Abstract
Plasmodium vivax circumsporozoite (CS) protein is a leading malaria vaccine candidate. We describe the characterization of specific immune responses induced in 21 malaria-naive volunteers vaccinated with long synthetic peptides derived from the CS protein formulated in Montanide ISA 720. Both antibody- and cell-mediated immune responses were analyzed. Antibodies were predominantly of IgG1 and IgG3 isotypes, recognized parasite proteins on the immunofluorescent antibody test, and partially blocked sporozoite invasion of hepatoma cell lines in vitro. Peripheral blood mononuclear cells from most volunteers (94%) showed IFN-γ production in vitro upon stimulation with both long signal peptide and short peptides containing CD8+ T-cell epitopes. The relatively limited sample size did not allow conclusions about HLA associations with the immune responses observed. In summary, the inherent safety and tolerability together with strong antibody responses, invasion blocking activity, and the IFN-γ production induced by these vaccine candidates warrants further testing in a phase II clinical trial.
Introduction
Plasmodium vivax, the second most important human malaria species, generates an estimated 60–70 million clinical cases worldwide every year, representing ∼20% of global malaria cases and 56% of cases outside Africa.1 Because clinical manifestations develop during asexual parasitic blood-stage multiplication, which occurs after the parasite has successfully completed its development in the liver, blocking parasite invasion or its further development in hepatocytes could thereby prevent subsequent clinical manifestations and further transmission to mosquitoes. Moreover, in the case of P. vivax, a pre-erythrocytic vaccine could prevent the development of hypnozoites and occurrence of clinical relapses, and thus would be an ideal vaccine for human use. Indeed, vaccination of humans using irradiated sporozoites has been shown to induce significant protection from experimental malaria parasite challenge,2–4 indicating that immunological responses can arrest parasite development before it appears in blood and clinical symptoms are produced. Because sera from humans and animals protected by this means recognize, among other molecules, the circumsporozoite (CS) protein that is abundantly expressed on the sporozoite surface and is involved in the process of hepatocyte invasion,5 this protein has been one of the most extensively studied malaria antigens.6,7
Several studies have shown that at the pre-erythrocytic stages, protection is mediated by both innate and acquired immune responses.8,9 Currently, it is thought that antibodies block sporozoite invasion of the liver and cytokines inhibit the intracellular parasite development contributing synergistically to protection.10 Production of interferon-gamma (IFN-γ) by either CD4+ or CD8+ T cells is considered the most important mechanism involved in protective pre-erythrocytic stage immunity.9,11,12
The CS proteins of various Plasmodium species have shown great promise in inducing protective immunity in animal models and humans.8,13–15 Recently, encouraging results were obtained in clinical trials conducted with a recombinant vaccine based on a fragment of the Plasmodium falciparum CS protein fused to hepatitis B surface antigen (RTS,S vaccine). This vaccine was shown to protect individuals from experimental infection with P. falciparum sporozoites,16 and it also protected semi-immune adults17 and children from natural infection in endemic areas.18–20 In these previous studies, RTS, S induced specific antibodies to the recombinant protein19,21–23 and elevated IFN-γ production in the protected subjects; both CD4+ and CD8+ T cells secreted IFN-γ specifically in response to CS protein peptides.24
During the last few years, we have devoted considerable effort to characterizing the P. vivax CS protein and to testing the protective potential of a peptide-based CS vaccine in preclinical and clinical studies.6 A phase I vaccine clinical trial conducted using long synthetic peptides (LSP) derived from the P. vivax CS protein formulated in Montanide ISA 720 indicated that the vaccine when tested in 69 volunteers was safe, well tolerated, and immunogenic. Most individuals produced immunoglobulin G (IgG) antibodies and significant levels of IFN-γ upon stimulation of peripheral blood monouclear cells (PBMC) with peptides derived from the CS protein.25 More recently, a chimeric P. vivax recombinant CS protein expressed in Escherichia coli was reported. Sera from individuals naturally exposed to malaria in endemic areas and from immunized mice displayed high antibody titers to the recombinant protein. This construct is also being considered as a vaccine candidate.26
Herein, we describe a detailed analysis of the immune responses induced in a subgroup of malaria-naive volunteers vaccinated with P. vivax CS derived LSP.
Materials and Methods
Blood samples.
Blood samples from a total of 21 (of 69) volunteers that participated in a previous randomized, dose-escalating, phase I clinical trial were analyzed.25 Volunteers were selected for this study because they had been immunized with the highest vaccine dose that resulted in the best immune response. Volunteers were healthy men and women, 18–33 years of age, with no history of malaria and who had been vaccinated at Months 0, 2, and 6 by intramuscular injection of 100 μg of either N, R, or C peptides corresponding to the P. vivax CS protein (VK210 variant)27 formulated in Montanide ISA 720 (Seppic, Paris, France). The original study protocol was approved by the corresponding Institutional Review Boards, and complied with the Declaration of Helsinki principles, Good Clinical Practices guidelines, and all pertinent Colombian regulations. Samples for immunological tests were analyzed before the first immunization (Day 0) and at three set points during and after the immunization phase (Months 3, 7, and 9).25
Peptides.
The LSP corresponding to the amino-terminal (N = 77 aa residues); the repeat (R = 48 aa residues); and the carboxyl-terminal (C = 70 aa residues) regions of the P. vivax CS protein were used for vaccination. In addition, 20-mer peptides covering the full sequence of the flanking regions28,29 and seven other peptides containing CTL motifs restricted by HLA-A*0201,30 HLA-B35, or HLA-B53 alleles were synthesized. Peptides were synthesized according to the solid-phase fluorenylmethoxycarbonyl (F-moc) method31 using an Applied Biosystem (Foster City, CA) synthesizer.32 Molecular mass and purity of peptides were assessed by MALDI-TOF mass spectrometry using a Voyager-DETM, RP Biospectrometry, (PerSpective Biosystems, Framingham, MA), and RP-HPLC (Waters, Milford, MA).
Total IgG to long, overlapping, and short peptides within N and C regions.
