Abstract
Vaccine development for Plasmodium vivax malaria is underway. A model to assess the protective efficacy of vaccine candidates in humans is urgently needed. Given the lack of continuous P. vivax cultures, we developed a system to infect Anopheles albimanus mosquitoes using blood from P. vivax-infected patients and determined parameters for challenge of malaria-naive volunteers by mosquito bite. Absence of co-infections in parasitized blood was confirmed by tests consistent with blood bank screening. A total of 119 experiments were conducted using batches of 900–4,500 mosquitoes fed by an artificial membrane feeding method. Optimal conditions for mosquito probing and infection were determined. Presence of oocyst and sporozoites were assessed on Days 7–8 and 14–15, respectively, and conditions to choose batches of infected mosquitoes for sporozoite challenge were established. Procedures to infect volunteers took a 2-hour period including verification of inoculum dose. Anopheles albimanus mosquitoes represent a valuable resource for P. vivax sporozoite challenge of volunteers.
Introduction
Because of the important economic burden of malaria and the failure of classical control measures, significant efforts are currently being invested in the identification of parasite antigens that may be developed as malaria vaccines to complement current control strategies. Several Plasmodium falciparum antigens are being pursued as vaccine candidates and numerous formulations are currently being assessed in pre-clinical and clinical studies.1–5 Continuous P. falciparum culture systems have significantly facilitated not only the identification of vaccine target antigens but also have allowed the establishment of well-standardized artificial membrane feeding assays (MFA) for mosquito infection and the production of live, mature, and infective sporozoites. Plasmodium falciparum sporozoite challenge methods have been successfully developed and routinely used to assess the protective efficacy of pre-erythrocytic malaria vaccines.6,7 The mosquito species used in these P. falciparum challenge studies are An. gambiae and An. stephensi, which are robust mosquitoes considered to be highly efficient for sporozoite production.6,8
Development of a similar challenge model for Plasmodium vivax has proven more difficult because of the lack of a continuous culture for this species and therefore the need to use freshly collected parasites. Most laboratories conducting vaccine studies are all located in non-endemic countries such as the United States and Europe and until now, no conditions for P. vivax malaria challenge trials have been reported in other malaria-endemic countries.
Previously, we have described the methods for developing a challenge model for infecting humans with P. vivax. This system regularly produces P. vivax-infected Anopheles mosquitoes that can be used to assess the protective efficacy of P. vivax candidate vaccines. Our Center has established and maintained An. albimanus mosquito colonies in both endemic and non-endemic sites of Colombia.9–11 This mosquito species is widely distributed in the American continent, ranging from southern regions of Mexico to northern Peru.12,13 This mosquito species has been found naturally infected by both P. vivax and P. falciparum and is considered a primary malaria vector in Latin America.12,14,15 Some reports indicate that An. albimanus is a less susceptible vector than several other anopheline species,16 i.e., it has been reported to be experimentally less susceptible to a P. falciparum Thai strain than An. quadrimaculatus or An. freeborni,17 to NF54 strain than An. stephensi, An. darling, and An. vestitipennis18 and to P. vivax Chesson strain than several other Anopheles species,19 and it appears to have shorter longevity than other Anopheles species.20 However, it is a mosquito species easy to colonize and breed, and it has been used for several years under laboratory conditions in different centers for malaria sporozoite production and transmission blocking studies.10,19,21
Herein, we describe studies for standardizing the continuous production of mature infective sporozoites in laboratory-reared An. albimanus mosquitoes, taking advantage of easy, routine access to blood from P. vivax malaria-infected patients. In addition, biosafety measures to avoid possible co-infections of naive volunteers who were challenged are described. This is considered a crucial step toward the development of a P. vivax sporozoite challenge system in human volunteers to evaluate subunit or attenuated whole malaria parasite vaccines.
Materials And Methods
Blood samples.
Plasmodium vivax-infected human blood samples were collected from patients with acute infections who presented at the outpatient clinic at the Immunology Institute headquarters in Buenaventura, a city located in a malaria-endemic region on the Pacific Coast of Colombia. Before enrollment in the study, written informed consent was obtained from all patients. The study protocol was previously reviewed and approved by the Institutional Review Board of the Universidad del Valle. Blood samples for diagnosis were collected by finger-prick and then blood was obtained from each selected donor volunteer by venipuncture using heparinized Vacutainers (10 mL) (Becton Dickinson, Franklin Lakes, NJ). Patients were immediately treated with antimalarials, following the standard therapeutic protocol approved by the Colombian Ministry of Social Protection. This comprised chloroquine (25 mg/kg) and primaquine (15 mg per 14 d).
Parasitemia.
Patients were diagnosed by microscopic examination of Giemsa-stained thick blood smears collected by finger-prick. Patients carrying more than 0.1% of parasitemia of asexual P. vivax parasites/μL, independently of the number of gametocytes, were invited to participate in the study as parasite donors.
Asexual and sexual stage parasites were counted using as a reference a total of 1,000 red blood cells, and both total parasitemia and gametocytemia were expressed as a percentage. Plasmodium vivax-infected blood was transported from the endemic area (Buenaventura) to the Immunology Institute in Cali, a city without malaria transmission. To avoid premature parasite exflagellation during transport, infected blood samples were maintained at 34–37°C using a temperature regulated device. The time required for transport of blood samples and the subsequent mosquito feeding process was ∼3–4 hrs.
Biosafety measures.
