Abstract
The purinergic signalling system, which uses purines and pyrimidines as chemical transmitters, and purinoceptors as effectors, is deeply rooted in evolution and development and is a pivotal factor in cell communication. The ATP and its derivatives function as a ‘danger signal' in the most primitive forms of life. Purinoceptors are extraordinarily widely distributed in all cell types and tissues and they are involved in the regulation of an even more extraordinary number of biological processes. In addition to fast purinergic signalling in neurotransmission, neuromodulation and secretion, there is long-term (trophic) purinergic signalling involving cell proliferation, differentiation, motility and death in the development and regeneration of most systems of the body. In this article, we focus on the latter in the immune/defence system, in stratified epithelia in visceral organs and skin, embryological development, bone formation and resorption, as well as in cancer.
Keywords: ATP, P2X and P2Y receptors, epithelia, immune cells, bone, cancer
ATP – The Universal Intercellular Signalling Molecule
The molecule of adenosine 5′-triphosphate or ATP was discovered 80 years ago simultaneously in Heidelberg and Boston by Lohman,1 Fiske and SubbaRow.2 Very soon afterwards, the central role of ATP in cell energetics was fully appreciated.3 In fact, the role of ATP in living matter is unique and without ATP we, in all probability, would not witness life in its present forms.
Indeed, the life forms, which we know on earth, are built around the genetic code that is stored in the relatively simple molecules of DNA and RNA, composed from the purine adenine and the pyrimidines, guanine, uracil and thymine. The purines and pyrimidines, as well as ATP and GTP, most likely appeared in the prebiotic period, with adenine derivatives being preferentially synthesised as a result of purely thermal reactions.4, 5 Very early in evolution, ATP was chosen as an energy substrate, thus shaping the metabolism of all forms of life.6 The preponderance of ATP stimulated the evolution of enzymes with preferential binding properties, and adenine nucleotides began to be used in various intracellular signalling cascades, such as, for example, the cAMP cascade.7 At the very same time, ATP probably became the first extracellular signalling molecule, because of its sheer availability. Indeed, as every cell contained high concentrations of ATP, cell damage inevitably results in the appearance of ATP gradients in the surrounding milieu, which thus became a universal ‘danger' signal. As a result, virtually every known cell or single-cell organism has a form of ATP sensitivity, and purinergic signalling represents the primordial form of chemical intercellular signalling.8
Although the intracellular signalling and metabolic roles for ATP were established quite early, its importance as an extracellular signalling molecule was acknowledged much later. The possible signalling role for AMP was postulated in 19299 and purinergic signalling (i.e., signalling mediated by purines and pyrimidines) was initially suggested in 1970, when ATP was identified as a transmitter in the autonomic nervous system.10 In 1972, the concept of purinergic nerves and purinergic transmission was formulated,11 and after initial resistance is now widely accepted and has a major role in both the nervous system12, 13, 14 and non-neuronal cells.15 Initially, the focus was on short-term purinergic signalling in neurotransmission, neuromodulation and secretion, but more recent studies have also established roles in long-term (trophic) signalling in cell proliferation, differentiation, motility and death in development and regeneration.16, 17
The Omnipresent Purinoceptors
The action of purines and pyrimidines is mediated through an extended family of purinoceptors,18, 19 generally divided into P1 adenosine receptors20 and P2 receptors for ATP and related nucleotides.21, 22 In the early 1990s, receptors for purines and pyrimidines were cloned and characterised12, 23, 24, 25 and it is currently recognised that there are four subtypes of P1 receptors (A1, A2A, A2B and A3), seven subtypes of P2X ligand-gated ion channel receptors (P2X1–7) and eight subtypes of P2Y G-protein-coupled receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y1426). P2 receptors appeared very early in evolution, for example, P2X receptors have been found in early prokaryotes;27, 28, 29 despite little sequence homology with later evolutionary forms of the receptors, the functional properties are very much conserved. Similarly, functional metabotropic (P2Y-like) receptors are present in Protozoa, and in the most primitive plants in which they regulate numerous vital functions.8, 30
The P1 and P2Y receptors are classical 7-transmembrane domain receptors, the action of which is mediated through G-proteins and numerous intracellular second messengers, including the cAMP and InsP3 cascades. In addition, some of these receptors are linked to membrane ion channels, thus mediating plasmalemmal ion fluxes and electrophysiological effects. P2X receptors are archetypal ligand-operated cationic channels,31, 32, 33 many of which have an appreciable Ca2+ permeability.34 The P2X channels are assembled (in a homo- or heteromeric manner) from seven subunits, designated as P2X1–P2X7, which determines the variability of their biophysical and pharmacological properties.
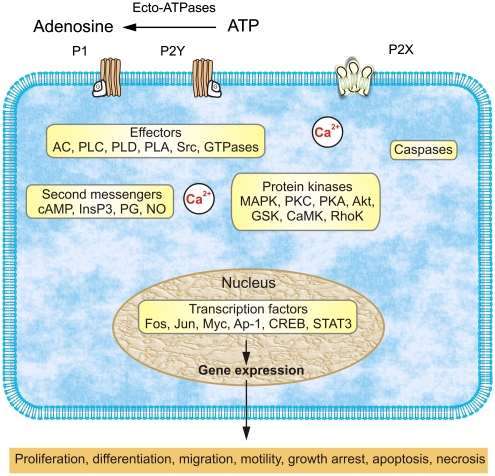
Probably because of their ancient origin, the extensive array of purinoceptors has a unique property of being extraordinarily widely distributed throughout living cells and tissues (Table 1). In contrast to all other chemical transmitters, which are, as a rule, segregated to certain cell types and certain functions, the receptors for purines and pyrimidines are found everywhere and as a matter of fact it is almost impossible to find a cell without sensitivity to ATP and its analogues. Indeed, purinoceptors are extensively present in the central nervous system, where they mediate fast synaptic transmission, provide for presynaptic inhibition and regulation of neuronal excitability and are particularly important for signalling in neuronal–glial circuitry, being one of the most important gliotransmitters.12, 13, 35, 36 In the peripheral nervous system, purinoceptors are involved in sensory37, 38 and autonomic functions.39 Purinoceptors are present in all peripheral tissues, being involved in the regulation of very different functions in the gut, kidneys, in the cardiovascular and respiratory systems, the immunological system, in blood cells, skin, bones and muscles.8, 15 Furthermore, the purinergic signalling system possesses another unique property – the release of the principal mediator, ATP, initiates the appearance of a trail of derivatives, ADP, AMP and adenosine, to which extracellular ATP rapidly degrades due to the activity of ectonucleotidases that represent an important component of purinergic signalling.13 As a result of the single event of ATP release from different cell types (which occurs through different concomitant mechanisms, including Ca2+-regulated exocytosis, membrane transporters and diffusion through large-permeability plasmalemmal channels40, 41), several classes of receptors (sometimes having opposite actions) are activated at effector cells. Finally, purinoceptors are linked to an extensive array of intracellular signalling cascades that underlie their long-term trophic effects (Figure 1).
