 1992; Lerouge et al., 1998). Glucosidases I and II sequentially remove specific glucosyl residues from glycan precursors attached to proteins as part of the trimming that occurs during the synthesis of membrane and secreted proteins. Mutational analyses show that both glucosidases are essential for normal embryo development in Arabidopsis (Arabidopsis thaliana; Boisson et al., 2001; Burn et al., 2002; Gillmor et al., 2002; Furumizu and Komeda, 2008; Soussillane et al., 2009). In conditional mutants that can germinate under permissive conditions, loss of glucosidase II from seedlings causes dramatic reduction in root elongation (Burn et al., 2002; Soussillane et al., 2009). Similarly, a weak mutant allele of the gene encoding glucosidase I permits germination but subsequent root growth is retarded (Furumizu and Komeda, 2008). Glucosidases I and II are inhibited by DNJ and some other glucosidase inhibitors (Peyrieras et al., 1983; Gross et al., 1986; Mega, 2004, 2005). In radish (Raphanus sativus) leaves treated with DNJ, there was a correlation between inhibition of trimming of glucosyl residues of N-linked glycans and inhibition of growth (Mega, 2005). Thus, the reduced growth of barley seedlings in the presence of DNJ, miglitol, and N-butyl DNJ may well be due to the inhibition by these compounds of glucosidases I and II.
The reasons why G1M does not inhibit root and shoot growth remain to be established. G1M may be a poor inhibitor of glucosidases I and II, but it is also possible that the more polar nature of this pseudodisaccharide relative to the monosaccharide iminosugar inhibitors renders it less prone to passive uptake by the roots.
Transgenic Studies Confirm that HvAGL97 Is Not Required for Starch Degradation or Seedling Growth
Transgenic barley seedlings expressing an RNAi cassette for HvAgl97 had reduced α-glucosidase activity when assayed with pNPG as a substrate. This assay probably overestimates the activity of HvAGL97, because the actions of inhibitors indicated that more than one enzyme in endosperm extracts metabolizes pNPG. G1M inhibited only half of the activity in extracts of control seedlings and none of the activity in transgenic seedlings. In contrast, DNJ inhibited all of the activity in both control and transgenic seedlings. We suggest that G1M specifically inhibits HvAGL97, whereas DNJ inhibits other glucosyl hydrolases as well. Taken as a whole, these data indicate that HvAGL97 activity is strongly reduced or absent in some of our transgenic lines.
Analysis of growth and metabolite levels in transgenic seedlings provides good evidence that HvAGL97 is the major enzyme involved in the conversion of maltose to Glc in the endosperm. The reduction relative to controls in the Glc-to-maltose ratio in four transgenic lines was comparable with that achieved by incubation of grains with inhibitors. It is interesting that although Glc levels were strongly reduced in these transgenic lines, there was no elevation of maltose. This was also true for cv Golden Promise seedlings incubated with N-butyl DNJ and G1M, although all four inhibitors caused elevated maltose levels in seedlings of cv Optic. It seems likely that maltose is being metabolized by another route when maltase is inhibited in cv Golden Promise. We suggest that it may be transported across the scutellum and metabolized within the embryo. Consistent with this idea, the barley and wheat SUT1 Suc transporters can transport maltose (Sivitz et al., 2005), and TaSUT1 is proposed to transport maltose from the endosperm to the scutellum during grain germination (Aoki et al., 2006).
None of the transgenic lines showed any alteration in starch degradation or seedling growth following germination, including those lines in which the Glc-to-maltose ratio was strongly reduced. Thus, we conclude that although AGL97 is probably responsible for most of the conversion of maltose to Glc in the endosperm, it is not required for normal starch degradation.