Although we had made an extensive search and consults with experts on the potential of Anophelines to transmit pathogens other than Plasmodium, and none of those included in blood bank screening have any chance to be transmitted by this mosquito, we decided to perform this screening to provide confidence to the volunteers. Blood samples collected to feed mosquitoes were therefore subjected to standard blood bank screening for infectious agents. Screening included co-infections produced by other plasmodia (no commercial polymerase chain reaction [PCR]), Hepatitis B (MEIA for HBsAgAbs by ABBOTT, Abbott Park, IL; reference value OD ≥ 2.0), Hepatitis C (HCV-3 Antibodies by ABBOTT; OD ≥ 1.0), Syphilis (Flocculation of reagin and cardiolipin coated coal particles by Biomerieux [Marcy l'Etoile, France] to check for dark aggregates), PET MicroELISA for gp46-I, p21-I, gp46-II by ABBOTT; reference value > cutoff and CHAGAS (MicroELISA for Trypanosoma cruzi rec proteins by IMMCO (IMMCO Diagnostics, INc., Buffalo, NY); reference value > cutoff), as described before by Herrera and others.22 Thirty mL of whole blood was collected using EDTA, heparin, and plain (without anticoagulant) Vacutainer tubes (Becton Dickinson). Next, the sample was divided into three aliquots: a 15 mL sample for mosquito feeding (Hep); 10 mL (EDTA), and a 5 mL (without anticoagulant) samples for routine screening against a panel of common infectious agents (viral, bacterial, parasitic) including confirmation of Plasmodium species (P. vivax, P. falciparum, P. malariae) by PCR.23 Blood screening was performed at the blood bank of the Clínica Valle del Lili in Cali. Mosquito lots that were infected with blood that contained other pathogens were euthanized by exposure to a CO2 atmosphere and immersion in 70% alcohol. Other biosafety measures included restricted access and handling of mosquitoes only within confinement space approved for challenge, biosafety suiting to minimize–prevent mosquito bites, and sterilization of mosquitoes before discarding them as biohazardous waste.
Mosquito infection.
The An. albimanus mosquito is easy to rear under laboratory conditions, and is susceptible to experimental infections with P. falciparum and P. vivax.9–11,21,24 Anopheles albimanus, Buenaventura strain, was reared in laboratory conditions under controlled temperature (24–27°C) and relative humidity (80–90%) as described previously9 and infections were performed according to a method standardized for production of high quality P. vivax sporozoites using gametocytes derived from infected humans and monkeys.11 Briefly, female An. albimanus, 3–4 d old, distributed in cages of 300 mosquitoes/cage, were infected with P. vivax parasite isolates using the MFA described by Graves.25 Before feeding, mosquitoes were subjected to fasting overnight. Blood samples were centrifuged at 500 × g for 3 min at 37°C and autologous plasma was removed. Packed red blood cells were washed with two volumes of serum-free RPMI 1640 medium (Sigma, St. Louis, MO) and were reconstituted to 50% hematocrit using equal volumes of pooled AB non-immune human serum obtained from the Red Cross blood bank. After blood feeding, mosquitoes were fed daily with a 10% sugar solution supplemented with 0.05% para-amino-benzoic-acid (PABA) to promote the sporogonic cycle.26 All cages were labeled with a code and date of infection. The day after blood feeding, females that did not have a blood meal were removed from their cages, while the remaining mosquitoes were maintained under strict biosafety standards at the temperature and humidity conditions described previously.
Assessment of mosquito infections.
Seven days after blood feeding, a sample of mosquitoes was dissected to examine microscopically the presence of oocysts in midgut preparations. Midguts were stained with 2% mercurochrome and examined as described by Eyles.27 The remaining mosquitoes were kept inside a biosafety room for infected mosquitoes and were maintained for another 7-day period. Thirty-six female mosquitoes from each batch were randomly grouped into nine lots of four each to determine sporozoite load, and frequencies and probabilities of obtaining infective mosquitoes. Salivary glands (6 lobes) from each mosquito were mounted on a glass cover-slip in a drop of phosphate-buffered saline (PBS), disrupted by applying pressure and then examined at 400× magnification. Sporozoite load was expressed as the weighted average of sporozoite load per batch; to calculate the average sporozoite loads, parasite density scores were defined as follows: 1+ (1–10 sporozoites); 2+ (11–100 sporozoites); 3+ (101–1,000 sporozoites); and 4+ (> 1,001 sporozoites).28 To assess the sporozoite load per batch, salivary glands were dissected and pooled to determine the mean sporozoite load. Pooled salivary glands were homogenized in a cell tissue grinder with PBS. The mixture was then centrifuged at 26 × g for 3 min to extract mosquito debris and cells. Supernatant was collected and sporozoite load counted using a Neubauer chamber. Positive mosquito batches were analyzed to determine the relationship between parasitemia, gametocytemia, and mosquito infection.
Mosquito probing time.
In anticipation of future human challenge trials, we attempted to estimate the time that mosquitoes would need to feed for full engorgement. Thus, taking into account that study participants would be infected using groups of 3, 6, or 9 mosquitoes, a total of 10 trials were carried out for each mosquito dose using batches with different percentages of sporozoites-infected mosquitoes (55.3%, 60.5%, and 71.0%).
Mosquitoes were placed into small cartons (7 × 7 × 7 cm) with meshed windows on three sides and the cover. A water-jacketed membrane feeder covered with stretched parafilm membrane was positioned onto the gauze mesh placed on top of each box as described for MFA and kept at 37°C.25 Mosquito biting time was measured immediately after placing the blood in the feeder. Mosquitoes and feeder were covered with a black cloth to limit mosquito disturbance. The degree of mosquito engorgement was assessed visually every 30 sec during 5 min until all mosquitoes were engorged. On the basis of previous observations, we decided to routinely include male mosquitoes in each cage to stimulate female feeding.
