Abstract
The phytohormone ethylene differentially regulates plant architecture and growth in both a light- and nutrient-dependent fashion. The modulation of plant development by ethylene in response to both external and internal signals can also generate tissue-specific differential responses. Here, we report that XAP5 CIRCADIAN TIMEKEEPER (XCT) is involved in blue light-dependent ethylene responses in the aerial tissues of Arabidopsis (Arabidopsis thaliana) seedlings. XCT was first identified as a circadian clock mutant with a short free-running period. The xct mutation also causes sugar-specific hypocotyl growth defects, in which mutants are short in blue light when grown on a sucrose-rich medium but tall when grown on sucrose-deficient medium. Our data suggest that the hypocotyl defects in blue light are not directly caused by defects in clock or light signaling but rather by enhanced ethylene responses. In blue light, xct mutants have a more active ethylene response pathway and exhibit growth phenotypes similar to the constitutive ethylene signaling mutant constitutive triple response1 (ctr1). xct mutants also have reduced ethylene emission, analogous to plants that have lost CTR1 function. Genetic analysis suggests that XCT negatively regulates ethylene responses downstream of ETHYLENE-INSENSITIVE3 in aerial tissues. However, XCT is not required for all ethylene-mediated processes, such as the inhibition of root growth. Thus, XCT acts downstream of a major transcriptional regulator in an organ-specific manner, playing an environment-dependent role in the regulation of plant growth.
As sessile organisms, plants must tightly control their growth in order to optimize and complete their life cycle. To this end, plants have evolved sophisticated mechanisms to modulate their growth in response to various internal and external stimuli. Studies in growth control of the hypocotyl, the plant embryonic stem, have revealed diverse molecular players involved in this process (Jiménez-Gómez and Maloof, 2009). The circadian clock, light signaling, and various phytohormones act to control growth of the hypocotyl as well as other organs (Vandenbussche et al., 2005, Nozue and Maloof, 2006). An emerging understanding of these pathways is beginning to shed light to how each acts independently and cooperatively to impact growth.
Light regulates plant growth and development in a process called photomorphogenesis. Plants perceive different qualities and quantities of light using a variety of photoreceptors. The phytochrome family in Arabidopsis (Arabidopsis thaliana) contains five photoreceptors, PHYTOCHROME A to PHYTOCHROME E, that perceive and respond to red and far-red light (Montgomery and Lagarias, 2002). The cryptochrome family, consisting of CRYPTOCHROME1 (CRY1) and CRY2, perceive and respond to blue light (Jiao et al., 2007). Both phytochromes and cryptochromes act as negative regulators of hypocotyl elongation and positive regulators of the photomorphogenic response (Holm et al., 2002). In this sense, light signaling, regardless of the quality of light perceived, generally acts to inhibit elongation growth.
Phytohormones affect virtually every aspect of plant growth and development, including the regulation of hypocotyl growth (Vandenbussche et al., 2005). One phytohormone involved in the development of a wide variety of plant organs is ethylene, a simple gaseous hydrocarbon that impacts every stage of plant growth and development (Kieber and Ecker, 1993; De Paepe and Van Der Straeten, 2005). Ethylene has differential effects on the growth of different plant organs (Vandenbussche et al., 2007). In dark-grown plants, ethylene initiates the classic triple response, which consists of the radial swelling and shortening of the hypocotyl, the inhibition of root growth, and the formation of an exaggerated apical hook (Kieber et al., 1993).
As well as being organ specific, ethylene responses are also influenced by growth conditions. For example, light quality affects ethylene responses: in monochromatic blue or white light, ethylene promotes hypocotyl elongation; in contrast, in monochromatic red light, ethylene inhibits hypocotyl elongation (Khanna et al., 2007; Vandenbussche et al., 2007). A further complication is that in light-grown plants, ethylene action is also dependent on the nutrient status of the growth medium (Smalle et al., 1997; Collett et al., 2000). Ethylene signaling promotes hypocotyl elongation when light-grown plants are maintained on minimal medium, but this effect is partially or totally masked on rich medium (Smalle et al., 1997). Other external factors, such as temperature, have also been reported to modulate the action of ethylene on hypocotyl growth (Collett et al., 2000). Therefore, the role of ethylene in regulating growth of the hypocotyl is extremely dependent on environmental conditions.
The biosynthesis of ethylene in planta begins with the stepwise conversion of l-Met to S-adenosyl-l-methionine to 1-aminocyclopropane-1-carboxylic acid (ACC) by the enzyme ACC SYNTHASE (ACS). The conversion of S-adenosyl-l-methionine to ACC is the rate-limiting step in the catalytic pathway to produce ethylene. ACC OXIDASE (ACO) then converts ACC into ethylene by an oxidation reaction (Chae and Kieber, 2005). Both ACS and ACO are present in multigene families in the Arabidopsis genome (as well as in other plant species), are regulated at the transcriptional and posttranscriptional levels, and contain a high degree of functional specificity (Tsuchisaka et al., 2009). Several characterized ethylene biosynthesis mutants fail to properly regulate ACS stability, which in general are short-lived proteins. For example, the ethylene-overproducing mutant ethylene overproducer1 (eto1) confers increased stability to the ACS5 protein, resulting in increased ethylene production (Chae et al., 2003; Chae and Kieber, 2005).
After ethylene is synthesized in the cytosol, it is perceived by endoplasmic reticulum membrane-localized ethylene receptors, of which there are five in Arabidopsis (Lin et al., 2009). In the absence of ethylene, the receptors are bound to a putative mitogen-activated protein kinase kinase kinase protein called CONSTITUTIVE TRIPLE RESPONSE1 (CTR1). CTR1 is a negative regulator of the entire pathway; thus, ethylene signaling is constitutively active in loss-of-function ctr1 mutants (Kieber et al., 1993). The interaction of ethylene with the ethylene receptors alters receptor activity, allowing the inactivation of CTR1 and thus relieving its repression of the pathway (Chen et al., 2007).
The perception of ethylene and subsequent inactivation of CTR1 activates a protein called ETHYLENE-INSENSITIVE2 (EIN2) through an unknown mechanism. EIN2 is an endoplasmic reticulum-localized transmembrane protein with unidentified function that is essential for the activation of EIN3 activity. EIN3 is a nucleus-localized transcription factor with several homologs, termed EIN3-LIKE proteins, or EILs (Chao et al., 1997; Solano et al., 1998; Lin et al., 2009). Loss of EIN3 results in full or partial ethylene insensitivity depending on the environmental condition, as EIN3 and the EILs have partially overlapping functions (Binder et al., 2007). EIN3 binds to the promoters of target genes called ETHYLENE RESPONSE FACTORS (ERFs). Despite an abundance of data supporting the molecular function of EIN3, very few ERFs have been functionally characterized and can have roles in other signaling pathways, such as abscisic acid signaling (Nakano et al., 2006). There are over 100 ERF or ERF-like genes in the Arabidopsis genome, and they themselves encode transcription factors. ERF transcription factors bind to the promoters of ethylene-regulated genes at an element containing a GCC motif and initiate the ethylene response at the molecular level, which includes both the activation and repression of target genes (Guo and Ecker, 2004; Gutterson and Reuber, 2004; Nakano et al., 2006).
Ethylene signaling is tightly regulated at every level, ranging from ethylene biosynthesis to downstream ethylene-mediated transcriptional regulation. ACS proteins function as both homodimers and heterodimers, providing some spatial and temporal specificity to ethylene production (Tsuchisaka et al., 2009). Ethylene receptors also function as protein complexes, and this may influence how and when ethylene can be perceived (Lin et al., 2009). CTR1 is involved in the activation of a mitogen-activated protein kinase signaling cascade involving MITOGEN-ACTIVATED PROTEIN KINASE KINASE9, MITOGEN-ACTIVATED PROTEIN KINASE3 (MPK3), and MPK6, which activate EIN3 independently of EIN2 (Yoo et al., 2008). EIN3 is also regulated by additional regulatory proteins, such as EIN3-BINDING F-BOX PROTEIN1 (EBF1) and EBF2, which destabilize EIN3 (Binder et al., 2007), as well as in response to different environmental conditions, such as sugar availability and light (Yanagisawa et al., 2003; Lee et al., 2006). Downstream of EIN3, little is known about how ERF proteins are regulated or how their target genes might be additionally regulated.