Antibody reactivity to synthetic peptides was assessed by enzyme-linked immunosorbent assay (ELISA) using long PvCS synthetic peptides (N, R-common, and C). To determine the fine specificity and to confirm recognition of previously identified B-cell epitopes, sera collected at Month 3 were tested against both 21 overlapping peptides covering the N- and C-terminal regions of the proteins and p8, p24, and p25 short peptides known to contain B-cell epitopes.28 Microplates were coated at a peptide concentration of 1 μg/mL and assays performed as described previously,28 using a microplate reader (MRX; Dynex Technologies, Inc., Chantilly, VA). Cut-off points were calculated as three standard deviations above the mean absorbance value at 405 nm (OD405) of negative control sera from healthy volunteers described previously. Pooled sera from semi-immune donors from malaria-endemic areas of Colombia were used as a positive control.
IgG isotypes.
Sera from immunized volunteers were tested for specific IgG isotype reactivity to the three LSP using a modified ELISA protocol previously described by Bouharoun-Tayoun and others.33 Briefly, 1 μg/mL peptide was used to coat the microplates and then blocked with phosphate-buffered saline (PBS) and Tween 20 fat-free milk. Plates were incubated with serum samples diluted 1:200 in PBS for 1 h, and then washed with PBS-Tween 20 and incubated with mouse monoclonal antibodies (MAbs) to human IgG1, clone NL 16; IgG3, clone ZG4; IgG4, clone GB78 from Skybio (Bedfordshire, England), and IgG2, clone HP-600, Sigma (St. Louis, MO) isotypes.
Finally, a peroxidase-conjugated goat anti-human IgG antibody (Kirkegaard and Perry Laboratories, Gaithersburg, MD) was added and after four washes, the substrate solution, containing 1 mg p-nitrophenyl phosphate (disodium; Sigma) per mL 0.1 M diethanolamine with 100 mM MgCl2, pH 9.8 was added. The reaction was stopped 1 h after with H2SO4 and absorbance was read at 405 nm. Results were expressed as a ratio of the OD mean of samples divided by the OD mean obtained from a pool of sera from healthy Colombian volunteers who had never been exposed to malaria.28 The MAbs used here were selected for their ability to recognize equally well the corresponding isotypes in sera from Caucasians, Black, or Asians, as described earlier in several studies with other malaria antigens.34–36
Immunofluorescent antibody test (IFAT).
Parasite recognition by anti-peptide antibodies was determined by IFAT using sera collected on Day 0 and after the third immunization. The IFAT antigen consisted of slides containing VK210 P. vivax sporozoites produced before by experimental infection of Anopheles albimanus mosquitoes and maintained cryopreserved.37,38 Briefly, sporozoites were fixed to multispot microscope slides with PBS containing 2% bovine serum albumin (BSA). Parasite spots were incubated with serial 2-fold dilutions of sera starting at 1:10. This reaction was developed with fluorescein-conjugated goat anti-human IgG (H+L) (Jackson Immunoresearch Laboratories, Inc., West Grove, PA) diluted at 1:100. Slides were examined under an epifluorescence microscope (Laborlux 2; Leitz GmbH & Co. KG, Wezlar, Germany), and antibody titers were determined as the reciprocal endpoint dilution that showed positive fluorescence.
Inhibition of sporozoite invasion (ISI) assay.
Human antibodies against N and C peptides were tested for the capacity to inhibit VK247 P. vivax sporozoite invasion of HepG2-A16 hepatoma cells in vitro. Inhibition induced by anti-R peptide antibodies could not be assessed because of the high prevalence of the VK247 variant parasites and the scarcity of VK210 in local endemic regions, that prevents the production of fresh, live sporozoites for in vitro cultures on due times. The HepG2-A16 cells were collected, washed, and resuspended in RPMI medium 1640 (Gibco, Grand Island, NY) and then plated on Labtek chamber slide system (NUNC, Rochester, NY) at a density of 8 × 104 cells/0.3 mL of William's medium supplemented with L-glutamine, Penicillin/Streptomycin (Gibco BRL, France), fetal bovine serum (FBS). Cultures were incubated overnight at 37°C in a 5% CO2 incubator. Before infection, the cells were irradiated at 3,000 rad, and the culture medium removed before addition of 5 × 104 P. vivax sporozoites diluted in 0.1 mL medium. Next, a 1/100 dilution of sera from volunteers immunized with N or C peptides was added to each well (in duplicate). Sporozoites were allowed to invade liver cells for 5 days, and were then washed with PBS pH 7.4 and fixed with cold methanol. Immuno-staining was performed using antibodies directed against parasite-specific HSP70, and secondary fluorescent Alexa 488-labeled anti-mouse antibodies (Molecular Probes, Invitrogen, Carlsbad, CA), diluted 1/200 and 1/600, respectively, to assess the presence and degree of liver-stage parasite maturation. Fluorescence was read using an epifluorescence microscope (Eclipse 50i; Nikon, Avon, MA). Percentage of invasion inhibition was calculated by the formula ([mean schizont number of negative control – mean schizont number of sample/mean schizont number of negative control] × 100).39
Enzyme-linked immunosorbent spot (ELIspot) assay for enumeration of peptide-specific IFN-γ-secreting cells.
The PBMC collected at Months 3, 7, and 9 were separated from whole blood using Ficoll Histopaque (Sigma-Aldrich, St. Louis, MO) density gradients, resuspended in RPMI 1640 medium, and used for enumerating IFN-γ-producing cells by the ELIspot assay. Cells were stimulated with the N- and C-LSP and with short peptides known to contain CD8+ epitopes with different binding motifs restricted by HLA class I alleles.25 Briefly, microtiter plates (Multiscreen MAHAS 4510, Millipore, Bedford, MA) were coated with anti-human IFN-γ MAb (1-D1K; Mabtech AB, Stockholm, Sweden) and incubated at 4°C overnight, and then blocked for 2 hrs at room temperature with RPMI medium containing 10% human AB serum. A suspension of 4 × 105 PBMC/well was mixed with 10 μg/mL of each synthetic peptide and incubated for 40 hrs at 37°C and 5% CO2. After plates were washed with PBS/0.05% Tween-20, 1 μg/mL biotinylated anti-IFN-γ MAb (7-B6-1; Mabtech AB) was added, and incubated for 3 hrs at room temperature. Next, streptavidine alkaline phosphatase (Boehringer Mannheim, Mannheim, Germany) diluted to 1:1,000 was added, followed by addition of 5-bromo-2-chloro-3-indolyl phosphatase/nitroblue tetrazolium (Sigma) as enzyme substrate. The number of dark blue spots appearing was determined by an automatic spot-counting system (Scanalytic, Fairfax, VA). The magnitude of the responses was expressed as the mean number of spot-forming cells per 106 PBMC collected on Months 3, 7, and 9.