Midgut and salivary gland dissection time.
The time required for mosquito dissection to confirm blood feeding and presence of sporozoites in salivary glands was estimated. These parameters were also determined for groups of 3, 6, and 9 mosquitoes and were performed after the probing time assessment.
Statistical analyses.
To determine mosquito infection and oocyst load per batch, the necessary number of mosquito dissections was estimated according to the equation for population sample size calculation, adjusted with finite Cochran's population formula (P = 71%, E = ±10% Z = 1.96).29 Results were expressed as the percentage of mosquitoes infected and the arithmetic mean of oocysts per batch. To determine sporozoite infection, salivary glands were dissected from 38 mosquitoes in each batch. We used binomial distribution to establish the probability of obtaining 2, 3, and 4 sporozoite-infected mosquitoes in boxes of four mosquitoes, which allowed us to predict how many biting rounds were necessary to reach the target infective bite dose required.22 Mosquito batches with sporozoite positivity ≥ 50% were randomly divided into nine groups of four females and sporozoite-infected mosquitoes (50 batches) were scored according to sporozoite infection rates: 50–59%, 60–69%, 70–79%, and ≥ 80%. Mosquito biting time was compared by analysis of variance followed by all pairwise multiple comparison procedure (Student-Newman-Keuls method); time for sporozoite load assessment was compared by Kruskal-Wallis one-way followed by all pairwise multiple comparison procedure (Dunn's method). Correlation between variables (parasitemia, gametocytemia, % oocyst-infected mosquitoes, % sporozoite-infected mosquitoes, mean oocyst and mean sporozoite load) was tested by using the correlation coefficients of Pearson or Spearman. Significance was determined for P = < 0.05.
Results
A total of 119 blood samples collected from the P. vivax-infected donors (confirmed positive by thick smears) were used for feeding an equal number of mosquito batches. Parasitemia in blood donors ranged from 0.1% to 2.1%, whereas gametocytemia ranged from 0.0% to 0.3%. Although we were unable to confirm microscopically the presence of gametocytes in 13 of 119 blood samples, these also resulted in oocyst mosquito infections.
Significant variability in the blood feeding rate of mosquito batches used to optimize the sporozoite production was observed (range = 41.7–100%; mean 85.9%). Similarly, the survival of infected mosquitoes in those batches was highly variable (range = 2.4–96.6%, mean 20.8%).
One hundred four blood feedings (87.4%) resulted in oocyst infections, whereas the remaining 15 (12.6%) failed to produce infections. There was significant variability in the oocyst counts, which ranged from a mean of 0.10–70.8 oocysts per mosquito. Similarly, there was also considerable variation in percentage of both oocyst (range = 1.4–100%) and sporozoites infection (range = 2.6–97.4%). Although total parasitemia positively correlated with gametocytemia (Spearman coefficient = 0.509, P = 0.0001), neither total parasitemia nor gametocytemia correlated with the percentage of sporozoite-infected mosquitoes (Spearman coefficient = 0.15, P = 0.13, Spearman coefficient = 0.11, P = 0.26; N = 104 samples).
Frequency of oocyst and sporozoite infections in the 104 mosquito batches, and mean values for mosquito infections are presented in Table 1. On average, 59.5% (±1.86 SE) of the mosquitoes were infected with a mean value of 11.6 ± 1.2 oocysts per mosquito. By Days 14–15, 48.5% ± 2.4 mosquito batches had developed sporozoite infection. The percentage of mosquitoes carrying oocysts correlated significantly with that of mosquitoes developing sporozoites (Pearson = 0.724, P = 0.0001; 104 mosquito lots). The mean number of oocysts per mosquito also had a significant correlation with sporozoite loads (expressed as weighted average of sporozoite per mosquito batch) in the corresponding lots (Spearman = 0.525, P < 0.0001; 89 mosquito lots).
Table 1.
Anopheles albimanus infection with Plasmodium vivax wild-type strains
| Oocyst infection | Sporozoite infection | |||
|---|---|---|---|---|
| % Infected mosquitoes | Arithmetic mean oocyst per mosquito | % Infected mosquitoes | Average sporozoite number/mosquito | |
| Mean* | 59.5 | 11.6 | 48.5 | 1,284 |
| SE* | 1.86 | 0.85 | 1.86 | 56.8 |
| Range* | 1.4–100 | 0.10–70.8 | 2.6–97.4 | 8.17–8,347 |
| Mean† | 75.1 | 18.8 | 70.7 | 1,957 |
| SE† | 2.1 | 1.99 | 1.95 | 310 |
| Range† | 36.0–100 | 2.10–70.8 | 52.6–97.4 | 49–8,347 |
Values for 104 mosquito batches.
Values for batches with ≥ 50% sporozoite-infected mosquitoes, N = 50 mosquito batches.
To increase the probability of having a sufficient numbers of infective mosquitoes at the time of human sporozoite challenge, lots with > 50% sporozoite-infected mosquitoes were selected; 50 of 104 mosquito batches exhibited this grade of infection (Table 1). These 50 batches were stratified into four levels according to the percentage of sporozoite-infected mosquitoes (1+–4+), and it was observed that the mean percentage of negative mosquitoes decreased from 44.4% to 11.7% in the 104 batches in this group of selected mosquito batches. Additionally, the frequency of mosquitoes with 3+ and 4+ grades of sporozoites increased (Table 2). Moreover, the correlation between the average number of oocysts and weighted average of sporozoite per batch in this smaller group of mosquito batches was maintained (Spearman coefficient = 0.332, P = 0.28; N = 44 mosquito batches). Analyses of the frequency of sporozoite loads (salivary glands) indicated that 11.9% of mosquitoes had a low density (1+); 22.9% had 2+; 21.5% had 3+; 14.7% had 4+; ∼29% of mosquitoes remained non-infected.