Glucosidase Inhibitors Reveal Complexity in Starch Degradation in Cereal Endosperm
Our data point to the existence in cereal endosperm of one or more as yet unidentified enzymes that are necessary for the degradation of starch granules. We argue above that DNJ, N-butyl DNJ, and miglitol inhibit the action of an enzyme necessary for starch degradation in addition to inhibiting HvAGL97 activity. We provide strong evidence that this unidentified activity is not α-amylase, the enzyme usually held to be responsible for starch granule degradation in cereal endosperm. The unidentified activity may well be a glucosidase. Evidence for this comes from the different effects of DNJ and G1M on pNPG hydrolysis in extracts of normal and transgenic seedlings. Taken together, these data suggest that about half of the pNPG-hydrolyzing activity is attributable to HvAGL97. The other half is attributable to an unknown glucosidase susceptible to inhibition by DNJ but not G1M. Work is in progress to identify and characterize this enzyme at the molecular level and to discover whether it is important for starch degradation.
Our results confirm and extend previous evidence of unidentified α-glucosidases in barley endosperm. During purification of α-glucosidase from extracts of germinating barley grains, Stark and Yin (1987) were able to separate activities that hydrolyzed maltose in preference to pNPG from activities that hydrolyzed pNPG in preference to maltose. Within each of these two fractions, they identified at least two biochemically separable activities. Purification of maltose-hydrolyzing activity from barley malt yields both HvAGL97 and a second activity of lower pI (Sun and Henson, 1990; Frandsen et al., 2000). However, no α-glucosidases other than HvAGL97 have been characterized at either the protein or the gene level thus far.
MATERIALS AND METHODS
Plant Material and Inhibitors
Grains of barley (Hordeum vulgare ‘Optic’) were harvested at maturity from barley plants grown in a controlled-environment room at 20°C and 60% relative humidity with a 16-h light period at approximately 200 μmol quanta m−2 s−1. Grains of cv Golden Promise were harvested at maturity from a controlled-environment room at 15°C day, 12°C night, 80% relative humidity, and light levels of 500 μmol m−2 s−1. Prior to use in experiments, grains were stored at 2°C to 4°C.
Transgenic barley plants expressing the RNAi cassette for HvAGL97 were generated using the high-throughput Agrobacterium tumefaciens-mediated transformation method described by Bartlett et al. (2008). The method uses immature embryos as a target tissue and hygromycin as a selectable marker. Independent transgenic plants were transferred to soil and grown to maturity in a glasshouse under natural daylight (approximately 12- to 16-h photoperiod). Grains harvested from these plants were stored at 2°C to 4°C prior to use in experiments. Twenty-five T1 grains from each of 10 independent transgenic lines were germinated, and the seedlings were tested by PCR for the presence of the hygromycin marker gene. Four or five plants positive for the hygromycin gene were tested by PCR for the silencing cassette (see below). Subsequent work (Fig. 7; Supplemental Fig. S2) was carried out on selected validated transgenic lines.
Compound libraries were obtained from Microsource Discovery Systems (http://www.msdiscovery.com/home.html) and Phytoquest (http://www.phytoquest.co.uk/). Compounds for follow-up studies were from the following suppliers: DNJ, (1S,6S,7R,8R)-octahydroindolizine-1,6,7,8-tetraol (castanospermine), gal-DNJ, 2,5-dideoxy-2,5-imino-d-mannitol, and 3-hydroxymethyl-1,2,7-trihydroxypyrrolizidine (australine) were from Industrial Research Limited; (3R,4R,5R)-5-(hydroxymethyl)-3,4-piperidinediol (isofagomine), kifunensine, N-butyl DNJ, N-cyclohexylpropyl DNJ, valienamine, N-carboxypentyl DNJ, G1M, acarbose, miglitol, and N-(n-nonyl) DNJ were from Toronto Research Chemicals; 7-O-β-d-glucopyranosyl-α-homonojirimycin was from Wako Pure Chemical Industries; and α-homonojirimycin was from Carbosynth.
Germination and Seedling Growth
Grains were sterilized by immersion for 20 min in bleach (0.5% available chlorine), rinsed 10 times in distilled water, and placed individually onto two layers of sterilized filter paper in the base of 100-mm2 wells of 25-well sterile plastic plates. The filter paper was prewetted with 400 μL of water or aqueous solution of inhibitor. Plates were covered with a lid sealed with micropore tape and incubated at 17°C in the dark.
