Abstract
We assessed the safety, tolerability, and immunogenicity of a mixture of three synthetic peptides derived from the Plasmodium vivax circumsporozoite protein formulated in Montanide ISA 720 or Montanide ISA 51. Forty healthy malaria-naive volunteers were allocated to five experimental groups (A–E): four groups (A–D) were immunized intramuscularly with 50 and 100 μg/dose injections of a mixture of N, R, and C peptides formulated in the two different adjuvants at 0, 2, and 4 months and one group was administered placebo. Vaccines were immunogenic, safe, well tolerated, and no serious adverse events related to the vaccine occurred. Seroconversion occurred in > 90% of the vaccines and antibodies recognized the sporozoite protein on immunofluorescent antibody test. Vaccines in Montanide ISA 51 showed a higher sporozoite protein recognition and interferon production. Results encourage further testing of the vaccine protective efficacy.
Introduction
A vaccine against pre-erythrocytic stages of malarial infection would be an ideal weapon to avoid clinical manifestations of disease because it would block the infection during the initial asymptomatic phase. Indeed, it has been extensively proven that animals1,2 and humans3,4 vaccinated with irradiated sporozoites become immune to experimental infections induced by sporozoites, and do not develop patent blood-stage infections or clinical malaria symptoms. Sera and cells from these individuals recognize proteins expressed on the sporozoites and the parasite liver forms5–9 that have been incriminated in this protection and therefore have been proposed as malaria vaccine candidates.10,11 Among them, the circumsporozoite (CS) protein that is abundantly expressed on the sporozoite surface has been shown to be involved in the process of parasite invasion to the hepatocyte12,13 and its immunological blockage prevents the development of malaria infection.6,7,14 The RTS-S vaccine based on a construct of the Plasmodium falciparum CS protein and the S antigen of human hepatitis B virus has proven to be immunogenic and partially protective in phase II studies conducted with human malaria-naive volunteers15–18 and in adults and children from malaria-endemic areas of Africa.19–22
Regarding the Plasmodium vivax CS protein, three long synthetic peptides (LSP) homologous to the amino (N), central repeat (R), and carboxyl (C) regions were initially evaluated in preclinical studies and showed high immunogenicity in mice and Aotus monkeys.23,24 On the basis of those studies the same LSPs were formulated in Montanide ISA 720 and assessed in phase Ia clinical trial conducted by the Malaria Vaccine and Drug Development Center (MVDC) in Cali, Colombia. Immunization with this formulation indicated to be safe, well tolerated, and immunogenic.25 All three peptides induced production of high titers of specific antibodies that cross-reacted with the protein on the parasite and production of interferon-gamma (IFN-γ) in most vaccinated subjects. Although the N peptide induced the highest antibody titers at three different doses tested (10, 30, and 100 μg/dose), peptides R and C were also immunogenic at high doses. In the search for an optimal vaccine formulation for human use we have conducted pre-clinical studies in mice, monkeys, and clinical trials in malaria-naive volunteers, and we performed a new series of studies to assess the safety and immunogenicity of a combination of the three peptides formulated either in Montanide ISA 720 or in Montanide ISA 51. The rationale for these mixtures was to determine the possibility of immunological interference among the different peptides or their potential synergism. These adjuvants were selected because they form stable water-in-oil emulsions and induced high antibody levels that lasted for up to 1 year in mice, rabbits, and monkeys in previous studies using recombinant malaria proteins.23,26–32 More recently, a recombinant P. vivax CS protein produced in Escherichia coli and formulated in Montanide ISA 720 showed to be highly immunogenic in mice.33 Several phase I clinical trials have been conducted using different malaria vaccine antigens in which these two adjuvants have been able to stimulate both humoral and cellular immune responses.34–37
We present here a phase I clinical trial conducted with the same P. vivax CS derived peptides formulated in two different adjuvants, and provide further safety and immunogenicity data as part of a clinical development plan that aims at developing vaccines to prevent malaria.
Materials and Methods
Study design and population.
This was a phase I double-blind, controlled vaccine trial, evaluating safety, tolerability, and immunogenicity of mixtures of N, R and C LSP derived from the P. vivax CS protein formulated in two adjuvants Montanide ISA 720 and Montanide ISA 51. The primary objective was to assess in malaria-naive adults, the safety and reactogenicity of these peptides formulated in the two adjuvants. Study protocol was approved by the Institutional Review Boards (IRB) of the Universidad del Valle and Centro Médico Imbanaco (CMI), and the study complied with Declaration of Helsinki principles, International Conference on Harmonization, Good Clinical Practices guidelines, and all pertinent Colombian regulations. We recruited 40 healthy men and women volunteers from Cali, Colombia, a city non-endemic for malaria. Volunteers were 19–41 years of age and had no history of malaria. During a period of 3 months a total of 100 volunteers were assessed for eligibility criteria to select a total of 40 volunteers willing to participate in the clinical trial. By consecutive allocation, eight participants were allocated to each of the five experimental groups (A–E): four groups (A–D) were immunized with the vaccine formulations at two different dose concentrations and formulated in two different adjuvants. A control group (E) was injected with placebo (saline solution) (Table 1). Volunteers and physicians responsible for immunization were kept blind using codes for volunteers and vaccine groups. Vaccines were prepared by individuals not involved with patient care. Syringes containing either vaccine or placebo were covered by opaque adhesive tape, precluding visualization of the contents by the study participants and clinical researchers.
Table 1.
Volunteer recruitment and immunization schedule
| Recruitment process | Volunteers asked to participate | Refused to participate | Not meet inclusion criteria | Reserved if needed | Volunteers briefed |
|---|---|---|---|---|---|
| Number of volunteers (n) | 100 | 51 | 7 | 2 | 40 |
| Montanide adjuvant | Immunogen | Consecutive allocation | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | ||
| ISA 51 | ISA 720 | ISA 51 | ISA 720 | Placebo | ||
| 1st dose | N + C | 100* (N = 8) | 100 (N = 8) | 200 (N = 8) | 200 (N = 8) | SS† (N = 8) |
| 2nd dose | N + R + C | 150 (N = 8) | 150 (N = 7) | 300 (N = 7) | 300 (N = 8) | SS (N = 8) |
| 3rd dose | N + R + C | 150 (N = 8) | 150 (N = 7) | 300 (N = 6) | 300 (N = 8) | SS (N = 8) |
μg/dose.
Saline solution.
All participants provided written informed consent and were notified that they were free to withdraw from the study at any time. Volunteers were excluded if they had diseases or medical conditions that would alter assessment of the vaccine, including infection by human immunodeficiency virus, hepatitis B, or hepatitis C; cardiac, hepatic, renal, or autoimmune disease; anemia, allergies, pregnancy, immunodeficiencies, existing antibodies to the P. vivax CS protein, history of splenectomy or any other condition that could increase the risk of an adverse outcome.
Intervention.