Table 1. Functional consequences of genetic deletion of purinoceptors.
| Receptor subtype | Phenotypereference |
|---|---|
| P1 Adenosine receptors | |
| A1 | (i) Behavioural phenotype: increased aggression and anxiety; decreased motor activity (ii) Neural phenotype: neuroprotection in newborns; hyperalgesia; no inhibition of synaptic transmission; decreased long-term potentiation; reduced hypoxia-associated decrease in neural activity and recovery after hypoxia (iii) Kidney phenotype: absent tubuloglomerular feedback (iv) Metabolic phenotype: increased insulin and glucagon secretion103, 104, 105, 106 |
| A2A | (i) Behavioural phenotype: increased aggression and anxiety; decreased exploratory activity; attenuated psychostimulant responses; decreased alcohol sensitivity and withdrawal; decreased amphetamine- and cocaine-induced locomotor response (ii) Neural phenotype: neuroprotection in adults; hypoalgesia (iii) Cardiovascular phenotype: increased blood pressure, heart rate and rennin activity (iv) Haemostatic phenotype: increased platelet aggregation; increased brain damage after focal ischaemia (v) Immunological phenotype: increased inflammatory response (vi) Sensory phenotype: decreased pain threshold107, 108, 109, 110, 111 |
| A2B | (i) Immunological phenotype: increased histamine release but decreased IL-13 release from mast cells112, 113 |
| A3 | (i) Behavioural phenotype: increased despair and motor activity (ii) Neural phenotype: reduced neuroprotection; hyperalgesia (iii) Immunological phenotype: attenuated lipopolysaccharide-induced TNFα production and adenosine-induced histamine release from mast cells; decreased neutrophil infiltration of damaged myocardium; decreased local inflammatory response (iii) Cardiovascular phenotype: decreased infarct size following ischaemic–reperfusion injury; loss of adenosine-induced cutaneous vasopermeability; i.v. adenosine produces and greater drop in blood pressure; increased tolerance to ischaemia; lower intraocular pressure114, 115, 116, 117, 118, 119 |
| P2X receptors | |
| P2X1 | (i) Kidney phenotype: absent tubuloglomerular feedback (ii) Reproductory phenotype: male infertility due to the reduction of sperm in the ejaculate and severely impaired contractility of vas deference (iii) haemostatic phenotype: reduced thrombosis associated with injury of the walls of small arterioles120, 121, 122, 123 |
| P2X2 | (i) Neural phenotype: impaired synaptic facilitation in hippocampal interneurones (ii) Sensory phenotype: impaired taste (iii) Chemosensory phenotype: affected excitation of afferent nerves in carotid body by hypoxia (iv) Gut phenotype: reduced peristalsis of the small intestine124, 125, 126 |
| P2X3 | (i) Sensory phenotype: affected nociception, impaired temperature sensitivity, impaired taste (ii) Urinary phenotype: affected voiding reflex39, 125, 127, 128, 129 |
| P2X2&P2X3 | (i) Sensory phenotype: affected nociception, impaired temperature sensitivity, severely impaired taste (ii) Chemosensory phenotype: reduced ventilatory responses to a decrease in the level of inspired O239, 125 |
| P2X4 | (i) Neural phenotype: reduced hippocampal LTP (ii) Sensory phenotype: reduced chronic pain (both inflammatory and neuropathic) (ii) Vascular phenotype: impaired flow-sensitivity of blood vessels; decrease in NO production by endothelial cells, decreased vasodilatation, higher blood pressure130, 131 |
| P2X7 | (i) Immunological phenotype: impaired immune response (ii) Sensory phenotype: reduced inflammatory and neuropathic chronic pain (iii) Exocrine phenotype: impaired saliva production (iv) Bone phenotype: abnormal bone formation and resorption25, 132, 133, 134 |
| P2Y receptors | |
| P2Y1 | (i) Haemostatic phenotype: mildly prolonged bleeding times (ii) Metabolic phenotype: increases systemic glucose levels21, 135 |
| P2Y2 | (i) Epithelial phenotype: abnormal secretion (ii) Bone phenotype: inhibited bone formation88, 136 |
| P2Y4 | (i) Epithelial phenotype: abnormal secretion21, 137 |
| P2Y6 | (i) Immunological phenotype: UDP-induced IL-6 and macrophage-inflammatory protein-2 release to lipopolysaccharide and macrophage UDP-induced inositol phosphate production are lost (ii) Cardiovascular phenotype: loss of endothelium-dependent UDP vasodilation138 |
| P2Y12 | (i) Haemostatic phenotype: prolonged bleeding time, inhibition of platelet aggregation to ADP, and resistance to arterial thrombosis139 |
Figure 1.
Overview of purinergic signalling mechanisms that regulate long-term, trophic effects. Extracellular nucleotides and nucleosides bind to purinoceptors coupled to signal-transducing effector molecules. Activation of effectors leads to generation of second messengers and/or stimulation of protein kinases that regulate expression of genes needed for long-term, trophic actions. Trophic action of P2X receptors can be mediated by increases in cytosolic Ca2+ concentration; activation of P2X7 receptors can also be coupled to protein kinase cascades and caspases that can mediate proliferation and apoptosis. Cell-specific and/or receptor subtype-specific differences are likely to account for variations in signalling pathways and functional outcomes. It should be noted that the list of elements is not meant to be all-inclusive. Other protein kinases, for example, MEK and PI3K, are upstream of the listed kinases involved in purinergic signalling, whereas others are downstream, for example, p70S6K. In addition, dashed arrows indicate that not all listed elements are activated by the upstream component, for example, not all P1 receptors are coupled to all listed effectors. AC, adenylyl cyclase; AP-1, activator protein-1; CaMK, calcium/calmodulin protein kinase; CREB, cAMP response element binding protein; DG, diacylglycerol; GSK, glycogen synthase kinase; InsP3, inositol trisphosphate; MAPKs, mitogen-activated protein kinases (including extracellular signal-regulated protein kinase (ERK), p38 MAPK, and stress-activated protein kinase (SAPK)/c-Jun NH2-terminal kinase (JNK)); MEK, MAPK/ERK kinase; NO, nitric oxide; PG, prostaglandin; PI3K, phosphoinositide 3-kinase; PLC, phosphatidylinositol-specific phospholipase C; PKA, protein kinase A; PKC, protein kinase C; PLD, phospholipase D; PLA, phospholipase A; STAT3, signal transducer and activator of transcription-3 (based on Figure 11 from Burnstock12 with permission from the American Physiological Society )
Purinergic Signalling Controls Biological Defence Systems
The ancient ‘damage signaller' characteristic of ATP is evolutionarily conserved in several systems of biological defence. First, ATP functions as one of the main mediators of pain, both in acute and chronic contexts, as indeed P2X2/3 receptors are involved in fast pain perception,38, 42 whereas P2X4 and P2Y12 receptors assume a leading role in the pathogenesis of neuropathic pain.43, 44, 45
Second, purinergic agonists regulate the immune response in various tissues. In particular, in the brain and spinal cord, activation of several types of purinoceptors (most notably P2X4, P2X7, P2Y6 and P2Y12), which occurs in a highly coordinated temporal sequence, controls motility and activation of microglia, thus being central to the brain immune response.46, 47 In particular, the P2X7 receptor seems to be critical for microglial activation by β-amyloid, being therefore pathologically relevant for Alzheimer's disease.48 Similarly, purinergic signalling is intimately involved in the activation of the peripheral immune response being not only a stimulator but also a precise regulator of the differentiation and function of immunocompetent cells,49, 50 as well as in driving chemotaxis of neutrophiles, eosinophiles, macrophages and mast cells.51, 52, 53
Third, ATP and its analogues are directly involved in tissue remodelling in response to injury and have a key role in the regulation of subsequent repair and regeneration. In the nervous system, stimulation of purinoceptors triggers astrogliosis, the generalised response of astrocytes to brain damage, which is characterised by cell proliferation and remodelling of the neural circuitry.54, 55 Reactive astrogliosis is instrumental for both formation of scar and limitation of the brain damaged area (through anisomorphic astrogliosis), as well as for post-insult remodelling and recovery of neural function (by isomorphic astrogliosis). The initial events in astroglial responses to purinergic signallers are often associated with P2Y1/2 receptor-mediated Ca2+ astroglial signalling in astrocytes,56, 57 which, depending on the context, is instrumental for glial Ca2+ excitability or can initiate long-term effects.58 These trophic/astrogliotic effects of P2 agonists (manifested by proliferative and morphological responses) were found both in vitro, in glial cultures, and in vivo, in nucleus accumbens of rats.17, 59, 60, 61 Similarly, purinergic signalling has a fundamental role in remodelling and healing of lesions in other tissues, including skin and bone. In the next part of this review, we will focus on the long-term trophic roles of purines and pyrimidines in cell proliferation, differentiation and death in the turnover of epithelial cells in skin and in cells lining visceral organs, in restenosis, embryological development, bone formation and resorption and cancer.