Another important regulator of plant development is the circadian clock, which influences growth via multiple pathways. For example, it regulates the production of auxin and ethylene and modulates plant sensitivity to multiple hormones (Thain et al., 2004; Covington and Harmer, 2007; Covington et al., 2008; Michael et al., 2008; Legnaioli et al., 2009; Rawat et al., 2009). In addition, the circadian clock directly controls the expression of growth-promoting transcription factors (Nozue et al., 2007). It is thus not surprising that mutations in central clock genes such as CIRCADIAN CLOCK ASSOCIATED1 (CCA1), LATE ELONGATED HYPOCOTYL, and TIMING OF CAB EXPRESSION1 (TOC1) also cause growth-related phenotypes (Nozue and Maloof, 2006).
Previously, we reported the molecular identification of XAP5 CIRCADIAN TIMEKEEPER (XCT), a novel gene with pleiotropic effects on plant growth and development (Martin-Tryon and Harmer, 2008). xct mutants have delayed greening, short-period circadian rhythms, and altered regulation of hypocotyl elongation. XCT is a nucleus-localized protein that is highly conserved across eukaryotes and yet has no known molecular function in any organism. Here, we report that XCT is a negative regulator of the blue light-mediated ethylene response in aerial tissues of Arabidopsis. Loss-of-function xct mutants have phenotypes qualitatively similar to but less severe than the strong constitutive ethylene signaling mutant ctr1-3. Our data strongly suggest that XCT does not act within the ethylene signaling pathway itself but rather acts as an organ-specific regulator of the ethylene response downstream of the transcription factor EIN3, perhaps introducing additional specificity to the ethylene response. Our data, therefore, suggest that XCT acts in the nucleus to modulate the activity of one or more transcription factors, providing important clues regarding the molecular function of XCT-like proteins in plants and other eukaryotes.
RESULTS
XCT Differentially Regulates Hypocotyl Elongation in a Sugar- and Light-Specific Manner
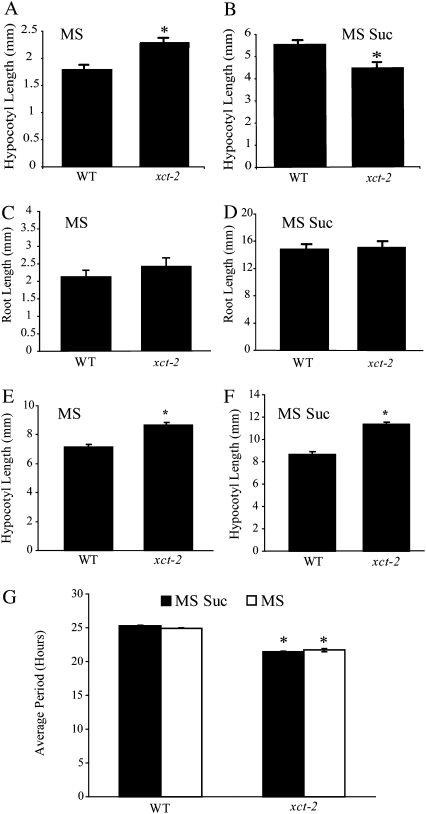
Since the molecular function of XCT is unknown, we decided to examine its biological role in the control of hypocotyl elongation, a well-studied model for growth control in plants. We previously reported that xct-2 mutants have a short hypocotyl in blue light but a tall hypocotyl in red light when grown on Murashige and Skoog (MS) medium supplemented with 3% Suc, a standard condition for circadian experiments (Martin-Tryon and Harmer, 2008). To investigate whether the presence of Suc in the medium might affect the growth phenotype, we assayed hypocotyl elongation in xct-2 grown on medium not supplemented with Suc. Surprisingly, when grown in blue light without the addition of exogenous Suc, xct-2 mutants had a tall hypocotyl relative to the wild type (Fig. 1A). We did not observe any significant difference in root growth between the wild type and xct mutants grown either plus or minus Suc (Fig. 1, C and D). XCT is expressed and produces a stable protein in the roots (Martin-Tryon and Harmer, 2008), so the lack of a root phenotype in xct mutants is not due to hypocotyl-specific expression. Therefore, XCT plays a sugar- and organ-dependent role in growth control in blue light.
Figure 1.
Phenotypic analysis of xct-2 growth patterns in the presence or absence of Suc. A and B, Hypocotyl length of the wild type (WT) and xct-2 (n = 35–40) grown under 3 μmol m−2 s−1 constant monochromatic blue light for 6 d on MS medium in the presence or absence of 3% Suc. C and D, Root length of the wild type and xct-2 (n = 25–30) grown under 3 μmol m−2 s−1 monochromatic blue light for 6 d on MS medium in the presence or absence of 3% Suc. E and F, Similar to A and B, but the experiments were performed in 20 μmol m−2 s−1 monochromatic red light. G, Average free-running period, assayed by monitoring bioluminescence of a ProCCR2::LUC reporter construct in each genotype (n = 12–15) grown under blue light on different medium conditions. All error bars represent se. Asterisks denote significant differences from the wild type under the same condition based on Student’s t test (P < 0.05). All data presented are representative of at least three independent experiments.
Given this surprising phenotype in blue light, we examined whether medium composition could alter the growth phenotype of xct mutants in red light. In this condition, xct mutants had elongated hypocotyls relative to the wild type regardless of the presence or absence of Suc in the growth medium (Fig. 1, E and F). Thus, the hypocotyl phenotype of xct mutants is sugar dependent in blue light but not in red light.
Since the circadian clock regulates the rhythmic growth of the hypocotyl (Nozue and Maloof, 2006), we hypothesized that the sugar-specific hypocotyl growth defect in xct-2 in blue light might be due to differential clock defects in this mutant when grown in the presence or absence of Suc. However, xct-2 plants grown in blue light had the same short-period phenotype both in the presence and absence of exogenous Suc (Fig. 1G). Since the presence or absence of sugar had no effect on the circadian phenotype but completely changed the nature of the hypocotyl phenotype, the growth phenotypes in xct-2 are likely not directly caused by the circadian defect.
Since the xct hypocotyl and clock phenotypes seemed independent, we reasoned that XCT might act in a light-signaling pathway to control hypocotyl elongation. An important mediator of both blue and red light signaling is the transcription factor ELONGATED HYPOCOTYL5 (HY5; Oyama et al., 1997; Nozue and Maloof, 2006). To determine whether XCT acts in HY5 signaling, we examined epistatic interactions between xct-2 and hy5-215. We found that the xct-2 and hy5-215 phenotypes were additive when plants were grown either in the presence or absence of Suc (Supplemental Fig. S1). This suggests that XCT does not act primarily in a HY5-regulated light signaling pathway, a conclusion supported by the normal sensitivity of xct mutants to increasing intensities of light (Martin-Tryon and Harmer, 2008).
We next hypothesized that the hypocotyl phenotypes might be related to an altered hormone-signaling pathway. It has been reported previously that ethylene regulates hypocotyl elongation in a differential manner, depending on the light environment and nutrient status of the growth medium (Pierik et al., 2006). Indeed, ctr1-3 mutants have a constitutively active ethylene signaling pathway and demonstrate both light quality- and nutrient-specific growth phenotypes (Vandenbussche et al., 2007) similar to xct-2. Therefore, we reasoned that the blue light hypocotyl phenotypes in xct-2 might be due to an overactive ethylene pathway.
