Abstract
In C3 plants, CO2 assimilation is limited by ribulose 1,5-bisphosphate (RuBP) regeneration rate at high CO2. RuBP regeneration rate in turn is determined by either the chloroplast electron transport capacity to generate NADPH and ATP or the activity of Calvin cycle enzymes involved in regeneration of RuBP. Here, transgenic tobacco (Nicotiana tabacum ‘W38’) expressing an antisense gene directed at the transcript of either the Rieske iron-sulfur protein of the cytochrome (Cyt) b6/f complex or the δ-subunit of chloroplast ATP synthase have been used to investigate the effect of a reduction of these complexes on chloroplast electron transport rate (ETR). Reductions in δ-subunit of ATP synthase content did not alter chlorophyll, Cyt b6/f complex, or Rubisco content, but reduced ETR estimated either from measurements of chlorophyll fluorescence or CO2 assimilation rates at high CO2. Plants with low ATP synthase content exhibited higher nonphotochemical quenching and achieved higher ETR per ATP synthase than the wild type. The proportional increase in ETR per ATP synthase complex was greatest at 35°C, showing that the ATP synthase activity can vary in vivo. In comparison, there was no difference in the ETR per Cyt b6/f complex in plants with reduced Cyt b6/f content and the wild type. The ETR decreased more drastically with reductions in Cyt b6/f complex than ATP synthase content. This suggests that chloroplast ETR is more limited by Cyt b6/f than ATP synthase content and is a potential target for enhancing photosynthetic capacity in crops.
Plants capture light energy with their light-harvesting systems, including chlorophylls (Chls) and carotenoids, and drive photosynthetic electron transport through the thylakoid membranes of the chloroplasts. Electrons excised from water in PSII are ultimately transferred to NADP+ via PSI, resulting in production of NADPH. This process is known as linear electron transport. At the same time, this linear electron transport that passes through the cytochrome (Cyt) b6/f complex generates a proton gradient across the thylakoid membrane (ΔpH; Allen, 2003). Together with the proton gradient generated by the water-splitting complex associated with PSII, these proton gradients enable ATP production by the ATP synthase complex and help to regulate nonphotochemical quenching (NPQ) of excitation energy (Müller et al., 2001). There is also a cyclic electron transport that depends on the PSI photochemical reactions and also passes through the Cyt b6/f complex. The cyclic electron transport can generate a ΔpH and drives ATP synthesis by ATP synthase without concomitant generation of NADPH (Shikanai, 2007).
ATP and NADPH generated by light reactions are utilized primarily in the Calvin cycle and photorespiratory cycle. The activity and regulation of the Cyt b6/f complex and the ATP synthase are thus key components determining the rate of NADPH and ATP production for CO2 fixation. Photosynthetic CO2 assimilation rate can be viewed as being limited either by the capacity of Rubisco to consume ribulose 1,5-bisP (RuBP; at lower CO2) or by the capacity of the chloroplast electron transport to generate ATP and NADPH for RuBP regeneration (at higher CO2; Farquhar et al., 1980). However, within this framework of limitations, significant uncertainties remain in our understanding of how electron transport and ATP synthesis are coordinated and affect electron transport capacity and photosynthesis (Baker et al., 2007).
Previous work has shown that a targeted reduction in Cyt b6/f complex content caused reductions in the chloroplast electron transport rate (ETR) and CO2 assimilation rate at 25°C (Price et al., 1995, 1998; Ruuska et al., 2000). Therefore, the electron flow through Cyt b6/f complex is considered to be a key rate-limiting step for RuBP regeneration at 25°C. However, there are few studies that have considered the role of chloroplast ATP synthase as a limiting factor for the thylakoid reactions. Recent studies have documented that the in vivo activity of ATP synthase is modulated, especially at low or high CO2 concentration where CO2 assimilation is restricted either by CO2 concentration or end product limitation (Kanazawa and Kramer, 2002; Kramer et al., 2004; Baker et al., 2007). The conductivity of proton efflux from the lumen (gH+) through the ATP synthase could be modulated to regulate the thylakoid proton motive force (pmf; Kramer et al., 2004), providing flexibility in the ratio of ATP production per H+.
We used transgenic tobacco (Nicotianna tabacum ‘W38’) plants expressing an antisense gene directed at the transcript of either the Rieske iron-sulfur (FeS) protein of the Cyt b6/f complex or the δ-subunit of chloroplast ATP synthase (Price et al., 1995) to investigate the effect that a reduction of these complexes has on chloroplast ETR and CO2 assimilation rate. Combined measurements of gas exchange and Chl fluorescence were made over a range of CO2 concentrations and leaf temperatures. We show that at high CO2 when the rate of RuBP regeneration limits CO2 assimilation, chloroplast ETR is more limited by Cyt b6/f than ATP synthase content and confirm that ATP synthase activity is modulated in vivo. We suggest that increasing Cyt b6/f content may be a useful biomolecular target for enhancing leaf photosynthesis for improved crop yield (von Caemmerer and Evans, 2010).