To optimize the vaccine dose, eligible participants were enrolled to receive three doses of vaccine containing peptide mixtures at a dose of 50 or 100 μg of each individual peptide, for a final dose of 150 or 300 μg, respectively, in a volume of 0.5 mL. The previous clinical trial had indicated that doses between 30 and 100 μg produced better responses than lower doses. The first immunization dose (given at Month 0) contained the peptides N and C only, whereas the two boosting doses (given at Months 2 and 4) contained all three (N, R, and C) peptides (Table 1). Vaccination was performed by intramuscular injection in the deltoid muscle, alternating arms with each injection. For safety reasons, participants assigned to the low vaccine dose groups were immunized first and only 2 weeks after initiation when no serious adverse events (SAE) had occurred, immunization of participants in the high dose was started. Half of the participants assigned to receive placebo were immunized along with each dose level group. Clinical monitors and the institutional review boards (IRBs) of the Universidad del Valle and IMC, evaluated the occurrence and severity of adverse events (AE) associated with immunization. The occurrence of more than three AE (severity grade 2 or higher) or one SAE related to the vaccine would have led to study termination. Participants who left the study were not replaced.
Vaccines.
The N polypeptide comprises amino acids 20–96 of CS protein and constitutes a 77-mer peptide, the R peptide is a hybrid 48-mer peptide, type I or common sequence (VK210) contains repeats of the GDRADGQPA motif, and the 72-mer C peptide is composed of amino-acid residues 301–372 of the CS protein. We synthesized the peptides under good laboratory practices (GLP) conditions at the Biochemistry Institute, University of Lausanne, Switzerland, and using solid-phase fluorenylmethoxycarbonyl (F-moc) chemistry.38 The C-terminal peptide containing 4-Cys was oxidized according to Verdini and others.39 Mass and purity of the peptides were assessed by high performance liquid chromatography and mass spectrometry and was higher than 90%.25 Peptides were lyophilized, packaged, and both sterility and pyrogenicity were tested. We mixed peptides N, R, and C in concentrations of 50 and 100 µg for each peptide for a final concentration of 150 or 300 µg/dose, and then 24 hrs before each immunization, the mixture was emulsified in either of the two adjuvants: Montanide ISA 51 or Montanide ISA 720 (Seppic Inc., Paris, France) and stored at 4°C according to manufacturer recommendations. Vaccine stability at this temperature is long lasting. Saline solution (Baxter, Deerfield, IL) was emulsified with the same adjuvants and used as placebo. Both vaccine and placebo were emulsified as described earlier.23 An independent monitor assessed peptide integrity at the beginning and the end of the study.
Safety.
The primary endpoints were the occurrence of solicited signs and symptoms during an 8-day period after each vaccination. Volunteers were encouraged to maintain contact with the research team by phone and whenever needed personally, at any time of the day, to inform the occurrence of any symptom during the 1-year study period. Volunteers were observed for 60 minutes after injection and evaluated by the study team at 8 and 24 hrs after injection and again on Day 7 after vaccination. Safety was assessed by a complete physical examination and clinical laboratory tests on all study participants during monthly visits up to Month 5 post immunization and followed up by phone between months 5 and 12. Blood samples were collected for complete blood count, sedimentation rate, prothrombin time, partial thromboplastin time, blood urea nitrogen, and serum glucose, total protein, albumin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and bilirubin. Immediately before each vaccination, we tested female volunteers for pregnancy using a beta human chorionic gonadotropin test. Solicited symptoms were grouped by local and systemic. Local symptoms included pain, swelling, and erythema, whereas systemic symptoms were fever, flu-like symptoms, headache, dizziness, nausea, emesis, and abdominal pain. Signs and symptoms were graded from mild to serious with a four level score. The AE were recorded throughout the study and were defined as any new or worsening sign or symptom of illness or an abnormal laboratory test during the protocol specified follow-up. Each AE was evaluated by the study clinician for its severity,40 and relatedness to vaccination and SAE were reported as required by the World Health Organization (WHO).41 The data safety monitoring board was constituted by an external clinical monitor and an internal clinical monitor who met three times during the study.
Measurement of humoral responses.
The immunological assays were performed using samples collected during the monthly visits. Antibody response was measured by enzyme-linked immunosorbent assay (ELISA) using as antigen the N, R, or C peptides (1 µg/mL), as described previously.42 The final reaction was read at 405 nm in a microplate reader (MRX; Dynex Technologies, Inc., Chantilly, VA). Cut-off points were calculated as three SD above the mean absorbance value at 405 nm of sera from healthy volunteers who had never been exposed to malaria. Controls were selected from a pool of sera from semi-immune blood donors (positive controls) and a pool of sera from malaria-naive donors (negative controls). Peptide-specific IgG isotypes were determined by modified ELISA using sera collected at Months 0 and 5 from immunized volunteers. Briefly, 1 µg/mL of peptide was used as antigen to coat microplates. Wells were blocked with phosphate buffer saline (PBS)-Tween 20 plus fat free milk and then incubated with the serum sample dilutions for 1 hr. Plates were washed with PBS-Tween 20 and then incubated with mouse anti-human IgG isotype antisera. The ELISA titers of < 1:200 were considered negative.
Anti-IgG1, IgG3, and IgG4 (Skibio, Bedfordshire, England) and anti-IgG2 (Sigma, St. Louis, MO) isotypes were used and a peroxidase conjugated goat anti-mouse IgG antibody (Kirkegaard and Perry Laboratories, Gaithersburg, MD) was added and the reaction read at 630 nm (OD630). Data were reported as ratio (OD mean of the sample, positive or negative controls divided by the cut-off point).
Parasite recognition by anti-peptide antibodies was determined by indirect immunofluorescent antibody test (IFAT) at 0, 3, and 5 months after enrollment using P. vivax sporozoites produced in Anopheles albimanus mosquitoes. Antibody titers were determined as the reciprocal of the end-point dilution that showed positive fluorescence as described previously.25 In addition, 50 sera from individuals naturally exposed to malaria were also evaluated for the presence of anti-P. vivax sporozoite and comparison with antibodies induced by vaccinations.
Enzyme-linked immunosorbent spot (ELIspot) assay for single cell release of IFN-γ.
Peripheral blood mononuclear cells (PBMC) collected after last immunization (Month 5) were separated from whole blood using Ficoll Histopaque (Sigma-Aldrich, St. Louis, MO) density gradients, and were resuspended in RPMI medium 1640 (Gibco, Grand Island, NY). The IFN-γ-producing cells were identified using a commercial IFN-γ ELIspot human kit (Mabtech AB, Stockholm, Sweden). Fresh PBMCs (4 × 105/well were then mixed with 10 μg/mL of each synthetic peptide and plates were incubated for 40 hrs at 37°C in an atmosphere of 5% CO2.25
Spots were counted with a spot counting system (Scanalytics, Fairfax, VA), and the results were expressed as the mean number of IFN-γ spot-forming cells (sfc) per 106 PBMC. Volunteers were considered responders if the number of sfc in their samples had increased from their own baseline level (before immunization on Day 0), any increase ≥ 5 sfc were considered positive.25
Statistical methods.