XCT Plays a Role in Ethylene Signaling
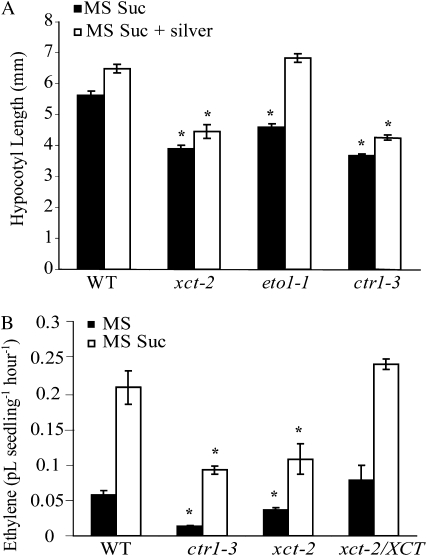
To determine whether XCT is involved in ethylene signaling, we analyzed xct-2 hypocotyl growth in blue light in the presence of an ethylene signaling inhibitor. Plants were grown under blue light for 6 d on MS medium containing Suc or medium containing both Suc and silver nitrate, an antagonist of the ethylene signaling pathway thought to act at the level of the ethylene receptors. The addition of 50 μm silver nitrate to the growth medium resulted in an increase of the hypocotyl height in wild-type plants (Fig. 2A), suggesting that ethylene inhibits hypocotyl growth in blue light in a Suc-rich medium. xct-2, like ctr1-3, exhibited a short hypocotyl relative to the wild type both in the presence and absence of silver nitrate. In contrast, the short-hypocotyl phenotype of the ethylene-overproducing mutant eto1-1 became indistinguishable from the wild type upon the addition of silver nitrate to the medium. This suggested that the xct phenotype was not due to ethylene overproduction.
Figure 2.
xct-2 and ctr1-3 have similar phenotypes. A, Hypocotyl length of various genotypes (n = 35–40) grown on MS Suc medium in the presence or absence of 50 μm silver nitrate for 6 d under constant monochromatic blue light. B, Ethylene emission levels (n = 200–400) of various genotypes grown in the presence or absence of 3% Suc. Seedlings were grown under blue light for 3 to 4 d, capped, and allowed to accumulate ethylene over 24 h in blue light, then sent for ethylene detection. All error bars represent se. Asterisks denote significant differences from the wild type (WT) under the same condition based on Student’s t test (P < 0.05). All data presented are representative of at least three independent experiments.
When grown on MS medium without added Suc, xct-2, eto1-1, and ctr1-3 were all significantly taller than the wild type (Supplemental Fig. S2), and the addition of silver to the medium did not have any significant effect on hypocotyl height in any of the genotypes. Consistent with a previous study (Vandenbussche et al., 2007), this suggests that ethylene signaling was not limiting for hypocotyl growth under these conditions.
Since the inability of silver nitrate to rescue the xct-2 phenotype suggested that these mutants might have some sort of ethylene signaling (as opposed to biosynthesis-related) defect, we measured ethylene emission levels in xct-2 in both the presence and absence of exogenous Suc in blue light. xct-2 mutants produced significantly less ethylene than the wild type when grown in either medium (Fig. 2B). This decrease in ethylene emission was completely rescued by the introduction of a genomic copy of XCT driven by its own promoter into the xct-2 mutant background (xct-2/XCT), indicating that the ethylene production defect was indeed solely due to the loss of XCT function. Similar to xct-2, ctr1-3 mutants displayed reduced ethylene emission levels both in the presence and absence of Suc (Fig. 2B), consistent with previous studies of dark-grown ctr1-3 plants (Kieber et al., 1993). This decrease in ethylene emission in mutants such as ctr1-3 that have increased ethylene signaling is likely due to negative feedback regulation on the production of ethylene. This is corroborated by the fact that ethylene-insensitive mutants actually produce more ethylene than wild-type plants (Thain et al., 2004). Taken together, these data suggested that XCT negatively regulates some aspect of the ethylene signaling pathway.
xct-2 Mutants Are Not Hypersensitive to Ethylene
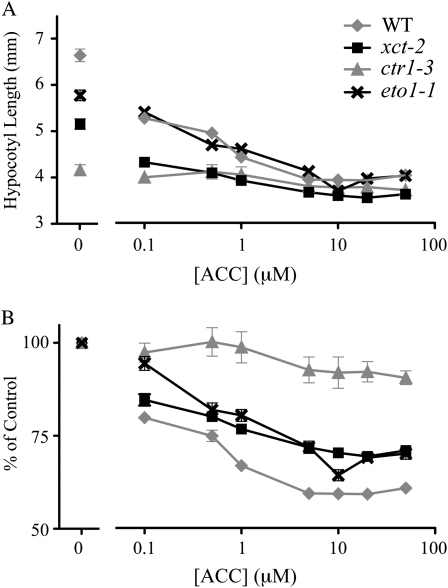
The similarity between the xct-2 and ctr1-3 phenotypes suggested that xct-2 mutants might have altered responsiveness to ethylene. Therefore, we examined the sensitivity of xct and ethylene signaling mutants to ACC, the precursor molecule to ethylene in the biosynthetic pathway. In the absence of ACC, the hypocotyls of xct-2, ctr1-3, and eto1-1 mutants grown in blue light on rich medium were all shorter than the wild type (Fig. 3A). When grown on plates containing the ethylene precursor, the ethylene-overproducing mutant eto1-1 was indistinguishable from the wild type, showing maximal growth inhibition at 5 μm ACC (Fig. 3A). In contrast, the lowest concentration of ACC tested (0.1 μm) was sufficient to cause maximal inhibition of hypocotyl growth in the constitutive ethylene signaling mutant ctr1-3. Hypocotyl elongation in xct-2 mutants, like the wild type, was maximally inhibited by 5 μm ACC (Fig. 3). When the hypocotyl lengths of ACC-treated plants are graphed as a percentage of control plants, it is clear that xct-2 mutants show reduced responsiveness to ACC (Fig. 3B).
Figure 3.
Inhibition of hypocotyl elongation by ACC. A, Hypocotyl length of various genotypes (n = 35–40) grown on MS Suc medium in constant blue light with the addition of various concentrations (0–50 μm) of ACC to the growth medium. All error bars represent se. B, Change in hypocotyl length in response to ACC as viewed in A, represented as the percentage of the hypocotyl length relative to untreated hypocotyls. All data presented are representative of at least three independent experiments. WT, Wild type.
Therefore, xct-2 is not hypersensitive to ethylene-induced inhibition of hypocotyl elongation in blue light. The observed reduced responsiveness could arise from a more active basal ethylene signaling pathway in xct-2, analogous to ctr1-3. However, the basal ethylene pathway in xct-2 is not as activated as in ctr1-3, given that ethylene-mediated inhibition of growth in xct-2 only saturated at 5 μm ACC. These data suggest that xct-2 mutants have an ethylene signaling defect in blue light unrelated to alterations in sensitivity to the hormone.
