Abstract
The p53 family member p63 has been shown to be critical for growth, proliferation and chemosensitivity. Here we demonstrate that the c-Abl tyrosine kinase phosphorylates the widely expressed ΔNp63α isoform and identify multiple sites by mass spectrometry in vitro and in vivo. Phopshorylation by c-Abl results in greater protein stability of both ectopically expressed and endogenous ΔNp63α. c-Abl phosphorylation of ΔNp63α induces its binding to Yes-associated protein (YAP) and silencing of YAP by siRNA reduces the c-Abl-induced increase of ΔNp63α levels. We further show that cisplatin induces c-Abl phosphorylation of ΔNp63α and its binding to YAP. Overexpression of ΔNp63α, but not the c-Abl phosphosites mutant, protects cells from cisplatin treatment. Finally, we demonstrate the rescue of p63 siRNA-mediated loss of viability with p63siRNA insensitive construct of ΔNp63α but not the phosphosites mutant. These results demonstrate that c-Abl phosphorylation of ΔNp63α regulates its protein stability, by inducing binding of YAP, and is critical for cell viability.
Keywords: p63, c-Abl, Yes-associated protein, cisplatin
The p53 family of transcription factors consists of three members, each transcribed from separate genes, p53, p63 and p73. p53 was the first family member to be discovered, and transgenic mice studies and mutational analyses in human cancers have implicated p53 to be one of the most important tumor suppressors.1 Functionally, p53 responds to a variety of stresses in the cell and acts to protect DNA from damage. p73 and p63 were discovered later and both preliminary transgenic studies and mutational analyses yielded little evidence for a direct role for these two family members in tumorigenesis.2, 3 Indeed, p73 and p63 were shown to have a role in development, with p73 thought to be pivotal for the formation of neural system and p63 necessary for skin development.4, 5, 6 However, recent studies have determined that p73 is critical for the response to DNA damage induced by chemotherapeutics and that p63 also has a vital role in protecting the female germline from radiation and chromosomal damage.7, 8 Moreover, p63 and p73 have been shown to cooperate with p53 in regulating both apoptosis and tumorigenesis.9, 10
The p63 and p73 genes can each be transcribed from two promoters, generating full-length transactivation domain containing TA isoforms or N-terminal truncated ΔN isoforms, which contain a short unique transactivation domain depending on promoter usage. Even greater complexity is generated by the fact that these isoforms can undergo alternative C-terminal splicing generating a total of 6 different p63 isoforms and at least 14 p73 isoforms.11 The expression level of each isoform differs in various tissues and stages of development and the overall combined activity of the p53 family in any given scenario will be dependent on the ratio of the isoforms present and their interaction.11 This was clearly demonstrated in a recent transgenic study in mice that demonstrated that p73 can be a tumor suppressor in it is own right, but only if the TA isoform is knocked out.12
Though the three family members are structurally homologous and can be activated by similar stresses to regulate a comparable set of genes, they are differentially regulated by upstream signaling. For example, p53 is phosphorylated by a host of serine/threonine kinases, whereas p73 is phosphorylated by the tyrosine kinase c-Abl upon DNA damage.13, 14, 15, 16 They also bind to and are degraded by different E3 ubiquitin ligases; MDM2 is well-characterized as one of the key regulators of p53 but not p73, whereas ITCH has recently been shown to control the stability of both p63 and p73 but does not bind p53.17, 18, 19
Another protein that binds p73 but not p53 is the Yes-associated protein (YAP).20 YAP binds p73 and promotes its transcription of pro-apoptotic genes, and this interaction has been shown to be negatively regulated by Akt and stimulated by DNA damage, in part, through c-Abl.21, 22, 23, 24 More recently, YAP has been shown to also stabilize p73 by displacing ITCH by competitive binding.25, 26
In this study, we demonstrate that the widely expressed ΔNp63α isoform of p63 is directly phosphorylated by c-Abl both in vitro and in vivo. Phosphorylation of ΔNp63α promotes increased binding to YAP and results in its increased stability. c-Abl activity can signal a wide spectrum of cellular processes including apoptosis, proliferation and differentiation.27 Though cisplatin induced c-Abl phosphorylation of TA p73 and YAP has previously been shown to be pro-apoptotic, here we show that c-Abl-phosphorylated ΔNp63α in head and neck cancer cells promotes greater proliferation and protection from cisplatin-induced cell death.