Table 2.
Frequency and sporozoite index in mosquito batches with ≥ 50% of mosquitoes carrying sporozoites
| % spz mosquitoes infection (average) | Range (%) | n | Sporozoite-infected mosquitoes proportion (frequency)* | Sporozoites mean/mosquito | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2++ | 3+++ | 4++++ | ||||
| 55% | 52.6–57.9 | 17 | 44.4 | 13.2 | 23.0 | 13.8 | 5.6 | 1,013 |
| 66% | 63.2–68.4 | 6 | 32.9 | 16.7 | 26.4 | 16.7 | 7.4 | 1,289 |
| 76% | 71.1–79.0 | 13 | 25.9 | 12.4 | 18.6 | 24.4 | 18.8 | 2,563 |
| 88% | 81.6–94.7 | 14 | 11.7 | 13.1 | 23.4 | 30.2 | 21.6 | 3,141 |
Frequency of mosquitoes with sporozoites load according to rate (1+ = 1–10 spz; 2++ = 11–100 spz; 3+++ = 101–1,000 spz; 4++++ = > 1,000 spz) n = batches number evaluated.
Random selection of a small number of mosquitoes, i.e., 3 ± 1, was carried out to determine the optimal number for human challenge. A total of four mosquitoes were selected from batches with a mean of 55–88% infected mosquitoes. Cages with 55% infected mosquitoes were confirmed to have the greatest probability of having two infected mosquitoes (0.4183). For batches with 66–76% infection rates, the greatest probability was to get 3 infected mosquitoes (0.4630 and 0.3761, respectively), and when the batch had 88% infected mosquitoes, 4 infected mosquitoes were found (0.6031) (Table 3).
Table 3.
Probability of obtaining 0–4 spz-infected mosquitoes in groups of 4 mosquitoes
| % spz mosquitoes | n† | Probabilities* | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| 55% | 153 | 0.0392 | 0.1699 | 0.4183 | 0.2810 | 0.0915 |
| 66% | 54 | 0.0000 | 0.0741 | 0.3148 | 0.4630 | 0.1481 |
| 76% | 117 | 0.0085 | 0.0256 | 0.2649 | 0.3760 | 0.3248 |
| 88% | 126 | 0.0000 | 0.0079 | 0.0476 | 0.3413 | 0.6032 |
Probability of obtaining 0, 1, 2, 3, and 4 positive mosquitoes in groups of 4 mosquitoes randomly selected.
Number of tested groups of 4 mosquitoes according to sporozoite load.
Mosquito probing time was not affected by the absence, presence, or percentage of sporozoite infection (55.3–71%) for groups of 3 (F = 1.38; P = 0.26), 6 (F = 2.94; P = 0.05), and 9 (F = 0.8; P = 0.50). On the basis of these results, probing times for groups of 3 mosquitoes were pooled regardless of mosquito sporozoite infection; the same procedure was followed for groups of 6 and 9 mosquitoes. Probing time was compared among groups of 3, 6, and 9 mosquitoes and the average probing times were 5.72, 9.69, and 18.15 min, respectively (Figure 1). These values were determined to be significantly different (F = 281.3, P < 0.0001, degrees of freedom [df] = 2) followed by a multiple-paired comparison (Student-Newman-Keuls method, P < 0.05).
Figure 1.
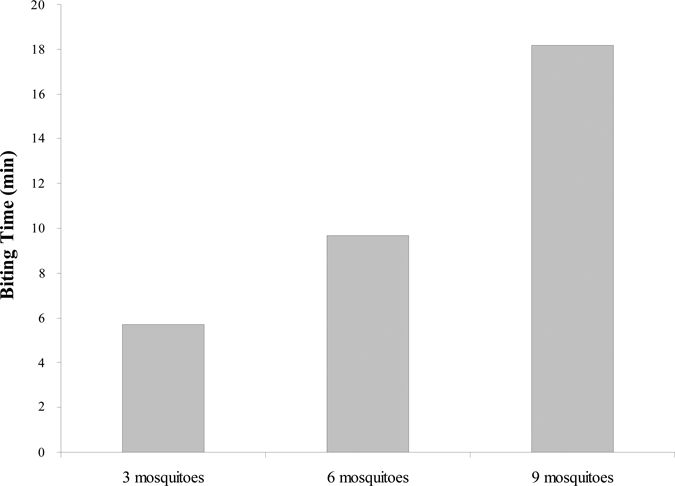
Probing time (min) of groups of 3, 6, and 9 Plasmodium vivax-infected Anopheles albimanus (mean and SD values, N = 40 data for each group, 5.72, SD 1.97; 9.69, SD 2.35; 18.16, SD 2.79).
Time required to dissect and microscopically confirm the presence of salivary gland infection was < 1 min/mosquito, therefore the lots of 3, 6, and 9 mosquitoes required 2.3, 4.7, and 6.8 min, respectively (Kruskal-Wallis, H = 93.9, 2 df, P < 0.0001, followed by Student-Newman-Keuls, P < 0.05, N = 38). A total of 216 mosquitoes with different degree of infection were used to determine the time required to assess sporozoite loads. The time needed for determination of sporozoite load decreased as the sporozoite density increased (0–4+). Significantly more time was required to determine negative (5.2 min) or 1+ (2.18 min) sporozoite-infected mosquitoes than that needed for mosquitoes with 2+ (1.67 min), 3+ (1.33 min), and 4+ (1.03 min) infections.
