Abstract
Malaria infection induces antibodies capable of suppressing the infectivity of gametocytes and gametes, however, little is known about the duration of the antibody response, the parasite specificity, and the role of complement. We report the analyses of the transmission-blocking (TB) activity of sera collected from 105 Plasmodium vivax-infected and 44 non-infected individuals from a malaria endemic region of Colombia, using a membrane feeding assay in Anopheles albimanus mosquitoes. In infected donors we found that TB activity was antibody dose dependent (35%), lasted for 2–4 months after infection, and in 70% of the cases different P. vivax wild isolates displayed differential susceptibility to blocking antibodies. Additionally, in a number of assays TB was complement-dependent. Twenty-seven percent of non-infected individuals presented TB activity that correlated with antibody titers. Studies here provide preliminary data on factors of great importance for further work on the development of TB vaccines.
Introduction
Acquisition of immunity to malaria is a slow and complex process that varies among individuals and develops with age and number of malaria episodes.1,2 In areas of high malaria transmission, individuals naturally exposed to malaria develop a certain degree of clinical immunity that is maintained by repeated exposure to malaria infections.3 This clinical immunity is mainly elicited by pre-erythrocytic and asexual parasite blood stages and reduces parasite burden in circulation and the related physiopathological effects of the parasite. Additionally, the sexual parasite stages can stimulate immune responses in the vertebrate host that are capable of blocking parasite fertilization and ookinete invasion to the mosquito midgut, preventing the sporogonic development of the parasite in the Anopheles mosquito. This blockage appears to be primarily mediated by antibodies and possibly other mediators, in what is called transmission-blocking (TB) immunity.4,5 Immunological factors such as complement and cytokines appear to contribute to this TB immunity,6–8 and together with antibodies are considered valuable in reducing the burden of malaria transmission, particularly in areas with low transmission intensity.
Although individuals with high antibody concentrations are more efficient suppressors of parasite transmission to the mosquito, low concentrations of specific antibodies appear to produce a paradoxical enhancing effect on parasite transmission.9,10 Natural TB activity for Plasmodium falciparum infections has been demonstrated but less is known about TB immunity in P. vivax, the most common malaria parasite in Latin America, Asia, and other regions where 132–391 million malaria cases are reported yearly.11 It has been found that in high P. vivax transmission areas of Sri Lanka only 22% of sera from infected patients living in endemic settings have TB activity, whereas 65% of sera from acute infected patients living in non-endemic areas are blockers.10,12 There is very limited information about when during the infection, and for how long the blocking activity remains in endemic areas. The duration of the TB activity in the latter study was < 4 months,10,12,13 and it was hypothesized that TB immunity depends on recent rather than on cumulative exposure to parasite.9 In low-to-moderate malaria P. vivax transmissions setting of Latin America, only two studies have been reported, one in Mexico and another in Colombia.14,15 Both studies indicated high TB activity in infected individuals at the time of malaria diagnosis, but neither addressed the question about duration.
In addition to the role of antibodies in TB, the effect of complement has been documented in several Plasmodium species. Using immune sera raised against whole parasites and monoclonal antibodies, the complement appears to become activated both through the classical and the alternative pathways.16 Moreover, it has been shown that both monoclonal antibodies and antibodies of humans exposed to P. falciparum malaria, which recognize specific parasite antigens such as Pfs230 and Pfs48/45, contain complement fixing immunoglobulin G (IgG) isotypes and display greater effect in the presence of complement.17,18 Furthermore, PfCCp4, a protein from a group of six P. falciparum gametocyte proteins, named PfCCps because it contains adhesive motives belonging to the Limulus coagulation factor (LCCL), was found to form a protein complex with Pfs230 that appears to be associated with a complement-mediated reduction of male gametogenesis.19 In P. vivax, 50% of sera collected in an endemic region of Sri Lanka, were much more suppressive of mosquito infectivity if complement was present,10 and C4 levels decreased during the course of acute malaria infection.20 Because of the scarcity of data in other endemic regions of the world, and to better understand both the role of IgG and complement, the longevity of these TB responses and their effect on naturally circulating parasite populations, we conducted longitudinal and cross-sectional surveys in a low to moderate malaria transmission area of the Colombian Pacific coast.
Materials and Methods
Study area and population.
This study was conducted in Buenaventura, the main port on the Pacific coast of Colombia, which is a malaria-endemic area with low to moderate malaria transmission of both P. vivax and P. falciparum parasites. The population from this region is predominantly (> 90%) composed of African descendents with variable percentages of Duffy positive (Fy+) individuals in each community, and therefore variable proportions of individuals are susceptible to P. vivax infection in different villages.14 A total of 127 volunteers were studied; 105 corresponded to patients acutely infected with P. vivax who presented at the outpatient clinic at the Immunology Institute headquarters in Buenaventura, whereas 44 were individuals exposed to malaria transmission, but non-infected at the time of the study. These latter volunteers were recruited in rural localities of Buenaventura (La Delfina, Zacarías, La Triana, and Zaragosa) from a larger group of 119 potential participants that were screened for antibodies against blood stages of P. vivax and for the prevalence of Fy antigen to determine their susceptibility to P. vivax infection. The study protocol was reviewed and approved by the Institutional Review Board of the Universidad del Valle. The patients were considered eligible if they were > 18 years of age, had a mono-infection with P. vivax as determined by microscopic blood examination, and provided a written informed consent. For non-infected volunteers from rural settings, the inclusion criteria were that individuals were positive for both Fy+ and for anti-P. vivax antibodies as ascertained by immunofluorescence antibody test (IFAT).