Results
Identification of in vitro and in vivo c-Abl phosphorylation of ΔNp63α
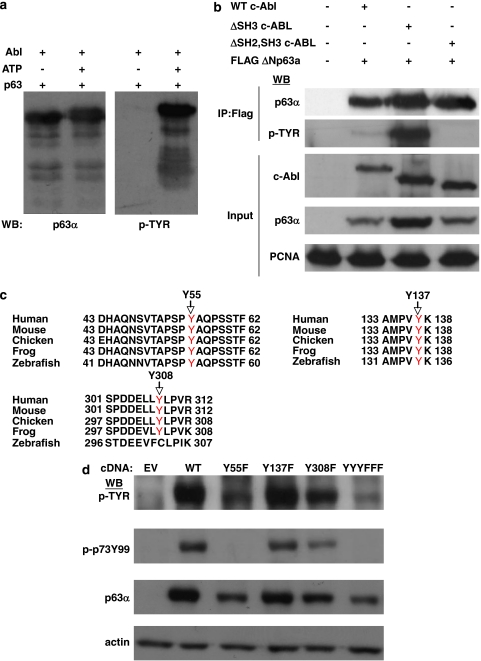
We noted that the c-Abl phosphorylation site identified in TAp73 is conserved in both TA and ΔN isoforms of p63. To gain insight into the possible role of c-Abl phosphorylation of p63, we focused on the widely expressed ΔNp63α isoform. We first assessed the direct phosphorylation of ΔNp63α by carrying out in vitro kinase assays employing recombinant proteins. As shown in Figure 1a, tyrosine phosphorylation as well as a visible band shift of p63 was detected in SDS-PAGE fractionation of c-Abl but not control kinase assays, in an ATP-dependent manner. Co-transfection in cells with either empty vector, full-length c-Abl, an activating SH3 truncated mutant c-Abl or an inactive SH2, SH3 truncation mutant c-Abl28 reveals similar c-Abl dependent, in vivo tyrosine phosphorylation of ΔNp63α (Figure 1b). Mass spectrometric analysis of ΔNp63α from in vitro kinase assays identified six putative, direct c-Abl-dependent tyrosine phosphorylation sites (Supplementary Figure S1a), whereas analysis of p63 immunoprecipitated from cells co-transfected with active c-Abl yielded seven tyrosine phosphorylation sites (Supplementary Figure S1b). There were three phosphorylated residues detected that overlapped from in vitro and in vivo analyses (Figure 1c). These were selected for further analyses to focus on sites that were most likely to be directly phosphorylated by c-Abl as well as being physiologically relevant. One of these sites, Y55, is the homologous site of c-Abl phosphorylation identified on p73. The second site, Y137, is also conserved in p73 but has not previously been reported to be phosphorylated, whereas the third site, Y308, is only found in p63 isoforms. The first two sites are conserved in p63 isoforms from zebrafish to human, whereas the third site is conserved from frog to human (Figure 1c). Phospho-deficient tyrosine to phenylalanine point mutant constructs of each site (Y55F, Y137F and Y308F), as well as a combined mutant construct (YYYFFF) were generated. Co-transfection of wild-type ΔNp63α and the mutants constructs with c-Abl demonstrated that each of the three sites were phosphorylated in vivo and that ablating all three is necessary for abrogating c-Abl-dependent tyrosine phosphorylation of p63 (Figure 1d). It is interesting to note that mutation analysis also revealed that a commercial antibody (p-p73Y99) to the c-Abl-phosphorylated tyrosine, Y99, of TAp73 cross-reacts specifically to the conserved tyrosine, Y55, on ΔNp63α.
Figure 1.