The main outcomes we evaluated were AE and SAE related to vaccination during the study period. An AE was considered related if it was determined to be possibly, probably, or most probably related to vaccination. Rates of related and other common AE were compared among experimental groups using Fisher's exact tests. Main comparisons of interest were between adjuvants and between vaccine doses. Antibody titers and IFN-γ production were compared among peptides, vaccine doses, and adjuvants at several points in the studies using Wilcoxon signed-rank and Kruskal-Wallis tests. Two-tailed, non-parametric P values ≤ 0.05 were considered significant.
Results
Study population.
A total of 100 people were asked to participate; of these 49 consented to screening. Finally, 40 participants willing to continue in the study (18 males and 22 females) were consecutively allocated to one of the five treatment groups, as shown in Table 1. Allocation started in the low-dose groups and once they were completed, and no SAEs were reported in response to the first vaccination dose, the study continued with the high dose. Groups were comparable in baseline demography, and clinical laboratory values did not show statistically significant differences (data not shown). One volunteer received a dose of tetanus vaccine 1 week after the first immunization and another one was immunized against rubella and smallpox at Day 68 of the study. These events were evaluated by the clinical monitor and the IRBs, and they both agreed that these two volunteers could continue in the study. Two other participants abandoned the study voluntarily, both after the first immunization, and a third participant abandoned after the second immunization.
Safety.
All three immunization doses were well tolerated by all participants from the low and high vaccine doses. Low grade pain (mild or moderate) at the injection site up to 48 hrs after injection was the most frequent AE related to immunization, followed by local swelling and local erythema (Table 2). We found no statistically significant differences of AEs between doses. When comparing between adjuvants, pain at injection site was the only difference significantly higher at the third dose of the injection with Montanide ISA 720. No significant differences were observed in the occurrence of the remainder AEs. When both adjuvants were taken together and compared with placebo, local pain was more frequent in the malaria immunized group in the first and third injections (P = 0.03). Although local swelling seemed to be more frequent in the immunized group compared with placebo, the difference did not reach statistical significance. Headache was the most common systemic AE with both adjuvants, but again its difference with the placebo group did not reach statistical significance.
Table 2.
Number of volunteers reporting vaccine-related adverse events per dose and adjuvant group
| Adverse event | No. doses | Montanide* | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ISA 51 | ISA 720 | |||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | ||
| n = | (16) | (15) | (15) | (16) | (15) | (14) | (N = 8) | |||
| Local | ||||||||||
| Injection site pain | 14 | 12 | 9 | 14 | 13 | 12† | 4 | 8 | 3 | |
| Swelling | 5 | 2 | 2 | 11 | 6 | 8 | 4 | 1 | 2 | |
| Erythema | 1 | 0 | 1 | 1 | 0 | 2 | 1 | 0 | 1 | |
| Systemic | ||||||||||
| Fever (> 37.5°C) | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 1 | 1 | |
| Headache | 3 | 2 | 1 | 5 | 4 | 0 | 2 | 2 | 0 | |
| Dizziness | 1 | 1 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | |
| Nausea/Emesis | 1 | 2 | 0 | 2 | 0 | 0 | 2 | 1 | 0 | |
| Abdominal pain | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | |
| Adenopathy (Axilar/cervical) | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
Montanide at 150 μg or 300 μg dose.
Two-tailed P values < 0.05 by Fisher exact test, for comparison among adverse events (AE) in adjuvant and placebo groups.
None of the participants experienced SAE related to the vaccine, whereas two volunteers showed SAE that were considered not related to immunization. In the placebo group, 7 days after first immunization one volunteer was diagnosed with chronic cholecystitis and another in the high dose group formulated in Montanide ISA 51 presented an acute episode of urolithiasis on Day 82 of the study. All laboratory tests for the remaining participants yielded normal values during the study period.
Humoral response.
Figure 1 shows antibody responses to each peptide. After the first immunization with mixtures containing the N and C peptides, 74% of participants seroconverted for peptide N, but none for peptide C. With the second immunization, which was performed with the mixture of the three LSP, 100% of the volunteers became positive to N peptide and 71% of volunteers seroconverted to C peptide. Few of the volunteers seroconverted to R peptide after this second immunization that corresponded to a priming dose for R, but 93% of the volunteers responded to this peptide after first boosting (third immunization). Antibody titers to R peptide reached comparable levels as those obtained with the three doses of C peptide in most study groups, except for the group immunized with the 150 μg/dose in Montanide ISA 51 adjuvant, which showed titers four times higher to C peptide than to R peptide. In general, 96% of volunteers produced specific IgG antibodies to all peptides after the third immunization with the three peptide mixtures. Volunteers responded earlier to N peptide, and all volunteers required only two immunizations to seroconvert. The N peptide also elicited the highest median titer (1:9,600) when administered at 300 μg dose in Montanide ISA 720 adjuvant.
Figure 1.

Enzyme-linked immunosorbent assay (ELISA) anti-peptide IgG titers upon vaccination with a mixture of N, R, and C peptides. Panels show responses to mixtures using 150 μg total peptide dose in (Row A) Montanide ISA 720 or (Row B) Montanide ISA 51 and to mixtures using 300 μg total peptide dose in (Row C) Montanide ISA 720 or (Row D) Montanide ISA 51. Immunizations were made in the 0, 2, and 4 months.
The response in low-dose groups seemed to be more rapid, but chance effect could not be securely discarded (100% versus 77.8%, P = 0.1). Antibody profiles in high-dose groups were very similar to those of the low-dose groups, and after the third immunization, all volunteers responded to all peptides. Comparison of antibody responses by dose showed no statistically significant differences (P = 0.16) or did comparison by adjuvant (P = 0.77), however, total antibody response to the N peptide differed from that of C and R peptides (P < 0.01). At 150-μg vaccine-dose, the antibody response was higher in Montanide ISA 51 than in Montanide ISA 720 group. With the 300 μg/dose all volunteers responded regardless of adjuvant.
The specific anti-peptide IgG isotypes response determined at Day 150, after the third immunization is presented in Table 3 related to peptide, adjuvant, and vaccine dose. The N peptide showed a stronger response for all isotypes than the other peptides, although it was more evident for IgG3, in contrast C peptide showed a more specific increase of IgG1. All four IgG isotypes specific for R peptide presented similar low titers in all groups. Although there was a difference among adjuvants, showing higher median IgG1 titers for this peptide in the Montanide ISA 51 group, this did not reach statistical significance, and there was no difference by peptide dose either. Concordant with all this, when the ratio of IgG1 plus IgG3 versus IgG2 plus IgG4 was evaluated, C peptide showed a higher ratio, followed by N peptide, but not a significant difference was observed between these two peptides (Figure 2).
Table 3.