Inhibition of Recombinant α-Glucosidase
Recombinant α-glucosidase was obtained as described by Naested et al. (2006) and stored at 2.54 mg mL−1 in 50 mm NaH2PO4, 300 mm NaCl, and 50% glycerol at −20°C.
Assays were performed on a 96-well microtiter plate using a plate reader. Each well contained a total of 100 μL, comprising 50 mm sodium acetate (pH 4.5), 5 mm CaCl2, 0.3 mm sodium azide, 0.6 mm pNPG, 0.24 μg of α-glucosidase, and inhibitor as specified in Figure 1. Plates were incubated for 60 min at 37°C, then 100 μL of 0.5 m ammonium bicarbonate was added. The plates were incubated for 20 min at room temperature with shaking. The amount of released p-nitrophenol was determined at a wavelength of 405 nm. In controls without inhibitor, pNPG hydrolysis over the 60-min incubation gave a change in optical density of approximately 0.6 units.
Enzyme Assays and Native PAGE on Seedling Extracts
Purified recombinant α-amylase was prepared according to Juge et al. (1996) and assayed using the copper-bicinchoniate method for detection of the release of reducing sugars from amylose. Purified β-amylase from barley was purchased from Megazyme (www.megazyme.com) and assayed using the dinitrosalicylic acid method for detection of the release of reducing sugars from soluble starch (Miller et al., 1960).
Extracts were prepared by grinding seedling material at 4°C with a pestle and mortar in 100 mm MOPS (pH 7.2), 1 mm EDTA, 1 mm dithiothreitol, and 10% (v/v) ethanediol at 600 μL per seedling. After centrifugation (15,000g, 4°C), the supernatant was desalted on a column of Sephadex G25 equilibrated with extraction medium.
α-Glucosidase was assayed as for the recombinant enzyme (see above). Linearity of the reaction was checked with respect to time and volume of extract.
α-Amylase was assayed using the Amylazyme kit (Megazyme) according to the manufacturer’s instructions, except that the ratio of extract to buffer was 1:200.
Native gels were 10 cm long, 1 mm thick, and contained 7.5% acrylamide and 2% (w/v) potato amylopectin. Gels were run at constant voltage so that the dye front reached the bottom in 4 h. They were incubated in 100 mm MES (pH 5.8), 1 mm MgCl2, 1 mm CaCl2, and 1 mm dithiothreitol for between 15 and 120 min after an initial wash in this medium, stained with Lugol’s iodine solution, and washed with water to remove excess iodine solution.
Measurement of Starch and Sugars
Seedlings or parts thereof were transferred rapidly to preweighed tubes at the temperature of liquid nitrogen, weighed, and stored at −80°C until analysis. In some experiments, each sample was from a single seedling; in others, seedlings were pooled to give a sample weight of 200 to 300 mg.
For single seedlings, frozen samples were mixed with 0.5 mL of 0.7 m perchloric acid in polycarbonate vials on dry ice, a stainless steel ball (1 cm diameter), prechilled on dry ice, was added, and the vials were capped. Samples were milled (GenoGrinder 2000; OPS Diagnostics) for 90 s at 1,500 strokes min−1. A further 1 mL of 0.7 m perchloric acid was added to each vial, and 1 mL of the homogenate was transferred to a clean tube. After 30 min at 4°C, tubes were centrifuged to pellet insoluble material.
Pooled samples of multiple seedlings (Fig. 1B) were mixed with 2 mL of 0.7 m perchloric acid and ground with a pestle and mortar. A further 2 mL of 0.7 m perchloric acid was used to wash the mortar and then added to the homogenate. After 30 min at 4°C, tubes were centrifuged to pellet insoluble material. The supernatant was removed, and the pellet was resuspended in 1 mL of perchloric acid and centrifuged again. The two supernatants were pooled.