RESULTS
CO2 Assimilation Rate and Physiological Components of Photosynthesis
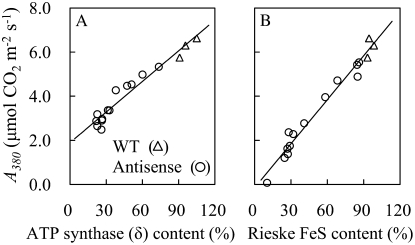
CO2 assimilation rate at 380 μmol mol−1 CO2 at high light (A380) was strongly decreased with reductions in the content of either the δ-subunit of ATP synthase complex or the Rieske FeS subunit of the Cyt b6/f complex (Fig. 1). However, the comparative extent of the reductions of A380 was greater in anti-Rieske FeS plants than in anti-ATP synthase (δ) plants.
Figure 1.
CO2 assimilation rates at 380 μL L−1 CO2 concentration at 1,200 μmol photons m−2 s−1 (A380) at 25°C in antisense plants with a variety of contents of δ-subunit of chloroplast ATP synthase (A) and in antisense plants with a variety of Rieske FeS contents (B). The regression lines are shown in each figure. A, y = (0.046 ± 0.003)x + (1.906 ± 0.16), R2 = 0.93. B, y = (0.067 ± 0.003)x − (0.149 ± 0.190), R2 = 0.97. Statistical comparison of regressions showed them to be significantly different at P < 0.000001. WT, Wild type.
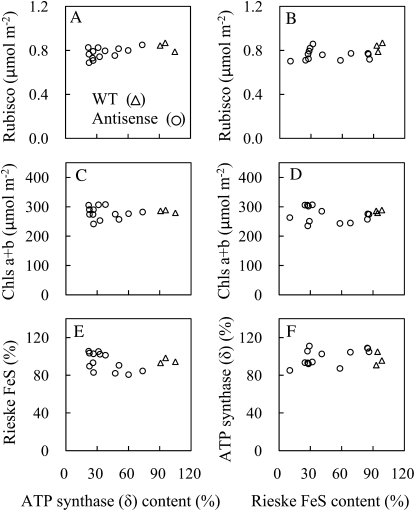
The contents of several photosynthetic components, including Rubisco and Chl, were similar among the wild type, anti-Rieske FeS plants, and anti-ATP synthase (δ) plants (Fig. 2). Chl a/b ratio in the wild type was 3.14 ± 0.06 and similar to anti-Rieske FeS plants (3.06 ± 0.15) and anti-ATP synthase (δ) plants (3.09 ± 0.15). It was previously shown that reduction in the ATP synthase (δ) subunit led to a reduction in ATP synthase (Price et al., 1995) and that the reduction on Rieske FeS protein led to a reduction in the complete Cyt b6/f complex (Price et al., 1998).We therefore assume that alterations in photosynthetic properties are primarily the result of the reduction in either ATP synthase or Cyt b6/f complex.
Figure 2.
Content of Rubisco, Chl, Rieske FeS protein of the Cyt b6/f complex, and δ-subunit of chloroplast ATP synthase in antisense plants with a variety of contents of δ-subunit of chloroplast ATP synthase (A, C, E, and G) and in antisense plants with a variety of Rieske FeS contents (B, D, F, and H). Contents of Rieske FeS protein and δ-subunit of chloroplast ATP synthase in antisense lines were shown as a percentage relative to the wild type (WT).
Chl Fluorescence
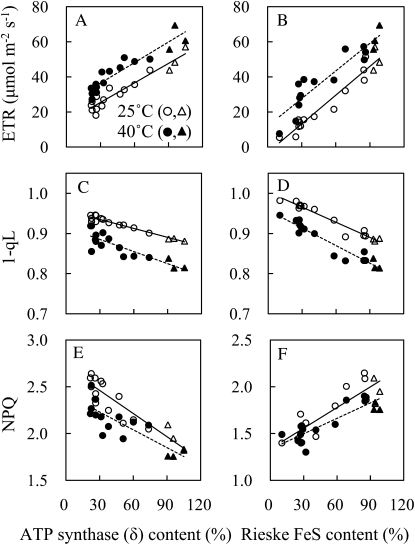
The ETR was greater at 40°C than at 25°C in the wild type. The parameter of 1−qL, which reflects the plastoquinone reduction state (Kramer et al., 2004; Baker et al., 2007), was lower at 40°C than at 25°C. NPQ was lower at 40°C than at 25°C (Fig. 3).