Isotype response to Plasmodium vivax CS protein
| Isotype | IgG1 | IgG2 | IgG3 | IgG4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR* | P† | Median | IQR | P | Median | IQR | P | Median | IQR | P | ||||||
| Peptide | N | 10.6 | 10.4 | 10.9 | 6.0 | 2.0 | 7.5 | 25.1 | 24.2 | 25.6 | 12.0 | 6.7 | 15.1 | ||||
| R | 0.9 | 0.6 | 1.4 | < 0.001 | 0.7 | 0.5 | 0.9 | < 0.001 | 0.9 | 0.8 | 1.5 | < 0.001 | 0.9 | 0.7 | 1.0 | < 0.001 | |
| C | 10.1 | 6.7 | 17.7 | 0.8 | 0.7 | 1.1 | 2.0 | 1.4 | 3.3 | 1.2 | 1.1 | 1.5 | |||||
| Adjuvant Motanide | ISA 51 | 10.3 | 1.8 | 10.9 | 0.06 | 1.0 | 0.8 | 2.6 | 0.53 | 2.6 | 1.4 | 24.6 | 0.18 | 1.4 | 1 | 7.8 | 0.13 |
| ISA 720 | 6.2 | 0.9 | 10.7 | 0.9 | 0.7 | 5.2 | 2.0 | 0.9 | 22.8 | 1.1 | 0.9 | 2.3 | |||||
| Doses μg | 150 | 8.7 | 0.9 | 10.9 | 0.70 | 0.9 | 0.7 | 3.4 | 0.79 | 2.5 | 0.9 | 22.8 | 0.75 | 1.2 | 1.0 | 3.8 | 0.76 |
| 300 | 10.1 | 2.4 | 10.9 | 0.9 | 0.7 | 2.6 | 1.9 | 1.1 | 24.6 | 1.3 | 0.9 | 11.0 | |||||
IQR = interquartile range.
Two-tailed, Kruskal-Wallis non-parametric P value.
Figure 2.
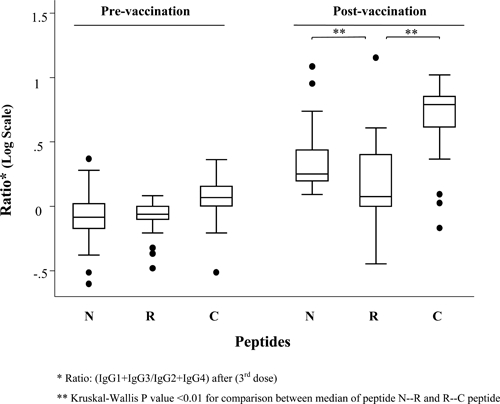
Ratio of immunoglobulin isotypes by time and peptide.
Sporozoite recognition.
Forty-three percent of the participants were (13/30) IFAT positive (titers ≥ 1:20) after the second immunization and 73% (21/29) after the third dose, without taking into account the type of adjuvant used. When responses to both adjuvants were compared after the third dose, 93% of participants receiving Montanide ISA 51 were IFAT positive as compared with 56% of those receiving Montanide ISA 720 (Table 4). Similarly, Montanide ISA 51 adjuvant resulted in higher sporozoite recognition antibody titers than Montanide ISA 720, independently of the vaccine dose (P = 0.01; two-tailed Kruskal-Wallis non-parametric P value, data not shown). In general, the two-dose immunization schedule using 150 μg/dose resulted in a lower level of anti-sporozoite antibodies when compared with titers after the third dose (P = 0.02). However, levels of anti-sporozoite antibodies in the groups vaccinated with both peptide doses formulated in Montanide ISA 51 were similar after the third immunization. At the higher dose, anti-sporozoite antibody levels were similar after two and three immunizations. Specificity of the antibody response was confirmed by negative results obtained by ELISA and IFAT, using sera from volunteers in the control group and from all other volunteers before immunization. Half of the individuals naturally exposed to malaria presented anti-P. vivax sporozoite antibodies titers ranging from 1:80 to 1:640. Most of them (10 individuals) had low antibody titers (1:80) similar to those observed in immunized volunteers.
Table 4.
Recognition of Plasmodium vivax sporozoites by immunofluorescence assay
| Montanide adjuvant | Dose ug | Immunzation | Natural infection* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Second | Third | P | No. of responders | Antibody titers | ||||||
| n | Median† | IQR‡ | n | Median† | IQR‡ | |||||
| ISA 51 | 150 | 7 | 0 | 0–10 | 7 | 40 | 40–320 | 0.02 | 10 | 80 |
| 300 | 7 | 20 | 20–80 | 7 | 20 | 20–80 | 0.25 | 5 | 160 | |
| 6 | 320 | |||||||||
| ISA 720 | 150 | 8 | 10 | 10–20 | 8 | 10 | 0–60 | 0.22 | 4 | 640 |
| 300 | 8 | 20 | 10–20 | 7 | 20 | 10–20 | 0.91 | 25 | Negatives | |
Anti-P. vivax sporozoite antibodies titers of 50 adults from malaria endemic regions.
Values are reciprocal dilution titers.
IRQ = interquartile ratio. Two-tailed, Wilcoxon signed-rank test non-parametric P value, H0: 2nd = 3rd.
Single-cell IFN-γ production.
Production of IFN-γ by PBMC varied among volunteers and immunization doses (Figure 3). The response to peptide N (median 140 sfc/106 PBMC) was significantly higher than to peptide R (median 80 sfc/106 PBMC) and peptide C (median 100 sfc/106 PBMC) (P = 0.001). Response induced by the 300 μg dose was higher than 150 μg dose (P = 0.001). When comparing groups by adjuvant, we found that the response to Montanide ISA 51 (median 20 sfc/106 PBMC) was higher than to Montanide ISA 720 (median 7 sfc/106 PBMC) (P = 0.05).
Figure 3.

IFN- γ production in response vaccination with a mixture of N, R, and C peptides. Median of sfc to each peptide independent of doses and adjuvant.