Blood collection and malaria diagnosis.
Blood samples for malaria diagnosis were collected by finger-prick for thick or thin blood smears. Slides were Giemsa stained and parasite species identified by an experienced microscopist. Asexual and sexual P. vivax parasites were quantified in 100 leukocytes and expressed in microliters assuming a mean leukocyte count of 8,000/μL.21 Whole blood samples were obtained by venipuncture of donors selected on the basis of P. vivax parasitemia and gametocytemia, using both heparinized (5 mL) (Becton Dickinson, Inc., NJ) and dry vacutainer tubes (10 mL).
Duffy blood group typing.
Blood was collected from potential volunteers in the endemic villages described previously and analyzed by a nested polymerase chain reaction (PCR) as described elsewhere.22 Briefly, a set of two primers, P1 (CCT TTT TCC TGA GTG TAGT) and P2 (GCA GAG CTG CCA GCG GAA GA) were used for the first PCR reaction .The amplification product (1 kb product) was then used as a template for a second reaction using a second set of primers: P38 (AGG CTT GTG CAG GCA GTG) and P39 (GGC ATA GGG ATA AGG GACT) to obtain a product of 221 bp. Finally, after Sty I enzyme digestion for 4 hrs at 37°C the reaction and digested products were separated by sodium dodecyl sulfate-polyacrylamide electrophoresis (SDS-PAGE). Gels were stained with silver nitrate for the presence of three bands: 62, 77, and 82 bp were considered as positive to Fy antigen, whereas the presence of four bands: 12, 62, 65 and 82 bp were considered as negative for the presence of Fy antigen.
Specific antimalarial antibody detection.
Sera from P. vivax non-infected volunteers were evaluated for the presence of specific antibody titers against blood-stage parasites by an IFAT.14 Air-dried smears of P. vivax parasites obtained from infected patients containing both sexual and asexual blood forms were placed onto multispot microscope slides and incubated with 2-fold serial dilutions of sera samples starting at 1:100 diluted in phosphate-buffered saline (PBS) for 30 min at room temperature (20–24°C). After washing with PBS this reaction was developed with fluorescein-conjugated goat antihuman IgG (H+L) (Jackson Immunoresearch Laboratories, Inc., West Grove, PA) diluted at 1:100 in Evans blue solution (diluted 1:5,000 in PBS), and incubated for 30 min. Slides were then washed, overlaid with glass cover slips using 30% glycerol, examined by UV microscopy, and scored for the presence or absence of fluorescent parasites in the test samples. Antibody titers were determined as the reciprocal of the end-point dilution that showed positive fluorescence.
Membrane feeding assays (MFA).
Whole blood was used to feed Anopheles albimanus mosquitoes of the Buenaventura strain, that was reared in laboratory conditions under controlled temperature (24–27°C) and relative humidity (80–90%),23 and mosquito infections were performed using a standardized MFA as described previously.14 Briefly, infected blood samples were maintained at 37°C to prevent exflagellation before the MFA and 400 µL aliquots of whole blood were centrifuged and their corresponding P. vivax-infected red blood cell fractions were washed with two volumes of serum-free RPMI 1640 medium (Sigma, St. Louis, MO). These fractions were reconstituted with 200 µL of test serum and the other with the same volume of normal AB human serum (control) at a 50% hematocrit. The AB serum was obtained from healthy blood donors without malaria experience, from the Red Cross blood bank in Cali. Reconstituted blood samples were immediately placed in the artificial membrane feeding device, with regulated temperature at 37°C and offered to batches of 110 female of An. albimanus, 3–4 d old. After 30 min of feeding, unfed mosquitoes were removed from the cages. Seven to 8 days after feeding, mosquitoes were dissected to examine the number of oocysts in both control and test mosquitoes, to determine the percentage of reduction in the proportion of infected mosquitoes and the reduction in the mean oocyst counts/mosquito. In these assays, 40 mosquitoes were dissected and midguts were stained with 2% mercurochrome.24 Batches with fewer than 40 mosquitoes surviving were discarded. The number of oocysts per mosquito midgut was counted for test and control serum groups.
Presence of TB activity in non-infected individuals.
From a total of 119 non-infected individuals, 82 were IFAT positive with titers ranging from 1:100–1:1,600 and 37 were negative. Forty-four out of 82 sera from individuals with specific antibodies to P. vivax were Fy+ and selected for this study. Eleven groups of four sera were tested by MFA for the presence of TB activity against one strain of P. vivax parasite, each obtained from infected donors. Because Fy− individuals do not develop the erythrocytic parasite phase, sera from Fy− were not tested.
Potency of TB activity.
The inhibitory potency of sera on oocyst development was assessed in the MFA using serial dilutions of test sera with different levels of TB activity selected from the 83 P. vivax-infected individuals (12 patients with a high and intermediate level of TB activity at each level and 13 patients with ≤ 50% of TB). Thirty-seven assays were carried out using undiluted and 2-fold sera dilutions from P. vivax-infected subjects starting in 1:2 and tested for transmission-blocking activity using autologous P. vivax parasites.
Influence of complement on parasite development.