Sugars and starch were assayed according to Hargreaves and ap Rees (1988) and Zeeman et al. (1998). The pellet was used for the assay of starch as Glc after solubilization and incubation with α-amyloglucosidase and α-amylase. The supernatant was neutralized and assayed for sugars. Soluble glucans were assayed as Glc after incubation of the neutralized supernatant α-amyloglucosidase and α-amylase and subtraction of the free Glc content.
Preparation of an RNAi Construct for HvAgl97
A 411-bp region of HvAgl97 was amplified from genomic DNA extracted from Optic using the 5′ primer HvAgl97TopoF1 (5′-CACCCTTCGTGCTCAGCAGGTC-3′) and the 3′ primer HvAgl97TILLR1 (5′-TGCGGGTAGGAGAAGAAGAG-3′); amplification was performed using the Pfu DNA polymerase (Stratagene; http://stratagene.gene-quantification.info/) to give a blunt-ended product. The 5′ end of HvAgl97TopoF1 contains the recognition sequence CACC, required for directional TOPO cloning of the resulting PCR product into the vector pENTR/D-TOPO (Invitrogen; http://www.invitrogen.com/), following the manufacturer’s instructions. The resulting plasmid, pENTR-HvAgl97, was used in a Gateway reaction (using Gateway LR Clonase II enzyme mix [Invitrogen]) with the vector pBract207 (sequence available at http://www.bract.org) to generate pBract207-HvAgl97. This vector contains two copies of the 411-bp HvAgl97 region in inverted orientation separated by two introns (Supplemental Fig. S3). The vector was transferred to Agrobacterium strain AGL1 together with the pSoup helper plasmid as described by Bartlett et al. (2008). The integrity of the vector was checked after electroporation by diagnostic enzymatic digests after extraction, transformation, and multiplication in Escherichia coli DH5α. A standard inoculum for transformation was prepared according to Tingay et al. (1997). Transformation, selection, and regeneration of transformed plants were as described by Bartlett et al. (2008).
PCR Confirmation of the Presence of the Hygromycin and Silencing Cassettes
To confirm the presence of the hygromycin cassette, seedlings from T1 and control (Golden Promise) plants were separated into endosperm and embryo fractions 10 d after imbibition. Genomic DNA was extracted from the embryo fraction using a DNeasy 96 Plant Kit (Qiagen; http://www.qiagen.com/) according to the manufacturer’s instructions. The hygromycin resistance gene was amplified using the primers HygF (5′-ACTCACCGCGACGTCTGTCG-3′) and HygR (5′-GCGCGTCTGCTGCTCCATA-3′) that amplify a 917-bp fragment of the HPT gene. The PCR conditions were as follows: 95°C for 1 min; 35 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min; followed by a 10-min final extension at 72°C and a 10°C hold. This assay was also used to confirm the transformed status of the primary transgenic plants on DNA extracted from young leaves according to Edwards et al. (1991).
To confirm the presence of the entire silencing cassette, two PCRs were carried out on DNA from young leaves. The primers UbiProF1 (5′-ATGCTCACCCTGTTGTTTGG-3′) and i18intronR1 (5′-CATCGTTGTATGCCACTGGA-3′) isolated a 723-bp fragment from the ubiquitin promoter into the first intron of the silencing cassette. Primers IV2intronF1 (5′-CCAAAATTTGTTGATGTGCAG-3′) and NosTermR1 (5′-TGTTTGAACGATCCTGCTTG-3′) isolated a 640-bp fragment from the second intron into the Nos terminator. The PCR conditions were as follows: 95°C for 5 min; 38 cycles of 95°C for 30 s, 60°C for 40 s, and 72°C for 45 s; followed by a 10°C hold.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Structures of compounds tested for inhibitory effects on recombinant α-glucosidase.
Supplemental Figure S2. Root and coleoptile lengths of seedlings of lines of barley transformed with an RNAi construct for α-glucosidase.