Figure 3.
Chl fluorescence parameters at 380 μL L−1 CO2 concentration at 1,200 μmol photons m−2 s−1 at 25°C (white symbols) or 40°C (black symbols) in antisense plants with a variety of contents of δ-subunit of chloroplast ATP synthase (A, C, and E), and in antisense plants with a variety of Rieske FeS contents (circles; B, D, and F). A and B, ETR. C and D, 1−qL that reflects the plastoquinone reduction state. E and F, NPQ. The data in the wild type are shown in triangles, whereas the data in antisense plants are shown in circles. The regression lines are shown in each figure. A, R2 = 0.92 at 25°C, R2 = 0.87 at 40°C. B, R2 = 0.94 at 25°C, R2 = 0.87 at 40°C. C, R2 = 0.95 at 25°C, R2 = 0.75 at 40°C. D, R2 = 0.95 at 25°C, R2 = 0.91 at 40°C. E, R2 = 0.83 at 25°C, R2 = 0.70 at 40°C. F, R2 = 0.77 at 25°C, R2 = 0.77 at 40°C.
Reduction in Rieske FeS contents greatly reduced ETR at 25°C and 40°C. These resulted in a concomitant overreduction of the plastoquinone pool (high 1−qL) and a low transthylakoid pH gradient (ΔpH; low NPQ), because of a reduced ability to transport protons across the thylakoid membrane. The reduction state of PSII centers could not be associated with a buildup of transthylakoid ΔpH, since NPQ was lower in anti-Rieske FeS plants than the wild type.
Reduction in ATP synthase (δ) contents increased NPQ, suggesting an increase in transthylakoid ΔpH due to a reduction in the flux of protons outwards through the ATP synthase. The limitation imposed on CO2 fixation by a reduction in ATP supply led to the reduction of potential for ETR, causing an increase in the reduction state of the plastoquinone pool (high 1−qL). The reduction state of PSII centers was associated with a buildup of transthylakoid ΔpH, since NPQ was greater in anti-ATP synthase (δ) plants than the wild type.
Activation State of NADP-Malate Dehydrogenase
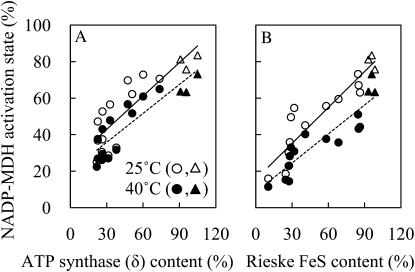
Activation state of NADP-malate dehydrogenase (MDH), which is an indicator of NADPH/NADP+ ratio, decreased by 7% to approximately 8% at 40°C compared to 25°C in the wild type (Fig. 4).
Figure 4.
NADP-MDH activation state at 380 μL L−1 CO2 concentration at 1,200 μmol photons m−2 s−1 at 25°C (black symbols) or 40°C (white symbols) in wild-type (triangles) and antisense plants with a variety of contents of δ-subunit of chloroplast ATP synthase (circles; A), and wild-type (triangles) and antisense plants with a variety of Rieske FeS contents (circles; B).
The anti-Rieske FeS plants had an impaired ETR and a low NPQ that restricts NADPH and ATP synthesis. The activation state of NADP-MDH strongly decreased in anti-Rieske FeS plants. In contrast, anti-ATP synthase (δ) plants had an impaired ETR and a high NPQ and the activation state of NADP-MDH was also strongly decreased.
NADP-MDH activity is correlated with the redox status of the stroma and NADPH availability (Scheibe and Stitt, 1988). Reductions in ETR via reductions in Rieske FeS protein caused decreases in 1−qL and NADP-MDH activation (Figs. 3 and 4). Therefore, in anti-Rieske FeS plants, an oxidation of the stroma could inhibit many of the redox-sensitive enzymes within the stroma, including several in the Calvin cycle. We also observed decreases in 1−qL and NADP-MDH activation at high temperature (Figs. 3 and 4), implying that the heat-induced decline in photosynthesis would be also affected by, at least, the redox status of the stroma (Schrader et al., 2004; Sharkey, 2005).
RuBP Regeneration and Chloroplast Electron Transport Capacity
CO2 assimilation rate (A) versus intercellular CO2 concentration (Ci) was measured to determine RuBP regeneration and/or electron transport-limited CO2 assimilation rate at high CO2, and these were used to calculate actual ETR (Jg) as described in “Materials and Methods” (Supplemental Figs. S1 and S2). The A-Ci curves showed a transition from Rubisco-limited A at lower CO2 concentrations to RuBP regeneration-limited A at higher CO2 concentrations at all leaf temperatures in all plants except those plants with the lowest ATP synthase (δ) or Rieske FeS content where CO2 assimilation was always electron transport limited.