To assess the role of complement in P. vivax TB activity, we performed MFA using both fresh and heat-inactivated (56°C for 30 min) sera collected from 44 P. vivax malaria-infected individuals in paired assays using as control a pool of heat-inactivated AB sera.
Duration of TB activity.
A cohort of 24 volunteers from previous studies presenting high TB activity (TB: 90–100%) were selected and followed up in a longitudinal study every 2 months during an 8-month period. Volunteers were asked if they had suffered any malaria episode since the previous visit and blood samples were collected and assessed by thick smear to evaluate malaria re-infection, and by MFA to determine TB activity. Only six volunteers could be followed up in five opportunities without presenting either P. vivax or P. falciparum infection; the remaining were re-infected or refused to continue their participation.
TB responses using heterologous parasites.
To evaluate the cross-blocking activity of sera using heterologous P. vivax parasites, 37 sera previously classified in two levels of blockage as described below (high TB: N = 25 and intermediate TB: N = 12) and were tested in five groups of six sera each and one group of seven sera. Each group was tested by MFA performed with three different isolates of P. vivax collected from infected donors for a total of 18 different isolates. Nine sera (8%) were excluded because of the low number of mosquitoes per lots.
Statistical analysis.
Both the percentage reduction in the proportion of infected mosquitoes and the percentage reduction in mean oocyst counts/mosquito were estimated using the formula: [(Xc − Xa)/Xc)] × 100, where X is the proportion or arithmetic mean in control (c) and autologous (a) sera.25
Sera were classified depending on the level of blocking according to the following categories: high transmission was considered when sera had 90–100% of transmission reduction; intermediate transmission, those reducing 50–89%; and low transmission, those with reductions between 0% and 49% were considered enhancers of transmission if they produced better infection rates than the normal control sera (< 0%).
The Sperman's correlation coefficient was used to evaluate in non-infected donors, the correlation between the intensity of the immune response at four defined antibody titers (< 1:100, 1:400, 1:800) and the corresponding values of TB activity.
A binomial test was used to estimate the probability of ≥ 90% of TB activity in sera with heterologous parasites strains, results were divided into two groups (TB activity ≥ 90% and ≤ 89%) and the Wilcoxon signed-rank test to assess differences between paired in batches fed sera with and without complement.
Results
Antibodies against P. vivax parasite blood forms and Fy antigen.
From 119 sera of non-infected volunteers studied, 82 sera presented positive titers of specific antibodies to P. vivax blood forms and 37 were negative when tested by IFAT at a 1:100 sera dilution. From the group of individuals with positive antibody titers, 44 were Fy+ and 38 Fy−, the latter sera were excluded from the study. Specific anti-P. vivax antibody titers ranged from 1:100–1:1,600 (data not shown).
Prevalence of TB activity in non-infected individuals.
TB activity, as measured by percentage of reduction in the proportion of infected mosquitoes in a given lot and the percentage reduction in the mean occyst counts/mosquito of 44 Fy+ non-infected individuals, are summarized in Table 1. Five (11.4%) of 44 individuals displayed high levels of TB activity (> 90% reduction) as determined by reduction in infected mosquitoes in the corresponding lot and in the other three individuals (6.8%) sera induced > 90% oocyst reduction. Intermediate levels of TB activity (50–89%) were also more frequent for reduction in the number of infected mosquitoes (15.9%) than for oocyst reduction (9.1%). Percentage of reduction in oocyts count (36.4%) was more frequently observed than reduction in the number of infected mosquitoes (30%) in the range of low levels of TB activity (0–49%). In contrast, most of the subjects presented an enhancing effect (< 0% reduction), 19 presented a reduction (43%) in percentage infected mosquitoes and 21 a reduction of (48%) oocyst counts. Boxplots of TB activity according to serum antibodies titers are shown in Figure 1. The mean of TB activity for antibody titers ≤ 1:100, 1:400, and ≥ 1: 800 were 5.0%, 67.0%, 50.0%, respectively. Statistical significance between TB activity and increased antibody titers was observed (Spearman's correlation coefficient = 0.5, P = 0.0022). None of the individuals followed up presented malaria re-infections during the follow-up period.
Table 1.
TB activity as reduction in percentage of infected mosquitoes and mean oocyst count/mosquito among P. vivax non-infected individuals
| TB categories* | No. (%) of non-infected individuals per TB category | |||
|---|---|---|---|---|
| Infected mosquitoes | Oocyst/mosquito | |||
| % Reduction | n† | % | n† | % |
| 90–100 | 5 | 11.4 | 3 | 6.8 |
| 50–89 | 7 | 15.9 | 4 | 9.1 |
| 0–49 | 13 | 29.5 | 16 | 36.4 |
| < 0 (enhancing) | 19 | 43.2 | 21 | 47.7 |
| Total | 44 | 100 | 44 | 100 |
Categories of transmission-blocking (TB) activity to classify the percentage reduction of infected.
Total number of control positive batches of mosquitoes of the 44 paired membrane feeding assays.
Figure 1.
