Abstract
Background.
In a robust and consistent manner, sustained caloric restriction (CR) has been shown to retard the aging process in a variety of animal species. Nonhuman primate studies suggest that CR may have similar effects in longer-lived species. The CALERIE (Comprehensive Assessment of the Long-term Effects of Reducing Intake of Energy) research program is the first systematic investigation of CR in nonobese human beings. In the phase 2 study, it is hypothesized that 2 years of sustained CR, involving a 25% reduction of ad libitum energy intake, results in beneficial effects similar to those observed in animal studies. This article presents the design and implementation of this study.
Methods.
The study is a multicenter, parallel-group, randomized controlled trial. A sample of 225 participants (22.0 ≤ body mass index [BMI] < 28.0 kg/m2) is being enrolled with 2:1 allocation to CR.
Results.
An intensive dietary and behavioral intervention was developed to achieve 25% CR and sustain it over the 2 years. Adherence is monitored using a doubly labeled water technique. Primary outcomes are resting metabolic rate and core temperature, and are assessed at baseline and at 6-month intervals. Secondary outcomes address oxyradical formation, cardiovascular risk markers, insulin sensitivity and secretion, immune function, neuroendocrine function, quality of life and cognitive function. Biologic materials are stored in a central repository.
Conclusions.
An intricate protocol has been developed to conduct this study. Procedures have been implemented to safeguard the integrity of the data and the conclusions drawn. The results will provide insight into the detrimental changes associated with the human aging process and how CR mitigates these effects.
Keywords: Aging, Caloric restriction, Resting metabolic rate, Markers of inflammation, Randomized controlled trial
PRIMARY aging is the inevitable deterioration of cells and tissues independent of illness and environmental factors. Secondary aging is the decline resulting from external influences such as disease and detrimental lifestyle. Both are important codeterminants of health (1). The attenuation of primary aging increases maximal life span, whereas delays in secondary aging mostly affect average life span. Caloric restriction (CR) is the only known intervention that slows both primary and secondary aging in laboratory animals.
Since the original discovery by McCay and colleagues (2) in the 1930s, CR has consistently been shown to extend life spans across a range of animal species including yeast, nematodes, flies, fish, and rodents (3,4). Although findings on lifespan in longer-lived species including nonhuman primates are as yet inconclusive (5), CR monkeys display a substantially reduced age-related morbidity (6–8). In humans, information is lacking from controlled trials of the effects of CR on surrogate markers believed to be indicative of primary aging (9). The only evidence comes from observational studies in longer-lived humans (10) and individuals who self-impose CR (11,12).
Although the exact mechanisms are not fully understood (4,13), CR has been shown in animal studies to reduce metabolic rate and oxidative damage and improve markers of age-related diseases such as insulin resistance for diabetes (14). Studies in rhesus monkeys suggest that prolonged CR opposes many age-associated pathophysiological changes (6,15,16). Because the benefits associated with prolonged CR are important to the health and survival of humans, it has become an important research objective to assess the feasibility, safety, and health-related effects of prolonged CR in well-controlled human trials.
CALERIE (Comprehensive Assessment of the Long-term Effects of Reducing Intake of Energy) is a research program performing the first clinical trials evaluating the effects of CR on the biomarkers of aging and longevity in nonobese human beings. Phase 1 of the program was conceived as site-specific, short-term pilot studies. The designs of these studies have been published elsewhere (9,17,18), and although they applied different eligibility criteria, different interventions (some involving exercise components), and focused on different outcome measures, they established the feasibility of a carefully controlled CR intervention in this population. Publications from these studies have detailed the short-term effects of CR on resting metabolic rate (RMR) (9), cardiovascular (19) and diabetes (20–22) risk factors, body composition (18,23), and cognitive function (24).
The following lessons were learned from these studies. First, an intricate and detailed screening process is required to screen out volunteers unlikely to persevere with the rigors of the CR intervention over the full 24 months. A carefully constructed CR intervention is essential but must be flexible enough to allow different approaches to achieve CR. Intensive contact at the beginning is critical when new habits are being formed and recidivism is likely. Equally, feedback must be provided to participants throughout the intervention so that corrections can be made while problems are small. A detailed quality control process must be applied to ensure that all study procedures are conducted consistently and reliably across sites and over time. Finally, although no safety issues emerged in these studies, a proactive safety surveillance protocol is no less important in a longer-term study of this intervention.