Stratified Squamous Epithelia
Stratified squamous epithelia in several sites, including two non-keratinised cell types, namely, rat cornea and oesophagus, and four keratinised cell types, soft palate, foot pad skin, vagina and tongue, showed heavy immunostaining of the P2X5 receptor associated with cell differentiation in spinous and granular cell layers, but not in basal cuboidal or keratinised outer layers. In contrast, there was heavy immunostaining of P2X7 receptors in the outer keratinised layer, perhaps associated with apoptotic cell death.62
Rapid turnover rates are found in the epithelium of the small intestine. The crypts contain undifferentiated progenitor cells from which almost all other epithelial cell types, including goblet cells and enterocytes, arise. The differentiated cells glide towards the villus tips where they are finally ejected into the lumen. In the rat, this whole process takes 3–4 days.63 P2X5 receptors are expressed on the narrow ‘stem' of villus goblet cells, whereas P2X7 receptor immunoreactivity is seen only on the membranes of enterocytes and goblet cells at the tip of the villus, where cells undergo apoptosis, before shedding into the lumen.64
Skin
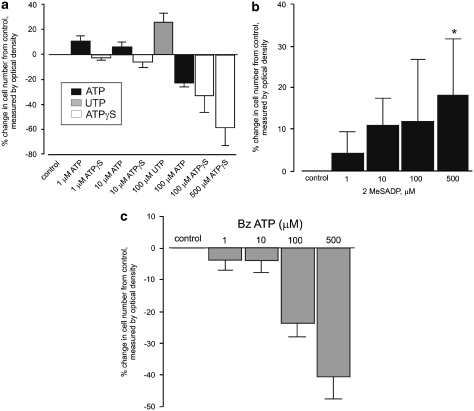
The expression of P2X5, P2X7 and P2Y2 receptor subtypes was studied in healthy human epidermal keratinocyes in relation to markers for proliferation (PCNA and Ki-67), differentiation (cytokeratin KIO and involucrin) and apoptosis (TUNEL and anti-caspase-3).65 It was shown that P2Y1 and P2Y2 receptors were immunoreactive in basal and parabasal keratinocytes, P2X5 receptor immunostaining within the stratum spinosum and P2X7 receptor immunostaining in the stratum corneum, associated with cell proliferation, differentiation and apoptotic cell death, respectively (Figure 2). Functional experiments on cultured keratinocytes were also carried out in this study, which showed the following: an increase in cell numbers in response to the P2Y1 receptor agonist 2-methylthio ADP and the P2Y2 receptor agonist UTP; and a significant decrease in cell numbers with the P2X5 receptor agonist ATPγS and the P2X7 receptor agonist BzATP (Figure 3). Later studies from this group examined the purinergic signalling profile in human fetal epidermis.66 They showed P2Y1 receptors in the basal layer of the developing epidermis associated with proliferation; P2X5 receptors predominantly in the basal and intermediate layers associated with differentiation; and P2X7 receptors in the periderm associated with apoptotic cell death. P2Y2 receptors were also found in the periderm, where they may have a role in chloride and fluid secretion into the amniotic fluid.
Figure 2.
Double labelling of P2Y1 and P2Y2 receptors with markers of proliferation shows colocalisation within a sub-population of basal and parabasal keratinocytes. Double labelling of P2X5 receptors with markers of differentiated keratinocytes shows colocalisation within the stratum spinosum, and double labelling of P2X7 receptors with markers of apoptosis in human leg skin shows colocalisation within the stratum corneum. (a) Ki-67 immunolabelling (a marker for proliferation) stained the nuclei (green) of a sub-population of keratinocytes in the basal and parabasal layers of the epidermis. P2Y1 receptor immunostaining (red) was found in the basal layer on cells also staining for Ki67. Scale bar 30 μm. (b) PCNA immunolabelling (a marker for proliferation) stained the nuclei (green) of a sub-population of keratinocytes. These nuclei were often distributed in clusters and found in the basal and parabasal layers of the epidermis. P2Y2 receptor immunostaining (red) was also expressed in basal and parabasal epidermal cells. Scale bar 30 μm. (c) P2X5 receptor immunostaining (red) showed overlap (yellow) with cytokeratin K10 (green), an early marker of keratinocyte differentiation. P2X5 receptors were present in the basal layer of the epidermis up to the mid-granular layer. Cytokeratin K10 was distributed in most suprabasal keratinocytes. The stratum basale stained only for P2X5 receptors, indicating that no differentiation was taking place in these cells. The colocalisation of P2X5 receptors and cytokeratin K10 appeared mainly in the cytoplasm of differentiating cells within the stratum spinosum and partly in the stratum granulosum. Note that the stratum corneum also stained for cytokeratin K10, which labelled differentiated keratinocytes, even in dying cells. Scale bar 30 μm. (d) P2X5 receptor immunostaining (red) showed overlap (yellow) with involucrin (green). P2X5 receptors were present in the basal layer of the epidermis up to the mid-granular layer. Note that the pattern of staining with involucrin was similar to that seen with cytokeratin K10, except that cells from the stratum basale up to the midstratum spinosum were not labelled with involucrin, which is a late marker of keratinocyte differentiation. Scale bar 30 μm. (e) TUNEL (green) labelled the nuclei of cells at the uppermost level of the stratum granulosum and P2X7 antibody (red) mainly stained cell fragments within the stratum corneum. Scale bar 15 μm. (f) Anti-caspase-3 (green) colocalised with areas of P2X7 receptor immunostaining (red) both at the junction of the stratum granulosum and within the stratum corneum. Areas of colocalisation were yellow. Note that the differentiating keratinocytes in the upper stratum granulosum were also positive for anti-caspase-3. Scale bar 15 μm (reproduced with permission from Greig et al.140)
Figure 3.