ΔNp63α is a direct target of the c-Abl tyrosine kinase. (a) In vitro kinase assay employing recombinant ΔNp63α and active, recombinant c-Abl was carried out as described in Experimental Procedures. Kinase reactions were fractionated by SDS-PAGE and analyzed by western blot with indicated antibodies (p63α=p63α isoform specific antibody). (b) 293 cells were co-transfected with indicated plasmids or relevant empty vector and Flag-ΔNp63α immunoprecipitated with Flag antibody. Immunoprecipitates and representative whole cell lysate (Input) were fractionated by SDS-PAGE and analyzed by western blot with indicated antibodies. (c) Sequences from peptides containing phosphorylated tyrosines which overlapped from mass spectrometric analysis of ΔNp63α of both in vivo and in vitro phosphorylation by c-Abl (see Supplementary Figure S1) were compared across species: human=Homo sapien; mouse=Mus musculus; chicken=Gallus gallus; frog=Xenopus laevis; zebrafish=Danio rerio. Phosphorylated tyrosine indicated in red. (d) 293 cells were transfected with c-Abl and either empty vector, ΔNp63α or indicated tyrosine to phenalanine mutant construct. Whole-cell lysates were fractionated by SDS-PAGE and analyzed by western blot with indicated antibodies (p-p73Y99=antibody to Tyrosine 99 phosphorylated p73, see text)
Endogenous ΔNp63α stability is regulated by c-Abl phosphorylation
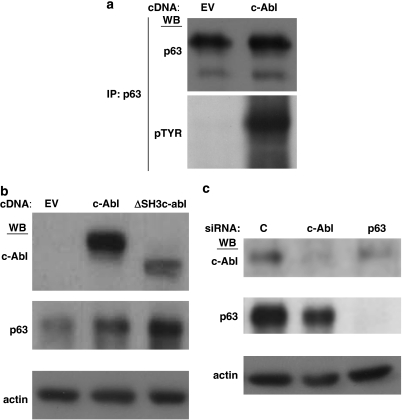
We noted that in Figure 1b, co-transfection of ΔNp63α with the more active ΔSH3 c-Abl mutant in 293 cells increased its protein stability relative to co-expression with the less active full-length c-Abl. Furthermore, in Figure 1d we see that the phosphosite mutants are markedly less stable than wild-type ΔNp63α, with the triple mutant exhibiting least stability. To assess whether c-Abl regulates endogenous ΔNp63α, we examined head and neck cancer cells, in which the ΔNp63α isoform is expressed at high levels and has been shown to be vital for proliferation, differentiation, growth and protection from p73-dependent apoptosis.29, 30, 31 We ectopically expressed c-Abl in the H357 head and neck cancer cell line and immunoprecipitated endogenous ΔNp63α with an α−isoform selective p63 antibody. Western blot with phosphotyrosine antibody from c-Abl, but not empty vector transfected cells, demonstrate robust tyrosine phosphorylation of ΔNp63α (Figure 2a). Similar to what was shown in 293 cells (Figure 1b), transfection of c-Abl increased expression of endogenous ΔNp63α over transfection of empty vector in the head and neck cancer cells (Figure 2b). This effect was further enhanced by transfection with the hyperactive ΔSH3 c-Abl construct (Figure 2b). In contrast, silencing endogenous c-Abl with siRNA leads to decreased expression of endogenous p63 (Figure 2c).
Figure 2.
ΔNp63α stability is regulated by c-Abl phosphorylation. (a) H357 head and neck cancer cells were transfected with indicated plasmids and immunoprecipitated with p63α antibody. Immunoprecipitates were analyzed with indicated antibodies (p63=pan p63 antibody). (b) H357 cells were transfected with indicated plasmids and whole-cell lysates were treated were analyzed with indicated antibodies. (c) H357 cells were transfected with indicated siRNA oligonucleotides as described in Experimental Procedures. Whole-cell lysates were analyzed with indicated antibodies
YAP binds to and stabilizes ΔNp63α in a c-Abl-dependent manner
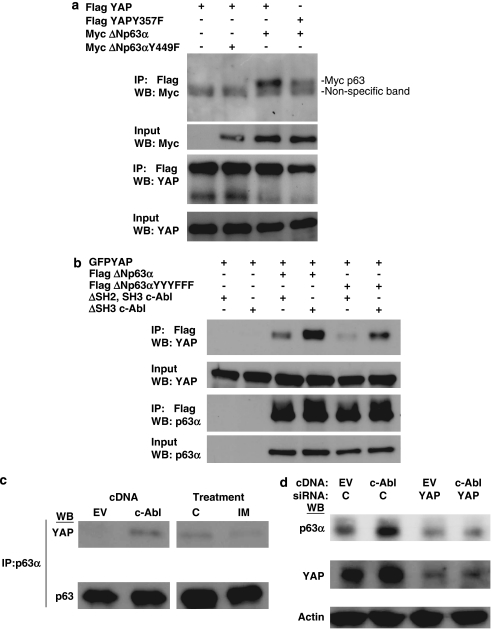
Recently, YAP was shown to bind to and stabilize TAp73.25, 26 Though YAP has not previously been demonstrated to bind p63 in vivo, the PPXY motif which YAP binds to in p73 is conserved in the longer p63 isoforms, including ΔNp63α.20 We first co-transfected 293 cells with mycΔNp63α or ΔNp63α Y449F (PPXY motif point mutant) and either FLAG-YAP or YAP Y357F (c-Abl phosphosite mutant) and immunoprecipitated with Flag beads to pull down YAP. We determined that ΔNp63α but not the PPXY motif point mutant binds YAP (Figure 3a lanes 2,3). YAP has also been shown to be a direct c-Abl substrate23 and we further show that the c-Abl phosphosite mutant, YAP Y357F, does not co-immunoprecipitate ΔNp63α as well as wild-type YAP (Figure 3a, lanes 3 and 4).