Discussion
Our results provide evidence that vaccination of malaria-naive volunteers with mixtures of LSP derived from the P. vivax CS protein formulated in Montanide ISA 720 and in Montanide ISA 51 is safe, well tolerated, and immunogenic. This study also confirms data previously reported by our group on the vaccination with similar P. vivax CS derived LSP individually formulated in Montanide ISA 720.25 In this regard AEs related to vaccination occurred with similar frequency in all groups and transient pain at the site of injection, which was described as similar to that caused by commercially available vaccines, was the most frequent complaint. Moreover, the trial indicated that although the peptide mixture as formulated did not appear to produce any synergism on immune responses, we expect an additive effect of cell-mediated immunity and antibodies, especially on their blocking activity on sporozoite invasion, which may be more effective than vaccination with individual peptides.43–45
The peptide mixtures induced antibody titers to each peptide that were similar to those from our previous trial, in which volunteers were immunized with single peptides. This suggests that there is no detectable interference in the peptide mixture. The strategy of initially giving a priming vaccine containing N and C peptides only was used to avoid the potential imbalance that might produce the immunodominant R fragment. It has been shown that after continued exposure to malaria, sera of individuals from endemic areas recognize the R region better than the N- and C-terminal regions,46 which may be caused by its repetitive structure, where the same B- and T-cell epitopes are repeated numerous times, whereas any individual epitope present in the N and C flanking regions is present only once in each CS molecule. This immuno-dominance may represent a “smoke screen” that diverts the response toward it, preventing the development of stronger responses to the flanking regions that contain functional cell-binding domains (regions I and II) required for parasite invasion.16,17
Although the use of the full-length protein may be considered desirable, besides its immunodominance, the P. vivax central repeat domain is highly polymorphic. Use of the LSP approach allows an easy, inexpensive, and accurate methodology to down-select protein fragments containing epitopes relevant in protection. Plasmodium falciparum RTS-S, the most advanced malaria vaccine candidate is based on a recombinant construct containing protein fragments similar to the P. vivax R and C fragments studied here. Although that construct might be the best selection for P. falciparum, results here indicate that other combinations, i.e., including the N fragment with other CS fragments or other antigens may be more effective in P. vivax. In this and in our previous vaccine trial, the C fragment was less immunogenic than N and R.24
Although the choice of protective protein fragment is critical, adjuvants are also fundamental. Montanide ISA 720 and Montanide ISA 51 represent a new generation of promising adjuvants, with an overall acceptable safety profile and strong B cell and Th stimulation capacity.47 Montanide ISA 51 is a mineral oil-based adjuvant, which has been used in more than 25 clinical trials, representing more than 4,000 patients, and 40,000 injections, showing that it is well tolerated.29 Local reactions such as pain, tenderness, and erythema have been the most commonly reported AEs with this adjuvant, whereas the most frequently reported AEs have been nausea, vomiting, and flu-like symptoms, although other studies have reported injection pain nodules and sterile abscesses. Their intensity usually have been classified as mild to moderate, and SAEs reported such as erythema nodosum-like syndrome and transient leukemoid cases, seems to be more related to the antigen used than attributable directly to the adjuvant.28,36,48 Montanide ISA 720 a squalene-based adjuvant, has also been tested in multiple malaria antigens clinical trials, which have reported from minor,25 to mild and moderate local reactogenicity37,49,50; however, a trial conducted with a malaria vaccine candidate formulated in this adjuvant had to be suspended because of the presence of SAEs.43
In this study, all formulations were immunogenic inducing both, specific anti-peptide antibodies and IFN-γ production. Importantly, even low LSP vaccine doses were enough to induce sero-conversion in 95% of the volunteers and sera from 86% of the participants recognized the native protein in sporozoites. Although IFAT titers may appear low, the high seroconversion is encouraging because in field conditions only a limited number of the individuals continuously exposed to P. vivax recognize sporozoites,51,52 even if they are continuously exposed to the complete mass of parasite proteins. Interestingly, similar antibody titers were indicated in a previous trial53 to induce significant in vitro sporozoites invasion inhibition.
Throughout our study, antibody responses to the N peptide developed a more homogeneous profile and were consistently stronger than those to the other peptides. As expected most volunteers produced anti-R-peptide antibodies only after a boosting dose on Month 4, thereafter they followed a trend comparable to that produced by peptide N. Similarly to our previous study, volunteers required one or two boosting doses to respond to the C-terminal peptide. Therefore, it seems that higher doses are necessary for responses to peptides R and C to reach a similar degree of maturity than induces peptide N. This may indicate the importance of attempting an even better balance among the different specificities that may be important for the protective capacity of the vaccine.
In general, antibody responses were similar in their pattern and titers to those obtained in volunteers immunized previously with individual peptides,25 and in preclinical trials in Aotus monkeys vaccinated with the same vaccine formulations.53 Moreover, titers were higher than those observed in sera of most people from P. vivax endemic areas of Colombia.42
As we expected, IgG isotype profile response was different for each peptide. The N peptide showed increased titers for all IgG isotypes, compared with C peptide that showed more defined IgG1 and IgG3 responses. This is certainly of interest, because of reports indicating that these are the main isotypes involved in protection against malaria infection, that IgG4 is non-protective and that IgG2 is not only non-protective but could even compete and interfere with the protective isotypes.54
Recognition of sporozoites by anti-peptide antibodies is encouraging because of the potential boosting effect of sporozoites on the humoral response upon natural exposure in endemic areas and the greater possibilities of a functional role of antibodies in blocking parasite invasion to hepatocytes. Although anti-sporozoite antibody titers were low, it has not yet been defined what antibody levels are needed for protection. Indeed, similar antibody titers were able to block in vitro parasite invasion.53 Furthermore, the fact that Montanide ISA 51 elicited a more vigorous anti-sporozoite response than Montanide ISA 720 formulations should be considered when moving forward in designing further clinical trials with these peptides.
Specific induction of IFN-γ production by T cells by all peptides but mainly by peptide N, particularly when formulated in Montanide ISA 51, is consistent with the higher antibody response; peptide N may contain stronger T-cell epitopes than in R and C peptides.55 Recently, the N-terminal region of the P. falciparum CS in combination with the C-terminal region had a better ability to inhibit sporozoite invasion to hepatocytes and recognition of short N-terminal peptide (PfCS65-110) by sera from children living in a malaria-endemic region was associated with protection from disease.56,57
In conclusion, vaccination of human volunteers with a combination of LSP derived from the P. vivax CS protein formulated Montanide ISA 720 or Montanide ISA 51 is safe, well tolerated, and immunogenic. Peptides formulated in Montanide ISA 51 produced better anti-sporozoite recognition antibody response, suggesting that the choice of adjuvant for the formulation of these vaccines is an important variable to be considered in future research.48 This study confirms LSP as a safe and immunogenic antigen. The recent development of a P. vivax challenge model for humans creates ideal conditions to assess these antigens in phase II studies (Herrera and others, 2011, in press).
ACKNOWLEDGMENTS
We acknowledge the technical support of Paola Santos, Angela M. Lenis, and Carolina Ramírez. UNDP/World Bank/World Health Organization, Special Program for Research, and Training in Tropical Diseases (WHO/TDR) provided us with valuable advice and clinical monitoring.
Footnotes
Financial support: This work was supported by World Health Organization Initiative for Vaccine Research (grant no. LA35735G), National Institute of Allergy and Infectious Diseases (NIAID grant no. 49486/TMRC), Colombian National Research Council, COLCIENCIAS (contract no. 487-2003), the Colombian Ministry for Social Protection (conv. 255-2004), and the Malaria Vaccine and Drug Development Center Foundation. Ricardo Palacios was supported by a studentship grant from the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq and Mario Chen-Mok by the National Institutes of Health (NIH contract no. NIH/NO1-AI-05403).
Authors' addresses: Sócrates Herrera, Olga Lucía Fernández, Omaira Vera, and Myriam Arévalo-Herrera, Instituto de Inmunología, Facultad de Salud, Universidad del Valle, Cali, Colombia and Malaria Vaccine and Drug Development Center, Cali, Colombia, E-mails: sherrera@inmuno.org, olgalufe@yahoo.com, ovlizcano@gmail.com, and marevalo@inmuno.org. William Cárdenas, Centro Médico Imbanaco, Unidad de Medicina Interna y de Epidemiología Clínica, Cali, Colombia, E-mail: cardenasnino@gmail.com. Oscar Ramírez, Fundación Clínica Valle del Lili, Cali, Colombia, E-mail: oramirez@fcvl.org. Ricardo Palacios, Division of Infectious Diseases, Federal University of São Paulo, Rua Napoleao de Barros, São Paulo, Brazil, E-mail: ricardopalacios@gmx.net. Mario Chen-Mok, Family Health International, Durham, NC, E-mail: mchen@fhi.org. Giampietro Corradin, Biochemistry Institute, University of Lausanne, Lausanne, Switzerland, E-mail: giampietro.corradin@unil.ch.