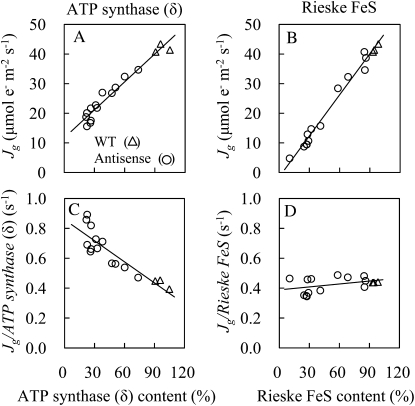
Reductions in contents either of ATP synthase (δ) or Rieske FeS led to a decrease in Jg at 25°C (Fig. 5, A and B). In anti-Rieske FeS plants, Jg per Rieske FeS content was constant irrespective of Rieske FeS content (Fig. 5D). However, in anti-ATP synthase (δ) plants, the Jg per ATP synthase (δ) content increased with reductions in ATP synthase (δ) content (Fig. 5C), indicating that in vivo ATP synthase activity of an individual ATP synthase complex was enhanced in anti-ATP synthase (δ) plants.
Figure 5.
The capacity of RuBP regeneration (Jg) and the Jg per ATP synthase (δ) content at 25°C in antisense plants with a variety of contents of δ-subunit of chloroplast ATP synthase (A and C) and the capacity of RuBP regeneration (Jg) and the Jg per Rieske FeS content at 25°C in antisense plants with a variety of Rieske FeS contents (B and D). Jg was calculated from measurements of CO2 assimilation rate at high CO2 as described in “Materials and Methods.” The regression lines are shown in each figure. Regression coefficient (R2). A, R2 = 0.95. B, R2 = 0.98. C, R2 = 0.80. D, R2 = 0.17. Statistical comparison of regressions shown in A and B showed them to be significantly different at P < 0.00001. WT, Wild type.
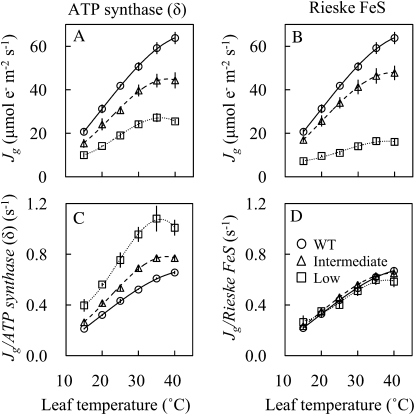
Temperature responses of Jg in anti-ATP synthase (δ) plants and anti-Rieske FeS plants are shown in Figure 6. We found that the temperature dependence of Jg/Rieske FeS content was the same for the wild type and plants with a range of Rieske FeS content. However, the temperature dependence of Jg/ATP synthase (δ) content varied with the ATP synthase levels of the plants being measured, being greatest at high temperature in transgenic plants with low ATP synthase content.
Figure 6.
Temperature responses of chloroplast electron transport (Jg) and the Jg per ATP synthase (δ) content in antisense plants with a variety of contents of δ-subunit of chloroplast ATP synthase (A and C) and temperature responses of Jg and the Jg per Rieske FeS content in antisense plants with a variety of Rieske FeS contents (B and D). Three groups were classified with respect to ATP synthase (δ) content or Rieske FeS content: Rieske FeS: the wild type (WT; approximately 100%), plants with intermediate Rieske FeS level (58% to approximately 85%), and plants with low Rieske FeS level (27% to approximately 29%); ATP synthase (δ): the wild type (approximately 100%), plants with intermediate ATP synthase (δ) level (48% to approximately 75%), and plants with low ATP synthase (δ) level (22% to approximately 26%). Data represent means ± se, n = 3 to approximately 4.
DISCUSSION
The data presented here show that there is a strong control of chloroplast electron transport and photosynthetic capacity by the level and activity of both the Cyt b6/f and ATP synthase complexes. However, the manner in which each complex does this and their relative contributions are distinctly different. It was clearly evident that the Cyt b6/f complex exhibited much tighter control of electron transport capacity and photosynthesis than that of the ATP synthase complex (Figs. 1 and 5). A significant basis for this appears to lie in the fact that there is a strong potential for an individual ATP synthase complex to modulate its proton conductance and ATP synthesis per electron transport, while the Cyt b6/f complex has much less flexibility (Figs. 5C and 6C).