Figure 3.
Yes-associated protein (YAP) binds to and stabilizes ΔNp63α in a c-Abl-dependent manner. (a) 293 cells were transfected with indicated plasmids and immunoprecipiated with Flag antibody. Immunoprecipitates were analyzed with indicated antibodies. (b) 293 cells were transfected with indicated plasmids and immunoprecipiated with Flag antibody. Immunoprecipitates were analyzed with indicated antibodies. (c) H357 cells were transfected with indicated plasmid (left panels) or treatments (right panels; c=control vehicle, IM=2 μM imatinib, 16 h) and immunoprecipitated with p63α antibody. Immunoprecipitates were analyzed with indicated antibodies. (d) H357 cells were reverse transfected with indicated siRNA oligonucloetide and forward transfected 24 h later with indicated plasmid. Whole-cell lysates were analyzed with indicated antibodies
We next examined the effect of c-Abl phosphorylation on the YAP–p63 interaction by co-transfecting FLAG ΔNp63α or ΔNp63αYYYFFF (combined c-Abl phosphosite mutant) and GFP–YAP along with either the inactive c-Abl truncation mutant (Δ SH2, SH3 c-Abl) or the hyperactive truncation mutant (Δ SH3 c-Abl). Co-immunoprecipitation of p63 and YAP in these cells revealed greatly diminished binding to YAP of the ΔNp63α phosphosite mutant compared with wild-type ΔNp63α (Figure 3b, lanes 3 and 5). Furthermore, expression of hyperactive c-Abl increased binding of YAP to wild-type ΔNp63α (Figure 3b, lanes 3 and 4). This effect was reduced but not completely abrogated for ΔNp63αYYYFFF (Figure 3b, lanes 5 and 6). These data, along with the YAP phosphosite mutant result (Figure 3a, lanes 3 and 4) indicate that phosphorylation of c-Abl on both YAP and p63 regulate their binding.
Binding of YAP and p63 is also regulated by c-Abl phosphorylation in the head and neck cancer cells. In a similar experiment to Figure 2a, we demonstrate that the binding of endogenous ΔNp63α and endogenous YAP is enhanced in c-Abl transfected H357 cells (Figure 3c, left). Conversely, treatment with the c-Abl selective inhibitior imatinib32, 33 decreased this interaction (Figure 3c, right). Silencing endogenous YAP in the head and neck cancer cells results in decreased endogenous p63 and prevents further c-Abl-dependent increase in p63 (Figure 3d). These results indicate that c-Abl phosphorylation stabilizes ΔNp63α by increasing its binding to YAP, similar to the effect of YAP on TAp73.23, 25, 26
Cisplatin stimulates c-Abl phosphorylation of ΔNp63α and its binding to YAP
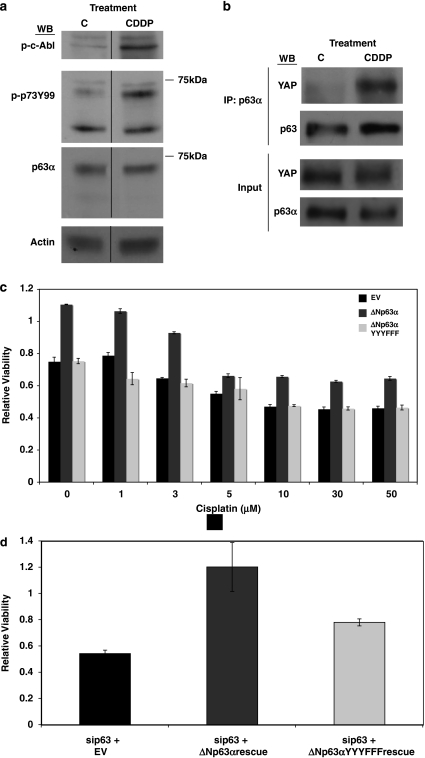