Reprint requests: Sócrates Herrera, Malaria Vaccine and Drug Development Center, Carrera 37 - 2Bis No. 5E - 08, Cali, Colombia, E-mail: sherrera@inmuno.org.
References
- 1.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 2.Clyde DF. Immunity to falciparum and vivax malaria induced by irradiated sporozoites: a review of the University of Maryland studies, 1971–75. Bull World Health Organ. 1990;68((Suppl)):9–12. [PMC free article] [PubMed] [Google Scholar]
- 3.Herrington D, Davis J, Nardin E, Beier M, Cortese J, Eddy H, Losonsky G, Hollingdale M, Sztein M, Levine M, Nussenzweig RS, Clyde D, Edelman R. Successful immunization of humans with irradiated malaria sporozoites: humoral and cellular responses of the protected individuals. Am J Trop Med Hyg. 1991;45:539–547. doi: 10.4269/ajtmh.1991.45.539. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, Gordon DM, Stoute JA, Church LW, Sedegah M, Heppner DG, Ballou WR, Richie TL. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 5.Egan JE, Hoffman SL, Haynes JD, Sadoff JC, Schneider I, Grau GE, Hollingdale MR, Ballou WR, Gordon DM. Humoral immune responses in volunteers immunized with irradiated Plasmodium falciparum sporozoites. Am J Trop Med Hyg. 1993;49:166–173. doi: 10.4269/ajtmh.1993.49.166. [DOI] [PubMed] [Google Scholar]
- 6.Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341:323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- 7.Tsuji M, Romero P, Nussenzweig RS, Zavala F. CD4+ cytolytic T cell clone confers protection against murine malaria. J Exp Med. 1990;172:1353–1357. doi: 10.1084/jem.172.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daubersies P, Thomas AW, Millet P, Brahimi K, Langermans JA, Ollomo B, BenMohamed L, Slierendregt B, Eling W, Van Belkum A, Dubreuil G, Meis JF, Guerin-Marchand C, Cayphas S, Cohen J, Gras-Masse H, Druilhe P. Protection against Plasmodium falciparum malaria in chimpanzees by immunization with the conserved pre-erythrocytic liver-stage antigen 3. Nat Med. 2000;6:1258–1263. doi: 10.1038/81366. [DOI] [PubMed] [Google Scholar]
- 9.Chappel JA, Rogers WO, Hoffman SL, Kang AS. Molecular dissection of the human antibody response to the structural repeat epitope of Plasmodium falciparum sporozoite from a protected donor. Malar J. 2004;3:28. doi: 10.1186/1475-2875-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman SL, Nussenzweig V, Sadoff JC, Nussenzweig RS. Progress toward malaria preerythrocytic vaccines. Science. 1991;252:520–521. doi: 10.1126/science.2020852. [DOI] [PubMed] [Google Scholar]
- 11.Taylor-Robinson AW. Immunity to liver stage malaria: considerations for vaccine design. Immunol Res. 2003;27:53–70. doi: 10.1385/IR:27:1:53. [DOI] [PubMed] [Google Scholar]
- 12.Cerami C, Frevert U, Sinnis P, Takacs B, Clavijo P, Santos MJ, Nussenzweig V. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell. 1992;70:1021–1033. doi: 10.1016/0092-8674(92)90251-7. [DOI] [PubMed] [Google Scholar]
- 13.Frevert U, Sinnis P, Cerami C, Shreffler W, Takacs B, Nussenzweig V. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J Exp Med. 1993;177:1287–1298. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida N, Nussenzweig RS, Potocnjak P, Nussenzweig V, Aikawa M. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science. 1980;207:71–73. doi: 10.1126/science.6985745. [DOI] [PubMed] [Google Scholar]
- 15.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, Wellde BT, Garcon N, Krzych U, Marchand M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 16.Sun P, Schwenk R, White K, Stoute JA, Cohen J, Ballou WR, Voss G, Kester KE, Heppner DG, Krzych U. Protective immunity induced with malaria vaccine, RTS,S is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. J Immunol. 2003;171:6961–6967. doi: 10.4049/jimmunol.171.12.6961. [DOI] [PubMed] [Google Scholar]
- 17.Kester KE, McKinney DA, Tornieporth N, Ockenhouse CF, Heppner DG, Jr, Hall T, Wellde BT, White K, Sun P, Schwenk R, Krzych U, Delchambre M, Voss G, Dubois MC, Gasser RA, Jr, Dowler MG, O'Brien M, Wittes J, Wirtz R, Cohen J, Ballou WR. A phase I/IIa safety, immunogenicity, and efficacy bridging randomized study of a two-dose regimen of liquid and lyophilized formulations of the candidate malaria vaccine RTS,S/AS02A in malaria-naive adults. Vaccine. 2007;25:5359–5366. doi: 10.1016/j.vaccine.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Kester KE, Cummings JF, Ockenhouse CF, Nielsen R, Hall BT, Gordon DM, Schwenk RJ, Krzych U, Holland CA, Richmond G, Dowler MG, Williams J, Wirtz RA, Tornieporth N, Vigneron L, Delchambre M, Demoitie MA, Ballou WR, Cohen J, Heppner DG., Jr Phase 2a trial of 0, 1, and 3 month and 0, 7, and 28 day immunization schedules of malaria vaccine RTS,S/AS02 in malaria-naive adults at the Walter Reed Army Institute of Research. Vaccine. 2008;26:2191–2202. doi: 10.1016/j.vaccine.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 19.Bojang KA, Milligan PJ, Pinder M, Vigneron L, Alloueche A, Kester KE, Ballou WR, Conway DJ, Reece WH, Gothard P, Yamuah L, Delchambre M, Voss G, Greenwood BM, Hill A, McAdam KP, Tornieporth N, Cohen JD, Doherty T. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358:1927–1934. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 20.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, Mandomando I, Spiessens B, Guinovart C, Espasa M, Bassat Q, Aide P, Ofori-Anyinam O, Navia MM, Corachan S, Ceuppens M, Dubois MC, Demoitie MA, Dubovsky F, Menendez C, Tornieporth N, Ballou WR, Thompson R, Cohen J. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–1420. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 21.Bejon P, Lusingu J, Olotu A, Leach A, Lievens M, Vekemans J, Mshamu S, Lang T, Gould J, Dubois MC, Demoitie MA, Stallaert JF, Vansadia P, Carter T, Njuguna P, Awuondo KO, Malabeja A, Abdul O, Gesase S, Mturi N, Drakeley CJ, Savarese B, Villafana T, Ballou WR, Cohen J, Riley EM, Lemnge MM, Marsh K, von Seidlein L. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359:2521–2532. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdulla S, Oberholzer R, Juma O, Kubhoja S, Machera F, Membi C, Omari S, Urassa A, Mshinda H, Jumanne A, Salim N, Shomari M, Aebi T, Schellenberg DM, Carter T, Villafana T, Demoitie MA, Dubois MC, Leach A, Lievens M, Vekemans J, Cohen J, Ballou WR, Tanner M. Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. N Engl J Med. 2008;359:2533–2544. doi: 10.1056/NEJMoa0807773. [DOI] [PubMed] [Google Scholar]