On the basis of these results, four mosquito batches were chosen for use in two human challenge trials; the first was aimed at establishing the minimal number of sporozoite-infected mosquitoes able to reproducibly induce human infections. The trial used cages with 4, 7, and 9 mosquitoes to deliver infective bite doses of 3 ± 1, 6 ± 1, and. 9 ± 1.22 The second trial was to determine if infection was dependent on the P. vivax strain used for mosquito feeding, using doses of 3 ± 1 mosquitoes shown to be capable of reproducibly infecting humans.30 All four batches produced infection in virtually all volunteers.
For human challenge trials a total of 33 infected mosquito batches were produced. Fifteen mosquito lots were infected over a 2-week period and were used in the first trial, which corresponded to 2–3 mosquito batches every other day.22 For the second trial a total of 18 mosquito batches were infected over a similar time period.30 Eleven batches were discarded because parasite donors presented infectious diseases (Hepatitis, Syphilis, and human T-lymphotrophic virus [HTLV]) (Table 4).
Table 4.
Characteristics of batches available in two human challenges. Percentages and means were calculated from 70 mosquitoes per batch
| Mosquito batches | Infectious screening | % Infected mosquitoes | Mean oocyst/mosquito | % Infected mosquitoes | Mean spz/mosquito |
|---|---|---|---|---|---|
| 1 | Neg | 85.0 | 21.1 | 97.3 | 824 |
| 2 | Neg | 85.5 | 22.5 | 94.7 | 20 |
| 3 | Neg | 71.0 | 18.1 | 86.5 | 1,043 |
| 4 | Neg | 88.1 | 18.2 | 84.2 | 2,034 |
| 5 | Neg | 72.0 | 38.9 | 78.9 | 3,773 |
| 6 | Neg | 69.7 | 45.5 | 68.4 | 883 |
| 7 | Neg | 76.0 | 15.4 | 63.2 | 4,582 |
| 8 | Neg | 69.7 | 5.4 | 55.3 | 407 |
| 9 | Neg | 57.9 | 6.4 | 15.8 | 20 |
| 10 | Neg | 52.6 | 3.6 | 47.4 | 55 |
| 11 | Neg | 53.9 | 4.6 | 44.7 | 407 |
| 12 | Neg | 38.5 | 2.5 | 47.4 | 48 |
| 13 | Neg | 46.1 | 2.7 | 21.1 | 146 |
| 14 | Neg | 23.7 | 1.2 | 31.6 | 429 |
| 15 | Neg | 62.9 | 2.6 | ND | ND |
| 16 | Neg | 74.3 | 7.2 | ND | ND |
| 17 | Neg | 18.9 | 0.2 | ND | ND |
| 18 | Neg | 13.3 | 0.4 | ND | ND |
| 19 | Neg | 4.0 | 0.2 | ND | ND |
| 20 | Neg | 0.0 | 0.0 | ND | ND |
| 21 | Neg | 6.6 | 0.4 | ND | ND |
| 22 | Neg | 1.3 | 2.9 | ND | ND |
| 23 | Hepatitis B | 78.7 | 20.9 | ND | ND |
| 24 | Hepatitis B | 84.0 | 9.4 | ND | ND |
| 25 | Hepatitis B | 90.0 | 43.2 | ND | ND |
| 26 | Hepatitis B | 10.5 | 0.5 | ND | ND |
| 27 | Hepatitis B | 59.2 | 3.9 | ND | ND |
| 28 | Hepatitis B | 48.7 | 2.9 | ND | ND |
| 29 | Hepatitis B, C | 5.3 | 3.9 | ND | ND |
| 30 | Syphilis | 6.7 | 0.1 | ND | ND |
| 31 | Syphilis | 78.9 | 22.4 | ND | ND |
| 32 | Syphilis + HTLV | 80.3 | 32.1 | ND | ND |
| 33 | HTLV 1-2 | 89.3 | 26.2 | ND | ND |
Altogether, for the two sporozoite challenge trials conducted so far by our group in humans, a total of eight lots fulfilled the criterion of having sporozoite infection ≥ 50% of the mosquitoes. Four of these lots were chosen to infect volunteers, and the rest were maintained as backup lots. In the first challenge the batch used had a total of 97.3% of the mosquitoes infected, whereas for the second challenge we used batches with 55.3%, 84.2%, and 94.7% of their mosquitoes infected (Table 4).
Discussion
This study shows that we could systematically infect An. albimanus mosquitoes with P. vivax derived from infected patients. More than 100 mosquito batches were infected and used to optimize the infection conditions to conduct human sporozoite challenge trials.22,30 During this process, biosafety measures were established and implemented to minimize volunteer exposure to mosquito bites and the potential for infection by other blood-borne pathogens. Furthermore, conditions for mosquito infection by artificial membrane feeding and mosquito biting of human volunteers were optimized. Similarly, conditions for salivary gland dissection to ensure that the precise number of mosquitoes and sporozoites used for human infection were standardized.