HLA determination.
Class I HLA haplotypes were determined by polymerase chain reaction-sequence-specific primers (PCR-SSP) analysis using a commercial kit (Biotest SSP typing kit, Dreieich, Germany) on whole blood collected in EDTA vacutainer tubes. The DNA was extracted by a short salting-out method40 and quantification was performed by measuring the absorbance at 260 nm and purity at 280 nm. The DNA oligotyping was carried out by SSP and the thermal cycler (Applied Biosystems, Carlsbad, CA) programmed following insert instructions. Amplified products were identified after electrophoresis on a 2% agarose gel; results were analyzed by a software system (Biotest).
Results
Antibody-mediated immune responses.
Although almost all (95%) of the volunteers vaccinated with individual LSP generated anti-peptide IgG antibodies against the N, R, and C regions of the P. vivax CS protein, different response profiles were observed. Total IgG antibody profiles to the non-repetitive N and C regions of the PvCS protein are presented in Figure 1. All volunteers (7/7) immunized with the N peptide elicited specific IgG antibody responses with mean titers ranging from 1,200 to 9,800; in contrast to volunteers immunized with the C peptide who developed significantly lower antibody titers with means ranging from 200 to 3,200 as determined by ELISA. The serum IgG isotype profiles of volunteers immunized with the N peptide indicated a predominant induction of IgG1 and IgG3, suggesting that this peptide contains epitopes, which are main targets of Th1 responses, whereas IgG1 presented higher titers (ratios > 10) in all volunteers (7/7) as compared with IgG3, which presented similar ratios in only a portion (3/7) of volunteers. Because of the low antibody titers to the C peptide, we could differentiate a similar isotype shift in only one volunteer.
Figure 1.
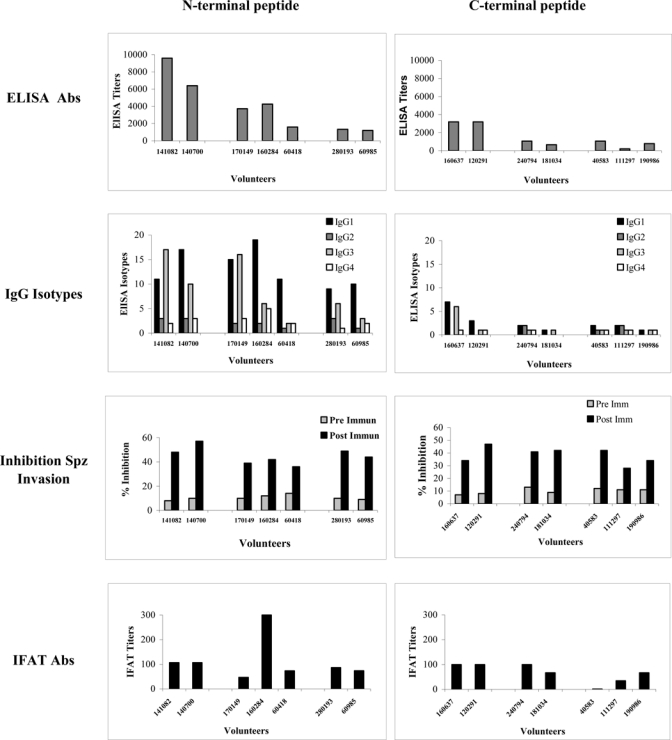
Antibody response profiles in human volunteers immunized with N and C peptides.
Specific anti-peptide antibodies were tested by IFAT to assess the recognition of the parasite protein. Independent of antibody titers, almost all sera recognized the native protein with mean IFAT values ranging from 50 to 300. Serum from only one individual vaccinated with the C peptide failed to recognize sporozoites.
Figure 2 depicts the kinetics of IgG antibody responses in sera from Months 3, 7, and 9 of volunteers vaccinated with the R peptide. Antibody titers ranged from 400 to 12,800 by ELISA and from 50 to 800 by IFAT. Two individuals developed high anti-sporozoite antibody levels that correlated with anti-peptide IgG1 and IgG3 responses.
Figure 2.
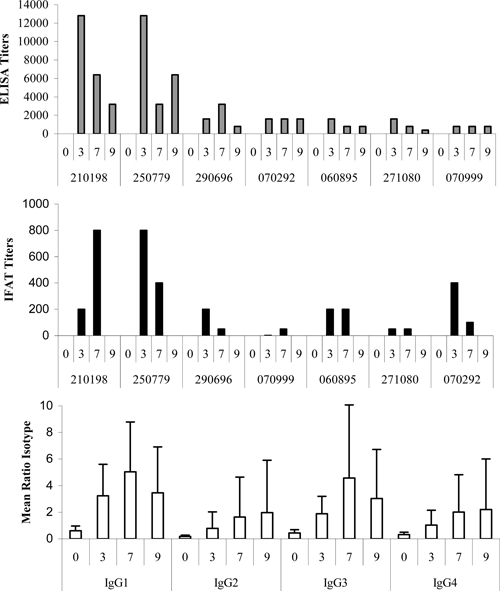
Antibody response profiles to the central repeat region.
Sporozoite invasion inhibit (ISI).
Functional activity of antibodies in sera of volunteers after the third immunization with N and C peptides were tested by ISI assays to determine the capacity of anti-N and anti-C antibodies to inhibit P. vivax sporozoite invasion of human hepatoma cells (HepG2À-16) in vitro. All sera resulted in strong inhibition of P. vivax sporozoite invasion as compared with pre-immune sera (Figure 1). Percentage of inhibition ranged between 36% and 57% (median = 46%) for volunteers immunized with the N peptide and between 32% and 52% (median = 38%) in volunteers immunized with the C peptide.