- 23.Herrera S, Bonelo A, Perlaza BL, Valencia AZ, Cifuentes C, Hurtado S, Quintero G, Lopez JA, Corradin G, Arevalo-Herrera M. Use of long synthetic peptides to study the antigenicity and immunogenicity of the Plasmodium vivax circumsporozoite protein. Int J Parasitol. 2004;34:1535–1546. doi: 10.1016/j.ijpara.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Herrera S, Perlaza BL, Bonelo A, Arevalo-Herrera M. Aotus monkeys: their great value for anti-malaria vaccines and drug testing. Int J Parasitol. 2002;32:1625–1635. doi: 10.1016/s0020-7519(02)00191-1. [DOI] [PubMed] [Google Scholar]
- 25.Herrera S, Bonelo A, Perlaza BL, Fernandez OL, Victoria L, Lenis AM, Soto L, Hurtado H, Acuna LM, Velez JD, Palacios R, Chen-Mok M, Corradin G, Arevalo-Herrera M. Safety and elicitation of humoral and cellular responses in colombian malaria-naive volunteers by a Plasmodium vivax circumsporozoite protein-derived synthetic vaccine. Am J Trop Med Hyg. 2005;73:3–9. doi: 10.4269/ajtmh.2005.73.3. [DOI] [PubMed] [Google Scholar]
- 26.Pye D, Vandenberg KL, Dyer SL, Irving DO, Goss NH, Woodrow GC, Saul A, Alving CR, Richards RL, Ballou WR, Wu MJ, Skoff K, Anders RF. Selection of an adjuvant for vaccination with the malaria antigen, MSA-2. Vaccine. 1997;15:1017–1023. doi: 10.1016/s0264-410x(96)00289-7. [DOI] [PubMed] [Google Scholar]
- 27.Peters BS, Jaoko W, Vardas E, Panayotakopoulos G, Fast P, Schmidt C, Gilmour J, Bogoshi M, Omosa-Manyonyi G, Dally L, Klavinskis L, Farah B, Tarragona T, Bart PA, Robinson A, Pieterse C, Stevens W, Thomas R, Barin B, McMichael AJ, McIntyre JA, Pantaleo G, Hanke T, Bwayo J. Studies of a prophylactic HIV-1 vaccine candidate based on modified vaccinia virus Ankara (MVA) with and without DNA priming: effects of dosage and route on safety and immunogenicity. Vaccine. 2007;25:2120–2127. doi: 10.1016/j.vaccine.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 28.O'Hagan DT, Valiante NM. Recent advances in the discovery and delivery of vaccine adjuvants. Nat Rev Drug Discov. 2003;2:727–735. doi: 10.1038/nrd1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aucouturier J, Ascarateil S, Dupuis L. The use of oil adjuvants in therapeutic vaccines. Vaccine. 2006;24((Suppl 2)):S2-44–45. doi: 10.1016/j.vaccine.2005.01.116. [DOI] [PubMed] [Google Scholar]
- 30.Arevalo-Herrera M, Solarte Y, Yasnot MF, Castellanos A, Rincon A, Saul A, Mu J, Long C, Miller L, Herrera S. Induction of transmission-blocking immunity in Aotus monkeys by vaccination with a Plasmodium vivax clinical grade PVS25 recombinant protein. Am J Trop Med Hyg. 2005;73:32–37. doi: 10.4269/ajtmh.2005.73.32. [DOI] [PubMed] [Google Scholar]
- 31.Arevalo-Herrera M, Castellanos A, Yazdani SS, Shakri AR, Chitnis CE, Dominik R, Herrera S. Immunogenicity and protective efficacy of recombinant vaccine based on the receptor-binding domain of the Plasmodium vivax Duffy binding protein in Aotus monkeys. Am J Trop Med Hyg. 2005;73:25–31. doi: 10.4269/ajtmh.2005.73.5_suppl.0730025. [DOI] [PubMed] [Google Scholar]
- 32.Valderrama-Aguirre A, Quintero G, Gomez A, Castellanos A, Perez Y, Mendez F, Arevalo-Herrera M, Herrera S. Antigenicity, immunogenicity, and protective efficacy of Plasmodium vivax MSP1 PV200l: a potential malaria vaccine subunit. Am J Trop Med Hyg. 2005;73:16–24. doi: 10.4269/ajtmh.2005.73.16. [DOI] [PubMed] [Google Scholar]
- 33.Bell BA, Wood JF, Bansal R, Ragab H, Cargo J, 3rd, Washington MA, Wood CL, Ware LA, Ockenhouse CF, Yadava A. Process development for the production of an E. coli produced clinical grade recombinant malaria vaccine for Plasmodium vivax. Vaccine. 2009;27:1448–1453. doi: 10.1016/j.vaccine.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 34.Hu J, Chen Z, Gu J, Wan M, Shen Q, Kieny MP, He J, Li Z, Zhang Q, Reed ZH, Zhu Y, Li W, Cao Y, Qu L, Cao Z, Wang Q, Liu H, Pan X, Huang X, Zhang D, Xue X, Pan W. Safety and immunogenicity of a malaria vaccine, Plasmodium falciparum AMA-1/MSP-1 chimeric protein formulated in montanide ISA 720 in healthy adults. PLoS ONE. 2008;3:e1952. doi: 10.1371/journal.pone.0001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malkin E, Hu J, Li Z, Chen Z, Bi X, Reed Z, Dubovsky F, Liu J, Wang Q, Pan X, Chen T, Giersing B, Xu Y, Kang X, Gu J, Shen Q, Tucker K, Tierney E, Pan W, Long C, Cao Z. A phase 1 trial of PfCP2.9: an AMA1/MSP1 chimeric recombinant protein vaccine for Plasmodium falciparum malaria. Vaccine. 2008;26:6864–6873. doi: 10.1016/j.vaccine.2008.09.081. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, Fay MP, Narum D, Rausch K, Miles AP, Aebig J, Orcutt A, Muratova O, Song G, Lambert L, Zhu D, Miura K, Long C, Saul A, Miller LH, Durbin AP. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS ONE. 2008;3:e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roestenberg M, Remarque E, de Jonge E, Hermsen R, Blythman H, Leroy O, Imoukhuede E, Jepsen S, Ofori-Anyinam O, Faber B, Kocken CH, Arnold M, Walraven V, Teelen K, Roeffen W, de Mast Q, Ballou WR, Cohen J, Dubois MC, Ascarateil S, van der Ven A, Thomas A, Sauerwein R. Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Alhydrogel, Montanide ISA 720 or AS02. PLoS ONE. 2008;3:e3960. doi: 10.1371/journal.pone.0003960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atherton E, Logan CJ, Sheppard RC. Peptide synthesis. II. Procedures for solid phase synthesis using N-fluorenylmethoxycarbonylamino-acids on polyamide supports: synthesis of substance P and of acyl carrier protein 65–74 decapeptide. J Chem Soc Perkin Trans. 1988;1:538–548. [Google Scholar]