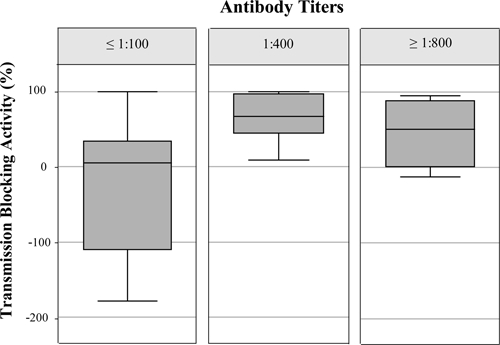
Boxplot of transmission-blocking (TB) activity according to serum antibody titers in non-infected subjects. The upper and lower quartiles of TB activity as percent of reduction in mean oocyst counts per mosquito. The central line is the median. The ends of the whiskers are 1.5 interquartile distance above the upper quartile to Plasmodium vivax in an immunofluorescent antibody test (IFAT).
Potency of TB activity.
From the total of 83 individuals acutely infected with P. vivax, 35.2% of the patient presented a high level (90–100%) of TB activity, as a reduction in percentage of oocyst count/mosquito, 31.4% of them had an intermediate level (50–89%), and 15.2% and 18.1% were in the range of low level (0–49%) and enhancing effect (< 0%), respectively. Figure 2 shows mean values of each dilution and the tendency of TB activity of the 37 samples studied. As observed, TB activity was maintained in all sera diluted up to 1:16 and an enhanced effect was observed in the following dilutions with the exception of sera with TB activity of 90–100%. Sera with TB activity less than 89% presented an enhancing effect when sera were diluted up to 1:8, whereas the opposite effect was observed in sera with an enhancing effect after sera dilution. As shown in Table 2 after sera dilution, samples with high and intermediate (> 50%) TB activity had the greater probability (0.43 and 0.40, respectively) to retain the TB activity in an intermediate level (50–89%) after sera dilution in contrast to sera with initial TB less that 50%, which had greater probability (0.69 and 0.55) to have a transmission enhancing effect upon dilution.
Figure 2.
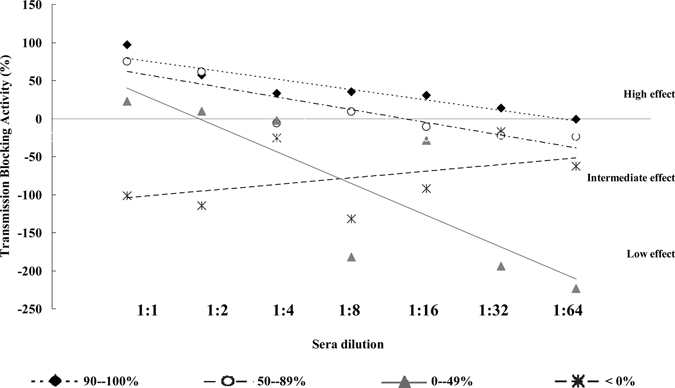
Potency of transmission-blocking (TB) activity, as a mean percentage in oocyst reduction, of 37 sera with a high level of transmission (90–100%) after 2-fold dilutions. Each dilution point corresponds to the mean value of sera stratified as a high level of transmission (90–100%), intermediate level of transmission (50–89%), low level of transmission (0–49%), and no transmission or enhancing effect (< 0%).
Table 2.
Probability of sera used in the potency assay to retain transmission-blocking (TB) or enhancing activities upon dilution
| TB categories (% reduction)* | Probability of sera to retain TB activity† | |||
|---|---|---|---|---|
| 90–100‡ | 50–89‡ | 0–49‡ | < 0‡ | |
| 90–100 | 0.06 | 0.02 | 0.00 | 0.00 |
| 50–89 | 0.43 | 0.40 | 0.05 | 0.06 |
| 0–49 | 0.24 | 0.36 | 0.26 | 0.39 |
| < 0 (enhancing) | 0.27 | 0.22 | 0.69 | 0.55 |
Categories of transmission-blocking activity to classify the percentage reduction of infected mosquitoes and oocyst count.
Analysis of the probablity of 37 sera at 2-fold dilution (1:2 to 1:64) to retain the ability of blocking at any of the TB categories.
Classification of percentage of transmission–blocking activity in 37 undiluted sera.
Influence of the complement system in parasite development.
In the total 44 paired samples studied it was found that 63.6% of lots fed with parasites in the presence of complement had a lower number of oocyst counts in contrast to 27.3% of lots fed with sera without complement. In the remaining samples (9.1%), both active and inactive complement led to similar oocyst counts (Figure 3).
Figure 3.
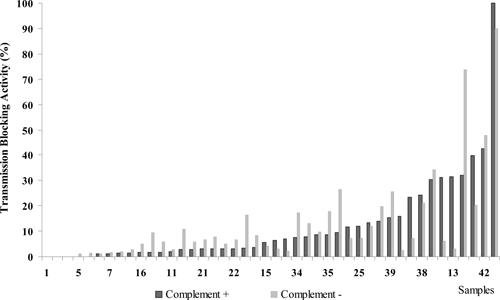
Role of complement in Plasmodium vivax transmission-blocking (TB) activity. A total of 44 paired assays using both fresh (Complement +) and heat-inactivated (Complement −) sera from P. vivax malaria-infected individuals were evaluated by membrane feeding assay (MFA). A pool of heat-inactivated AB sera was used as control.
No statistically significant difference was found between the TB of the group of sera with complement and the paired samples without complement. Results indicated no significant difference in the percentage of infected mosquitoes (Wilcoxon signed-rank, P = 0.246) or in the oocyst means (P = 0.226). However, a detailed analysis of the paired assays with sera that presented high TB activity (21/44), indicated that almost half of them (9/21) drastically decreased their TB capacity in the absence of complement, whereas the remaining did not present significant changes. Surprisingly, an analysis of 23 sera with low TB activity showed that four sera depleted of complement presented a significant increase (mean = 56%) in TB, whereas the remaining maintained the low TB levels (< 50%) or decreased it.