Given the lack of continuous P. vivax cultures, parasites were obtained from donors who presented at an outpatient clinic located in an area of unstable low malaria transmission. It had been previously demonstrated that sera from infected individuals display variable transmission blocking immunity ranging from complete blockage to transmission enhancing may affect the outcome of the mosquito infection, however, here we succeeded to easily and reproducibly infect most An. albimanus batches.11,21 Infections could be successfully achieved in most cases even though blood was transported from the field to the insectary in a non-endemic site, which represented several hours of in vitro incubation of parasites and serum antibodies. It is likely that because we used gels pre-warmed to 37°C during parasite transportation to prevent premature exflagellation, some of the parasites produced lower or no infections caused by transmission blocking antibodies. Mosquito infections were variable; nevertheless, a significant number of mosquito batches (50%) developed infections with > 50% of mosquitoes sporozoites positive and thereby suitable for use in human challenge trials.22,30
Although mosquito infections in this study were highly variable, the process can be considered reliable and reproducible taking into account that multiple confounding factors, including the genetic polymorphism of the P. vivax strain used for mosquito feeding, variability in gametocytemia levels, and degree of gametocyte maturation. Success of infections did not seem to depend on the level of parasitemia or gametocytemia, which is in agreement with previous studies using An. dirus where a poor correlation between infectivity and P. vivax gametocyte density was observed.31 Parasite genetic analyses to determine the potential parasite selection by mosquito were not carried out. Although the criteria that predict the degree of infectivity of mosquitoes are unknown32 despite the great variability of P. vivax infections in An. albimanus, our results allowed optimal preparation and screening of mosquito batches before sporozoite challenge studies.
Another important issue was that of blood specimen biosafety, which was ensured by using blood bank screening for infectious agents.22 Because mosquito feeding and blood screening were performed simultaneously, mosquito lots fed on blood samples that presented evidence or risk of concomitant infections, such as P. falciparum, hepatitis B or hepatitis C viruses, syphilis, or others were discarded.
The significant positive correlation between percent oocyst infection and percent sporozoite infection found is in agreement with a previous report using An. tessellatus infected with P. vivax.33 Because of the positive profile of mosquito batches with ≥ 50% of mosquitoes infected, both in terms of percentage and sporozoite loads, any batches with < 50.0% of oocyst-infected mosquitoes and < 7.55 oocysts per mosquito were withdrawn. Oocyst level of infection (percentage of infected mosquitoes and oocyst mean) as determined on Day 7 was in general used as a predictor for scheduling a sporozoite challenge trial a week later. However, percentage of infected mosquitoes and sporozoite loads were confirmed on Day 14 and only mosquito lots with ≥ 50% of sporozoite-infected mosquitoes for human challenge studies were considered, i.e., 52% and 97% for the first and second challenge trials, respectively. Although sporozoite inoculum is likely to be independent of gland sporozoite load,34 we arbitrarily decided to select only mosquito lots with ≥ 2+.
Small numbers of mosquitoes required significantly different feeding times to full engorgement. Groups of 2–10 mosquitoes spent from 5 to 18 min feeding; this time was similar for infected and non-infected mosquitoes that had been described previously for An. stephensi.35 These parameters were important to guarantee successful human infections within the shortest possible probing time. However, to facilitate the challenge procedure we decided not to attach to a fix biting dose, but to a range, e.g. (3 + 1), so that even with suboptimal infection rates (< 80%) one could attain the target biting dose within a single mosquito biting round. In the first sporozoite challenge trial, a mosquito lot with 97% infection rate, most volunteers completed their biting dose during a single biting round.22
Total time invested in salivary gland dissection and sporozoite load confirmation ranged from 2.3–6.8 min, thus the lower the sporozoite loads the longer the microscopic examination. Therefore, the time required for challenge of volunteers with groups of 3, 6, and 9 mosquitoes was ∼9, 16, and 26 min, respectively (without sporozoite load determination).
In conclusion, this study has confirmed the high susceptibility of An. albimanus to co-indigenous P. vivax infections and its effectiveness in infecting malaria-naive volunteers after appropriate standardization of methodology.
ACKNOWLEDGMENTS
We thank the Programa de Enfermedades Tropicales (PET) and the community of Buenaventura for providing infected samples for this study, and Bermans Murrain, Juana Vergara, Zuleyma Castillo, Bonny Lobaton, Einer Celorio, Efigenia Gonzalez, Sandra Preciado, and Carmen Murillo from the Malaria Vaccine and Drug Development Center for their technical assistance during these studies.
Footnotes
Financial support: This work was supported by WHO-IVR and the World Health Organization/Tropical Diseases Research Special Program (Research Capability Strengthening contract no. LA-35735G), the National Institute of Allergy and Infectious Diseases through Tropical Medicine Research Centers (grant no. 49486), National Heart, Lung and Blood Institute (NHLBI), Bethesda, COLCIENCIAS and the Colombian Ministry for Social Protection (contract nos. 253-2005 and 207-2007) and through an International Center of Excellence for Malaria Research NIAID/ICEMR grant no U 19AI089702.
Authors' addresses: Yezid Solarte, Myriam Arévalo-Herrera, and Sócrates Herrera, Instituto de Inmunología, Edificio de Microbiología, Facultad de Salud, Universidad del Valle and Malaria Vaccine and Drug Development Center, Cali, Colombia, E-mails: ysolarte@inmuno.org, marevalo@inmuno.org, and sherrera@inmuno.org. María R. Manzano, Departamento de Ciencias Agrícolas, Universidad Nacional, Palmira, Colombia, E-mail: mrmanzanom@palmira.unal.edu.co. Leonardo Rocha and Hugo Hurtado, Malaria Vaccine and Drug Development Center, Cali, Colombia, E-mails: lrocha94@hotmail.com and hhurtado01@gmail.com. Mark A. James, Department of Tropical Medicine, Tulane University, New Orleans, LA, E-mail: mjames@tulane.edu.