Fine specificity of antibodies responses.
Total IgG responses to N- or C-LSP and to 21 overlapping peptides derived from the PvCS protein are presented in Figure 3. Most of the individuals (4/7) developed antibodies directed to the N-terminal region with high antibody titers (> 6,000), specifically against peptides p8, p9, and p10 with titers ranging from 1,600 to 12,800. Two of seven individuals vaccinated with the C-LSP presented antibody titers ranging between 200 and 3,200; 6/7 individuals recognized peptide p21 with low antibody titers (400–800). None of the remaining short overlapping peptides from both N and C induced significant antibody titers (200–400).
Figure 3.
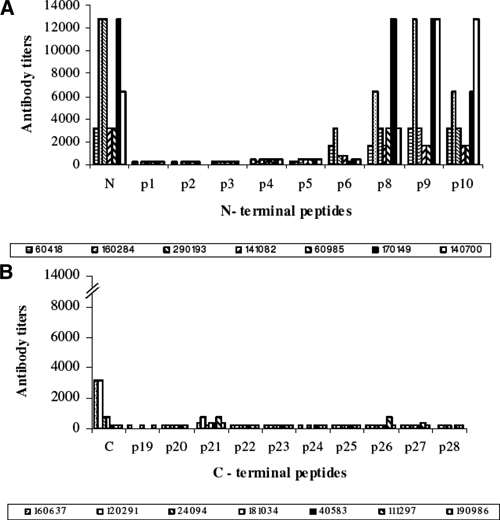
Comparison of immunoglobulin G (IgG) antibody titers to defined B-cell epitopes located in (A) N- and (B) C-terminal regions.
Cell-mediated immune responses.
Table 1 shows mean values of IFN-γ of three analyses and the peak value found at any time point in the study. Cells from 94% of the volunteers showed IFN-γ responses, regardless of the peptide. Six of seven volunteers in the N-peptide group and 5/7 of the group immunized with C peptide were able to produce an IFN-γ response. A greater and more uniform response was observed with the N-peptide group as compared with those vaccinated with peptide C.
Table 1.
Mean values of interferon gamma (IFN-γ) response of volunteers on stimulation with LSP and peptides restricted to different HLA alleles from the N- and C-terminal regions of the Plasmodium vivax circumsporozite (CS) protein
| Volunteers | HLA class I typing | N* | Pv6† | Pv8‡ | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | A2,24 | B35,38 | 9 (26) | 16 (30) | 6 (13) | |||
| 2 | A24,0 | B40,57 | 11 (25) | 30 (85) | 38 (107) | |||
| 3 | A1,23 | B15,48 | 24 (29) | 15 (32) | 4 (7) | |||
| 4 | A24,0 | B35,40 | 73 (120) | 26 (29) | 51 (132) | |||
| 5 | A24,32 | B8,52 | 0 | 19 (28) | 2 (4) | |||
| 6 | A24,0 | B35,38 | 39 (116) | 25 (51) | 20 (52) | |||
| C§ | Pv1¶ | Pv3¶ | Pv5¶ | Pv7¶ | Pv9‖ | |||
| 8 | A24,29 | B35,38 | 31 (93) | 0 | 22 (66) | 80 (39) | 38 (115) | 0 |
| 9 | A24,0 | B40,57 | 6 (10) | 13 (14) | 12 (25) | 5 (7) | 13 (24) | 7 (16) |
| 10 | A24,0 | B15,48 | 38 (111) | 0 | 0 | 26 (38) | 0 | 51 (65) |
| 11 | A24,29 | B35,38 | 0 | 0 | 3 (9) | 13 (25) | 0 | 0 |
| 12 | A24,5 | B15,44 | 0 | 0 | 34 (102) | 82 (205) | 16 (48) | 16 (47) |
| 13 | A2,24 | B40,57 | 6 (8) | 0 | 0 | 15 (24) | 0 | 1 (13) |
| 14 | A24,0 | B35,38 | 27 (65) | 52 (72) | 31 (50) | 4 (10) | 107 (121) | 201 (208) |
N-terminal long synthetic peptide (LSP).
HLA-A2 Pv CTL epitope.
HLA-B35 restricted peptide.
C-terminal long synthetic peptide (LSP).
HLA-A2 Pv restricted epitodes.
HLA-B53 restricted peptide.
The PBMC of immunized volunteers were also stimulated in vitro with short peptides restricted by different HLA class I molecules. Pv6 and Pv8 contained sequences located within the N-terminal region, whereas Pv1, Pv3, Pv5, Pv7, and Pv9 were all located in the C-terminal region. All short peptides from the N-terminal region were recognized by all volunteers independent of HLA haplotype.
Peptide Pv8, restricted to HLA-B35, stimulated IFN-γ production in three volunteers typed as HLA-B35; one presented with the highest response, whereas the other two individuals produced lower responses.
Pv1, Pv3, Pv5, Pv6, and Pv7 are all HLA-A*0201-restricted epitopes; two volunteers carried this HLA allele. These two individuals recognized some of these peptides (Pv6 and Pv5), but not the others. It is striking that Pv5 was recognized by all volunteers independent of their haplotypes. Pv9 contained an epitope predicted to be restricted by HLA-B53, although none of the volunteers displayed this HLA allele. Pv9 stimulated IFN-γ production in four other volunteers carrying different HLA-B alleles among which one volunteer (V-14) produced the greatest response. In general, more individuals reacted to peptides than originally predicted according to their haplotypes.
Discussion
Vaccination of malaria-naive volunteers with LSP derived from the P. vivax CS protein formulated in Montanide ISA 720 has previously been shown to be safe, well tolerated, and highly immunogenic.25 Vaccination induced rapid antibody responses after the first immunization, which was frequently boosted by a second immunizing dose, with antibody levels remaining stable for about 1 year. This trend had been previously observed in multiple preclinical trials conducted by our group using different vaccines formulated in Montanide ISA 720.6,41 Antibody responses were consistently more vigorous in volunteers immunized with peptide N than in volunteers immunized with peptides R or C. The bias of antibody responses toward the N-terminal region of the P. vivax CS protein has also been observed in primates vaccinated with the same peptides29 and during natural P. vivax and P. falciparum infections.28,42 However, on the basis of preclinical studies, the C-terminal region of the P. falciparum CS was selected to be included in the Pf-RTS,S vaccine candidate. Results of this study favor the selection of the N- terminal region of the P. vivax protein as a subunit vaccine candidate.