Supplemental Figure S3. T-DNA region of the plasmid used for barley transformation.
Supplemental Table S1. Characterization of the transgenic lines used in Figure 7.
Supplementary Material
Acknowledgments
We thank Andrew Davies (John Innes Centre) for the photographs of germinating grains and Simone Dedola and Christian Ruzanski (both John Innes Centre) for help with experiments on inhibition of the recombinant enzyme.
References
- Al Kazaz M, Desseaux V, Marchis-Mouren G, Prodanov E, Santimone M. (1998) The mechanism of porcine pancreatic α-amylase: inhibition of maltopentaose hydrolysis by acarbose, maltose and maltotriose. Eur J Biochem 252: 100–107 [DOI] [PubMed] [Google Scholar]
- Aoki N, Scofield GN, Wang XD, Offler CE, Patrick JW, Furbank RT. (2006) Pathway of sugar transport in germinating wheat seeds. Plant Physiol 141: 1255–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai M, Sumida M, Fukuhara K, Kainosho M, Murao S. (1986) Isolation and characterization of amylase inhibitors, (glucose)n·deoxynojirimycin. Agric Biol Chem 50: 639–644 [Google Scholar]
- Asano N, Kato A, Kizu H, Matsui K, Watson AA, Nash RJ. (1996) Calystegine B4, a novel trehalase inhibitor from Scopolia japonica. Carbohydr Res 293: 195–204 [DOI] [PubMed] [Google Scholar]
- Bamforth CW. (2009) Current perspectives on the role of enzymes in brewing. J Cereal Sci 50: 353–357 [Google Scholar]
- Bartlett JG, Alves SC, Smedley M, Snape JW, Harwood WA. (2008) High-throughput Agrobacterium-mediated barley transformation. Plant Methods 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson M, Gomord V, Audran C, Berger N, Dubreucq B, Granier F, Lerouge P, Faye L, Caboche M, Lepiniec L. (2001) Arabidopsis glucosidase I mutants reveal a critical role of N-glycan trimming in seed development. EMBO J 20: 1010–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn JE, Hurley UA, Birch RJ, Arioli T, Cork A, Williamson RE. (2002) The cellulose-deficient Arabidopsis mutant rsw3 is defective in a gene encoding a putative glucosidase II, an enzyme processing N-glycans during ER quality control. Plant J 32: 949–960 [DOI] [PubMed] [Google Scholar]
- Dunn G. (1974) A model for starch breakdown in higher plants. Phytochemistry 13: 1341–1346 [Google Scholar]
- Edwards K, Johnstone C, Thompson C. (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19: 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox GP, Panozzo JF, Li CD, Lance RCM, Inkerman PA, Henry RJ. (2003) Molecular basis of barley quality. Aust J Agric Res 54: 1081–1101 [Google Scholar]
- Frandsen TP, Lok F, Mirgorodskaya E, Roepstorff P, Svensson B. (2000) Purification, enzymatic characterization, and nucleotide sequence of a high-isoelectric-point alpha-glucosidase from barley malt. Plant Physiol 123: 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen TP, Svensson B. (1998) Plant α-glucosidases of the glycoside hydrolase family 31: molecular properties, substrate specificity, reaction mechanism, and comparison with family members of different origin. Plant Mol Biol 37: 1–13 [DOI] [PubMed] [Google Scholar]
- Furumizu C, Komeda Y. (2008) A novel mutation in KNOPF uncovers the role of α-glucosidase I during post-embryonic development in Arabidopsis thaliana. FEBS Lett 582: 2237–2241 [DOI] [PubMed] [Google Scholar]