Building on these lessons, the phase 2 study is conceived as a single-protocol, multicenter, randomized controlled trial (RCT) investigating the effects of sustained CR over a 2-year interval. The purpose of this article was to report its main design features. We describe the study population and eligibility criteria, provide an overview of the CR intervention, summarize the outcome evaluations and the frequency with which they are carried out, and describe monitoring procedures to safeguard participant safety.
SPECIFIC AIMS AND HYPOTHESES
The overall aim of the CALERIE phase 2 study is to test the hypothesis that 2 years of sustained CR, involving a 25% reduction of ad libitum (AL) energy intake (EI), in healthy men aged 21–50 years (inclusive) and healthy women aged 21–47 years (inclusive) results in the same beneficial effects as observed in animals subjected to similar levels of CR. The primary hypotheses is that CR results in metabolic adaptation as defined by (a) a reduction in RMR adjusted for changes in body composition and (b) a reduction in core body temperature. A reduction in metabolic rate has been proposed as one of the mechanisms by which CR slows aging, possibly by reducing oxidative damage associated with EI in excess of body energy needs. Secondary aims are to test whether CR reduces serum triiodothyronine concentrations and reduces inflammation as determined by plasma tumor necrosis factor-α concentration. Triiodothyronine is a potential mediator of the predicted metabolic adaptation and provides insight into the mechanism of this hypothesized primary adaptation to CR. Reduction of inflammation is one of the adaptive responses suggested to mediate the salutary effects of CR on the aging process in rodents. The study is also investigating the safety implications of sustained CR in humans. A number of exploratory aims address the mechanisms by which CR mediates its biologic effects at cellular and subcellular levels. They are summarized in Table 1. Biologic samples (plasma, biopsy samples, circulating cells, and urine) are being stored in a repository for future mechanistic studies.
Table 1.
Exploratory Aims in CALERIE (Comprehensive Assessment of the Long-term Effects of Reducing Intake of Energy)
| Exploratory aims are designed to test hypotheses on the effects of 2 y of caloric restriction (CR) in humans. The following measures capture the effects of CR at the structural and physiological levels |
| 1. Changes body composition (fat mass, lean mass), including bone mineral density. Changes in caloric intake during CR impact body composition. As such, measuring body composition is important as a covariate for the primary aim (core temperature and resting metabolic rate) in CALERIE phase 2. Intra-abdominal fat is an important covariate for the interpretation of the atherosclerosis and type 2 diabetes measures. Bone mineral density is important to assess the safety of CR. |
| 2. Changes serum hormones, including DHEAS, cortisol, thyroid-stimulating hormone, thyroid-binding globulin, growth hormone, leptin, adiponectin, angiotension II, norepinephrine, sex hormones, and hormone-binding protein levels. Changes in hormone levels could provide clues regarding the mechanisms by which some of the effects of CR are mediated. |
| 3. Lowers plasma growth factor concentrations, including insulin-like growth factor-1 (IGF-1), platelet-derived growth factor-AB and transforming growth factor-β. A reduction in growth factor levels has been hypothesized to play an important role in mediating the effects of CR on longevity and protection against the development of malignancies. |
| 4. Decreases the intermediate risk factors that are predictive of developing atherosclerosis and type 2 diabetes as evidenced by improvements in circulating inflammatory cytokines and CRP, serum lipid and lipoprotein levels, lowering serum insulin and glucose levels, reduction in abdominal fat, lowering of blood pressure, and reduction of 10-y CHD risk using comprehensive population-based risk models. These adaptations are likely to be among the most important protective effects of CR against secondary aging. |
| 5. Reduces oxidative stress, as reflected in urinary levels of isoprostanes, dinitrotyrosine, and 2-deoxyguanosine levels. CR has been shown to lower oxidative stress in rodents, and this is thought to be one of the mechanisms by which CR slows aging. |
| 6. Modulates immune function as reflected by a change in lymphocyte count, delayed-type hypersensitivity, and response to vaccine. Short-term CR has been shown to impact immune function. These studies are important because immune function impacts many other physiological and cellular systems. In addition, changes in the immune system may determine responses to environmental pathogens, thus affecting mortality. |
| To explore the effects of CR on cellular structure and function, CALERIE is collecting and processing tissue samples as a means of determining the cell structure, signaling, gene expression, and pathways that mediate the beneficial effects of CR. |