Reprint requests: Sócrates Herrera, Malaria Vaccine and Drug Development Center, Carrera 37 - 2Bis No. 5E - 08, Cali, Colombia, E-mail: sherrera@inmuno.org.
References
- 1.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, Mandomando I, Spiessens B, Guinovart C, Espasa M, Bassat Q, Aide P, Ofori-Anyinam O, Navia MM, Corachan S, Ceuppens M, Dubois MC, Demoitie MA, Dubovsky F, Menendez C, Tornieporth N, Ballou WR, Thompson R, Cohen J. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–1420. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 2.Moreno CA, Rodriguez R, Oliveira GA, Ferreira V, Nussenzweig RS, Moya Castro ZR, Calvo-Calle JM, Nardin E. Preclinical evaluation of a synthetic Plasmodium falciparum MAP malaria vaccine in Aotus monkeys and mice. Vaccine. 1999;18:89–99. doi: 10.1016/s0264-410x(99)00184-x. [DOI] [PubMed] [Google Scholar]
- 3.Roggero MA, Weilenmann C, Bonelo A, Audran R, Renggli J, Spertini F, Corradin G, Lopez JA. Plasmodium falciparum CS C-terminal fragment: preclinical evaluation and phase I clinical studies. Parassitologia. 1999;41:421–424. [PubMed] [Google Scholar]
- 4.Mullen GE, Giersing BK, Ajose-Popoola O, Davis HL, Kothe C, Zhou H, Aebig J, Dobrescu G, Saul A, Long CA. Enhancement of functional antibody responses to AMA1-C1/Alhydrogel, a Plasmodium falciparum malaria vaccine, with CpG oligodeoxynucleotide. Vaccine. 2006;24:2497–2505. doi: 10.1016/j.vaccine.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 5.Ballou WR, Arévalo-Herrera M, Carucci D, Richie TL, Corradin G, Diggs C, Druilhe P, Giersing BK, Saul A, Heppner DG, Kester KE, Lanar DE, Lyon J, Hill AV, Pan W, Cohen JD. Update on the clinical development of candidate malaria vaccines. Am J Trop Med Hyg. 2004;71:239–247. [PubMed] [Google Scholar]
- 6.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, Gordon DM, Stoute JA, Church LW, Sedegah M, Heppner DG, Ballou WR, Richie TL. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 7.Epstein JE, Rao S, Williams F, Freilich D, Luke T, Sedegah M, de la Vega P, Sacci J, Richie TL, Hoffman SL. Safety and clinical outcome of experimental challenge of human volunteers with Plasmodium falciparum-infected mosquitoes: an update. J Infect Dis. 2007;196:145–154. doi: 10.1086/518510. [DOI] [PubMed] [Google Scholar]
- 8.Silvie O, Semblat JP, Franetich JF, Hannoun L, Eling W, Mazier D. Effects of irradiation on Plasmodium falciparum sporozoite hepatic development: implications for the design of pre-erythrocytic malaria vaccines. Parasite Immunol. 2002;24:221–223. doi: 10.1046/j.1365-3024.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- 9.Hurtado S, Salas ML, Romero JF, Zapata JC, Ortiz H, Arévalo-Herrera M, Herrera S. Regular production of infective sporozoites of Plasmodium falciparum and P. vivax in laboratory-bred Anopheles albimanus. Ann Trop Med Parasitol. 1997;91:49–60. doi: 10.1080/00034983.1997.11813111. [DOI] [PubMed] [Google Scholar]
- 10.Salas ML, Romero JF, Solarte Y, Olano V, Herrera MA, Herrera S. Development of sporogonic cycle of Plasmodium vivax in experimentally infected Anopheles albimanus mosquitoes. Mem Inst Oswaldo Cruz. 1994;89((Suppl 2)):115–119. doi: 10.1590/s0074-02761994000600024. [DOI] [PubMed] [Google Scholar]
- 11.Arévalo-Herrera M, Solarte Y, Zamora F, Mendez F, Yasnot MF, Rocha L, Long C, Miller LH, Herrera S. Plasmodium vivax: transmission-blocking immunity in a malaria-endemic area of Colombia. Am J Trop Med Hyg. 2005;73:38–43. doi: 10.4269/ajtmh.2005.73.5_suppl.0730038. [DOI] [PubMed] [Google Scholar]
- 12.Frederickson EC. Bionomics and Control of Anopheles albimanus. Washington, DC: Pan-American Health Organization; 1993. p. 76. [Google Scholar]
- 13.Rubio-Palis Y. Anopheles (Nyssorhynchus) de Venezuela: Taxonomiìa, bionomiìa, ecologiìa e importancia meìdica. Maracay, Venezuela: Escuela Malariología y Saneamiento Ambiental; 2000. [Google Scholar]
- 14.Rubio-Palis Y, Zimmerman RH. Ecoregional classification of malaria vectors in the neotropics. J Med Entomol. 1997;34:499–510. doi: 10.1093/jmedent/34.5.499. [DOI] [PubMed] [Google Scholar]
- 15.Quiñones ML. Estado de la susceptibilidad al DDT de los principales vectores de malaria en Colombia y su aplicación epidemiológica. Colombia Med. 1987;18:19–24. [Google Scholar]
- 16.Vaughan JA, Noden BH, Beier JC. Sporogonic development of cultured Plasmodium falciparum in six species of laboratory-reared Anopheles mosquitoes. Am J Trop Med Hyg. 1994;51:233–243. doi: 10.4269/ajtmh.1994.51.233. [DOI] [PubMed] [Google Scholar]