Recognition of the native sporozoite protein by sera from most volunteers (20/21) as determined by IFAT is encouraging and indirectly confirms the proper conformation of the peptides. The fact that sera of one of the immunized volunteers did not recognize the native protein is striking. Although it may indicate recognition of linear sequences of the LSP that are not surface-exposed, i.e., sequestered, in the native protein, it is surprising that the same serum was able to inhibit parasite invasion of hepatoma cells in vitro.
It is remarkable that sera from all volunteers immunized with the N peptide primarily recognized peptides p8, p9, and p10 with high antibody titer ranging from 2,000 to 12,800. These three peptides span a 40 aa sequence in the N-terminal region (aa position 71–111) that contains a dominant B-cell epitope p8 (aa 71–90) and the highly conserved RI region (KLKQP), which is known to be involved in parasite binding to hepatocytes.43 This fragment, which is preferentially recognized by sera of individuals naturally exposed to malaria in endemic areas of Colombia,28 contains a protein cleavage site that has been shown to be critical for P. falciparum sporozoites invasion.44,45
Therefore, antibodies may play an important role in blocking the required protein cleavage site. The partial invasion inhibition produced by antibodies in response to the various protein fragments could be mediated by different mechanisms, and thus exert an additive effect that could generate a more robust blockade of the parasite in vivo.
All sera were capable of at least partial inhibition of invasion, suggesting that both regions RI and RII-plus located in peptides N and C, respectively, participate independently in sporozoite invasion,43 and that antibodies directed to these regions may interfere with ligand-receptor interactions leading to inhibition of hepatocyte invasion. Inhibition of sporozoite invasion by sera with low ELISA or IFAT antibody titers may indicate other functional factors such as IgG isotype or fine specificity of antibodies are important. Alternatively, sporozoite inhibition may have also been affected in part by cytokines or other factor(s) present in sera as described previously in studies on P. falciparum.5,46
Moreover, it is interesting that all three peptides induced stronger IgG1 and IgG3 antibody responses, suggesting that the antibody response is Th1-dependent, and may play an important protective role in immune phagocytosis of sporozoites by monocytes and macrophages.47 Both IgG1 and IgG3 have high affinities for Fc receptors, fix complement, and are preferentially induced during Th1-type responses. In addition, antibody-dependent cell inhibition of parasite growth, known to be associated with IgG1 and IgG3 in inhibiting asexual blood-stage development, has not been sufficiently emphasized as a possible protective mechanism against pre-erythrocytic stages.48 Indeed, the pattern of antibody responses to each of the three peptides in this study was consistent with responses induced in studies with mice immunized with repeat and non-repeat regions of the P. vivax CS protein formulated in different adjuvants, in which the predominant IgG2a/IgG2b and IgG1 murine isotypes are known to be cytophilic.49 Although different MAbs may bind to the respective human IgG subclass with different affinity and specificity, we standardized the ELISA for each IgG subclass to ascertain approximately the same sensitivity for each subclass, as described for previous studies with other malaria antigens.34–36
The PBMC from most volunteers were able to secrete IFN-γ from T cells upon in vitro stimulation with peptides restricted to different HLA class I molecules; no association could be found with specific HLA-restricting alleles. Although we had several peptides containing HLA-A*0201-restricted epitopes (Pv1, Pv3, Pv5, Pv6, and Pv7) and two volunteers carrying HLA-A*0201 allelic haplotypes, IFN-γ levels were low in these volunteers but variable responses in individuals with unrelated HLA haplotypes. It is likely caused by cross-reactivity between these peptides with HLA-A24, which was found to be the most frequent allele found in the study (N = 13/14). None of the peptides included in this study had been predicted to be recognized in the context of HLA-A24, however previous studies showed that not only a broad cross-reactivity within the A2 subgroup, but also cross-reactivity with A24, A26, A28, and A29.50,51 Most peptides in our study contained amino acid sequences closely related to those known to be recognized by HLA-A24.51 This may explain why most volunteers of our study produced IFN-γ with or without known peptide-HLA matching. Our results appear to be in contrast to those of a recent study, where only one of nine HLA-A24 volunteers, previously exposed to P. falciparum malaria responded to five of 14 pre-erythrocytic stage epitopes predicted to be recognized in the context of that allele.52 In general, it would be important that a P. vivax pre-erythrocytic malaria vaccine stimulate CD4+ and CD8+ IFN-γ-secreting-cells that could play a significant protective role. Identification of other potential CD8+ epitopes restricted to different HLA molecules and further characterization of the cellular mechanisms involved in protective immunity are required.
In conclusion, these vaccine formulations induced highly specific antibodies reactive with the parasite and that functionally blocked parasite invasion in vitro. Together with specific induction of IFN-γ production, these immune mechanisms may play a synergistic role in the protection of the volunteers from sporozoite challenge. On the basis of their safety, high tolerability, and immunogenicity, these vaccine candidates are being proposed for a phase II clinical trial to determine protective efficacy.
ACKNOWLEDGMENTS
We acknowledge the invaluable collaboration of the study participants, the support of Leonel Gulloso for statistical analysis and Vincent Ganne (Seppic, France) for his collaboration in supplying the Montanide ISA 720 adjuvant. We also acknowledge the technical assistance of Claudia García in preparing the manuscript.