Genes Positively Regulated by Ethylene Have Increased Expression in xct-2
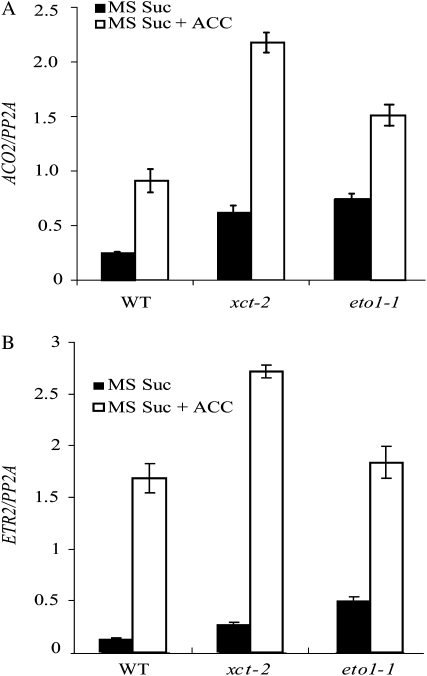
If basal ethylene signaling is increased in xct, we would expect genes positively regulated by ethylene to have higher expression levels even in the absence of exogenous ACC. To investigate this, we used quantitative reverse transcription (qRT)-PCR to examine mRNA levels of various ethylene-responsive genes in the presence or absence of ACC. In xct-2 mutants grown on MS Suc medium without exogenous ACC, the expression levels of ACO2 and ETHYLENE RESPONSE2 (ETR2) were indeed higher in xct-2 relative to the wild type (Fig. 4). Similarly, the expression of these genes was elevated in eto1-1 relative to the wild type. We observed similar gene expression patterns in these genotypes when grown on MS medium without added Suc (Supplemental Fig. S3). These data strongly suggest that basal activity of the ethylene pathway is elevated in xct-2 mutants.
Figure 4.
Analysis of ethylene-regulated gene expression. qRT-PCR analysis of ACO2 (A) and ETR2 (B) in various genotypes in response to ACC treatment. Plants were grown under constant blue light for 5 d on MS Suc supplemented with or without 20 μm ACC. Plants were harvested on day 5, and RNA was extracted with subsequent cDNA synthesis and analysis. All error bars represent se. All data presented are representative of at least two independent experiments, with each sample in each experiment containing three technical replicates for analysis. WT, Wild type.
We next examined the effect of chronic ACC treatment on the expression of these genes in both wild-type and mutant backgrounds. For this assay, plants were grown and harvested as described previously, but 20 μm ACC was added to the medium. Growth in the presence of ACC increased the expression of ethylene-induced genes in all genotypes analyzed (Fig. 4). For eto1-1, the fold induction of ethylene-regulated genes in response to ACC was reduced relative to the wild type. In xct-2, the chronic ethylene treatment resulted in a higher expression of these genes relative to the wild type, but with a similar fold change given the higher basal level of expression. This strong induction of ethylene-induced genes in xct-2 is very different from the constitutively highly expressed and ethylene-unresponsive regulation reported in ctr1 mutants (Kieber et al., 1993). When the wild type, xct-2, and eto1-1 were grown on medium without added Suc, ACC treatment caused induction of ACO2 and ETR2 expression (Supplemental Fig. S3). Interestingly, although xct-2 mutants grown on Suc medium supplemented with saturating levels of ACC had higher levels of both ACO2 and ETR2 expression than the wild type (Fig. 4), this enhanced expression was not observed in the absence of Suc (Supplemental Fig. S3). This enhanced response to ACC only in the presence of Suc suggests that XCT may be involved in both sugar response and ethylene signaling pathways.
XCT Functions Downstream of EIN3 to Regulate Ethylene Responses in Aerial Tissues
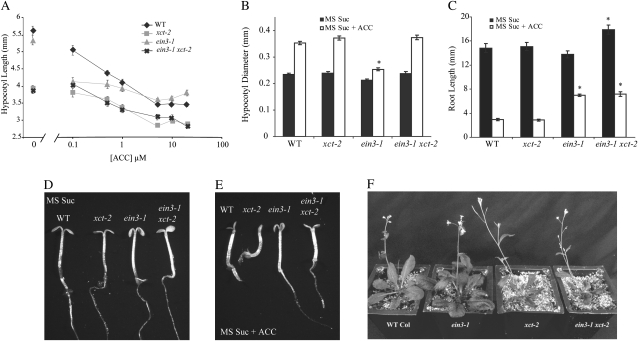
Our data suggested that XCT normally functions to repress the ethylene pathway somewhere downstream of the ethylene receptors. To further delineate where XCT acts within this pathway, we examined the genetic interactions between XCT and EIN3, an important transcriptional regulator of the ethylene response. We first characterized the hypocotyl response of ein3-1 xct-2 double mutants grown with different concentrations of ACC on MS Suc medium. As seen in Figure 3, hypocotyl elongation in wild-type and xct-2 plants was inhibited by ACC under these conditions (Fig. 5A). ein3-1 single mutants lacked a strong hypocotyl phenotype in the absence of exogenous ACC and showed normal responsiveness to low concentrations of ACC. However, unlike the wild type, they were fairly insensitive to increasing concentrations of ACC. The residual response to ethylene in this mutant background is likely attributed to the activity of EIN3-like proteins, known as the EILs. Interestingly, the ein3-1 xct-2 double mutant was indistinguishable from xct-2 single mutants at all concentrations of ACC, including its complete absence. Thus, the loss of XCT in an ein3-1 mutant restored ethylene responsiveness, genetically placing XCT downstream of EIN3.
Figure 5.
XCT is epistatic to EIN3. A, Hypocotyl lengths of various genotypes (n = 35–40) grown under blue light for 6 d on MS Suc supplemented with increasing concentrations of ACC. B, Hypocotyl diameter of various genotypes (n = 35–40) grown under blue light for 6 d on MS Suc supplemented with or without 10 μm ACC. C, Root length of various genotypes (n = 25–30) grown under blue light for 6 d on MS Suc supplemented with or without 20 μm ACC. D and E, Six-day-old plants grown under constant blue light on MS Suc supplemented with (E) or without (D) 10 μm ACC. F, Five-week-old plants grown under long-day conditions (18 h of light and 6 h of dark) in soil under 40 μmol m−2 s−1 white light. All error bars represent se. Asterisks denote significant differences from the wild type (WT) under the same condition based on Student’s t test (P < 0.05). All data presented are representative of at least three independent experiments.
We next examined the effect of exogenous ACC on additional traits in these genotypes. In wild-type plants, the addition of ACC induced radial hypocotyl thickening, a hallmark of the ethylene response (Fig. 5B). xct-2 had a hypocotyl diameter similar to the wild type both in the presence and absence of exogenous ACC, while ein3-1 was almost completely insensitive to the ethylene-induced radial expansion of the hypocotyl. However, the ein3-1 xct-2 double mutant had a normal response to ethylene-induced hypocotyl thickening, further demonstrating that loss of XCT restored ethylene sensitivity to ein3-1 mutants. Another phenotype strongly affected by ethylene is cotyledon expansion and unfolding. We found that ACC inhibited these processes in the wild type, as expected, but that these responses were exaggerated in xct-2 mutants (Fig. 5, D and E). Although the ein3-1 single mutant was largely insensitive to this response, the ein3-1 xct-2 double mutants treated with ACC behaved more similar to the wild type, suggesting that loss of XCT partially rescued this ein3-1 mutant phenotype. Altogether, these data indicated that loss of XCT restores ethylene responsiveness to ein3 mutants in aerial tissues.
Another aspect of the ethylene response is the inhibition of root growth. Inhibition of root growth in response to ACC was robust in both wild-type and xct-2 plants (Fig. 5C). In contrast, ein3-1 mutants showed reduced responsiveness to ACC in this assay, similar to the effects of ACC on hypocotyl elongation in this mutant. The ein3-1 xct-2 double mutants also showed reduced responsiveness to ACC, with the root length after ACC treatment being indistinguishable from the ein3-1 single mutant. These data, along with the lack of a root phenotype in xct-2 single mutants grown in the presence or absence of ACC, suggest that XCT is not involved in the ethylene response in root tissue despite its expression in this organ (Martin-Tryon and Harmer, 2008).