Duration of TB activity in individuals carrying TB antibodies.
A trend on decreasing TB activity was observed in individuals followed up for 8 months. Only one volunteer (code 630) was able to maintain TB levels of up to 40% during the five assays performed and variability of TB activity was observed in the remaining volunteers during a period of 2–8 months, with TB decreasing tendency (Figure 4).
Figure 4.
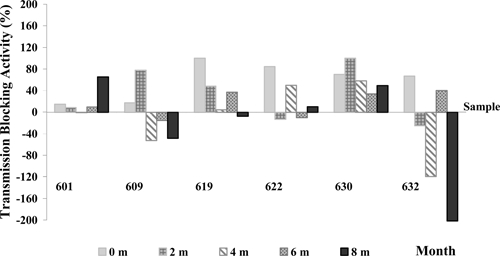
Longitudinal study of transmission-blocking (TB) immunity in Plasmodium vivax-infected subjects (N = 6) followed every 2 months during 8 months using MFA. Positive TB values indicate blockage, whereas negative values indicate parasite transmission enhancing.
TB activity on heterologous P. vivax strains.
Variability of TB activity in An. albimanus was observed in the sera of the two groups with higher activity that displayed different TB activity to heterologous P. vivax parasite strains. From the 25 sera with TB activity 90–100% on the autologous parasite 44.9% showed a similar level of blocking activity on heterologous parasites, 26% maintained high TB (50–89%) on the three tested isolates, whereas 20.3% presented significantly lower blockage (0–49%) in one or more of the heterologous parasites.
From the 12 sera with TB activity in the range 50–89% with the autologous parasite, TB activity showed similar TB effect with heterologous parasites in 30% of the cases, 20% of the assays indicated an increase of TB activity (90–100%), and in 50% of tests the TB decreased their effect with heterologous parasites (Table 3). No statistical differences were found between TB activities on autologous parasites as compare with heterologous parasites among the different TB categories as determined by a binomial test. The probability of a serum to maintain high TB activity (≥ 90%) on its autologous parasite when tested with heterologous parasites was of 0.5. For sera with TB activities in the range of 50–89% this probability was 0.4.
Table 3.
Evaluation of sera from Plasmodium vivax-infected individuals with high and intermediate levels of transmission-blocking (TB) activity against heterologous P. vivax parasites
| Code of sera with TB activity 90–100% | Transmission-blocking activity (%) | Code of sera with TB activity 50–89% | Transmission-blocking activity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Autologous parasite* | Heterologous parasite† | Autologous parasite* | Heterologous parasite† | ||||||
| 1 | 2 | 3 | 1 | 2 | 3 | ||||
| 54 | 100 | 100 | 100 | nd | 360 | 89.4 | 3.0 | −206.1 | 17.1 |
| 70 | 100 | 100 | 90.4 | nd | 368 | 89.2 | 20.5 | −94.4 | 80.3 |
| 68 | 100 | 100 | 81.3 | nd | 471 | 88.0 | 95.4 | 83.2 | 90.8 |
| 76 | 100 | 100 | 78.0 | nd | 360 | 87.4 | 57.8 | 88.0 | 32.4 |
| 107 | 100 | 100 | 37.8 | nd | 353 | 86.8 | 24.9 | −35.6 | 50.7 |
| 168 | 100 | 55.0 | 64.3 | nd | 366 | 83.1 | 41.0 | −81.6 | 55.8 |
| 79 | 100 | 80.9 | 39.8 | nd | 71 | 82.0 | 88.1 | 59.5 | nd |
| 333 | 100 | 100 | 96.9 | 100 | 278 | 59.6 | 100 | 100 | 100 |
| 385 | 100 | 99.6 | 93.6 | 93.0 | 345 | 58.7 | 81.1 | 48.0 | 99.3 |
| 370 | 100 | 51.1 | 98.4 | 55.4 | 346 | 57.9 | 100 | 65.4 | 97.4 |
| 372 | 100 | 80.3 | 97.8 | 100 | 349 | 53.5 | 6.6 | −142.0 | 2.4 |
| 477 | 100 | −75.7 | 100 | 41.5 | 468 | 53.3 | 93.8 | 98.7 | 78.5 |
| 357 | 100 | 15.6 | 15.4 | 56.1 | |||||
| 463 | 100 | 83.9 | 100 | 95.2 | |||||
| 362 | 99.7 | 17.8 | −76.6 | 41.5 | |||||
| 402 | 99.6 | 36.3 | 13.1 | 53.8 | |||||
| 288 | 98.7 | 100 | 100 | 100 | |||||
| 340 | 98.0 | 100 | 100 | 94.8 | |||||
| 341 | 98.0 | 100 | −15.7 | 100 | |||||
| 453 | 98.0 | 67.4 | 97.3 | 60.0 | |||||
| 383 | 97.1 | 65.5 | 100 | 79.0 | |||||
| 451 | 96.2 | 100 | 91.7 | 83.0 | |||||
| 280 | 95.7 | 91.9 | 61.9 | 100 | |||||
| 412 | 95.7 | 79.0 | 30.5 | 84.7 | |||||
| 352 | 92.1 | nd | −91.4 | 42.7 | |||||
Percentage of TB activity of sera using autologous P. vivax parasite.