- 17.Collins WE, Warren M, Skinner JC, Richardson BB, Kearse TS. Infectivity of the Santa Lucia (El Salvador) strain of Plasmodium falciparum to different anophelines. J Parasitol. 1977;63:57–61. [PubMed] [Google Scholar]
- 18.Grieco JP, Achee NL, Roberts DR, Andre RG. Comparative susceptibility of three species of Anopheles from Belize, Central America, to Plasmodium falciparum (NF-54) J Am Mosq Control Assoc. 2005;21:279–290. doi: 10.2987/8756-971X(2005)21[279:CSOTSO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Collins WE, Warren M, Contacos PG, Skinner JC, Richardson BB, Kearse TS. The Chesson strain of Plasmodium vivax in Aotus monkeys and anopheline mosquitoes. J Parasitol. 1980;66:488–497. [PubMed] [Google Scholar]
- 20.Kiszewski A, Mellinger A, Spielman A, Malaney P, Sachs SE, Sachs J. A global index representing the stability of malaria transmission. Am J Trop Med Hyg. 2004;70:486–498. [PubMed] [Google Scholar]
- 21.Ramsey JM, Salinas E, Rodriguez MH. Acquired transmission-blocking immunity to Plasmodium vivax in a population of southern coastal Mexico. Am J Trop Med Hyg. 1996;54:458–463. doi: 10.4269/ajtmh.1996.54.458. [DOI] [PubMed] [Google Scholar]
- 22.Herrera S, Fernandez O, Manzano MR, Murrain B, Vergara J, Blanco P, Palacios R, Velez JD, Epstein JE, Chen-Mok M, Reed ZH, Arévalo-Herrera M. Successful sporozoite challenge model in human volunteers with Plasmodium vivax strain derived from human donors. Am J Trop Med Hyg. 2009;81:740–746. doi: 10.4269/ajtmh.2009.09-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 1993;58:283–292. doi: 10.1016/0166-6851(93)90050-8. [DOI] [PubMed] [Google Scholar]
- 24.Olano V, Carillo MP, Espinal CA. Estudios de infectividad de la especie Anopheles albimanus Wiedemann, 1820 (Diptera: Culicidae) cepa Cartagena, con plasmodios humanos. Biomedica. 1985;5:5–10. [Google Scholar]
- 25.Graves PM. Studies on the use of a membrane feeding technique for infecting Anopheles gambiae with Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1980;74:738–742. doi: 10.1016/0035-9203(80)90189-3. [DOI] [PubMed] [Google Scholar]
- 26.Peters W, Ramkaran AE. The chemotherapy of rodent malaria, XXXII. The influence of p-aminobenzoic acid on the transmission of Plasmodium yoelii and P. berghei by Anopheles stephensi. Ann Trop Med Parasitol. 1980;74:275–282. [PubMed] [Google Scholar]
- 27.Eyles DE. A stain for malarial oocysts in temporary preparations. J Parasitol. 1950;36:501. [PubMed] [Google Scholar]
- 28.Chulay JD, Schneider I, Cosgriff TM, Hoffman SL, Ballou WR, Quakyi IA, Carter R, Trosper JH, Hockmeyer WT. Malaria transmitted to humans by mosquitoes infected from cultured Plasmodium falciparum. Am J Trop Med Hyg. 1986;35:66–68. doi: 10.4269/ajtmh.1986.35.66. [DOI] [PubMed] [Google Scholar]
- 29.Kish L. Survey Sampling. New York: John Wiley & Sons Inc; 1965. [Google Scholar]
- 30.Herrera S, Solarte Y, Jordan A, Echavarria J, Rocha L, Palacios R, Ramirez O, Velez J, Epstein J, Richie T, Arévalo-Herrera M. Consistent safety and infectivity in sporozoite challenge model of Plasmodium vivax in malaria-naive human volunteers. Am J Trop Med Hyg. 2011;84 (Suppl 2):4–11. doi: 10.4269/ajtmh.2011.09-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sattabongkot J, Maneechai N, Rosenberg R. Plasmodium vivax: gametocyte infectivity of naturally infected Thai adults. Parasitol. 1991;102:27–31. doi: 10.1017/s0031182000060303. [DOI] [PubMed] [Google Scholar]
- 32.Rickman LS, Jones TR, Long GW, Paparello S, Schneider I, Paul CF, Beaudoin RL, Hoffman SL. Plasmodium falciparum-infected Anopheles stephensi inconsistently transmit malaria to humans. Am J Trop Med Hyg. 1990;43:441–445. doi: 10.4269/ajtmh.1990.43.441. [DOI] [PubMed] [Google Scholar]
- 33.Gamage-Mendis AC, Rajakaruna J, Weerasinghe S, Mendis C, Carter R, Mendis KN. Infectivity of Plasmodium vivax and P. falciparum to Anopheles tessellatus; relationship between oocyst and sporozoite development. Trans R Soc Trop Med Hyg. 1993;87:3–6. doi: 10.1016/0035-9203(93)90396-8. [DOI] [PubMed] [Google Scholar]
- 34.Ponnudurai T, Lensen AH, van Gemert GJ, Bolmer MG, Meuwissen JH. Feeding behaviour and sporozoite ejection by infected Anopheles stephensi. Trans R Soc Trop Med Hyg. 1991;85:175–180. doi: 10.1016/0035-9203(91)90012-n. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Sina B, Rossignol PA. Probing behaviour and sporozoite delivery by Anopheles stephensi infected with Plasmodium berghei. Med Vet Entomol. 1992;6:57–61. doi: 10.1111/j.1365-2915.1992.tb00036.x. [DOI] [PubMed] [Google Scholar]