We also examined these seedling phenotypes on medium not supplemented with exogenous Suc. In the absence of ACC, xct-2 mutants had longer hypocotyls than the wild type or ein3-1 (Supplemental Fig. S4A). Similar to their growth patterns on Suc medium (Fig. 5A), the wild type and ein3-1 were not significantly different from each other on Suc-deficient medium. Low doses of ACC promoted hypocotyl elongation in the wild type and xct-2, but ein3-1 showed little response under these conditions. ein3-1 xct-2 double mutants had an unexpected phenotype: in the absence of ACC, they were significantly taller than xct-2 mutants, indicating a synergistic genetic interaction between these two loci (Supplemental Fig. S4A). Addition of ACC caused inhibition of hypocotyl elongation in ein3-1 xct-2, rather than the promotion of growth seen in the wild type and xct-2 or the unresponsiveness seen in ein3-1. This response to ACC in ein3-1 xct-2 was similar to that of wild-type plants grown on MS medium containing Suc (Fig. 5A). Thus, loss of XCT function restored ethylene responsiveness to ein3-1 seedlings in this assay as well, albeit in an unexpected manner.
We next monitored other seedling phenotypes in plants grown on MS without exogenous Suc. Unlike plants grown on MS supplemented with Suc, the ein3-1 xct-2 double mutants had thicker hypocotyls than either single mutant or the wild type even without the addition of ACC, a synergistic effect similar to their hypocotyl phenotype (Supplemental Fig. S4, A and B). Inhibition of root growth in response to ACC was impaired in both ein3-1 single and ein3-1 xct-2 double mutants (Supplemental Fig. S4C), similar to the phenotypes seen in plants grown on MS plus Suc (Fig. 5C). ein3-1 xct-2 double mutants showed a partial restoration of ACC effects on cotyledons relative to the unresponsive ein3-1 single mutants, with ACC treatment causing a small reduction in cotyledon expansion and unfolding in ein3-1 xct-2 (Supplemental Fig. S4, D and E). Although the genetic interactions between xct-2 and ein3-1 are more complex in plants grown on MS without Suc than on plants grown on MS with Suc, in general xct-2 was epistatic to ein3-1 in aerial tissues but not in roots, as loss of XCT fails to suppress the ein3-1 phenotype in roots.
We next wanted to determine the nature of the genetic interaction between ein3-1 and xct-2 in plants grown in more natural conditions and at different life stages. Therefore, we examined the adult morphology of ein3-1 xct-2 double mutants and the respective controls in 5-week-old plants grown in soil in long days. As seen in Figure 5F, xct-2 single mutants were smaller than wild-type plants, and conversely, ein3-1 mutants were larger than wild-type plants. However, the ein3-1 xct-2 double mutants were indistinguishable from xct-2 single mutants. Thus, we found that xct-2 was epistatic to ein3-1, at least in aerial tissues, in adult plants as well as in seedlings. Taken together, these data indicate that XCT acts in the ethylene pathway downstream of the major transcription factor EIN3.
DISCUSSION
This study was prompted by the unexpected finding that xct-2 plants grown in blue light had a sugar-dependent phenotype, displaying long hypocotyls when grown in the absence of Suc but short hypocotyls when grown in its presence (Fig. 1, A and B). This sugar dependence is very reminiscent of the nutrient-dependent effects that ethylene has on blue light-grown plants, promoting hypocotyl elongation in plants grown in minimal medium (Smalle et al., 1997; Supplemental Fig. S4A) but inhibiting elongation in plants grown on rich medium (Collett et al., 2000; Figs. 3 and 5A). Since the regulation of hypocotyl elongation by ethylene is more pronounced in plants grown in blue light than in red light (Vandenbussche et al., 2007), we focused our attention on a potential role for XCT in the regulation of ethylene signaling in plants grown in blue light.
XCT Specifically Regulates Ethylene Responses in Aerial Tissues
Examining ethylene-responsive phenotypes in seedlings, we found that XCT normally represses ethylene responses in aerial but not root tissues (Fig. 5). This specificity is not unique, as mutations in many other genes affect either a subset of ethylene responses or cause light- or tissue-specific ethylene-related defects. Two genes, WEAK ETHYLENE INSENSITIVE2 (WEI2) and WEI7, which encode different subunits of a Trp biosynthesis enzyme, regulate ethylene responses specifically in the roots (Stepanova et al., 2005). The enhanced ethylene response1 (eer1) mutant, which may modulate the activity of CTR1, shows altered ethylene signaling specifically in the hypocotyl of etiolated seedlings (Larsen and Chang, 2001; Larsen and Cancel, 2003). eer2 mutants show plant-wide enhanced ethylene responses, but only when grown in the light (De Paepe et al., 2005).
The specificity of the ethylene response can also arise at the level of ERF activity. When plants overexpressing ERF1, a direct target of EIN3, are grown in the dark, they exhibit a classic constitutive ethylene response but do not form an exaggerated apical hook (Solano et al., 1998). This suggests that distinct molecular players downstream of EIN3 control different physiological aspects of ethylene action. Our own data indicate that XCT modulates ethylene responses in both a tissue- and light-specific manner. We have previously reported that etiolated xct mutants lack noticeable phenotypes (Martin-Tryon and Harmer, 2008), unlike many other ethylene signaling mutants that show obvious phenotypes as dark-grown seedlings (Guzmán and Ecker, 1990). We now demonstrate that XCT affects ethylene responses in shoots but not in roots (Figs. 1 and 5).
In addition, we found that XCT differentially affects ethylene responses within a single organ, the hypocotyl. Hypocotyl length in both the absence and presence of exogenous ACC is altered in xct-2 mutants relative to the wild type (Fig. 5A; Supplemental Fig. S4A). In contrast, hypocotyl diameter is normal in both untreated and ACC-treated xct-2 seedlings (Fig. 5B; Supplemental Fig. S4B). Therefore, xct-2 mutants appear to have specific alterations in ethylene signaling that affect the elongation but not the radial expansion of hypocotyls. Hypocotyl elongation may be primarily controlled by epidermal cells (Savaldi-Goldstein et al., 2007), whereas changes in hypocotyl diameter in response to ethylene may be primarily regulated by vascular and cortical cells (Sánchez-Bravo et al., 1992). This suggests that downstream components of the ethylene signaling pathway, such as the ERFs, may be differentially required in these cell types and that XCT likely affects only a subset of these signaling molecules.
Early characterization and cloning of ethylene mutants in Arabidopsis revealed a relatively linear pathway from ethylene perception to ethylene response (Chao et al., 1997). As more data have become available, it is now clear that the ethylene signaling pathway at all levels, ranging from biosynthesis to induction of far-downstream genes, incorporates multiple levels of regulation and specificity (Lin et al., 2009). As phytohormones affect every aspect of plant growth and development, it is not surprising that their signaling pathways must be tightly regulated in order to achieve the appropriate biological response. XCT may now be added to the list of regulators of ethylene signaling that act in a complex and tissue-specific manner.
XCT Is a Pleiotropic Protein with Separable Functions in the Circadian Clock and Ethylene Response
Although the hypocotyl phenotypes of xct mutants are sugar and light dependent (Fig. 1, A, B, E, and F), the short-period circadian phenotype in these plants is not altered by the growth medium or light quality (Fig. 1G; Martin-Tryon and Harmer, 2008). This strongly suggests that the hypocotyl phenotypes are not a consequence of altered clock function. It has been reported previously that wild-type plants grown in either the presence or absence of Suc had subtle but significant differences in the free-running period of leaf movement rhythms when assayed in constant light (Knight et al., 2008). We did not observe any significant differences in the free-running period of ProCCR2::LUC in wild-type plants grown either on Suc-rich or Suc-deficient medium when assayed under constant blue light (Fig. 1G). It is possible that we did not detect the subtle Suc-mediated changes in free-running period due to differences in growth medium, temperature, or light intensity. Alternatively, it may be that Suc affects the period of leaf movement rhythms but does not affect the period of rhythmic CCR2 expression.