Footnotes
Financial support: This work was supported by grants from the Instituto Colombiano “Francisco José de Caldas” para la Ciencia y la Tecnología COLCIENCIAS, the Colombian Ministry for Social Protection (contract no. 216-2006) and the Health Department of the Valle del Cauca, Cali, Colombia. The UNDP/World Bank/World Health Organization, Special Programme for Research and Training in Tropical Diseases (WHO/TDR) provided us with valuable advice and clinical monitoring. This study was supported by the provision of facilities by the Tropical Medicine Research Center (TMRC-Cali) (NIAID contract no. 49486-01). R. Palacios was supported by a student grant from the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Authors' addresses: Myriam Arévalo-Herrera, Liliana Soto, Blanca Liliana Perlaza, Nora Céspedes, Omaira Vera, Ana Milena Lenis, Anilza Bonelo, and Sócrates Herrera, Instituto de Inmunología, Edificio de Microbiología, Facultad de Salud, Universidad del Valle and Centro Internacional de Vacunas, Cali, Colombia, E-mails: marevalo@inmuno.org, lsoto@inmuno.org, bperlaza@inmuno.org, ncespedes@inmuno.org, ovlizcano@gmail.com, ana_lenis@yahoo.com, anbonelo@yahoo.com, and sherrera@inmuno.org. Giampietro Corradin, Biochemistry Institute, University of Lausanne, Lausanne, Switzerland, E-mail: giampietro.corradin@unil.ch.
Reprint requests: Sócrates Herrera, Malaria Vaccine and Drug Development Center, Carrera 37 - 2Bis No. 5E - 08, Cali, Colombia, E-mail: sherrera@inmuno.org.
References
- 1.Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- 2.Clyde DF. Immunity to falciparum and vivax malaria induced by irradiated sporozoites: a review of the University of Maryland studies, 1971–75. Bull World Health Organ. 1990;68((Suppl)):9–12. [PMC free article] [PubMed] [Google Scholar]
- 3.Herrington D, Davis J, Nardin E, Beier M, Cortese J, Eddy H, Losonsky G, Hollingdale M, Sztein M, Levine M, Nussenzweig RS, Clyde D, Edelman R. Successful immunization of humans with irradiated malaria sporozoites: humoral and cellular responses of the protected individuals. Am J Trop Med Hyg. 1991;45:539–547. doi: 10.4269/ajtmh.1991.45.539. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, Gordon DM, Stoute JA, Church LW, Sedegah M, Heppner DG, Ballou WR, Richie TL. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 5.Hollingdale MR, Nardin EH, Tharavanij S, Schwartz AL, Nussenzweig RS. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells; an in vitro assay of protective antibodies. J Immunol. 1984;132:909–913. [PubMed] [Google Scholar]
- 6.Herrera S, Corradin G, Arévalo-Herrera M. An update on the search for a Plasmodium vivax vaccine. Trends Parasitol. 2007;23:122–128. doi: 10.1016/j.pt.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Sharma S, Pathak S. Malaria vaccine: a current perspective. J Vector Borne Dis. 2008;45:1–20. [PubMed] [Google Scholar]
- 8.Nardin E, Zavala F, Nussenzweig V, Nussenzweig RS. Pre-erythrocytic malaria vaccine: mechanisms of protective immunity and human vaccine trials. Parassitologia. 1999;41:397–402. [PubMed] [Google Scholar]
- 9.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 10.Doolan DL, Martinez-Alier N. Immune response to pre-erythrocytic stages of malaria parasites. Curr Mol Med. 2006;6:169–185. doi: 10.2174/156652406776055249. [DOI] [PubMed] [Google Scholar]
- 11.Mellouk S, Green SJ, Nacy CA, Hoffman SL. IFN-gamma inhibits development of Plasmodium berghei exoerythrocytic stages in hepatocytes by an L-arginine-dependent effector mechanism. J Immunol. 1991;146:3971–3976. [PubMed] [Google Scholar]
- 12.Mellouk S, Maheshwari RK, Rhodes-Feuillette A, Beaudoin RL, Berbiguier N, Matile H, Miltgen F, Landau I, Pied S, Chigot JP, Friedman RM, Mazier D. Inhibitory activity of interferons and interleukin 1 on the development of Plasmodium falciparum in human hepatocyte cultures. J Immunol. 1987;139:4192–4195. [PubMed] [Google Scholar]
- 13.Egan JE, Weber JL, Ballou WR, Hollingdale MR, Majarian WR, Gordon DM, Maloy WL, Hoffman SL, Wirtz RA, Schneider I, Woollett GR, Young JF, Hockmeyer WT. Efficacy of murine malaria sporozoite vaccines: implications for human vaccine development. Science. 1987;236:453–456. doi: 10.1126/science.3551073. [DOI] [PubMed] [Google Scholar]
- 14.Graves P, Gelband H. Vaccines for preventing malaria. Cochrane Database Syst Rev. 2003:CD000129. doi: 10.1002/14651858.CD000129. [DOI] [PubMed] [Google Scholar]
- 15.Moorthy V, Smith PG, Kieny MP. A vaccine against malaria: a substantial step forward. Lancet. 2009;373:1411–1412. doi: 10.1016/S0140-6736(09)60802-3. [DOI] [PubMed] [Google Scholar]
- 16.Stoute JA, Kester KE, Krzych U, Wellde BT, Hall T, White K, Glenn G, Ockenhouse CF, Garcon N, Schwenk R, Lanar DE, Sun P, Momin P, Wirtz RA, Golenda C, Slaoui M, Wortmann G, Holland C, Dowler M, Cohen J, Ballou WR. Long-term efficacy and immune responses following immunization with the RTS,S malaria vaccine. J Infect Dis. 1998;178:1139–1144. doi: 10.1086/515657. [DOI] [PubMed] [Google Scholar]