| 7. Skeletal muscle. To explore the effects of CR to: |
| • Increase the expression of SIRT1 and FOXO and decrease the expression of type II deiodinase |
| • Lower growth factor expression (IGF-1) |
| • Reduce protein glycation |
| • Alter the capacity of metabolic pathways (PDHK) |
| CR changes the expression of key genes, activation/deactivation of signaling systems. Taken together, these changes result in beneficial downstream changes in structure and function. These effects occur in animals and humans and in diverse tissues including skeletal muscle. The latter tissue is responsible for a large portion of the whole-body energy consumption. Understanding the effects of CR in skeletal muscle will provide key contextual information for the interpretation of the primary aim of the study—metabolic adaptation. |
| 8. Adipose tissue biopsies. To explore the effects of CR to: |
| • Reduce adipocyte size and lower the adipose tissue content of inflammatory cytokines and markers of inflammation as measured by the gene expression (quantitative reverse transcription polymerase chain reaction) of amyloid A, interleukin-6, resistin, adiponectin, leptin, MCP-1, CD68, and MAC-2 and immunohistochemistry to quantify adipose tissue macrophage infiltration content |
| • Change the levels of expression of mitochondrial and lipogenic genes |
| • Increase the capacity for free radical scavenging (superoxide dismutase) |
| There is evidence that a major beneficial effect of CR is a reduction in fat mass, particularly visceral fat, and adipose tissue is now recognized as a contributor to whole-body inflammation. These measures will explore the effects of long-term CR on adipose tissue. Adipose tissue is important for the regulation of whole-body energy partitioning (vis-à-vis metabolic and hormonal systems) and will provide key contextual information for the interpretation of the primary aim of the study—metabolic adaptation. |
| 9. Psychological factors, including quality of life, cognitive function, and affective status. The complex endocrine, physiological, and cellular changes that occur during CR theoretically may impact these two domains in both a positive and a negative fashion. As such, they represent important information to determine the safety and benefits of long-term CR. |
| 10.Physical functioning status, including VO2max and muscular strength and endurance. Changes in caloric intake during CR impact body composition. How these changes impact physical functioning will determine the ultimate desirability of CR in humans and the overall interpretation of the data generated in phase 2 CALERIE. |
Note: CHD = coronary heart disease; CRP = C-reactive protein; DHEAS = dehydroepiandrosterone; MCP-1= Monocyte colony protein-1; PDHK= Pyruvate dehydrogenase kinase.
METHODS
Key Design Considerations
Almost all of the data in animal studies were obtained when CR had been sustained for long durations of time (relative to the life span of the organism), with body weight relatively stable for much of the CR period (25–27). Outcomes observed during the initial weight loss phase in this study will likely parallel results commonly reported in short-term weight loss studies in overweight individuals (including the CALERIE phase 1 studies) and are therefore unlikely to produce new information. Sustained CR beyond the point when weight stability is firmly established, however, provides key data on whether CR effects become stable or whether early effects are transitory. From the phase 1 results, even with successful adherence, it may take a year (or longer) for the initial weight loss to transition to weight stability. Thus, a 2-year intervention period was selected to provide a significant period of weight stability.
Based upon findings from animal studies, a higher level of CR might be expected to result in greater biologic effects. However, CALERIE participants are not obese, and some are not even overweight, so that the health-related motivation to adhere to the intervention may be weaker for these participants (28). Thus, 25% CR was selected as optimal because it is likely to be sustained at a constant level over the entire 2 years yet at the same time produce measurable physiological effects.
The CALERIE phase 2 study is therefore structured as an intensive dietary and behavioral intervention focused on achieving and maintaining 25% CR throughout the 2-year interval. The intervention staff includes psychologists and registered dietitians, and their role is to deliver the CR intervention in a structured and consistent manner across the sites and over time. The intensity of the behavioral components of CALERIE exceeds that in most other CR studies due to the duration of the study and the importance of achieving the target CR level in the first year. Thus, frequent participant contact is provided throughout the intervention. Our approach is outlined in the following and described in detail in a separate manuscript.