Although there is a connection between ethylene signaling and the circadian clock, the roles for XCT in each pathway appear molecularly separable. Ethylene emission in Arabidopsis is regulated by the circadian clock (Thain et al., 2004). However, ethylene-insensitive mutants have normal circadian rhythms (Thain et al., 2004) and treatment of plants with ACC causes only minor changes in a subset of clock outputs (Hanano et al., 2006), indicating that ethylene emission is a clock output that does not feed back to affect the central clock. Mutation of clock genes may cause changes in both rhythms of ethylene emission and ethylene levels: ethylene emissions in the clock mutant cca1-ox are both arrhythmic and elevated, while in contrast, toc1-2 mutants emit ethylene with a short period but at normal levels (Thain et al., 2004). xct mutants have a short period similar to toc1 yet emit reduced levels of ethylene compared with the wild type (Fig. 2B). Our data, therefore, suggest that the mechanisms responsible for the ethylene and circadian defects in xct mutants are molecularly distinct, suggesting that XCT functions in more than one biological pathway.
Genetic data also support the idea that XCT has separable molecular functions. We have previously reported that xct-1, an ethyl methanesulfonate (EMS) allele of XCT, and xct-2, a putative null allele, have very similar circadian phenotypes. However, xct-2, but not xct-1, exhibits delayed greening (Martin-Tryon and Harmer, 2008), indicating that the clock and greening phenotypes are also separable. The xct-1 mutation is predicted to cause a loss of three amino acids from the XCT protein, suggesting that different regions of the XCT protein could have unique molecular functions.
XCT Function May Be Linked to Transcriptional Regulation
We have shown that ein3-1 phenotypes in aerial tissues require functional XCT (Fig. 5; Supplemental Fig. S4), effectively placing XCT downstream of EIN3 in the ethylene signaling pathway. An alternative explanation might be that XCT normally restricts the function of genes with analogous functions to EIN3, such as the EIL transcription factors. In total, the phenotypic data suggest that XCT, like CTR1, negatively regulates ethylene signaling but that, unlike CTR1, XCT acts downstream of EIN3 or related transcription factors. Thus, XCT may be thought of more specifically as a regulator of ethylene responses.
XCT may act in other signaling pathways as well: notably, ACC inhibits hypocotyl elongation in xct-2 ein3-1 double mutants grown on MS medium without Suc but does not have this effect in the wild type or the single mutants (Supplemental Fig. S4A). In contrast, ACC causes inhibition of hypocotyl elongation in all genotypes grown on MS plus Suc (Fig. 5A). This suggests that there may be functional connections between XCT, EIN3, and sugar signaling. Indeed, EIN3 protein stability is negatively regulated by sugar, and these signaling pathways are known to have cross talk (León and Sheen, 2003; Yanagisawa et al., 2003).
Given the genetic interactions between XCT and EIN3 and that they both encode nucleus-localized proteins, it is reasonable to assume that XCT acts within the nucleus. It is unlikely that XCT directly regulates the stability of EIN3. If this were the case, we would expect to see more global and drastic effects on ethylene signaling than are present in xct-2 single mutants. Indeed, loss-of-function mutants for EBF1 and EBF2, which directly negatively regulate the stability of EIN3, have severe constitutive ethylene activity phenotypes analogous to ctr1 mutants (Binder et al., 2007). Similarly, given that overexpression of EIL1 confers global constitutive ethylene responses (Chao et al., 1997), it is unlikely that XCT directly regulates EIL1 or similar proteins. However, XCT might affect the stability of a subset of the ERFs, thus indirectly altering transcriptional regulation. The cloning and characterization of eer5 has revealed an important role for the CONSTITUTIVE PHOTOMORPHOGENIC9 signalosome, which regulates protein stability, in the resetting and maintenance of the ethylene signaling pathway without altering EIN3 stability (Christians et al., 2008).
Another possible biochemical function of XCT could be as a transcriptional coregulator of EIN3-regulated genes, either by itself or as part of a larger complex. Additional transcriptional regulation of ethylene-responsive genes downstream of or in concert with EIN3 is not unprecedented. EER4, which encodes a TFIID-interacting transcription factor, is required for the accurate induction of ERF1 transcript and thus is important for the canonical ethylene response (Robles et al., 2007). We have shown that ethylene-regulated genes are misregulated in xct mutants (Fig. 4; Supplemental Fig. S3), although it is unknown whether this effect is direct or indirect.
CONCLUSION
In summary, we have shown that XCT functions in the ethylene signaling pathway downstream of EIN3, a major transcriptional regulator of ethylene responses. XCT and EIN3 are both nucleus-localized proteins. However, unlike EIN3, XCT is well conserved across eukaryotes, suggesting that it may act in processes essential for normal growth and development. Indeed, knockdown of the XAP5-like gene in Caenorhabditis elegans is embryo lethal (Piano et al., 2002), and the human homolog may be involved in disease states (Chiurazzi et al., 2001). Although nothing is currently known about the biological role of XCT-like proteins in other organisms, we now demonstrate that XCT functions genetically downstream of a transcription factor in a signaling pathway that impacts every stage of plant growth and development. In future work, it will be interesting to determine how XCT separately affects the function of at least two separate and distinct plant signaling networks: the circadian clock and ethylene signaling.
MATERIALS AND METHODS
Plant Growth Conditions for Hypocotyl and Root Growth Assays
Arabidopsis (Arabidopsis thaliana) seeds were surface sterilized by incubation in 70% ethanol for 5 min, followed by incubation in 100% ethanol for 10 min, and finally a thorough water wash of the seeds to eliminate residual ethanol. Sterile seeds were plated on 1× MS growth medium (RPI Research Products International), pH 5.7, containing 0.7% (w/v) agar (Sigma-Aldrich) supplemented with or without 3% (w/v) Suc (EMD Chemicals). Plates were wrapped in aluminum foil and transferred to 4°C for 3 to 4 d to cold stratify the seeds. Following cold stratification, plates were moved to white light (cool-white fluorescent bulbs, 55 μmol m−2 s−1) for 6 h to induce germination. Following a 6-h white light treatment, plates were wrapped with aluminum foil and kept in constant darkness for 18 h. At the end of the dark treatment, plates were transferred to their respective light quality conditions for 5 d of growth in constant monochromatic light. Hypocotyls were harvested on day 6 (the light/dark pulse is counted as day 1 of the experiment) and measured. Both monochromatic blue and red light were achieved using LED SnapLites (Quantum Devices) with a fluence rate of 3 μmol m−2 s−1 for blue light and 20 μmol m−2 s−1 for red light. All experiments were done at room temperature. Hypocotyls were harvested onto a transparency, scanned, and measured using the analysis software ImageJ (National Institutes of Health). All hypocotyl growth data presented are representative of at least three independent biological replicates. Error bars indicate se.
For the hypocotyl growth assays involving silver nitrate as the pharmacological ethylene signaling inhibitor, silver nitrate (Sigma Aldrich) was dissolved in water, filter sterilized, and added to a final concentration of 50 μm per MS plate. For ACC dose-response assays, ACC (Sigma-Aldrich) was dissolved in water, filter sterilized, and added to its respective final concentration on the MS plates. Hypocotyl growth and analysis in these assays are the same as described above.
Experiments for root growth assays were performed similarly to those for hypocotyl assays. Seeds were placed on square plates, and seedlings were allowed to grow vertically in constant blue light for 6 d. All experiments were performed at 22°C to 24°C.