Numbers (1, 2, 3) correspond to different parasite samples (heterologous strains) used for the three TB assays; nd = not determined.
Discussion
Among the factors that contribute to malaria reduction in endemic areas, TB immune mechanisms appear to play a significant role, particularly in areas of low and intermediate transmission intensity, whereas a limited impact is considered to occur in areas of high malaria transmission intensity.26 Immunity to malaria sexual stages is considered to be primarily mediated by antibodies that block malaria transmission by preventing the sporogonic development in Anopheles mosquito. TB immunity however, is likely to be dependent on the contribution of serum complement factors and cytokines,6–8 which all together may contribute to a significant reduction of malaria transmission in endemic areas and become the subject of investigation in the search for a TB malaria vaccine.
This study confirms the results obtained previously, regarding the presence of P. vivax TB activity in malaria endemic communities of the Colombian Pacific coast. In this study, and in the former one,14 similar percentages of sera (35% high and 32% intermediate level, respectively) from P. vivax acutely infected individuals showed TB activity (≥ 50% inhibition). In the present study we had the opportunity to determine the difference of TB activity in infected14 and non-infected individuals from the same region. In contrast to the high prevalence of TB activity found in acutely infected individuals, only 6.8% and 9.1% of sera from non-infected volunteers maintained similar levels of TB activity (high and intermediate levels, > 50%) (Table 1).
This may indicate that the presence of the parasite during infection increases the blocking factors, i.e., antibodies and other immune effectors responsible for TB activity, and as suggested in previous studies in Sri Lanka that blocking activity appears to depend on recent rather than on cumulative exposure to the parasite.10,12 In this community, infected individuals also presented significantly higher specific anti-P. vivax antibodies (24%, ≥ 1:3,200)14 than non-infected volunteers (20.6% up to 1:1,600). The fact that parasite exposure triggers an efficient antibody boosting highlights the contribution of anti-parasite immune response to produce TB. The potential of infection in boosting the antibody responses primed would be of great importance for TB vaccines that would be boosted each time the volunteer is exposed to the parasite. In contrast, it was observed that in the group of non-infected individuals that presented low antimalarial antibody titers 73% did not display TB but rather a transmission enhancing effect. This is also in agreement with results from previous studies, where it was shown that sera with low levels of specific antimalarial antibodies rather than blocking induced transmission enhancing.9 We could not conclusively state that the enhancing phenomenon is produced by antibodies but there is a direct correlation between a decrease in antibody titers and TB (Figure 1). Antibody potency studies have indicated a rapid reduction of blocking activity at sera dilutions of 1:4 and 1:16. This is directly associated with the longevity of antibody responses; here, we followed six individuals during an 8-month period without malaria infection and observed that the duration of detectable antibodies was 2–4 months, shorter than that found in previous studies in Sri Lanka (4–6 months).13 This divergence may be explained by a difference in P. vivax malaria transmission intensity in these two malaria endemic settings. Sri Lanka is considered one of the most P. vivax malaria-endemic areas of Asia and individuals are likely to be more frequently exposed to P. vivax infection and likely more immune12,15 than subjects of the Pacific Cost of Colombia.14 Alternatively, it is likely caused by the diversity of P. vivax strains circulating in both endemic regions. A similar phenomenon has been reported in previous studies using either monoclonal antibodies,27 with sera from individuals living in malaria endemic areas10,15 or sera from monkeys infected with Plasmodium cynomolgy.28 It has been suggested that sera without antibodies may contain substances that increased the number of oocysts, producing this enhancing effect.8,29,30
In addition to antibodies, complement has been associated with TB activity. In P. falciparum studies, it has been previously reported that complement contributes to TB.7,17 Here, we could not clearly establish an association between complement and TB. The paired MFA carried out with and without complement showed that in most cases there was greater TB in the presence of complement, however in a number of cases, complement did not appear to contribute to the blocking activity, and indeed in some cases (four) the depletion of complement increased the TB. More conclusive results in P. vivax may be obtained once specific TB antigens become available. In previous studies with P. falciparum, it was found that antibodies to proteins such as Pfs230 and Pf48/45, considered potential vaccine targets, were all of complement-fixing antibodies.7
An additional finding in this study was the fact that a significant number of sera (44.9%) presented the same TB activity effect (90–100%) on autologous and heterologous parasites. This was associated with greater antibody titers. Instead, for sera with lower levels of TB activity (50–89%), there was greater variability in TB to different parasite isolates. This has also been demonstrated using a set of monoclonal antibodies against several epitopes of TB antigens,31 suggesting that different target antigens may be able to induce antibodies of different TB efficacy. It has also been found that other factors, such as the numbers of gametocytes and the isolate, may influence the effect of an antibody on the infectivity of an isolate.12,31 These factors will be more easily approached once specific antigens with TB potential become available.
In conclusion, studies here indicate that malaria TB activity is greater in infected patients than in non-infected individuals and that besides great variability in susceptibility of different parasite strains to the blocking effect of sera, when antibody titers are sufficiently high, most strains are blocked. Additionally, as antibodies naturally decrease or get diluted, TB declines or sera produce transmission enhancing. Moreover, complement in some instances contributed to TB, most likely depending on the IgG isotype participating in the parasite blockage. Taking into consideration that the study areas in Colombia have low to middle malaria transmission intensity, the TB activity recorded was significant and apparently greater than in Mexico and Sri Lanka. Assessing TB activity under similar experimental conditions in different epidemiological settings would greatly contribute to understanding the diversity of TB and contribute to vaccine development.