- Gillmor CS, Poindexter P, Lorieau J, Palcic MM, Somerville C. (2002) α-Glucosidase I is required for cellulose biosynthesis and morphogenesis in Arabidopsis. J Cell Biol 156: 1003–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross V, Tran-Thi TA, Schwarz RT, Elbein AD, Decker K, Heinrich PC. (1986) Different effects of the glucosidase inhibitors 1-deoxynojirimycin, N-methyl-1-deoxynojirimycin and castanospermine on the glycosylation of rat alpha 1-proteinase inhibitor and alpha 1-acid glycoprotein. Biochem J 236: 853–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves JA, ap Rees T. (1988) Turnover of starch and sucrose in roots of Pisum sativum. Phytochemistry 27: 1627–1629 [Google Scholar]
- Henson CA, Sun Z. (1996) Barley seed α-glucosidases: their characteristics and roles in starch degradation. Saddler JN, Penner MH, , Enzymatic Degradation of Insoluble Carbohydrates, ACS Symposium Series 168. American Chemical Society, Washington, DC, pp 51–58 [Google Scholar]
- Hill GA, Macdonald DG, Lang X. (1997) α-Amylase inhibition and inactivation in barley malt during cold starch hydrolysis. Biotechnol Lett 19: 1139–1141 [Google Scholar]
- Jørgensen OB. (1963) Barley malt α-glucosidase. II. Studies on the substrate specificity. Acta Chem Scand 17: 2471–2478 [Google Scholar]
- Juge N, Andersen JS, Tull D, Roepstorff P, Svensson B. (1996) Overexpression, purification, and characterization of recombinant barley alpha-amylases 1 and 2 secreted by the methylotrophic yeast Pichia pastoris. Protein Expr Purif 8: 204–214 [DOI] [PubMed] [Google Scholar]
- Konishi Y, Okamoto A, Takahashi J, Aitani M, Nakatani N. (1994) Effect of Bay m 1099, an α-glucosidase inhibitor, on starch metabolism in germinating wheat seeds. Biosci Biotechnol Biochem 58: 135–139 [DOI] [PubMed] [Google Scholar]
- Lerouge P, Cabanes-Macheteau M, Rayon C, Fischette-Lainé AC, Gomord V, Faye L. (1998) N-Glycoprotein biosynthesis in plants: recent developments and future trends. Plant Mol Biol 38: 31–48 [PubMed] [Google Scholar]
- Manners DJ. (1974) Some aspects of the enzymatic degradation of starch. Pridham JB, , Plant Carbohydrate Biochemistry. Academic Press, London, pp 109–144 [Google Scholar]
- Maruo S, Kyotani Y, Yamamoto H, Miyazaki K, Ogawa H, Sakai T, Kojima M, Ezure Y. (1993) Effects of moranoline, 4-O-α-D-glucopyranosylmoranoline and their N-substituted derivatives on thermostability of cyclodextrin glycosyltransferase, glucoamylase, and β-amylase. Biosci Biotechnol Biochem 57: 1294–1298 [DOI] [PubMed] [Google Scholar]
- Mega T. (2004) Conversion of the carbohydrate structures of glycoproteins in roots of Raphanus sativus using several glycosidase inhibitors. J Biochem 136: 525–531 [DOI] [PubMed] [Google Scholar]
- Mega T. (2005) Glucose trimming of N-glycan in endoplasmic reticulum is indispensable for the growth of Raphanus sativus seedling (kaiware radish). Biosci Biotechnol Biochem 69: 1353–1364 [DOI] [PubMed] [Google Scholar]
- Miller GL, Blum R, Glennon WE, Burton AL. (1960) Measurement of carboxymethylcellulase activity. Anal Biochem 1: 127–132 [Google Scholar]
- Muslin EH, Kanikula AM, Clark SE, Henson CA. (2000) Overexpression, purification, and characterization of a barley α-glucosidase secreted by Pichia pastoris. Protein Expr Purif 18: 20–26 [DOI] [PubMed] [Google Scholar]
- Naested H, Kramhøft B, Lok F, Bojsen K, Yu S, Svensson B. (2006) Production of enzymatically active recombinant full-length barley high pI α-glucosidase of glycoside family 31 by high cell-density fermentation of Pichia pastoris and affinity purification. Protein Expr Purif 46: 56–63 [DOI] [PubMed] [Google Scholar]