c-Abl is activated by a number of upstream stimuli, including DNA damage.27 The chemotherapeutic agent cisplatin induces c-Abl phosphorylation of TAp73, as well as YAP, to induce apoptosis.13, 14, 16, 23 ΔNp63 is thought to counter TAp73 in chemotherapy-induced apoptosis in cancer cells and ΔNp63–TAp73 interaction has recently been shown to be regulated by c-Abl activity in breast cancer.31, 34, 35 More recently, it has been shown that c-Abl can phosphorylate and regulate the pro-apoptotic TAp63 in mouse oocytes upon cisplatin treatment.36 However, direct phosphorylation of ΔNp63 by cisplatin-induced c-Abl has not been demonstrated. We show that cisplatin treatment in H357 head and neck cells leads to an increase in endogenous c-Abl phosphorylation (Figure 4a), as detected by an antibody to phosphorylated c-Abl, and a concomitant increase in ΔNp63α phosphorylation (Figure 4a), as detected by the cross-reacting p-p73Y99 antibody (Figure 4a). Molecular weight comparison with the p63α antibody signal demonstrates that the cisplatin-induced p-p73Y99 signal is indeed p63α in these cells (Figure 4a). Cisplatin treament also induces increased binding of endogenous YAP to ΔNp63 (Figure 4b), similar to ectopic expression of c-Abl in these cells (Figure 3c).
Figure 4.
c-Abl phosphorylation of ΔNp63α regulates viability. (a) H357 cells were treated with 50 μM cisplatin (CDDP) or control vehicle for 6 h and whole cell lysates analyzed with indicated antibodies (p-c-Abl=phosphorylated c-Abl). (b) H357 cells were treated as in (a) and immunoprecipitated with p63α. Immunoprecipitates and input were analyzed with indicated antibodies. (c) H357 cells were transfected with indicated plasmids and treated it with indicated doses of cisplatin for 48 h. Viability was measured by MTS assay as described in Experimental Procedures. (d) H357 cells were reverse transfected with p63 siRNA oligonucleotide and then forward transfected with indicated plasmids (Rescue=insensitive to p63 siRNA oligonucleotide). Viability was measured by MTS as in (c) 48 h after plasmid transfection. Data are presented in (c) and (d) as mean±S.E.M.
c-Abl phosphorylation of ΔNp63α regulates viability in the head and neck cancer cells
To investigate the effect of ΔNp63α expression on cisplatin sensitivity and its regulation by c-Abl phosphorylation, we ectopically expressed either the empty vector ΔNp63α or the YYYFFF phosphosites mutant in H357 cells. ΔNp63α but not the c-Abl phosphosites mutant protected the head and neck cancer cells from loss of viability at all doses of cisplatin (Figure 4a). Noting that the overexpression of ΔNp63α but not the phospho-deficient construct resulted in increased viability in untreated cells relative to empty vector, we next examined the effect of endogenous c-Abl phosphorylation on ΔNp63α in unstimulated cells. Endogenous p63 was silenced by siRNA in the head and neck cells and either empty vector ΔNp63α or phospho-deficient ΔNp63α constructs resistant to the siRNA oligo (Supplementary Figure S2) were transfected. As shown in Figure 4b, loss of viability ensuing from p63siRNA was rescued by wild-type ΔNp63α but not the c-Abl phosphosites mutant. These results indicate that both DNA damage stimulated and unstimulated c-Abl activity is important in regulating ΔNp63α to maintain viability in the head and neck cancer cells.