ACKNOWLEDGMENTS
We thank the Programa de Enfermedades Tropicales (PET) and community of Buenaventura for providing infected samples for this study; Juana Vergara, Zuleyma Castillo, and Sandra Preciado (Serology and Entomology Unit, Malaria Vaccine Development Branch) for technical assistance during these experiments and Claudia Garcia for technical support with the manuscript.
Footnotes
Financial support: This work was supported by the National Institute of Allergy and Infectious Diseases through Tropical Medicine Research Centers NIAID/TMRC grant no. 49486 and through an International Center of Excellence for Malaria Research NIAID/ICEMR grant no U 19AI089702, the Instituto Colombiano para el Desarrollo de la Ciencia y Tecnología ‘Francisco José de Caldas' COLCIENCIAS grant no. 6124-05-17594, and the Ministry for Social Protection/Colciencias grant no. 2304-04-19524. John Beier was partially supported by the Abess Center for Ecosystem Science and Policy, University of Miami.
Authors' addresses: Myriam Arévalo-Herrera, Yezid Solarte, and Sócrates Herrera, Instituto de Inmunología, Edificio de Microbiología, Facultad de Salud, Universidad del Valle and Malaria Vaccine and Drug Development Center, Cali, Colombia, E-mails: marevalo@inmuno.org, ysolarte@inmuno.org, and sherrera@inmuno.org. Leonardo Rocha and Diego Álvarez, Malaria Vaccine and Drug Development Center, Cali, Colombia, E-mails: lrocha94@hotmail.com and kensof@gmail.com. John C. Beier, Department of Epidemiology and Public Health, Miller School of Medicine, Clinical Research Building, Miami, FL, E-mail: jbeier@med.miami.edu.
Reprint requests: Sócrates Herrera, Malaria Vaccine and Drug Development Center, Carrera 37 - 2Bis No. 5E - 08, Cali, Colombia, E-mail: sherrera@inmuno.org.
References
- 1.Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baird JK. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol Today. 1995;11:105–111. doi: 10.1016/0169-4758(95)80167-7. [DOI] [PubMed] [Google Scholar]
- 3.Hviid L. Naturally acquired immunity to Plasmodium falciparum malaria in Africa. Acta Trop. 2005;95:270–275. doi: 10.1016/j.actatropica.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Targett GA, Greenwood BM. Malaria vaccines and their potential role in the elimination of malaria. Malar J. 2008;7((Suppl 1)):S10. doi: 10.1186/1475-2875-7-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauerwein RW. Malaria transmission-blocking vaccines: the bonus of effective malaria control. Microbes Infect. 2007;9:792–795. doi: 10.1016/j.micinf.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Munesinghe YD, Mendis KN, Carter R. Anti-gamete antibodies block transmission of human vivax malaria to mosquitoes. Parasite Immunol. 1986;8:231–238. doi: 10.1111/j.1365-3024.1986.tb01035.x. [DOI] [PubMed] [Google Scholar]
- 7.Healer J, McGuinness D, Hopcroft P, Haley S, Carter R, Riley E. Complement-mediated lysis of Plasmodium falciparum gametes by malaria-immune human sera is associated with antibodies to the gamete surface antigen Pfs230. Infect Immun. 1997;65:3017–3023. doi: 10.1128/iai.65.8.3017-3023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naotunne TS, Karunaweera ND, Del Giudice G, Kularatne MU, Grau GE, Carter R, Mendis KN. Cytokines kill malaria parasites during infection crisis: extracellular complementary factors are essential. J Exp Med. 1991;173:523–529. doi: 10.1084/jem.173.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousema JT, Drakeley CJ, Sauerwein RW. Sexual-stage antibody responses to P. falciparum in endemic populations. Curr Mol Med. 2006;6:223–229. doi: 10.2174/156652406776055140. [DOI] [PubMed] [Google Scholar]
- 10.Mendis KN, Munesinghe YD, de Silva YN, Keragalla I, Carter R. Malaria transmission-blocking immunity induced by natural infections of Plasmodium vivax in humans. Infect Immun. 1987;55:369–372. doi: 10.1128/iai.55.2.369-372.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 12.Gamage-Mendis AC, Rajakaruna J, Carter R, Mendis KN. Transmission blocking immunity to human Plasmodium vivax malaria in an endemic population in Kataragama, Sri Lanka. Parasite Immunol. 1992;14:385–396. doi: 10.1111/j.1365-3024.1992.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 13.Ranawaka MB, Munesinghe YD, de Silva DM, Carter R, Mendis KN. Boosting of transmission-blocking immunity during natural Plasmodium vivax infections in humans depends upon frequent reinfection. Infect Immun. 1988;56:1820–1824. doi: 10.1128/iai.56.7.1820-1824.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arévalo-Herrera M, Solarte Y, Zamora F, Mendez F, Yasnot MF, Rocha L, Long C, Miller LH, Herrera S. Plasmodium vivax: transmission-blocking immunity in a malaria-endemic area of Colombia. Am J Trop Med Hyg. 2005;73:38–43. doi: 10.4269/ajtmh.2005.73.5_suppl.0730038. [DOI] [PubMed] [Google Scholar]