ATP Synthase Activity Varies in Vivo
When the ATP synthase complex content was reduced, the evidence clearly indicates that the actual chloroplast ETR per ATP synthase complex increased and this was greatest at high temperature (Fig. 5). However, the increased rate of ATP synthase does not fully compensate for the reduced amount of ATP synthase, but ATP synthase activity goes faster when there is less of it. This supports the notion that the activity of an ATP synthase complex can vary in vivo when ATP synthase content is reduced. This change in activity could be due to changes in substrate availability (stromal ADP, inorganic phosphate [Pi], and trans-thylakoid pmf), the activation state of the complex, or the proton stoichiometry per ATP.
There have been several reports about the nature of the modulation of ATP synthase activity. For example, the ATP synthase is regulated by pmf and by reduction of γ-subunit thiols via thioredoxin (Kramer and Crofts, 1989; Ort and Oxborough, 1992; Fischer et al., 2000; McCarty, 2005; McCallum and McCarty, 2007). We found that reductions in ATP synthase content increased NPQ and probably the transthylakoid ΔpH as previously reported (Price et al., 1995). It has also been suggested that ATP synthase senses the status of stromal metabolites either directly or indirectly (Kramer et al., 2004) and it has been suggested that its activity can be modulated by altering Pi levels (Kanazawa and Kramer, 2002; Takizawa et al., 2008). A number of Calvin cycle enzyme activities are regulated by the chloroplast ferredoxin-thioredoxin pathway (e.g. Rubisco via Rubisco activase in Supplemental Fig. S3; GA3PDH, sedoheptulose-1,7-bisphosphatase, FBPase, PRK). The reduction in NADP-MDH activation state with reduction in ATP synthase content suggests that the activity of these redox-regulated enzymes of the Calvin cycle may also have decreased. This would decrease ATP consumption such that changes in ADP and Pi levels may not be as large as otherwise expected similar to what was observed for plants with reduced Cyt b6/f complex (Ruuska et al., 2000). Nevertheless, both substrate availability and/or the activation state of the ATP synthase complex via thioredoxin system could be enhanced in plants with low ATP synthase contents.
Recent estimations of proton stoichiometry indicated that the H+/ATP ratio is 4.66 (Baker et al., 2007). Interestingly, there have been reports that the proton stoichiometry in ATPase may vary depending on environmental conditions in Escherichia coli (Schemidt et al., 1995, 1998). Thus, it may also be possible that the proton stoichiometry in ATP synthase varied between the wild type and anti-ATP synthase line, since their physiological states (e.g. transthylakoid ΔpH) were different.
Cyt b6/f Content Is Rate Limiting for Chloroplast Electron Transport
The Cyt b6/f complex has a unique role in chloroplast electron transport, as it can act in both linear electron transport (production of ATP and NADPH) and cyclic electron transport (ATP generation only). There was a strong linear relationship between chloroplast ETR and Cyt b6/f content such that electron transport per Cyt b6/f content was the same for plants with a large range of Rieske FeS content (Figs. 1 and 5) similar to previous observations (Price et al., 1995, 1998).
The photosynthetic model of Farquhar et al. (1980) suggests that CO2 assimilation in C3 plants is limited by the rate of RuBP regeneration at high CO2 and that RuBP regeneration rate in turn is determined by either the chloroplast electron transport capacity to generate NADPH and ATP or the activity of Calvin cycle enzymes involved in regeneration of RuBP. There have been a number of studies using transgenic plants to investigate whether Calvin enzymes limit the rate of RuBP regeneration and only sedoheptulose-1,7-bisphosphatase has been suggested as a possible candidate for a rate-limiting step (for review, see Raines, 2003, 2006).
To our knowledge, this is the first time that the dependence of ETR on Cyt b6/f content and ATP synthase content have been compared. Our results can be interpreted to suggest that measurements of CO2 assimilation rate at high CO2 can be used to infer Cyt b6/f content of leaves (see also Niinemets and Tenhunen, 1997; Yamori et al., 2010a). The assumption that RuBP regeneration rate is limited by chloroplast ETR and Cyt b6/f content rather than ATPase content may provide a robust mechanism for scaling carbon uptake from leaf photosynthesis to canopies, and ecosystems. This approach would be complementary to the common practice of using the initial slope of the CO2 response curve to quantify the Rubisco content of leaves (von Caemmerer and Farquhar, 1981; Long and Bernacchi, 2003; Yamori et al., 2006b, 2010a).