- Nomura T, Kono Y, Akazawa T. (1969) Enzymic mechanism of starch breakdown in germinating rice seeds. II. Scutellum as the site of sucrose synthesis. Plant Physiol 44: 765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman AM. (2002) The advantages of using natural substrate-based methods in assessing the roles and synergistic interactions of barley malt starch-degrading enzymes. J Inst Brew 108: 204–212 [Google Scholar]
- Oudjeriouat N, Moreau Y, Santimone M, Svensson B, Marchis-Mouren G, Desseaux V. (2003) On the mechanism of α-amylase: acarbose and cyclodextrin inhibition of barley amylase isozymes. Eur J Biochem 270: 3871–3879 [DOI] [PubMed] [Google Scholar]
- Palmiano EP, Juliano BO. (1972) Biochemical changes in the rice grain during germination. Plant Physiol 49: 751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrieras N, Bause E, Legler G, Vasilov R, Claesson L, Peterson P, Ploegh H. (1983) Effects of the glucosidase inhibitors nojirimycin and deoxynojirimycin on the biosynthesis of membrane and secretory glycoproteins. EMBO J 2: 823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissons MJ, MacGregor AW. (1994) Hydrolysis of barley starch granules by α-glucosidases from malt. J Cereal Sci 19: 161–169 [Google Scholar]
- Sivitz AB, Reinders A, Ward JM. (2005) Analysis of the transport activity of barley sucrose transporter HvSUT1. Plant Cell Physiol 46: 1666–1673 [DOI] [PubMed] [Google Scholar]
- Søgaard M, Kadziola A, Haser R, Svensson B. (1993) Site-directed mutagenesis of histidine 93, aspartic acid 180, glutamic acid 205, histidine 290, and aspartic acid 291 at the active site and tryptophan 279 at the raw starch binding site in barley α-amylase 1. J Biol Chem 268: 22480–22484 [PubMed] [Google Scholar]
- Soussillane P, D’Alessio C, Paccalet T, Fichette AC, Parodi AJ, Williamson R, Plasson C, Faye L, Gomord V. (2009) N-Glycan trimming by glucosidase II is essential for Arabidopsis development. Glycoconj J 26: 597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JR, Yin XS. (1987) Evidence for the presence of maltase and α-glucosidase isoenzymes in barley. J Inst Brew 93: 108–112 [Google Scholar]
- Sun Z, Henson CA. (1990) Degradation of native starch granules by barley α-glucosidases. Plant Physiol 94: 320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun ZT, Henson CA. (1991) A quantitative assessment of the importance of barley seed α-amylase, β-amylase, debranching enzyme, and α-glucosidase in starch degradation. Arch Biochem Biophys 284: 298–305 [DOI] [PubMed] [Google Scholar]
- Tibbot BK, Henson CA, Skadsen RW. (1998) Expression of enzymatically active, recombinant barley α-glucosidase in yeast and immunological detection of α-glucosidase from seed tissue. Plant Mol Biol 38: 379–391 [DOI] [PubMed] [Google Scholar]
- Tibbot BK, Skadsen RW. (1996) Molecular cloning and characterization of a gibberellin-inducible, putative alpha-glucosidase gene from barley. Plant Mol Biol 30: 229–241 [DOI] [PubMed] [Google Scholar]
- Tingay S, McElroy D, Kalla R, Fleg S, Wang M, Thornton S, Brettell RIS. (1997) Agrobacterium tumefaciens-mediated barley transformation. Plant J 11: 1369–1376 [Google Scholar]
- Winchester B, Fleet GWJ. (1992) Amino-sugar glycosidase inhibitors: versatile tools for glycobiologists. Glycobiology 2: 199–210 [DOI] [PubMed] [Google Scholar]
- Zeeman SC, Northrop F, Smith AM, Rees T. (1998) A starch-accumulating mutant of Arabidopsis thaliana deficient in a chloroplastic starch-hydrolysing enzyme. Plant J 15: 357–365 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.