- 17.Bojang KA, Milligan PJ, Pinder M, Vigneron L, Alloueche A, Kester KE, Ballou WR, Conway DJ, Reece WH, Gothard P, Yamuah L, Delchambre M, Voss G, Greenwood BM, Hill A, McAdam KP, Tornieporth N, Cohen JD, Doherty T. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358:1927–1934. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 18.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, Mandomando I, Spiessens B, Guinovart C, Espasa M, Bassat Q, Aide P, Ofori-Anyinam O, Navia MM, Corachan S, Ceuppens M, Dubois MC, Demoitie MA, Dubovsky F, Menendez C, Tornieporth N, Ballou WR, Thompson R, Cohen J. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–1420. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 19.Aponte JJ, Aide P, Renom M, Mandomando I, Bassat Q, Sacarlal J, Manaca MN, Lafuente S, Barbosa A, Leach A, Lievens M, Vekemans J, Sigauque B, Dubois MC, Demoitie MA, Sillman M, Savarese B, McNeil JG, Macete E, Ballou WR, Cohen J, Alonso PL. Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet. 2007;370:1543–1551. doi: 10.1016/S0140-6736(07)61542-6. [DOI] [PubMed] [Google Scholar]
- 20.Moorthy VS, Reed Z, Smith PG. Clinical trials to estimate the efficacy of preventive interventions against malaria in paediatric populations: a methodological review. Malar J. 2009;8:23. doi: 10.1186/1475-2875-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macete E, Aponte JJ, Guinovart C, Sacarlal J, Ofori-Anyinam O, Mandomando I, Espasa M, Bevilacqua C, Leach A, Dubois MC, Heppner DG, Tello L, Milman J, Cohen J, Dubovsky F, Tornieporth N, Thompson R, Alonso PL. Safety and immunogenicity of the RTS,S/AS02A candidate malaria vaccine in children aged 1–4 in Mozambique. Trop Med Int Health. 2007;12:37–46. doi: 10.1111/j.1365-3156.2006.01754.x. [DOI] [PubMed] [Google Scholar]
- 22.Macete EV, Sacarlal J, Aponte JJ, Leach A, Navia MM, Milman J, Guinovart C, Mandomando I, Lopez-Pua Y, Lievens M, Owusu-Ofori A, Dubois MC, Cahill CP, Koutsoukos M, Sillman M, Thompson R, Dubovsky F, Ballou WR, Cohen J, Alonso PL. Evaluation of two formulations of adjuvanted RTS, S malaria vaccine in children aged 3 to 5 years living in a malaria-endemic region of Mozambique: a Phase I/IIb randomized double-blind bridging trial. Trials. 2007;8:11. doi: 10.1186/1745-6215-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bojang KA, Olodude F, Pinder M, Ofori-Anyinam O, Vigneron L, Fitzpatrick S, Njie F, Kassanga A, Leach A, Milman J, Rabinovich R, McAdam KP, Kester KE, Heppner DG, Cohen JD, Tornieporth N, Milligan PJ. Safety and immunogenicty of RTS,S/AS02A candidate malaria vaccine in Gambian children. Vaccine. 2005;23:4148–4157. doi: 10.1016/j.vaccine.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Sun P, Schwenk R, White K, Stoute JA, Cohen J, Ballou WR, Voss G, Kester KE, Heppner DG, Krzych U. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. J Immunol. 2003;171:6961–6967. doi: 10.4049/jimmunol.171.12.6961. [DOI] [PubMed] [Google Scholar]
- 25.Herrera S, Bonelo A, Perlaza BL, Fernandez OL, Victoria L, Lenis AM, Soto L, Hurtado H, Acuna LM, Velez JD, Palacios R, Chen-Mok M, Corradin G, Arévalo-Herrera M. Safety and elicitation of humoral and cellular responses in colombian malaria-naive volunteers by a Plasmodium vivax circumsporozoite protein-derived synthetic vaccine. Am J Trop Med Hyg. 2005;73:3–9. doi: 10.4269/ajtmh.2005.73.3. [DOI] [PubMed] [Google Scholar]
- 26.Yadava A, Sattabongkot J, Washington MA, Ware LA, Majam V, Zheng H, Kumar S, Ockenhouse CF. A novel chimeric Plasmodium vivax circumsporozoite protein induces biologically functional antibodies that recognize both VK210 and VK247 sporozoites. Infect Immun. 2007;75:1177–1185. doi: 10.1128/IAI.01667-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arévalo-Herrera M, Herrera S. Plasmodium vivax malaria vaccine development. Mol Immunol. 2001;38:443–455. doi: 10.1016/s0161-5890(01)00080-3. [DOI] [PubMed] [Google Scholar]
- 28.Arévalo-Herrera M, Roggero MA, Gonzalez JM, Vergara J, Corradin G, Lopez JA, Herrera S. Mapping and comparison of the B-cell epitopes recognized on the Plasmodium vivax circumsporozoite protein by immune Colombians and immunized Aotus monkeys. Ann Trop Med Parasitol. 1998;92:539–551. [PubMed] [Google Scholar]
- 29.Herrera S, Bonelo A, Perlaza BL, Valencia AZ, Cifuentes C, Hurtado S, Quintero G, Lopez JA, Corradin G, Arévalo-Herrera M. Use of long synthetic peptides to study the antigenicity and immunogenicity of the Plasmodium vivax circumsporozoite protein. Int J Parasitol. 2004;34:1535–1546. doi: 10.1016/j.ijpara.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Arévalo-Herrera M, Valencia AZ, Vergara J, Bonelo A, Fleischhauer K, Gonzalez JM, Restrepo JC, Lopez JA, Valmori D, Corradin G, Herrera S. Identification of HLA-A2 restricted CD8(+) T-lymphocyte responses to Plasmodium vivax circumsporozoite protein in individuals naturally exposed to malaria. Parasite Immunol. 2002;24:161–169. doi: 10.1046/j.1365-3024.2002.00449.x. [DOI] [PubMed] [Google Scholar]
- 31.Atherton E, Logan CJ, Sheppard RC. Procedures for solid-phase synthesis using N-fluorenylmethoxycarbonylamino-acids on polyamide supports. Synthesis of substance P and of acyl carrier protein 65–74 decapeptide. J Chem Soc, Perkin Trans. 1981;1:538–546. [Google Scholar]
- 32.Roggero MA, Servis C, Corradin G. A simple and rapid procedure for the purification of synthetic polypeptides by a combination of affinity chromatography and methionine chemistry. FEBS Lett. 1997;408:285–288. doi: 10.1016/s0014-5793(97)00441-9. [DOI] [PubMed] [Google Scholar]