Mutant Alleles and Genotyping
All Arabidopsis wild-type and mutant seeds used are of ecotype Columbia. The xct-2 mutants are SALK T-DNA insertion mutants and were genotyped as described previously (Martin-Tryon and Harmer, 2008). The hy5-215 allele is an EMS allele and was described previously (Oyama et al., 1997). The ein3-1 mutation was genotyped using the cleaved-amplified polymorphic sequence (CAPS) method to detect the single-nucleotide change in this allele as described previously (Binder et al., 2007). The eto1-1 mutants are previously described EMS mutants (Guzmán and Ecker, 1990) and were obtained through the Arabidopsis Biological Resource Center. We genotyped this allele using the derived CAPS method beginning with PCR of genomic DNA using the primers 5′-GCAACACAACTTGACCCTCTT-3′ and 5′-GGGAGAATCCCTCAGAAAGG-3′. The resulting PCR product was subjected to restriction digestion using TaqI. An induced mutation in the first primer creates a TaqI recognition site in the wild-type product, but this site is absent in the eto1-1 allele. The 162-bp ETO1 product from the wild-type background was cut by TaqI, resulting in fragments of 140 and 22 bp, whereas this enzyme did not cut the eto1-1 product. The ctr1-3 mutant has been described previously (Kieber et al., 1993). We genotyped this allele using the derived CAPS method using the following primers: 5′-AATTGATTTACCCTGTCGAA-3′ and 5′-GACTGGCTATCGGAGAAATA-3′. Following PCR of genomic DNA and digestion of product with the restriction enzyme NlaIII, the wild-type CTR1 produces a fragment of 402 bp, while the ctr1-3 product is cut by NlaIII, producing bands of 333 and 69 bp (Anandkumar Surendrarao and Caren Chang, personal communication). All double mutants were obtained via genetic crossing and identified by PCR screening of F2 progeny following F1 self-fertilization.
Ethylene Emission Measurements
Ethylene measurements were essentially performed as described by Thain et al. (2004), with modifications. A total of 200 to 400 seeds were surface sterilized and sown in a 10-mL chromatography vial containing 5 mL of one-half-strength MS (Duchefa) with 0% or 3% Suc (VWR) and 0.8% plant tissue culture agar (LabM). The vial was kept for 2 d at 4°C in darkness and subsequently exposed to white light for 6 h at 21°C to stimulate germination. Seedlings were allowed to grow for 3 to 4 d in 3 μmol m−2 s−1 blue light (470-nm Dragontape light-emitting diodes; Osram). The vials were capped, left in blue light for another 24 h, and subsequently flushed with hydrocarbon-free air (Air Liquide). Ethylene in the head space was detected with an ETD-300 photoacoustic ethylene detector (Sensor Sense). Three independent sets of biological material were used for calculating mean values. The experiments were done twice with highly similar results.
RNA Extraction and qRT-PCR
For gene expression analysis, seedling germination and growth conditions were as described for the blue light hypocotyl experiment. Seedlings were grown in 3 μmol m−2 s−1 blue light either on MS or MS + 3% Suc plates or on these plates supplemented with 20 μm ACC. On day 5 of growth, approximately 30 to 40 seedlings of each genotype were harvested together during subjective afternoon. Total RNA was isolated from 5-d-old seedlings grown in monochromatic blue light using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol and cleaned by sodium acetate/ethanol precipitation. A quantity of 500 ng to 1 μg of total RNA from each sample was used in first-strand cDNA synthesis using an oligo(dT)18 primer and SuperScriptII (Invitrogen) reverse transcriptase per the manufacturer’s instructions. The cDNA was diluted 1:5, and 2 μL of this working cDNA was used as a template in a 20-μL qRT-PCR reaction. The reagents and their concentrations used in the qRT-PCR master mix are as described previously (Martin-Tryon and Harmer, 2008). Each cDNA sample was run in triplicate using an iCycler iQ (Bio-Rad), and the data were analyzed with iCycler iQ Optical Systems Software version 3.1 (Bio-Rad). Relative expression values for experimental genes are presented normalized to PP2A expression level. Melt curve analysis was done following product amplification to confirm that cDNA product was specifically and solely amplified. Error bars indicate the sd of relative expression level calculated using the standard curve method (ABI Prism). Expression data presented are representative of at least three independent biological replicates.
The sequences of the PP2A primers are as described previously (Martin-Tryon and Harmer, 2008). For ACO2, we designed and used 5′-CTCCTCTCAACCACTCTATTGTCATC-3′ and 5′-GGCTCCTTGGGCTGAAACTTG-3′. For ETR2, we designed and used 5′-CGGCGGCTATGGGTTAGG-3′ and 5′-GAGCGTGGTGGTCAGGAG-3′.
Circadian Period Assay and Quantification
Arabidopsis seeds were sterilized and plated as described for hypocotyl growth assays. Following cold stratification, plates were released into 12-h/12-h white light/dark cycles (cool-white fluorescent bulbs, 55 μmol m−2 s−1) for 7 d at constant 22°C. After 7 d in light/dark cycles, plants were sprayed with 3 mm d-luciferin (Biosynth) and then sent into an ORCA II ER CCD camera (Hamamatsu) for imaging of luciferase bioluminescence at constant 20 μmol m−2 s−1 blue light (LED SnapLites; Quantum Devices). All seeds for this assay harbor a ProCCR2::LUC reporter construct, allowing the CCD camera to monitor the real-time expression of luciferase under the control of the CCR2 promoter in each genetic background analyzed. After 5 d of luciferase imaging in constant blue light, images were analyzed using the MetaMorph software (Molecular Devices) to produce quantitative luciferase bioluminescence data. These bioluminescence data over time were then fit to a cosine wave using the Fourier Fast Transform-Nonlinear Least Squares program (Plautz et al., 1997), allowing for estimations of various circadian parameters, including free-running period.
Seedling Imaging
Six-day-old blue light-grown seedlings were imaged using a Zeiss Stemi SV 11 dissecting microscope. Images were taken with the use of a QImaging camera and processed using the software QCapture Pro.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Analysis of hy5-215 xct-2 double mutant growth patterns on various medium conditions.
Supplemental Figure S2. Hypocotyl response to silver nitrate when grown on MS medium.
Supplemental Figure S3. Ethylene-regulated gene expression in plants grown on MS medium.
Supplemental Figure S4. Analysis of ein3-1 xct-2 double mutant growth patterns on MS medium.
Supplementary Material
Acknowledgments
We are thankful to Anandkumar Surendrarao and Mariana Pereira for providing seeds and reagents used for some of the ethylene-related experiments. We are also thankful to Mark Belmonte and Ryan Kirkbride for technical assistance with seedling imaging as well as Jose Jimenez-Gomez for technical assistance with figure preparation. Anne Britt and Clark Lagarias provided intriguing and critical discussion of the experiments and their implications.