Enhancing C3 Photosynthesis
It has been argued that a new green revolution is needed in world agriculture to increase crop yields for food security (Fischer and Edmeades, 2010). Increasing leaf photosynthetic capacity provides one attractive avenue to drive increases in crop yields (see Long et al., 2006; Peterhansel et al., 2008). In a future high-CO2 world, C3 photosynthesis will be increasingly limited by RuBP regeneration. The observation that the introduction of a parallel electron carrier between Cyt f and PSI through the expression of Porphyra Cyt c6 in Arabidopsis (Arabidopsis thaliana) conferred more rapid electron flow in vitro and enhanced plant growth (Chida et al., 2007), supports the notion of the strong control that intersystem electron transport through the Cyt b6/f complex has on photosynthetic capacity. Research is needed to explore how the levels of Cyt b6/f and ATP synthase complexes are regulated in the thylakoid membrane and what strategies may be employed to increase their content. This will be challenging given that both complexes contain both nuclear and chloroplast encoded subunits and that there appears to be strong posttranscriptional control of complex synthesis and assembly in the chloroplast (Leister, 2003).
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Tobacco (Nicotiana tabacum ‘W38’) plants and the progeny of several transformants of anti-Rieske FeS tobacco and anti-ATP synthase tobacco that have reduced amounts of the chloroplast Cyt b6/f and ATP synthase were grown in controlled environmental growth cabinets (Price et al., 1995). Plants were grown at irradiance of 60 to approximately 80 μmol m−2 s−1 with a photoperiod of 20 h and ambient CO2 concentration. The day/night air temperatures were 30°C/25°C, and the relative humidity was 70%. Plants were grown in 5-L pots in garden mix containing approximately 2 g L−1 of a slow-release fertilizer (Osmocote, Scotts) and watered daily. The low irradiance was selected to minimize the differences in the growth rate of plants and the capacity of CO2 assimilation at the growth condition (Ruuska et al., 2000).
Gas Exchange and Fluorescence Measurements
CO2 gas exchange of leaves was measured with a portable gas exchange system (LI-6400, LI-COR). The whole portable gas exchange system was enclosed in a temperature-controlled cabinet (Yamori et al., 2005, 2006a, 2008, 2009, 2010b). The CO2 assimilation rate (A) versus intercellular CO2 concentration (Ci) was measured at a light intensity of 1,200 μmol photons m−2 s−1 under several measurement temperatures. A-Ci curves were fitted with the C3 photosynthesis model (Farquhar et al., 1980), using the Rubisco kinetic constants and temperature dependencies in tobacco (Bernacchi et al., 2001). CO2 assimilation rates at high CO2 and measured rates of dark respiration (Rd) were used to calculate actual rates of chloroplast electron transport required to satisfy NADPH consumption (Jg [μmol m−2 s−1]):
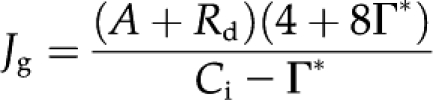 |
where Ci (μmol mol−1) is intercellular CO2 and Γ* (μmol mol−1) is the CO2 compensation point in the absence of day respiration (von Caemmerer and Farquhar 1981).
Chl a fluorescence was also determined by an integrated fluorescence chamber head (LI-6400, LI-6400-40 leaf chamber fluorometer, LI-COR). The quantum yield of PSII [ΦPSII = (Fm′ − F′)/Fm′], photochemical quenching [qP = (Fm′ − F′)/(Fm′ − Fo′)], NPQ [NPQ = (Fm − Fm′)/Fm′], and the fraction of PSII centers in the open state (with QA oxidized; qL = qP × [Fo′/F′]) were calculated (Baker et al., 2007; Baker, 2008). The ETR was calculated as ETR = 0.5 × absI × ΦPSII, where 0.5 is the fraction of absorbed light reaching PSII and absI is absorbed irradiance taken as 0.85 of incident irradiance (Genty et al., 1989).
Determinations of Rieske FeS of Cyt b6/f Complex, δ-Subunit of ATP Synthase, Rubisco Activase, and Chl
Immediately after the measurements of gas exchange, leaf discs were taken and immersed in liquid nitrogen and stored at −80°C. The frozen leaf sample was ground in liquid nitrogen and homogenized in an extraction buffer (Yamori and von Caemmerer, 2009; Yamori et al., 2010a). The content of Rieske FeS of Cyt b6/f complex and δ-subunit of ATP synthase was quantified by immunoblotting with anti-Rieske FeS antibody and anti-ATP synthase (δ) antibody (Agrisera). Rubisco activase was also quantified by immunoblotting with anti-activase antibody (Yamori and von Caemmerer, 2009). Chl was extracted in 80% (v/v) acetone and determined (Porra et al., 1989). The leaf extract of one wild-type leaf was selected as a standard (100%) and included as a dilution series on gels. The protein content of other samples was referenced against this standard.