Study Organization
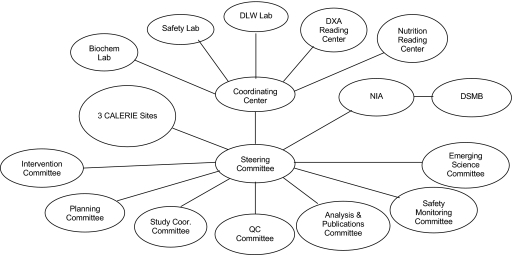
The study is funded by the National Institute on Aging of the U.S. National Institutes of Health, and the full organizational structure is depicted in Figure 1. There are three clinical sites located at the Pennington Biomedical Research Center, Washington University Medical Center, and Tufts University. The Duke Clinical Research Institute serves as the coordinating center (CC). A steering committee is the main governing body and consists of the principal investigators of the clinical sites and the coordinating center as well as the National Institute on Aging project scientist. Other committees include a planning committee, quality control committee, intervention design and delivery committee, and so on. A variety of specialized procedures are conducted with study participants and interpreted at central laboratories and reading centers. A full description of all CALERIE constituents is found in Appendix 1.
Figure 1.
Organizational structure for the CALERIE (Comprehensive Assessment of the Long-term Effects of Reducing Intake of Energy) research group. Note: Biochem Lab = biochemistry laboratory; DLW = doubly labeled water; DSMB = data and safety monitoring board; DXA = dual-energy x-ray absorptiometry; NIA = National Institute on Aging; Study Coor = study coordinators; QC = quality control.
Basic Study Design
The CALERIE phase 2 study is designed as a multicenter, parallel-group, randomized controlled trial. A sample of 225 participants is being enrolled and assigned at random to the CR intervention or the AL control group. A 2:1 allocation ratio in favor of CR is being applied to maximize the number of individuals receiving the intervention of greater scientific interest. There is no gradual ramping of CR, and the 25% energy reduction applies for the entire 2-year period. CR participants are permitted to vary the composition of their diets as needed or desired provided that nutritional adequacy is maintained. A comprehensive set of outcome evaluations is carried out before randomization, with follow-up evaluations at months 1, 3, 6, 9, 12, 18, and 24 after starting the intervention. It is expected that 10% of study participants will drop out or be withdrawn from the intervention in each of the 2 follow-up years, so that a sample of approximately 180 participants is expected to complete the study.
Study Population and Eligibility Criteria
Healthy individuals from both genders and all races are eligible to participate. Men must be between 21 and 50 years of age, whereas women must be between 21 and 47 years of age (to avoid menopause in most women), and all participants must have a BMI in the range 22.0 ≤ BMI < 28.0 kg/m2.
The lower age limit was chosen because adult height in normal healthy individuals is attained by age 18 and peak bone mineral accrual usually occurs by age 21 (29,30). The upper limit was chosen because in animal studies, data from CR initiated after 50% of average life span (the human equivalent of 38 years) are sparse and conflicting. However, to ensure an adequate recruitment base, the upper limit is restricted to the ages shown previously. Consistent with animal studies (31), the upper BMI limit was selected to exclude obese individuals. The lower BMI limit was selected primarily for safety reasons. That is, the commonly used standard of underweight is BMI less than or equal to 18.5 kg/m2, and the lower limit provides an adequate buffer from this threshold as participants lose weight over the 2-year intervention.
Exclusion criteria are detailed in Table 2. In general, volunteers are ineligible if they have significant medical conditions (eg, history or clinical manifestation of cardiovascular disease and diabetes), abnormal laboratory markers (eg, elevated potassium, or hemoglobin and hematocrit levels below their respective lower limits of normal), psychiatric or behavioral problems (eg, history or clinical manifestation of any eating disorders, Beck Depression Inventory [BDI] (32) score ≥20), and regular use of medications except oral contraceptives. Women must practice an acceptable form of contraception; breast-feeding and pregnant women are excluded.
Table 2.