- 33.Bouharoun-Tayoun H, Druilhe P. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect Immun. 1992;60:1473–1481. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Druilhe P, Tall A, Sokhna C. Worms can worsen malaria: towards a new means to roll back malaria? Trends Parasitol. 2005;21:359–362. doi: 10.1016/j.pt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Oeuvray C, Theisen M, Rogier C, Trape JF, Jepsen S, Druilhe P. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect Immun. 2000;68:2617–2620. doi: 10.1128/iai.68.5.2617-2620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soe S, Theisen M, Roussilhon C, Aye KS, Druilhe P. Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect Immun. 2004;72:247–252. doi: 10.1128/IAI.72.1.247-252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurtado S, Salas ML, Romero JF, Zapata JC, Ortiz H, Arévalo-Herrera M, Herrera S. Regular production of infective sporozoites of Plasmodium falciparum and P. vivax in laboratory-bred Anopheles albimanus. Ann Trop Med Parasitol. 1997;91:49–60. doi: 10.1080/00034983.1997.11813111. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez JM, Hurtado S, Arévalo-Herrera M, Herrera S. Variants of the Plasmodium vivax circumsporozoite protein (VK210 and VK247) in Colombian isolates. Mem Inst Oswaldo Cruz. 2001;96:709–712. doi: 10.1590/s0074-02762001000500023. [DOI] [PubMed] [Google Scholar]
- 39.Renia L, Vigario AM, Belnoue E. Inhibition of sporozoite invasion. The double-staining assay. Methods Mol Med. 2002;72:507–516. doi: 10.1385/1-59259-271-6:507. [DOI] [PubMed] [Google Scholar]
- 40.Nasiri H, Forouzandeh M, Rasaee MJ, Rahbarizadeh F. Modified salting-out method: high-yield, high-quality genomic DNA extraction from whole blood using laundry detergent. J Clin Lab Anal. 2005;19:229–232. doi: 10.1002/jcla.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrera S, De Plata C, Gonzalez M, Perlaza BL, Bettens F, Corradin G, Arévalo-Herrera M. Antigenicity and immunogenicity of multiple antigen peptides (MAP) containing P. vivax CS epitopes in Aotus monkeys. Parasite Immunol. 1997;19:161–170. doi: 10.1046/j.1365-3024.1997.d01-193.x. [DOI] [PubMed] [Google Scholar]
- 42.Bongfen SE, Ntsama PM, Offner S, Smith T, Felger I, Tanner M, Alonso P, Nebie I, Romero JF, Silvie O, Torgler R, Corradin G. The N-terminal domain of Plasmodium falciparum circumsporozoite protein represents a target of protective immunity. Vaccine. 2009;27:328–335. doi: 10.1016/j.vaccine.2008.09.097. [DOI] [PubMed] [Google Scholar]
- 43.Ying P, Shakibaei M, Patankar MS, Clavijo P, Beavis RC, Clark GF, Frevert U. The malaria circumsporozoite protein: interaction of the conserved regions I and II-plus with heparin-like oligosaccharides in heparan sulfate. Exp Parasitol. 1997;85:168–182. doi: 10.1006/expr.1996.4134. [DOI] [PubMed] [Google Scholar]
- 44.Coppi A, Pinzon-Ortiz C, Hutter C, Sinnis P. The Plasmodium circumsporozoite protein is proteolytically processed during cell invasion. J Exp Med. 2005;201:27–33. doi: 10.1084/jem.20040989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ancsin JB, Kisilevsky R. A binding site for highly sulfated heparan sulfate is identified in the N terminus of the circumsporozoite protein: significance for malarial sporozoite attachment to hepatocytes. J Biol Chem. 2004;279:21824–21832. doi: 10.1074/jbc.M401979200. [DOI] [PubMed] [Google Scholar]
- 46.Fries LF, Gordon DM, Schneider I, Beier JC, Long GW, Gross M, Que JU, Cryz SJ, Sadoff JC. Safety, immunogenicity, and efficacy of a Plasmodium falciparum vaccine comprising a circumsporozoite protein repeat region peptide conjugated to Pseudomonas aeruginosa toxin A. Infect Immun. 1992;60:1834–1839. doi: 10.1128/iai.60.5.1834-1839.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliveira GA, Wetzel K, Calvo-Calle JM, Nussenzweig R, Schmidt A, Birkett A, Dubovsky F, Tierney E, Gleiter CH, Boehmer G, Luty AJ, Ramharter M, Thornton GB, Kremsner PG, Nardin EH. Safety and enhanced immunogenicity of a hepatitis B core particle Plasmodium falciparum malaria vaccine formulated in adjuvant Montanide ISA 720 in a phase I trial. Infect Immun. 2005;73:3587–3597. doi: 10.1128/IAI.73.6.3587-3597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Druilhe P, Perignon JL. Mechanisms of defense against P. falciparum asexual blood stages in humans. Immunol Lett. 1994;41:115–120. doi: 10.1016/0165-2478(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 49.Thomas BE, Manocha M, Haq W, Adak T, Pillai CR, Rao DN. Modulation of the humoral response to repeat and non-repeat sequences of the circumsporozoite protein of Plasmodium vivax using novel adjuvant and delivery systems. Ann Trop Med Parasitol. 2001;95:451–472. doi: 10.1080/00034980120072275. [DOI] [PubMed] [Google Scholar]
- 50.Fruci D, Rovero P, Falasca G, Chersi A, Sorrentino R, Butler R, Tanigaki N, Tosi R. Anchor residue motifs of HLA class-I-binding peptides analyzed by the direct binding of synthetic peptides to HLA class I alpha chains. Hum Immunol. 1993;38:187–192. doi: 10.1016/0198-8859(93)90539-d. [DOI] [PubMed] [Google Scholar]
- 51.Tanigaki N, Fruci D, Chersi A, Falasca G, Tosi R, Butler RH. HLA-A2-binding peptides cross-react not only within the A2 subgroup but also with other HLA-A-locus allelic products. Hum Immunol. 1994;39:155–162. doi: 10.1016/0198-8859(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 52.Tippawangkosol P, Duangchanda T, Ubalee R, Ruengweerayut R, Hirayama K, Na-Bangchang K. Identification of HLA-A24 restricted pre-erythrocytic stage specific T-cell epitopes using Plasmodium falciparum synthetic peptides: a preliminary study. Southeast Asian J Trop Med Public Health. 2009;40:10–17. [PubMed] [Google Scholar]