Determinations of Rubisco Catalytic Sites, Rubisco Activation State, and NADP-MDH Activation State
Samples used for the Rubisco activation assay and chloroplast NADP-MDH assay were collected from a leaf equilibrated at steady-state conditions in the gas exchange chamber. After gas exchange had reached the steady-state rate for at least 30 min at a given leaf temperature, the leaf section in the chamber was taken out and immediately frozen in liquid nitrogen. Rubisco catalytic sites and Rubisco activation state were determined by the stoichiometric binding of 14C-carboxy-arabinitol-P2, whereas chloroplast NADP-MDH activation state was assayed by monitoring NADH oxidation at 340 nm (Yamori and von Caemmerer, 2009).
Statistical Analysis
Statistical comparison of the regressions shown in Figures 1, A and B, and 5, A and B, were analyzed with a separate slopes model using the software package Statistica.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. CO2 response of CO2 assimilation rate and ETR at 25°C.
Supplemental Figure S2. CO2 response of CO2 assimilation rate and ETR at 40°C.
Supplemental Figure S3. Rubisco activation state, Rubisco, and Rubisco activase content.
Supplementary Material
Acknowledgments
We would like to thank Dr. John Evans for his generous advice and Simon Dwyer for help with the statistical analysis.
References
- Allen JF. (2003) Cyclic, pseudocyclic and noncyclic photophosphorylation: new links in the chain. Trends Plant Sci 8: 15–19 [DOI] [PubMed] [Google Scholar]
- Baker NR. (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59: 89–113 [DOI] [PubMed] [Google Scholar]
- Baker NR, Harbinson J, Kramer DM. (2007) Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ 30: 1107–1125 [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP. (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ 24: 253–259 [Google Scholar]
- Chida H, Nakazawa A, Akazaki H, Hirano T, Suruga K, Ogawa M, Satoh T, Kadokura K, Yamada S, Hakamata W, et al. (2007) Expression of the algal cytochrome c6 gene in Arabidopsis enhances photosynthesis and growth. Plant Cell Physiol 48: 948–957 [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Caemmerer SV, Berry JA. (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90 [DOI] [PubMed] [Google Scholar]
- Fischer RAT, Edmeades GO. (2010) Breeding and cereal yield progress. Crop Sci 50: S85–S98 [Google Scholar]
- Fischer S, Graber P, Turina P. (2000) The activity of the ATP synthase from Escherichia coli is regulated by the transmembrane proton motive force. J Biol Chem 275: 30157–30162 [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Kanazawa A, Kramer DM. (2002) In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc Natl Acad Sci USA 99: 12789–12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer DM, Avenson TJ, Edwards GE. (2004) Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci 9: 349–357 [DOI] [PubMed] [Google Scholar]
- Kramer DM, Crofts AR. (1989) Activation of the chloroplast ATPase measured by the electrochromic change in leaves of intact plants. Biochim Biophys Acta 976: 28–41 [Google Scholar]
- Leister D. (2003) Chloroplast research in the genomic age. Trends Genet 19: 47–56 [DOI] [PubMed] [Google Scholar]
- Long SP, Bernacchi CJ. (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot 54: 2393–2401 [DOI] [PubMed] [Google Scholar]
- Long SP, Zhu XG, Naidu SL, Ort DR. (2006) Can improvement in photosynthesis increase crop yields? Plant Cell Environ 29: 315–330 [DOI] [PubMed] [Google Scholar]
- McCallum JR, McCarty RE. (2007) Proton flux through the chloroplast ATP synthase is altered by cleavage of its gamma subunit. Biochim Biophys Acta 1767: 974–979 [DOI] [PubMed] [Google Scholar]
- McCarty RE. (2005) ATP synthase of chloroplast thylakoid membranes: a more in depth characterization of its ATPase activity. J Bioenerg Biomembr 37: 289–297 [DOI] [PubMed] [Google Scholar]
- Müller P, Li XP, Niyogi KK. (2001) Non-photochemical quenching: a response to excess light energy. Plant Physiol 125: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets U, Tenhunen JD. (1997) A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ 20: 845–866 [Google Scholar]
- Ort DR, Oxborough K. (1992) In situ regulation of chloroplast coupling factor activity. Annu Rev Plant Physiol Plant Mol Biol 43: 269–291 [Google Scholar]
- Peterhansel C, Niessen M, Kebeish RM. (2008) Metabolic engineering towards the enhancement of photosynthesis. Photochem Photobiol 84: 1317–1323 [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975: 384–394 [Google Scholar]