Discussion
In cancer, c-Abl has been implicated as an oncogene in a number of malignancies with unregulated kinase activity leading to hyperproliferaton and transformation.37 In contrast, c-Abl has also been well-characterized as a DNA damage-stimulated kinase, activating cell death downstream of chemotherapeutic agents.27 For the latter, TAp73 and its interacting regulator YAP, have been demonstrated to be key substrates for c-Abl.23 Activation of ΔN isoforms of both p63 and p73 has been shown to counter the pro-apoptotic signaling of TAp73, as well as p53.11 YAP has been demonstrated to be pro-apoptotic in a number of studies, by binding and activating TAp73, and has recently been implicated as a tumor suppressor in certain breast cancers.21, 22, 23, 24, 25, 26, 38 Here we show that c-Abl phosphorylates ΔNp63α, leading to its increased stability, in part by inducing binding to YAP, to signal increased proliferation as well as to counter cisplatin-induced cell death in the head and neck cancer cells. YAP has also been shown to be pro-growth, most convincingly in the development of Drosophila melanogaster, at least, in part, by binding and activating the TEAD transcription factor.39, 40 It may be that YAP can also stimulate proliferation in other systems by binding and stabilizing ΔNp63α.
Expression of the different p63 and p73 isoforms vary across different cell types but beyond mRNA levels, post-translational modifications such as phosphorylation, as well as acetylation, sumoylation and ubiquitylation, determine their overall expression. The different isoforms themselves may interact and compete for binding on DNA.11 Furthermore, interacting partners such as YAP themselves are regulated by similar post-translational modifications.22, 23 Gonfloni et al36 have recently implicated cisplatin-induced c-Abl phosphorylation to be stimulate the pro-apoptotic TAp63 isoform in chemotherapy-induced cell death in oocytes, which complements our present study showing that c-Abl phosphorylation of the pro-proliferative ΔNp63α isoform is protective. These findings clearly illustrate that to fully understand the role of p63 and p73 in particular systems, the integration of the activities of all the isoforms must be assessed. Further work is required to determine the spatial and temporal kinetics of formation of the dynamic complexes of p63 and p73 family members and their regulatory proteins.
Materials and Methods
Cell culture
HEK293 cell line and human breast cancer cell line MCF7 were obtained from the Cancer Research UK Central Cell Service (Clare Hall, South Mimms, UK) and were cultured in 10% CO2 in DMEM supplemented with 10% foetal calf serum (FCS, Sigma, St. Louis, MO, USA). H357, an oral squamous cell carcinoma head and neck cancer cell line, was kindly given by Dr. Gareth Thomas, Institute of Cancer, Barts and The London School of Medicine, and was cultured in KGM medium supplemented with 10% FCS.
Transfections
Cells for immunoprecipitation were seeded at 1.5 × 106 in 10 cm dish, at 1 × 105 in a 6-well plate for straight western blot and at 1 × 104 for 96-well plate, 18–24 h before transfection. 1 μg of each plasmid was used for 10 cm dish, 200 ng of each for 6-well plate and 100 ng of each for 96 well plate using Effectene (Qiagen) for 48 h according to manufacturer's instructions. Plasmids, cloning and mutagenesis information are described in detail in Supplementary Materials and Methods. siRNA transfections were carried out in reverse with Interferin (Polyplus Transfection, Illkirch, France) at 10 nM final concentration. siGENOME Non-Targeting siRNA#2 (D001210-02-20) as siRNA control, p63 siGENOME siRNA (D003330-05-0005), c-Abl OnTARGET plus SMARTpool (L-003100-00-0005) are from Dharmacon (Lafayette, CO, USA). YAP siRNA-targeting sequence was described previously.21
Expression of GST–ΔNp63α
Cultures of E.coli (DH5a) were transformed with pGEX 6p1-ΔNp63α plasmid and induced to express recombinant protein using IPTG (0.5 mg/ml) over 4 h at 37°C. Cells were lysed using PBST buffer and isolated using glutathione-Sepharose beads.
In vitro kinase assay
A total of 40 μl of GST-ΔNp63α isolated on sepharose beads was used as substrate and recombinant Abl (New England Biolabs, Beverly, MA, USA) was used as kinase for in vitro kinase assay and carried out according to the manufacturer's instructions. In all, 200 U of Abl were used in the assay.
Immunoprecipitation
Np40 lysis buffer (Sodium chloride 150 mM, NP-40 1%, Tris pH 8.0 50 mM) was used to extract protein from cell pellet; 1 mM DTT, protease inhibitor cocktail and phosphatase inhibitor (Roche, Basel, CH, Switzerland) were added into lysis buffer. Protein A Sepharose 4B (Sigma) and p63α (H129) antibody was use for p63 immunoprecipitation. Flag immunoprecipitation was carried out using Flag M2 beads (Sigma) following the manufacturer's instructions.