At 48 h after application of drugs to primary human keratinocyte cultures. (a) ATP (1–10 μM) and UTP (100 μM) cause an increase in cell number, whereas ATPγS (100–500 μM) and ATP (100 μM) cause a significant decrease. Results represent the mean of eight experiments. *P<0.001 compared with that of control. (b) 2MeSADP (500 μM) causes a significant increase in cell number. Results represent the mean of eight experiments. *P<0.05 compared with that of control. (c) BzATP (100–500 μM) causes a significant decrease in cell number. Results represent the mean of nine experiments. *P<0.001 compared with that of control. Error bars represent mean±S.E.M (reproduced with permission from Greig et al.140)
In a study on purinergic signalling in wound healing67 in regenerating epidermis of denervated wounds, P2Y1 receptor protein expression was significantly increased in keratinocytes, whereas P2Y2 receptor protein expression was significantly decreased. However, NGF treatment of denervated wounds reduced the expression of P2Y1 receptors and enhanced the expression of P2Y2 receptors. In innervated wounds, NGF treatment enhanced both P2X5 and P2Y1 receptor proteins in keratinocytes. P2X7 receptors were absent in all experimental wound healing processes.
P2X5 and P2X7 receptors were shown to be present in human warts and CIN612 organotypic raft cultures of human papillomavirus-infected keratinocytes and may provide a novel approach for the treatment of warts.68 P2Y1, P2Y2 and P2X5 receptors are expressed on human anagen hair follicles, with P2Y1 receptors present in proliferating cells in the outer root sheath and bulb, whereas P2X5 receptors were present on the inner and outer root sheaths and medulla, and were associated with differentiation.65 P2Y2 receptors were found in living cells at the edge of the cortex/medulla. P2X7 receptors were not present.
Cancer
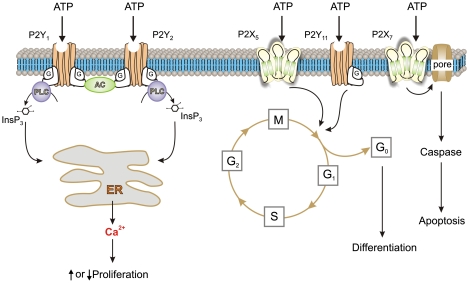
There were early reports of the beneficial effect of ATP in the treatment of cancer,69 and analysis of the purinergic receptor subtypes involved in the development of tumours in the prostate,70 bladder,71 melanoma,72, 73 breast74, 75, 76 and other organs has been described (see review by White and Burnstock77). Again, it was shown that P2Y1 and P2Y2 receptors were expressed and involved in cell proliferation, P2X5 receptors were involved in differentiation (and were therefore antiproliferative) and P2X7 receptors were involved in cell death (Figure 4). Human melanomas express functional P2X7 receptors that mediate the apoptotic functions of ATP,72 whereas P2Y1 and P2Y2 receptor agonists caused a decrease and increase in cell numbers, respectively.73 In human squamous cell carcinoma, P2Y2, P2X5 and P2X7 receptors seem to be associated with proliferation, differentiation and cell death, respectively.78
Figure 4.
Schematic diagram illustrating the different mechanisms by which P2 receptor subtypes might alter cancer cell function. P2Y1 and P2Y2 receptors could affect the rate of cell proliferation through altering the intracellular levels of cAMP by modulating adenylyl cyclase (AC) or by increasing intracellular calcium levels through the phospholipase C (PLC) pathway. P2X5 and P2Y11 receptor activation might switch the cell cycle from proliferation into a state of differentiation. The P2X7 receptor activates the apoptotic caspase enzyme system (redrawn from White and Burnstock77 with permission from Elsevier)
In high-grade bladder cancer, using the HT-1376 cell line, P2X5 and P2Y11 receptors were shown to mediate the antineoplastic effects of ATP, whereas P2X7 receptors mediated apoptotic cell death.71 Similar results are described for cell lines of hormone-refractory prostate cancer79 and ATP was shown to reduce the in vivo growth of advanced hormone-refractory prostate cancer implanted into mice.80
Finally, several clinical trials have demonstrated that systemic administration of ATP may have beneficial effects (prolongation of survival and reduced cachexia) in inoperable lung cancer patients (for details see White and Burnstock77).
Long-Term Purinergic Signalling in Embryological Development
The transient appearance of P2 receptors during both embryological and postnatal development suggests that ATP is involved in the sequential proliferation, differentiation, motility and death of cells during the complex events involved in development12, 81, 82. For example, a novel P2Y8 receptor was cloned in Xenopus embryos and was shown to be transiently expressed in the neural plate and tube from stages 13–18 and again at stage 28, when secondary neurulation occurs in the tail bud, suggesting an involvement of this receptor in the development of the nervous system.83 P2Y1 receptors were transiently expressed in the limb buds of chick embryos and were shown to mediate rapid cell proliferation.84 Changes in expression in P2X receptor subtypes during postnatal development of cerebellum have been described.85 Transient changes in P2X receptor subtype expression during the development of skeletal muscle have been described.86 P2X5 receptors were present during the early development of the myotube, followed by P2X6 receptor expression and then, during the development of the neuromuscular junction, P2X2 receptors were expressed. In the chicken retina, ATP-evoked Ca2+ transients were strongest as early as E3 and were drastically reduced at E11–13.5.87 Nucleotide signalling in development probably involves a cross-talk between several other signalling pathways, including growth factors, cytokines and extracellular matrix components.82
Trophic Purinergic Signalling in Bone Formation and Resorption
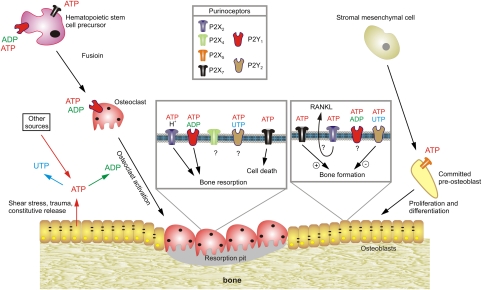
Activation of P2Y1 receptors by ADP stimulates osteoclast activity and bone resorption (Figure 5), whereas ATP and UTP signalling through P2Y2 receptors in osteoblasts inhibits bone growth and mineralisation.88 More recently, P2X7 receptors have been shown to have trophic regulatory roles in bone formation and resorption.89, 90 P2X7 receptor activation of osteoblasts enhances differentiation and bone formation,91 whereas P2X7 receptor activation of osteoclasts results in apoptosis and bone resorption.92, 93, 94
Figure 5.