- Price GD, von Caemmerer S, Evans JR, Siebke K, Anderson JM, Badger MR. (1998) Photosynthesis is strongly reduced by antisense suppression of chloroplastic cytochrome bf complex in transgenic tobacco. Aust J Plant Physiol 25: 445–452 [Google Scholar]
- Price GD, Yu J-W, von Caemmerer S, Evans JR, Chow WS, Anderson JM, Hurry V, Badger MR. (1995) Chloroplast cytochrome b6/f and ATP synthase complexes in tobacco: transformation with antisense RNA against nuclear-encoded transcripts for the Rieske FeS and ATP polypeptides. Aust J Plant Physiol 22: 285–297 [Google Scholar]
- Raines CA. (2003) The Calvin cycle revisited. Photosynth Res 75: 1–10 [DOI] [PubMed] [Google Scholar]
- Raines CA. (2006) Transgenic approaches to manipulate the environmental responses of the C3 carbon fixation cycle. Plant Cell Environ 29: 331–339 [DOI] [PubMed] [Google Scholar]
- Ruuska SA, Andrews TJ, Badger MR, Price GD, von Caemmerer S. (2000) The role of chloroplast electron transport and metabolites in modulating Rubisco activity in tobacco: insights from transgenic plants with reduced amounts of cytochrome b/f complex or glyceraldehyde 3-phosphate dehydrogenase. Plant Physiol 122: 491–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R, Stitt M. (1988) Comparison of NADP-malate dehydrogenase activation, QA reduction and O2 evolution in spinach leaves. Plant Physiol Biochem 26: 473–481 [Google Scholar]
- Schemidt RA, Hsu DKW, Deckers-Hebestreit G, Altendorf K, Brusilow WSA. (1995) The effects of an atpE ribosome-binding site mutation on the stoichiometry of the c subunit in the F1F0 ATPase of Escherichia coli. Arch Biochem Biophys 323: 423–428 [DOI] [PubMed] [Google Scholar]
- Schemidt RA, Qu J, Williams JR, Brusilow WSA. (1998) Effects of carbon source on expression of F0 genes and on the stoichiometry of the c subunit in the F1F0 ATPase of Escherichia coli. J Bacteriol 180: 3205–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader SM, Wise RR, Wacholtz WF, Ort DR, Sharkey TD. (2004) Thylakoid membrane responses to moderately high leaf temperature in pima cotton. Plant Cell Environ 27: 725–735 [Google Scholar]
- Sharkey TD. (2005) Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, Rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ 28: 269–277 [Google Scholar]
- Shikanai T. (2007) Cyclic electron transport around photosystem I: genetic approaches. Annu Rev Plant Biol 58: 199–217 [DOI] [PubMed] [Google Scholar]
- Takizawa K, Kanazawa A, Kramer DM. (2008) Depletion of stromal P(i) induces high “energy-dependent” antenna exciton quenching (q(E)) by decreasing proton conductivity at CF(O)-CF(1) ATP synthase. Plant Cell Environ 31: 235–243 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR. (2010) Enhancing C3 photosynthesis. Plant Physiol 154: 589–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387 [DOI] [PubMed] [Google Scholar]
- Yamori W, Evans JR, Von Caemmerer S. (2010a) Effects of growth and measurement light intensities on temperature dependence of CO(2) assimilation rate in tobacco leaves. Plant Cell Environ 33: 332–343 [DOI] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Hanba YT, Terashima I. (2006a) Effects of internal conductance on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant Cell Physiol 47: 1069–1080 [DOI] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Hikosaka K, Terashima I. (2009) Cold-tolerant crop species have greater temperature homeostasis of leaf respiration and photosynthesis than cold-sensitive species. Plant Cell Physiol 50: 203–215 [DOI] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Hikosaka K, Terashima I. (2010b) Phenotypic plasticity in photosynthetic temperature acclimation among crop species with different cold tolerances. Plant Physiol 152: 388–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Kashino Y, Terashima I. (2008) The role of electron transport in determining the temperature dependence of the photosynthetic rate in spinach leaves grown at contrasting temperatures. Plant Cell Physiol 49: 583–591 [DOI] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Terashima I. (2005) Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant Cell Environ 28: 536–547 [Google Scholar]
- Yamori W, Suzuki K, Noguchi K, Nakai M, Terashima I. (2006b) Effects of Rubisco kinetics and Rubisco activation state on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant Cell Environ 29: 1659–1670 [DOI] [PubMed] [Google Scholar]
- Yamori W, von Caemmerer S. (2009) Effect of Rubisco activase deficiency on the temperature response of CO2 assimilation rate and Rubisco activation state: insights from transgenic tobacco with reduced amounts of Rubisco activase. Plant Physiol 151: 2073–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.