- 15.Ramsey JM, Salinas E, Rodriguez MH. Acquired transmission-blocking immunity to Plasmodium vivax in a population of southern coastal Mexico. Am J Trop Med Hyg. 1996;54:458–463. doi: 10.4269/ajtmh.1996.54.458. [DOI] [PubMed] [Google Scholar]
- 16.Touray MG, Seeley DC, Jr, Miller LH. Plasmodium gallinaceum: differential lysis of two developmental stages of malaria sporozoites by the alternative pathway of complement. Exp Parasitol. 1994;78:294–301. doi: 10.1006/expr.1994.1031. [DOI] [PubMed] [Google Scholar]
- 17.Read D, Lensen AH, Begarnie S, Haley S, Raza A, Carter R. Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement-fixing. Parasite Immunol. 1994;16:511–519. doi: 10.1111/j.1365-3024.1994.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 18.Roeffen W, Geeraedts F, Eling W, Beckers P, Wizel B, Kumar N, Lensen T, Sauerwein R. Transmission blockade of Plasmodium falciparum malaria by anti-Pfs230-specific antibodies is isotype dependent. Infect Immun. 1995;63:467–471. doi: 10.1128/iai.63.2.467-471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scholz SM, Simon N, Lavazec C, Dude MA, Templeton TJ, Pradel G. PfCCp proteins of Plasmodium falciparum: gametocyte-specific expression and role in complement-mediated inhibition of exflagellation. Int J Parasitol. 2008;38:327–340. doi: 10.1016/j.ijpara.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Siddique ME, Ahmed S. Serum complement C4 levels during acute malarial infection and post-treatment period. Indian J Pathol Microbiol. 1995;38:335–339. [PubMed] [Google Scholar]
- 21.Shute GT. In: Principles and Practice of Malariology. Wernsdorfer H, McGregor IA, editors. London: Longman Group UK Limited; 1988. pp. 781–814. (The microscopic diagnosis of malaria). [Google Scholar]
- 22.Tournamille C, Le Van Kim C, Gane P, Cartron JP, Colin Y. Molecular basis and PCR-DNA typing of the Fya/fyb blood group polymorphism. Hum Genet. 1995;95:407–410. doi: 10.1007/BF00208965. [DOI] [PubMed] [Google Scholar]
- 23.Hurtado S, Salas ML, Romero JF, Zapata JC, Ortiz H, Arévalo-Herrera M, Herrera S. Regular production of infective sporozoites of Plasmodium falciparum and P. vivax in laboratory-bred Anopheles albimanus. Ann Trop Med Parasitol. 1997;91:49–60. doi: 10.1080/00034983.1997.11813111. [DOI] [PubMed] [Google Scholar]
- 24.Eyles DE. A stain for malarial oocysts in temporary preparations. J Parasitol. 1950;36:501. [PubMed] [Google Scholar]
- 25.Lensen A, van Druten J, Bolmer M, van Gemert G, Eling W, Sauerwein R. Measurement by membrane feeding of reduction in Plasmodium falciparum transmission induced by endemic sera. Trans R Soc Trop Med Hyg. 1996;90:20–22. doi: 10.1016/s0035-9203(96)90464-2. [DOI] [PubMed] [Google Scholar]
- 26.Boudin C, Diop A, Gaye A, Gadiaga L, Gouagna C, Safeukui I, Bonnet S. Plasmodium falciparum transmission blocking immunity in three areas with perennial or seasonal endemicity and different levels of transmission. Am J Trop Med Hyg. 2005;73:1090–1095. [PubMed] [Google Scholar]
- 27.Peiris JS, Premawansa S, Ranawaka MB, Udagama PV, Munasinghe YD, Nanayakkara MV, Gamage CP, Carter R, David PH, Mendis KN. Monoclonal and polyclonal antibodies both block and enhance transmission of human Plasmodium vivax malaria. Am J Trop Med Hyg. 1988;39:26–32. doi: 10.4269/ajtmh.1988.39.26. [DOI] [PubMed] [Google Scholar]
- 28.Naotunne TD, Rathnayake KD, Jayasinghe A, Carter R, Mendis KN. Plasmodium cynomolgi: serum-mediated blocking and enhancement of infectivity to mosquitoes during infections in the natural host, Macaca sinica. Exp Parasitol. 1990;71:305–313. doi: 10.1016/0014-4894(90)90035-b. [DOI] [PubMed] [Google Scholar]
- 29.Karunaweera ND, Carter R, Grau GE, Kwiatkowski D, Del Giudice G, Mendis KN. Tumour necrosis factor-dependent parasite-killing effects during paroxysms in non-immune Plasmodium vivax malaria patients. Clin Exp Immunol. 1992;88:499–505. doi: 10.1111/j.1365-2249.1992.tb06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karunaweera ND, Wijesekera SK, Wanasekera D, Mendis KN, Carter R. The paroxysm of Plasmodium vivax malaria. Trends Parasitol. 2003;19:188–193. doi: 10.1016/s1471-4922(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 31.Premawansa S, Peiris JS, Perera KL, Ariyaratne G, Carter R, Mendis KN. Target antigens of transmission blocking immunity of Plasmodium vivax malaria. Characterization and polymorphism in natural parasite isolates. J Immunol. 1990;144:4376–4383. [PubMed] [Google Scholar]