- 39.Verdini A, Terenzi S, Brossard V, Roggero M, Corradin G. Oxidative folding of synthetic polypeptides S-protected as tert-butylthio derivatives. J Pept Sci. 2008;14:1271–1282. doi: 10.1002/psc.1067. [DOI] [PubMed] [Google Scholar]
- 40.National Cancer Institute . Common Toxicity Criteria. 1998. pp. 1–30. version 2.0. [Google Scholar]
- 41.WHO . Workbook for Clinical Monitor. TDR/PRD Standard Operating Procedures (SOPs) and Guidelines. Geneva, Switzerland: World Health Organization; 2002. pp. 281–288. [Google Scholar]
- 42.Arevalo-Herrera M, Roggero MA, Gonzalez JM, Vergara J, Corradin G, Lopez JA, Herrera S. Mapping and comparison of the B-cell epitopes recognized on the Plasmodium vivax circumsporozoite protein by immune Colombians and immunized Aotus monkeys. Ann Trop Med Parasitol. 1998;92:539–551. [PubMed] [Google Scholar]
- 43.Moore AC, Hutchings CL. Combination vaccines: synergistic simultaneous induction of antibody and T-cell immunity. Expert Rev Vaccines. 2007;6:111–121. doi: 10.1586/14760584.6.1.111. [DOI] [PubMed] [Google Scholar]
- 44.Wang R, Charoenvit Y, Daly TM, Long CA, Corradin G, Hoffman SL. Protective efficacy against malaria of a combination sporozoite and erythrocytic stage vaccine. Immunol Lett. 1996;53:83–93. doi: 10.1016/s0165-2478(96)02610-7. [DOI] [PubMed] [Google Scholar]
- 45.Hutchings CL, Birkett AJ, Moore AC, Hill AV. Combination of protein and viral vaccines induces potent cellular and humoral immune responses and enhanced protection from murine malaria challenge. Infect Immun. 2007;75:5819–5826. doi: 10.1128/IAI.00828-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrera S, Gomez A, Vera O, Vergara J, Valderrama-Aguirre A, Maestre A, Mendez F, Wang R, Chitnis CE, Yazdani SS, Arevalo-Herrera M. Antibody response to Plasmodium vivax antigens in Fy-negative individuals from the Colombian Pacific coast. Am J Trop Med Hyg. 2005;73:44–49. doi: 10.4269/ajtmh.2005.73.44. [DOI] [PubMed] [Google Scholar]
- 47.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Audran R, Cachat M, Lurati F, Soe S, Leroy O, Corradin G, Druilhe P, Spertini F. Phase I malaria vaccine trial with a long synthetic peptide derived from the merozoite surface protein 3 antigen. Infect Immun. 2005;73:8017–8026. doi: 10.1128/IAI.73.12.8017-8026.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saul A, Lawrence G, Allworth A, Elliott S, Anderson K, Rzepczyk C, Martin LB, Taylor D, Eisen DP, Irving DO, Pye D, Crewther PE, Hodder AN, Murphy VJ, Anders RF. A human phase 1 vaccine clinical trial of the Plasmodium falciparum malaria vaccine candidate apical membrane antigen 1 in Montanide ISA720 adjuvant. Vaccine. 2005;23:3076–3083. doi: 10.1016/j.vaccine.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 50.Gregson AL, Oliveira G, Othoro C, Calvo-Calle JM, Thorton GB, Nardin E, Edelman R. Phase I trial of an alhydrogel adjuvanted hepatitis B core virus-like particle containing epitopes of Plasmodium falciparum circumsporozoite protein. PLoS ONE. 2008;3:e1556. doi: 10.1371/journal.pone.0001556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tapchaisri P, Dekumyoy P, Asavanich A, Chongsa-nguan M, Tharavanij S, Harinasuta T. Antibodies against circumsporozoite proteins of Plasmodium falciparum induced by natural infection. Southeast Asian J Trop Med Public Health. 1985;16:355–364. [PubMed] [Google Scholar]
- 52.Kremsner PG, Neifer S, Zotter GM, Bienzle U, Rocha RM, Maracic M, Clavijo P, Nussenzweig RS, Cochrane AH. Prevalence and level of antibodies to the circumsporozoite proteins of human malaria parasites, including a variant of Plasmodium vivax, in the population of two epidemiologically distinct areas in the state of Acre, Brazil. Trans R Soc Trop Med Hyg. 1992;86:23–27. doi: 10.1016/0035-9203(92)90423-a. [DOI] [PubMed] [Google Scholar]
- 53.Arévalo-Herrera M, Vera O, Castellanos A, Cespedes N, Soto L, Corradin G, Herrera S. Preclinical vaccine study of Plasmodium vivax circumsporozoite protein derived-synthetic polypeptides formulated in Montanide ISA 720 and Montanide ISA 51 adjuvants. Am J Trop Med Hyg. 2011;84((Suppl 2)):21–27. doi: 10.4269/ajtmh.2011.10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garraud O, Mahanty S, Perraut R. Malaria-specific antibody subclasses in immune individuals: a key source of information for vaccine design. Trends Immunol. 2003;24:30–35. doi: 10.1016/s1471-4906(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 55.Arevalo-Herrera M, Valencia AZ, Vergara J, Bonelo A, Fleischhauer K, Gonzalez JM, Restrepo JC, Lopez JA, Valmori D, Corradin G, Herrera S. Identification of HLA-A2 restricted CD8(+) T-lymphocyte responses to Plasmodium vivax circumsporozoite protein in individuals naturally exposed to malaria. Parasite Immunol. 2002;24:161–169. doi: 10.1046/j.1365-3024.2002.00449.x. [DOI] [PubMed] [Google Scholar]
- 56.Bongfen SE, Ntsama PM, Offner S, Smith T, Felger I, Tanner M, Alonso P, Nebie I, Romero JF, Silvie O, Torgler R, Corradin G. The N-terminal domain of Plasmodium falciparum circumsporozoite protein represents a target of protective immunity. Vaccine. 2009;27:328–335. doi: 10.1016/j.vaccine.2008.09.097. [DOI] [PubMed] [Google Scholar]
- 57.Coppi A, Pinzon-Ortiz C, Hutter C, Sinnis P. The Plasmodium circumsporozoite protein is proteolytically processed during cell invasion. J Exp Med. 2005;201:27–33. doi: 10.1084/jem.20040989. [DOI] [PMC free article] [PubMed] [Google Scholar]