Schematic diagram illustrating the potential functions of extracellular nucleotides and P2 receptors in modulating bone cell function. ATP released from osteoclasts (e.g., through shear stress or constitutively) or from other sources, can be degraded to adenosine 5′-diphosphate (ADP) or converted into uridine 5′-triphosphate (UTP) through ecto-nucleotidases. All three nucleotides can function separately on specific P2 receptor subtypes, as indicated by the colour coding. ATP is a universal agonist, whereas UTP is only active at the P2Y2 receptor and ADP is only active at the P2Y1 receptor. ADP acting on P2Y1 receptors seems to stimulate both the formation (i.e., fusion) of osteoclasts from haematopoietic precursors and the resorptive activity of mature osteoclasts. For the latter, a synergistic action of ATP and protons has been proposed by the P2X2 receptor. ADP could also stimulate resorption indirectly through actions on osteoclasts, which in turn release pro-resorptive factors (e.g., receptor activator of nuclear factor κB ligand, RANKL) ATP at high concentrations might facilitate fusion of osteoclast progenitors through P2X7 receptor pore formation or induce cell death of mature osteoclasts through P2X7 receptors. In osteoblasts, ATP, through P2X5 receptors, might enhance proliferation and/or differentiation. By contrast, UTP, through P2Y2 receptors, is a strong inhibitor of bone formation by osteoblasts. For some receptors (e.g., P2X4 and P2Y2 receptors on osteoclasts or P2X2 receptors on osteoblasts), evidence for expression has been found but their role is still unclear (based on schemes from Hoebertz et al.88)
Long-Term Trophic Actions of Purines and Pyrimidines in the Pathogenesis of Atherosclerosis and Post-Angioplasty Restenosis
ATP and UTP, acting through P2Y2 receptors, cause proliferation of vascular smooth muscle cells and proliferation of endothelial cells through P2Y1 receptors. Adenosine acting through A2 receptors inhibits smooth muscle proliferation but stimulates endothelial cell proliferation.95 The increase in vascular smooth muscle and endothelial cells in both atherosclerosis and hypertension may be mediated by the trophic actions of purines and pyrimidines released from nerves and endothelial cells96, 97, 98 and in post-angioplasty restenosis.99 P2Y4 receptors seem to be regulators of angiogenesis.100 ATP increases DNA synthesis and migration of vascular endothelial cells in vasa vasorum in diseased pulmonary vessels.101 Diabetic patients express microvascular disease characterised by an increased wall–lumen ratio, mainly because of an increase in vascular smooth muscle cells, and have higher rates of restenosis after angioplasty. High glucose-induced release of ATP exerts an effect on P2Y receptors to stimulate vascular smooth muscle cell growth.102
Conclusions
Purinergic signalling, mediated by ATP, related nucleotides and adenosine, operates in all types of tissues and cells. Purinergic agonists mediate fast cell signalling, and exert numerous long-term trophic effects, involved in regulation of cell replication, proliferation, differentiation and death. It is hoped that there will be further exploration of the roles of this primitive and widespread signalling system in cell biology.
Conflict of interest
The authors declare no conflict of interest.
Glossary
- ADP
adenosine diphosphate
- AMP
adenosine monophosphate
- ATP
adenosine 5′-triphosphate
- ATPγS
adenosine 5′-O-(3-thiotriphosphate)
- BzATP
2′-&3′-O-(4-benzoyl-benzoyl)-ATP
- DNA
deoxyribose nucleic acid
- cAMP
cyclic adenosine monophosphate
- GTP
guanosine triphosphate
- IL
interleukin
- InsP3
inosine trisphosphate
- LTP
long-term potentiation
- 2-MeSADP
2-methylthio ADP
- NGF
nerve growth factor
- NO
nitrous oxide
- TNF-α
tumour necrosis factor-α
- UTP
uridine 5′-triphosphate
References
- Lohmann K. Uber die Pyrophosphatfraktion im Muskel. Naturwissenschaften. 1929;17:624–625. [Google Scholar]
- Fiske CH, SubbaRow Y. Phosphorous compounds of muscle and liver. Science. 1929;70:381–382. doi: 10.1126/science.70.1816.381.b. [DOI] [PubMed] [Google Scholar]
- Lippman F. Metabolic generation and utilization of phosphate bond energy. Enzymology. 1941;1:99. [Google Scholar]
- Ponnamperuma C, Sagan C, Mariner R. Synthesis of adenosine triphosphate under possible primitive earth conditions. Nature. 1963;199:222–226. doi: 10.1038/199222a0. [DOI] [PubMed] [Google Scholar]
- Waldrop MM. Did life really start out in an RNA world. Science. 1989;246:1248–1249. doi: 10.1126/science.2479985. [DOI] [PubMed] [Google Scholar]
- Wilson JE. Some thoughts on the evolutionary basis for the prominent role of ATP and ADP in cellular energy metabolism. J Theor Biol. 1984;111:615–623. doi: 10.1016/s0022-5193(84)80257-x. [DOI] [PubMed] [Google Scholar]
- Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem. 1958;232:1077–1091. [PubMed] [Google Scholar]
- Burnstock G, Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol (Oxf) 2009;195:415–447. doi: 10.1111/j.1748-1716.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- Drury AN, Szent-Györgyi A. The physiological activity of adenine compounds with special reference to their action upon mamalian heart. J Physiol (London) 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- North RA, Verkhratsky A. Purinergic transmission in the central nervous system. Pflugers Arch. 2006;452:479–485. doi: 10.1007/s00424-006-0060-y. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- Abbracchio MP, Burnstock G. Purinergic signalling: pathophysiological roles. Jpn J Pharmacol. 1998;78:113–145. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G.A basis for distinguishing two types of purinergic receptorIn: Straub RW, Bolis L (eds).Cell Membrane Receptors for Drugs and Hormones: A Multidisciplinary Approach Raven Press: New York; 1978107–118. [Google Scholar]
- Burnstock G, Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor. Gen Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, et al. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SJ, Cao L, Young MT, North RA. Permeation properties of a P2X receptor in the green algae Ostreococcus tauri. J Biol Chem. 2008;283:15122–15126. doi: 10.1074/jbc.M801512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SJ, Parkinson K, Young MT, Cao L, Thompson CR, North RA. An intracellular P2X receptor required for osmoregulation in Dictyostelium discoideum. Nature. 2007;448:200–203. doi: 10.1038/nature05926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow MJ, Traynor D, Fisher PR, Ennion SJ. Purinergic-mediated Ca2+ influx in Dictyostelium discoideum. Cell Calcium. 2008;44:567–579. doi: 10.1016/j.ceca.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G, Roux SJ.Extracellular nucleotides: ancient signaling molecules Plant Sci 2009177239244. [Google Scholar]
- Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature. 2009;460:599–604. doi: 10.1038/nature08218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Krishtal OA, Verkhratsky A. P2X receptors and synaptic plasticity. Neuroscience. 2009;158:137–148. doi: 10.1016/j.neuroscience.2008.03.076. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Krishtal O, Verkhratsky A. Ionotropic P2X purinoreceptors mediate synaptic transmission in rat pyramidal neurones of layer II/III of somato-sensory cortex. J Physiol. 2002;542:529–536. doi: 10.1113/jphysiol.2002.021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Krishtal OA, Burnstock G. Purinoceptors on neuroglia. Mol Neurobiol. 2009;39:190–208. doi: 10.1007/s12035-009-8063-2. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purines and sensory nerves. Handb Exp Pharmacol. 2009;194:333–392. doi: 10.1007/978-3-540-79090-7_10. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic receptors and pain. Curr Pharm Des. 2009;15:1717–1735. doi: 10.2174/138161209788186335. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Wood JD, Burnstock G. Purinergic signalling in autonomic control. Trends Neurosci. 2009;32:241–248. doi: 10.1016/j.tins.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Verkhratsky A, North RA. Vesicular release of ATP at central synapses. Pflugers Arch. 2006;452:589–597. doi: 10.1007/s00424-006-0061-x. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Verkhratsky A, North RA. Quantal release of ATP in mouse cortex. J Gen Physiol. 2007;129:257–265. doi: 10.1085/jgp.200609693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. A unifying purinergic hypothesis for the initiation of pain. Lancet. 1996;347:1604–1605. doi: 10.1016/s0140-6736(96)91082-x. [DOI] [PubMed] [Google Scholar]
- Inoue K.ATP receptors of microglia involved in pain Novartis Found Symp 2006276263–272.discussion 273–281. [PubMed] [Google Scholar]
- Inoue K, Tsuda M. Microglia and neuropathic pain. Glia. 2009;57:1469–1479. doi: 10.1002/glia.20871. [DOI] [PubMed] [Google Scholar]
- Inoue K, Tsuda M, Koizumi S. ATP receptors in pain sensation: involvement of spinal microglia and P2X4 receptors. Purinergic Signal. 2005;1:95–100. doi: 10.1007/s11302-005-6210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009;32:79–87. doi: 10.1016/j.tins.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Sanz JM, Chiozzi P, Ferrari D, Colaianna M, Idzko M, Falzoni S, et al. Activation of microglia by amyloid b requires P2X7 receptor expression. J Immunol. 2009;182:4378–4385. doi: 10.4049/jimmunol.0803612. [DOI] [PubMed] [Google Scholar]
- Trautmann A. Extracellular ATP in the immune system: more than just a ‘danger signal'. Sci Signal. 2009;2:pe6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Burgers JA, Schweizer RC, Koenderman L, Bruijnzeel PL, Akkerman JW. Human platelets secrete chemotactic activity for eosinophils. Blood. 1993;81:49–55. [PubMed] [Google Scholar]
- Verghese MW, Kneisler TB, Boucheron JA. P2U agonists induce chemotaxis and actin polymerization in human neutrophils and differentiated HL60 cells. J Biol Chem. 1996;271:15597–15601. doi: 10.1074/jbc.271.26.15597. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F. Dr. Jekyll/Mr. Hyde: the dual role of extracellular ATP. J Auton Nerv Syst. 2000;81:59–63. doi: 10.1016/s0165-1838(00)00114-4. [DOI] [PubMed] [Google Scholar]
- Giaume C, Kirchhoff F, Matute C, Reichenbach A, Verkhratsky A. Glia: the fulcrum of brain diseases. Cell Death Differ. 2007;14:1324–1335. doi: 10.1038/sj.cdd.4402144. [DOI] [PubMed] [Google Scholar]
- Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, et al. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Moller T, Voitenko N, Kettenmann H, Verkhratsky A. ATP-induced cytoplasmic calcium mobilization in Bergmann glial cells. J Neurosci. 1995;15:7861–7871. doi: 10.1523/JNEUROSCI.15-12-07861.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S, Scherer J, Kettenmann H, Verkhratsky A. Activation of P2-purinoreceptors triggered Ca2+ release from InsP3-sensitive internal stores in mammalian oligodendrocytes. J Physiol. 1995;483:41–57. doi: 10.1113/jphysiol.1995.sp020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbracchio MP, Verderio C.Pathophysiological roles of P2 receptors in glial cells Novartis Found Symp 200627691–103.discussion 103–112, 275–181. [PubMed] [Google Scholar]
- Abbracchio MP, Ceruti S. Roles of P2 receptors in glial cells: focus on astrocytes. Purinergic Signal. 2006;2:595–604. doi: 10.1007/s11302-006-9016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbracchio MP, Saffrey MJ, Hopker V, Burnstock G. Modulation of astroglial cell proliferation by analogues of adenosine and ATP in primary cultures of rat striatum. Neuroscience. 1994;59:67–76. doi: 10.1016/0306-4522(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Franke H, Krugel U, Schmidt R, Grosche J, Reichenbach A, Illes P. P2 receptor-types involved in astrogliosis in vivo. Br J Pharmacol. 2001;134:1180–1189. doi: 10.1038/sj.bjp.0704353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschel-Stewart U, Bardini M, Robson T, Burnstock G. Localisation of P2X5 and P2X7 receptors by immunohistochemistry in rat stratified squamous epithelia. Cell Tissue Res. 1999;296:599–605. doi: 10.1007/s004410051321. [DOI] [PubMed] [Google Scholar]
- Madara JL.Functional morphology of epithelium of the small intestineIn: Fields H, Frizzell RA, Schultz SG (eds).Handbook of Physiology, Section 6: The Gastrointestinal System Volume IV: Intestinal Absorption and Secretion American Physiological Society: Bethesda, MD; 199183–120. [Google Scholar]
- Groschel-Stewart U, Bardini M, Robson T, Burnstock G. P2X receptors in the rat duodenal villus. Cell Tissue Res. 1999;297:111–117. doi: 10.1007/s004410051338. [DOI] [PubMed] [Google Scholar]
- Greig AV, Linge C, Burnstock G. Purinergic receptors are part of a signalling system for proliferation and differentiation in distinct cell lineages in human anagen hair follicles. Purinergic Signal. 2008;4:331–338. doi: 10.1007/s11302-008-9108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig AV, Linge C, Cambrey A, Burnstock G. Purinergic receptors are part of a signaling system for keratinocyte proliferation, differentiation, and apoptosis in human fetal epidermis. J Invest Dermatol. 2003;121:1145–1149. doi: 10.1046/j.1523-1747.2003.12567.x. [DOI] [PubMed] [Google Scholar]
- Greig AV, James SE, McGrouther DA, Terenghi G, Burnstock G. Purinergic receptor expression in the regeneration epidermis in a rat model of normal and delayed wound healing. Exp Dermatol. 2003;12:860–871. doi: 10.1111/j.0906-6705.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- Greig AV, Cuthill S, Linge C, Clayton E, Burnstock G. P2X5 and P2X7 receptors in human warts and CIN-612 organotypic raft cultures of human papillomavirus infected keratinocytes. Purinergic Signal. 2006;2:509–515. doi: 10.1007/s11302-005-5035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport E. Treatment of human tumor cells with ADP or ATP yields arrest of growth in the S phase of the cell cycle. J Cell Physiol. 1983;114:279–283. doi: 10.1002/jcp.1041140305. [DOI] [PubMed] [Google Scholar]