References
- Binder BM, Walker JM, Gagne JM, Emborg TJ, Hemmann G, Bleecker AB, Vierstra RD. (2007) The Arabidopsis EIN3 binding F-box proteins EBF1 and EBF2 have distinct but overlapping roles in ethylene signaling. Plant Cell 19: 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae HS, Faure F, Kieber JJ. (2003) The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell 15: 545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae HS, Kieber JJ. (2005) Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci 10: 291–296 [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Chen YF, Shakeel SN, Bowers J, Zhao XC, Etheridge N, Schaller GE. (2007) Ligand-induced degradation of the ethylene receptor ETR2 through a proteasome-dependent pathway in Arabidopsis. J Biol Chem 282: 24752–24758 [DOI] [PubMed] [Google Scholar]
- Chiurazzi P, Hamel BC, Neri G. (2001) XLMR genes: update 2000. Eur J Hum Genet 9: 71–81 [DOI] [PubMed] [Google Scholar]
- Christians MJ, Robles LM, Zeller SM, Larsen PB. (2008) The eer5 mutation, which affects a novel proteasome-related subunit, indicates a prominent role for the COP9 signalosome in resetting the ethylene-signaling pathway in Arabidopsis. Plant J 55: 467–477 [DOI] [PubMed] [Google Scholar]
- Collett CE, Harberd NP, Leyser O. (2000) Hormonal interactions in the control of Arabidopsis hypocotyl elongation. Plant Physiol 124: 553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington MF, Harmer SL. (2007) The circadian clock regulates auxin signaling and responses in Arabidopsis. PLoS Biol 5: e222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington MF, Maloof JN, Straume M, Kay SA, Harmer SL. (2008) Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biol 9: R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paepe A, De Grauwe L, Bertrand S, Smalle J, Van Der Straeten D. (2005) The Arabidopsis mutant eer2 has enhanced ethylene responses in the light. J Exp Bot 56: 2409–2420 [DOI] [PubMed] [Google Scholar]
- De Paepe A, Van Der Straeten D. (2005) Ethylene biosynthesis and signaling: an overview. Vitam Horm 72: 399–430 [DOI] [PubMed] [Google Scholar]
- Guo H, Ecker JR. (2004) The ethylene signaling pathway: new insights. Curr Opin Plant Biol 7: 40–49 [DOI] [PubMed] [Google Scholar]
- Gutterson N, Reuber TL. (2004) Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr Opin Plant Biol 7: 465–471 [DOI] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanano S, Domagalska MA, Nagy F, Davis SJ. (2006) Multiple phytohormones influence distinct parameters of the plant circadian clock. Genes Cells 11: 1381–1392 [DOI] [PubMed] [Google Scholar]
- Holm M, Ma LG, Qu LJ, Deng XW. (2002) Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev 16: 1247–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Lau OS, Deng XW. (2007) Light-regulated transcriptional networks in higher plants. Nat Rev Genet 8: 217–230 [DOI] [PubMed] [Google Scholar]
- Jiménez-Gómez JM, Maloof JN. (2009) Plant research accelerates along the (bio)informatics superhighway: symposium on plant sensing, response and adaptation to the environment. EMBO Rep 10: 568–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Shen Y, Marion CM, Tsuchisaka A, Theologis A, Schäfer E, Quail PH. (2007) The basic helix-loop-helix transcription factor PIF5 acts on ethylene biosynthesis and phytochrome signaling by distinct mechanisms. Plant Cell 19: 3915–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ, Ecker JR. (1993) Ethylene gas: it’s not just for ripening any more! Trends Genet 9: 356–362 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Knight H, Thomson AJ, McWatters HG. (2008) Sensitive to freezing6 integrates cellular and environmental inputs to the plant circadian clock. Plant Physiol 148: 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PB, Cancel JD. (2003) Enhanced ethylene responsiveness in the Arabidopsis eer1 mutant results from a loss-of-function mutation in the protein phosphatase 2A A regulatory subunit, RCN1. Plant J 34: 709–718 [DOI] [PubMed] [Google Scholar]
- Larsen PB, Chang C. (2001) The Arabidopsis eer1 mutant has enhanced ethylene responses in the hypocotyl and stem. Plant Physiol 125: 1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Deng XW, Kim WT. (2006) Possible role of light in the maintenance of EIN3/EIL1 stability in Arabidopsis seedlings. Biochem Biophys Res Commun 350: 484–491 [DOI] [PubMed] [Google Scholar]
- Legnaioli T, Cuevas J, Mas P. (2009) TOC1 functions as a molecular switch connecting the circadian clock with plant responses to drought. EMBO J 28: 3745–3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León P, Sheen J. (2003) Sugar and hormone connections. Trends Plant Sci 8: 110–116 [DOI] [PubMed] [Google Scholar]
- Lin Z, Zhong S, Grierson D. (2009) Recent advances in ethylene research. J Exp Bot 60: 3311–3336 [DOI] [PubMed] [Google Scholar]
- Martin-Tryon EL, Harmer SL. (2008) XAP5 CIRCADIAN TIMEKEEPER coordinates light signals for proper timing of photomorphogenesis and the circadian clock in Arabidopsis. Plant Cell 20: 1244–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael TP, Breton G, Hazen SP, Priest H, Mockler TC, Kay SA, Chory J. (2008) A morning-specific phytohormone gene expression program underlying rhythmic plant growth. PLoS Biol 6: e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery BL, Lagarias JC. (2002) Phytochrome ancestry: sensors of bilins and light. Trends Plant Sci 7: 357–366 [DOI] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H. (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140: 411–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL, Maloof JN. (2007) Rhythmic growth explained by coincidence between internal and external cues. Nature 448: 358–361 [DOI] [PubMed] [Google Scholar]
- Nozue K, Maloof JN. (2006) Diurnal regulation of plant growth. Plant Cell Environ 29: 396–408 [DOI] [PubMed] [Google Scholar]
- Oyama T, Shimura Y, Okada K. (1997) The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev 11: 2983–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano F, Schetter AJ, Morton DG, Gunsalus KC, Reinke V, Kim SK, Kemphues KJ. (2002) Gene clustering based on RNAi phenotypes of ovary-enriched genes in C. elegans. Curr Biol 12: 1959–1964 [DOI] [PubMed] [Google Scholar]
- Pierik R, Tholen D, Poorter H, Visser EJ, Voesenek LA. (2006) The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci 11: 176–183 [DOI] [PubMed] [Google Scholar]
- Plautz JD, Straume M, Stanewsky R, Jamison CF, Brandes C, Dowse HB, Hall JC, Kay SA. (1997) Quantitative analysis of Drosophila period gene transcription in living animals. J Biol Rhythms 12: 204–217 [DOI] [PubMed] [Google Scholar]
- Rawat R, Schwartz J, Jones MA, Sairanen I, Cheng Y, Andersson CR, Zhao Y, Ljung K, Harmer SL. (2009) REVEILLE1, a Myb-like transcription factor, integrates the circadian clock and auxin pathways. Proc Natl Acad Sci USA 106: 16883–16888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles LM, Wampole JS, Christians MJ, Larsen PB. (2007) Arabidopsis enhanced ethylene response 4 encodes an EIN3-interacting TFIID transcription factor required for proper ethylene response, including ERF1 induction. J Exp Bot 58: 2627–2639 [DOI] [PubMed] [Google Scholar]
- Sánchez-Bravo J, Ortuño AM, Pérez-Gilabert M, Acosta M, Sabater F. (1992) Modification by ethylene of the cell growth pattern in different tissues of etiolated lupine hypocotyls. Plant Physiol 98: 1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaldi-Goldstein S, Peto C, Chory J. (2007) The epidermis both drives and restricts plant shoot growth. Nature 446: 199–202 [DOI] [PubMed] [Google Scholar]
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Van Der Straeten D. (1997) Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA 94: 2756–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM. (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17: 2230–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thain SC, Vandenbussche F, Laarhoven LJ, Dowson-Day MJ, Wang ZY, Tobin EM, Harren FJ, Millar AJ, Van Der Straeten D. (2004) Circadian rhythms of ethylene emission in Arabidopsis. Plant Physiol 136: 3751–3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchisaka A, Yu G, Jin H, Alonso JM, Ecker JR, Zhang X, Gao S, Theologis A. (2009) A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics 183: 979–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Vancompernolle B, Rieu I, Ahmad M, Phillips A, Moritz T, Hedden P, Van Der Straeten D. (2007) Ethylene-induced Arabidopsis hypocotyl elongation is dependent on but not mediated by gibberellins. J Exp Bot 58: 4269–4281 [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Verbelen JP, Van Der Straeten D. (2005) Of light and length: regulation of hypocotyl growth in Arabidopsis. Bioessays 27: 275–284 [DOI] [PubMed] [Google Scholar]
- Yanagisawa S, Yoo SD, Sheen J. (2003) Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425: 521–525 [DOI] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Tena G, Xiong Y, Sheen J. (2008) Dual control of nuclear EIN3 by bifurcate MAPK cascades in C2H4 signalling. Nature 451: 789–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.