Western blot
Protein extracts were fractionated by SDS-PAGE and transferred to PVDF membrane (Immobilon-P, Millipore, Billerica, MA, USA). Anti- actin, c-Myc (A14), p63α (H129), pan-p63 (4A4), tubulin, YAP (H-125) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), phospho p73 (Tyr99) antibody, phospho-c-Abl (Tyr245) (Cell Signalling Technology, Beverly, MA, USA), c-Abl (Ab-3) antibody (Calbiochem, San Diego, CA, USA) and phosphotyrosine (4G10) antibody (Upstate, Millipore) were used as manufacture's recommendation; mouse anti-human proliferating cell nuclear antigen (PCNA) antibody (Research Monoclonal Antibody Service, Cancer Research UK, 1 : 1000). Chemiluminescence detected by (Amersham ECL Plus, GE Healthcare, Chalfont St. Giles, UK) or (SuperSignal West Pico Substrate, Pierce, Rockford, IL, USA) and the membranes were exposed to X-ray film.
Mass-spectrometry of phosphorylation sites of ΔNp63α
Recombinant GST ΔNp63α from c-Abl in vitro kinase assays or FLAG–ΔNp63α immunoprecipitated from c-Abl co-transfected 293 cells were fractionated by SDS-PAGE and ΔNp63α band excised, digested by indicated enzymes and subjected to mass spectrometric analysis at the Taplin Mass Spectrometry Facility (Harvard University, Cambridge, MA, USA).
Cell viability
A total of 1 × 104 H357 cells were seeded in 96-well dishes and transfected and treated as indicated and cell survival determined by MTS assay according to the manufacturer's instructions (Promega, Madison, WI, USA).
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This work was funded by Cancer Research UK and the Barts and The London Charity.
Glossary
- ΔN
N-terminal truncated
- ΔSH2, SH3
src homology domains 2, 3 truncated
- ΔSH3
src homology domain 3 truncated
- IPTG
isopropyl β--thiogalactopyranoside
- siRNA
short, interfering RNA
- TA
transactivation domain
- YAP
yes-associated protein
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Supplementary Material
References
- Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov. 2008;7:979–987. doi: 10.1038/nrd2656. [DOI] [PubMed] [Google Scholar]
- Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- Yang A, Walker N, Bronson R, Kaghad M, Oosterwegel M, Bonnin J, et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404:99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- Irwin MS, Kondo K, Marin MC, Cheng LS, Hahn WC, Kaelin WG., Jr Chemosensitivity linked to p73 function. Cancer Cell. 2003;3:403–410. doi: 10.1016/s1535-6108(03)00078-3. [DOI] [PubMed] [Google Scholar]
- Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–628. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- Flores ER, Sengupta S, Miller JB, Newman JJ, Bronson R, Crowley D, et al. Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell. 2005;7:363–373. doi: 10.1016/j.ccr.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Flores ER, Tsai KY, Crowley D, Sengupta S, Yang A, McKeon F, et al. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature. 2002;416:560–564. doi: 10.1038/416560a. [DOI] [PubMed] [Google Scholar]
- Murray-Zmijewski F, Lane DP, Bourdon JC. p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ. 2006;13:962–972. doi: 10.1038/sj.cdd.4401914. [DOI] [PubMed] [Google Scholar]
- Tomasini R, Tsuchihara K, Wilhelm M, Fujitani M, Rufini A, Cheung CC, et al. TAp73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes Dev. 2008;22:2677–2691. doi: 10.1101/gad.1695308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agami R, Blandino G, Oren M, Shaul Y. Interaction of c-Abl and p73alpha and their collaboration to induce apoptosis. Nature. 1999;399:809–813. doi: 10.1038/21697. [DOI] [PubMed] [Google Scholar]
- Gong JG, Costanzo A, Yang HQ, Melino G, Kaelin WG, Jr, Levrero M, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- Olsson A, Manzl C, Strasser A, Villunger A. How important are post-translational modifications in p53 for selectivity in target-gene transcription and tumour suppression. Cell Death Differ. 2007;14:1561–1575. doi: 10.1038/sj.cdd.4402196. [DOI] [PubMed] [Google Scholar]
- Yuan ZM, Shioya H, Ishiko T, Sun X, Gu J, Huang YY, et al. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature. 1999;399:814–817. doi: 10.1038/21704. [DOI] [PubMed] [Google Scholar]