- Janssens R, Boeynaems JM. Effects of extracellular nucleotides and nucleosides on prostate carcinoma cells. Br J Pharmacol. 2001;132:536–546. doi: 10.1038/sj.bjp.0703833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbir M, Ryten M, Thompson C, Mikhailidis D, Burnstock G. Purinergic receptor-mediated effects of ATP in high-grade bladder cancer. BJU Int. 2008;101:106–112. doi: 10.1111/j.1464-410X.2007.07286.x. [DOI] [PubMed] [Google Scholar]
- White N, Butler PE, Burnstock G. Human melanomas express functional P2X7 receptors. Cell Tissue Res. 2005;321:411–418. doi: 10.1007/s00441-005-1149-x. [DOI] [PubMed] [Google Scholar]
- White N, Ryten M, Clayton E, Butler P, Burnstock G. P2Y purinergic receptors regulate the growth of human melanomas. Cancer Lett. 2005;224:81–91. doi: 10.1016/j.canlet.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Dixon CJ, Bowler WB, Fleetwood P, Ginty AF, Gallagher JA, Carron JA. Extracellular nucleotides stimulate proliferation in MCF-7 breast cancer cells via P2-purinoceptors. Br J Cancer. 1997;75:34–39. doi: 10.1038/bjc.1997.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow IF, Thomson J, Davidson J, Shennan DB. The effect of a hyposmotic shock and purinergic agonists on K+(Rb+) efflux from cultured human breast cancer cells. Biochim Biophys Acta. 2005;1712:52–61. doi: 10.1016/j.bbamem.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Vandewalle B, Hornez L, Revillion F, Lefebvre J. Effect of extracellular ATP on breast tumor cell growth, implication of intracellular calcium. Cancer Lett. 1994;85:47–54. doi: 10.1016/0304-3835(94)90237-2. [DOI] [PubMed] [Google Scholar]
- White N, Burnstock G. P2 receptors and cancer. Trends Pharmacol Sci. 2006;27:211–217. doi: 10.1016/j.tips.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Greig AV, Linge C, Healy V, Lim P, Clayton E, Rustin MH, et al. Expression of purinergic receptors in non-melanoma skin cancers and their functional roles in A431 cells. J Invest Dermatol. 2003;121:315–327. doi: 10.1046/j.1523-1747.2003.12379.x. [DOI] [PubMed] [Google Scholar]
- Shabbir M, Ryten M, Thompson C, Mikhailidis D, Burnstock G. Characterization of calcium-independent purinergic receptor-mediated apoptosis in hormone-refractory prostate cancer. BJU Int. 2008;101:352–359. doi: 10.1111/j.1464-410X.2007.07293.x. [DOI] [PubMed] [Google Scholar]
- Shabbir M, Thompson C, Jarmulowiczc M, Mikhailidis D, Burnstock G. Effect of extracellular ATP on the growth of hormone-refractory prostate cancer in vivo. BJU Int. 2008;102:108–112. doi: 10.1111/j.1464-410X.2008.07578.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G.Purinergic signalling in developmentIn: Abbracchio MP, Williams M (eds).Handbook of Experimental Pharmacology, Volume 151/I Purinergic and Pyrimidinergic Signalling I – Molecular, Nervous and Urinogenitary System Function Springer-Verlag: Berlin; 200189–127. [Google Scholar]
- Zimmermann H. Nucleotide signaling in nervous system development. Pflugers Arch. 2006;452:573–588. doi: 10.1007/s00424-006-0067-4. [DOI] [PubMed] [Google Scholar]
- Bogdanov YD, Dale L, King BF, Whittock N, Burnstock G. Early expression of a novel nucleotide receptor in the neural plate of Xenopus embryos. J Biol Chem. 1997;272:12583–12590. doi: 10.1074/jbc.272.19.12583. [DOI] [PubMed] [Google Scholar]
- Meyer MP, Clarke JD, Patel K, Townsend-Nicholson A, Burnstock G. Selective expression of purinoceptor cP2Y1 suggests a role for nucleotide signalling in development of the chick embryo. Dev Dyn. 1999;214:152–158. doi: 10.1002/(SICI)1097-0177(199902)214:2<152::AID-AJA5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Burnstock G. Changes in expression of P2X purinoceptors in rat cerebellum during postnatal development. Brain Res Dev Brain Res. 2005;156:147–157. doi: 10.1016/j.devbrainres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Ryten M, Hoebertz A, Burnstock G. Sequential expression of three receptor subtypes for extracellular ATP in developing rat skeletal muscle. Dev Dyn. 2001;221:331–341. doi: 10.1002/dvdy.1147. [DOI] [PubMed] [Google Scholar]
- Sugioka M, Fukuda Y, Yamashita M. Ca2+ responses to ATP via purinoceptors in the early embryonic chick retina. J Physiol. 1996;493:855–863. doi: 10.1113/jphysiol.1996.sp021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebertz A, Arnett TR, Burnstock G. Regulation of bone resorption and formation by purines and pyrimidines. Trends Pharmacol Sci. 2003;24:290–297. doi: 10.1016/S0165-6147(03)00123-8. [DOI] [PubMed] [Google Scholar]
- Grol MW, Panupinthu N, Korcok J, Sims SM, Dixon SJ. Expression, signaling, and function of P2X7 receptors in bone. Purinergic Signal. 2009;5:205–221. doi: 10.1007/s11302-009-9139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Meyer R, Duncan RL, Turner CH. P2X7 nucleotide receptor plays an important role in callus remodeling during fracture repair. Calcif Tissue Int. 2009;84:405–412. doi: 10.1007/s00223-009-9237-7. [DOI] [PubMed] [Google Scholar]
- Panupinthu N, Rogers JT, Zhao L, Solano-Flores LP, Possmayer F, Sims SM, et al. P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: a signaling axis promoting osteogenesis. J Cell Biol. 2008;181:859–871. doi: 10.1083/jcb.200708037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartland A, Ginty AF, Gallagher JA, Bowler WB. Activation of P2X7 receptors expressed by human osteoclastoma modulates bone resorption. Calci Tissue Int. 1999;64:S56. [Google Scholar]
- Korcok J, Sims SM, Dixon SJ. P2X7 nucleotide receptors act through two distinct mechanisms to regulate osteoclast survival. J Bone Miner Res. 2004;19:S418–S419. [Google Scholar]
- Ohlendorff SD, Tofteng CL, Jensen JE, Petersen S, Civitelli R, Fenger M, et al. Single nucleotide polymorphisms in the P2X7 gene are associated to fracture risk and to effect of estrogen treatment. Pharmacogenet Genomics. 2007;17:555–567. doi: 10.1097/FPC.0b013e3280951625. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Solini A. P2 receptors: new potential players in atherosclerosis. Br J Pharmacol. 2002;135:831–842. doi: 10.1038/sj.bjp.0704524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Involvement of purinergic signaling in cardiovascular diseases. Drug News Perspect. 2003;16:133–140. doi: 10.1358/dnp.2003.16.3.876886. [DOI] [PubMed] [Google Scholar]
- Seye CI, Kong Q, Yu N, Gonzalez FA, Erb L, Weisman GA. P2 receptors in atherosclerosis and postangioplasty restenosis. Purinergic Signal. 2006;2:471–480. doi: 10.1007/s11302-006-9015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horckmans M, Lantz N, Dol-Gleizes F, Savi P, Gachet C, Boeynaems JM, et al. Role of P2Y4 nucleotide receptor in angiogenesis and inflammation. Purinergic Signal. 2008;4:S118. [Google Scholar]
- Woodward H, Roedersheimer M, Stenmark K, Gerasimovskaya E. Extracellular ATP as a pro-angiogenic factor for systemic microvascvular endothelial cellsation. Purinergic Signal. 2008;4:S120. [Google Scholar]
- Nilsson J, Nilsson LM, Chen YW, Molkentin JD, Erlinge D, Gomez MF. High glucose activates nuclear factor of activated T cells in native vascular smooth muscle. Arterioscler Thromb Vasc Biol. 2006;26:794–800. doi: 10.1161/01.ATV.0000209513.00765.13. [DOI] [PubMed] [Google Scholar]
- Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, et al. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1362–R1367. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L, Fernandez-Teruel A, Escorihuela RM, Fredholm BB, Tobena A, Pekny M, et al. Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur J Neurosci. 2002;16:547–550. doi: 10.1046/j.1460-9568.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Nicoll RA, Schmitz D. Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc Natl Acad Sci USA. 2003;100:14397–14402. doi: 10.1073/pnas.1835831100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JM, Chen JF, Schwarzschild MA, Apasov S, Smith PT, Caldwell C, et al. Gene dose effect reveals no Gs-coupled A2A adenosine receptor reserve in murine T-lymphocytes: studies of cells from A2A-receptor-gene-deficient mice. Biochem J. 2001;354:123–130. doi: 10.1042/0264-6021:3540123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Beilstein M, Xu YH, Turner TJ, Moratalla R, Standaert DG, et al. Selective attenuation of psychostimulant-induced behavioral responses in mice lacking A(2A) adenosine receptors. Neuroscience. 2000;97:195–204. doi: 10.1016/s0306-4522(99)00604-1. [DOI] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, et al. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- Naassila M, Ledent C, Daoust M. Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J Neurosci. 2002;22:10487–10493. doi: 10.1523/JNEUROSCI.22-23-10487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Kovarova M, Chason KD, Nguyen M, Koller BH, Tilley SL. Enhanced mast cell activation in mice deficient in the A2b adenosine receptor. J Exp Med. 2007;204:117–128. doi: 10.1084/jem.20061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Dikov MM, Blackburn MR, et al. Effect of A2B adenosine receptor gene ablation on proinflammatory adenosine signaling in mast cells. J Immunol. 2008;180:7212–7220. doi: 10.4049/jimmunol.180.11.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila MY, Stone RA, Civan MM. Knockout of A3 adenosine receptors reduces mouse intraocular pressure. Invest Ophthalmol Vis Sci. 2002;43:3021–3026. [PubMed] [Google Scholar]
- Cerniway RJ, Yang Z, Jacobson MA, Linden J, Matherne GP. Targeted deletion of A3 adenosine receptors improves tolerance to ischemia-reperfusion injury in mouse myocardium. Am J Physiol Heart Circ Physiol. 2001;281:H1751–H1758. doi: 10.1152/ajpheart.2001.281.4.H1751. [DOI] [PubMed] [Google Scholar]
- Guo Y, Bolli R, Bao W, Wu WJ, Black RG, Jr, Murphree SS, et al. Targeted deletion of the A3 adenosine receptor confers resistance to myocardial ischemic injury and does not prevent early preconditioning. J Mol Cell Cardiol. 2001;33:825–830. doi: 10.1006/jmcc.2001.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA. Disruption of the A3 adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem. 2000;275:4429–4434. doi: 10.1074/jbc.275.6.4429. [DOI] [PubMed] [Google Scholar]
- Tilley SL, Wagoner VA, Salvatore CA, Jacobson MA, Koller BH. Adenosine and inosine increase cutaneous vasopermeability by activating A3 receptors on mast cells. J Clin Invest. 2000;105:361–367. doi: 10.1172/JCI8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Makaritsis K, Francis CE, Gavras H, Ravid K. A role for the A3 adenosine receptor in determining tissue levels of cAMP and blood pressure: studies in knock-out mice. Biochim Biophys Acta. 2000;1500:280–290. doi: 10.1016/s0925-4439(99)00111-8. [DOI] [PubMed] [Google Scholar]
- Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, Cho K, et al. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J Neurosci. 2006;26:9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest. 2003;112:1895–1905. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- Hechler B, Lenain N, Marchese P, Vial C, Heim V, Freund M, et al. A role of the fast ATP-gated P2X1 cation channel in thrombosis of small arteries in vivo. J Exp Med. 2003;198:661–667. doi: 10.1084/jem.20030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Gittermann D, Cockayne DA, Jones A. ATP modulation of excitatory synapses onto interneurons. J Neurosci. 2003;23:7426–7437. doi: 10.1523/JNEUROSCI.23-19-07426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Bian X, Ren J, DeVries M, Schnegelsberg B, Cockayne DA, Ford AP, et al. Peristalsis is impaired in the small intestine of mice lacking the P2X3 subunit. J Physiol. 2003;551:309–322. doi: 10.1113/jphysiol.2003.044172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Sperlagh B, Illes P. P2X3 receptor involvement in pain states. Mol Neurobiol. 2007;36:165–183. doi: 10.1007/s12035-007-0033-y. [DOI] [PubMed] [Google Scholar]
- Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, et al. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–1017. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain. 2009;5:28. doi: 10.1186/1744-8069-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, et al. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12:133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Pochet S, Garcia-Marcos M, Seil M, Otto A, Marino A, Dehaye JP. Contribution of two ionotropic purinergic receptors to ATP responses in submandibular gland ductal cells. Cell Signal. 2007;19:2155–2164. doi: 10.1016/j.cellsig.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, et al. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- Lenain N, Freund M, Leon C, Cazenave JP, Gachet C. Inhibition of localized thrombosis in P2Y1-deficient mice and rodents treated with MRS2179, a P2Y1 receptor antagonist. J Thromb Haemost. 2003;1:1144–1149. doi: 10.1046/j.1538-7836.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Lazarowski E, Homolya L, Boucher RC, Koller BH, Grubb BR. Effect of loss of P2Y2 receptor gene expression on nucleotide regulation of murine epithelial Cl− transport. J Biol Chem. 1999;274:26461–26468. doi: 10.1074/jbc.274.37.26461. [DOI] [PubMed] [Google Scholar]
- Ghanem E, Robaye B, Leal T, Leipziger J, Van Driessche W, Beauwens R, et al. The role of epithelial P2Y2 and P2Y4 receptors in the regulation of intestinal chloride secretion. Br J Pharmacol. 2005;146:364–369. doi: 10.1038/sj.bjp.0706353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, Boeynams JM, et al. Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol. 2008;74:777–784. doi: 10.1124/mol.108.046904. [DOI] [PubMed] [Google Scholar]
- Conley PB, Delaney SM. Scientific and therapeutic insights into the role of the platelet P2Y12 receptor in thrombosis. Curr Opin Hematol. 2003;10:333–338. doi: 10.1097/00062752-200309000-00002. [DOI] [PubMed] [Google Scholar]
- Greig AV, Linge C, Terenghi G, McGrouther DA, Burnstock G. Purinergic receptors are part of a functional signaling system for proliferation and differentiation of human epidermal keratinocytes. J Invest Dermatol. 2003;120:1007–1015. doi: 10.1046/j.1523-1747.2003.12261.x. [DOI] [PubMed] [Google Scholar]