- Balint E, Bates S, Vousden KH. Mdm2 binds p73 alpha without targeting degradation. Oncogene. 1999;18:3923–3929. doi: 10.1038/sj.onc.1202781. [DOI] [PubMed] [Google Scholar]
- Rossi M, Aqeilan RI, Neale M, Candi E, Salomoni P, Knight RA, et al. The E3 ubiquitin ligase Itch controls the protein stability of p63. Proc Natl Acad Sci USA. 2006;103:12753–12758. doi: 10.1073/pnas.0603449103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, De Laurenzi V, Munarriz E, Green DR, Liu YC, Vousden KH, et al. The ubiquitin-protein ligase Itch regulates p73 stability. EMBO J. 2005;24:836–848. doi: 10.1038/sj.emboj.7600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strano S, Munarriz E, Rossi M, Castagnoli L, Shaul Y, Sacchi A, et al. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J Biol Chem. 2001;276:15164–15173. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- Lapi E, Di Agostino S, Donzelli S, Gal H, Domany E, Rechavi G, et al. PML, YAP, and p73 are components of a proapoptotic autoregulatory feedback loop. Mol Cell. 2008;32:803–814. doi: 10.1016/j.molcel.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Levy D, Adamovich Y, Reuven N, Shaul Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of pro-apoptotic genes in response to DNA damage. Mol Cell. 2008;29:350–361. doi: 10.1016/j.molcel.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E, et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol Cell. 2005;18:447–459. doi: 10.1016/j.molcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Danovi SA, Rossi M, Gudmundsdottir K, Yuan M, Melino G, Basu S. Yes-associated protein (YAP) is a critical mediator of c-Jun-dependent apoptosis. Cell Death Differ. 2008;15:217–219. doi: 10.1038/sj.cdd.4402226. [DOI] [PubMed] [Google Scholar]
- Levy D, Adamovich Y, Reuven N, Shaul Y. The Yes-associated protein 1 stabilizes p73 by preventing Itch-mediated ubiquitination of p73. Cell Death Differ. 2007;14:743–751. doi: 10.1038/sj.cdd.4402063. [DOI] [PubMed] [Google Scholar]
- Shaul Y, Ben-Yehoyada M. Role of c-Abl in the DNA damage stress response. Cell Res. 2005;15:33–35. doi: 10.1038/sj.cr.7290261. [DOI] [PubMed] [Google Scholar]
- Pluk H, Dorey K, Superti-Furga G. Autoinhibition of c-Abl. Cell. 2002;108:247–259. doi: 10.1016/s0092-8674(02)00623-2. [DOI] [PubMed] [Google Scholar]
- Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, Hill DE, et al. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci USA. 2000;97:5462–5467. doi: 10.1073/pnas.97.10.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena AM, Shalom-Feuerstein R, di Val Cervo PR, Aberdam D, Knight RA, Melino G, et al. miR-203 represses ‘stemness' by repressing DeltaNp63. Cell Death Differ. 2008;15:1187–1195. doi: 10.1038/cdd.2008.69. [DOI] [PubMed] [Google Scholar]
- Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- Nagar B, Hantschel O, Young MA, Scheffzek K, Veach D, Bornmann W, et al. Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell. 2003;112:859–871. doi: 10.1016/s0092-8674(03)00194-6. [DOI] [PubMed] [Google Scholar]
- DeYoung MP, Johannessen CM, Leong CO, Faquin W, Rocco JW, Ellisen LW. Tumor-specific p73 up-regulation mediates p63 dependence in squamous cell carcinoma. Cancer Res. 2006;66:9362–9368. doi: 10.1158/0008-5472.CAN-06-1619. [DOI] [PubMed] [Google Scholar]
- Leong CO, Vidnovic N, DeYoung MP, Sgroi D, Ellisen LW. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. J Clin Invest. 2007;117:1370–1380. doi: 10.1172/JCI30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonfloni S, Di Tella L, Caldarola S, Cannata SM, Klinger FG, Di Bartolomeo C, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15:1179–1185. doi: 10.1038/nm.2033. [DOI] [PubMed] [Google Scholar]
- Lin J, Arlinghaus R. Activated c-Abl tyrosine kinase in malignant solid tumors. Oncogene. 2008;27:4385–4391. doi: 10.1038/onc.2008.86. [DOI] [PubMed] [Google Scholar]
- Yuan M, Tomlinson V, Lara R, Holliday D, Chelala C, Harada T, et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15:1752–1759. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.