Exclusion Criteria
| Medical exclusion criteria |
| • History or clinical manifestation of cardiovascular disease or an elevated blood pressure (>140/90 mm Hg) |
| • Abnormal resting electrocardiogram demonstrating: type II second- or third-degree heart block; ventricular ischemia; left bundle branch block, cardiac hypertrophy by any criteria, QRS complex >100 ms in duration; abnormal QTc interval, supraventricular tachycardia of any type but not including APCs, or ventricular arrhythmia of any type (including VPCs >60/min) |
| • BMD t score at the total hip, femoral neck, or total spine (L1–L4) is less than or equal to −2.3 at the first DXA scan during the baseline visit |
| • History or clinical manifestation of diabetes |
| • History or clinical manifestation of cholelithiasis |
| • History of anaphylaxis, severe allergies, or asthma |
| • History or clinical manifestation of any other significant metabolic, hematologic, pulmonary, cardiovascular, gastrointestinal, neurological, immune, hepatic, renal, urological disorders, or cancer that, in opinion of the investigator, would make the candidate ineligible for the study |
| • History of stomach or intestinal surgery (except appendectomy) or major abdominal, thoracic, or non-peripheral vascular surgery within 1 y prior to the randomization date |
| • Any disease or condition that seriously affects body weight and/or body composition |
| Laboratory exclusion criteria |
| • Potassium level above the upper limit of normal at the screening visit confirmed by a test repeated within 2 wk |
| • Hemoglobin, hematocrit, red blood cells, or iron level below the lower limit of normal at the screening visit confirmed by a test repeated within 2 wk |
| • Evidence of active liver disease or ALT levels >1.5 times the upper limit of normal |
| • Low-density lipoprotein cholesterol level ≥190 mg/dL at the screening visit |
| Psychiatric and behavioral exclusion criteria |
| • Individuals who practice a vegan dietary lifestyle |
| • History or clinical manifestation of any eating disorder as determined by IDED-IV when the ratings for each of the diagnostic criteria are rated as “3” or more. Potential participants will also be excluded if they are experiencing a subthreshold eating disorder (defined as IDED-IV ratings of ≥3 on 5 of the 8 combined symptoms for bulimia nervosa and anorexia nervosa) |
| • Any history of pharmacological treatment for a psychiatric disorder within 1 y prior to the randomization date or a history of more than one episode of a pharmacological treatment for a psychiatric disorder within lifetime |
| • History of drug or alcohol abuse (up to 14 drinks a week are allowed) within the past 2 y |
| • Individuals who present with a Beck Depression Inventory (29) score ≥20 at screening or baseline |
| Medication exclusion criteria |
| • Short-term (less than a month) treatment with steroids within 6 mo prior to the randomization date |
| • Treatment with steroids for more than a month within 5 y prior to the randomization date |
| • Regular use of other medications, except contraceptives |
| Other exclusion criteria |
| • Individuals who participated in the CALERIE (Comprehensive Assessment of the Long-term Effects of Reducing Intake of Energy) phase 1 studies |
| • Individuals who have lost or gained ≥3 kg over the past 6 mo |
| • A volunteer must be either a never-smoker of tobacco products or an ex-smoker who quit completely at least 12 mo prior to the screening visit |
| • Individuals who donated blood within 30 d prior to the randomization date |
| • Concurrent participation in any other interventional study |
| • Breast-feeding or pregnant women, or women intending to become pregnant before the scheduled end of the intervention |
| • Individuals who were engaged in a regular program of physical fitness involving some kind of heavy physical activity (eg, jogging, running, or riding fast on a bicycle for ≥30 min) five or more times per week over the past year |
| • Unwilling to be assigned at random to the CR or control intervention |
| • Unwilling or unable to adhere to the rigors of the CR intervention over the entire 2-y intervention period |
| • Individuals who are unable or unwilling to discontinue dietary supplements or adhere to the alcohol consumption restrictions during the study |
| • Unwilling or unable to adhere to the rigors of the data collection and clinical evaluation schedule over the entire 2-y follow-up period |
Note: ALT = Alanine aminotransferase; APCs = Atrial premature contractions; CR = caloric restriction; DXA = dual-energy x-ray absorptiometry; IDED-IV = Interview for the Diagnosis of Eating Disorders-IV.
Individuals engaged in heavy physical activity (eg, jogging, running, or bicycling) for 30 minutes or more, five or more times per week, are also excluded to avoid the confounding effects of an increase in energy expenditure. Specifically, from the phase 1 studies, we anticipate that 25% CR might lead to an intentional decrease in regular physical activity, so that individuals training for competitive sporting events are excluded. The resulting change in activity energy expenditure would complicate and confound the determination of EI (and study adherence) using the doubly labeled water (DLW) method over the course of the study. Volunteers partaking at the recommended level of physical activity according to the Physical Activity Guidelines for Americans (150 minutes of moderate-intensity exercise or 75 minutes of vigorous-intensity exercise per week) would not meet this standard and are not excluded from the study.
Recruitment and Screening Strategies
Recruitment is continuous, and includes media advertising, direct mail, health promotion events, databases, Internet Web sites, and referral sources. An initial telephone screening requests the volunteer’s age, height, and weight (from which BMI is calculated), as well as basic medical information. Volunteers who are clearly ineligible are screened out at this point. Then, a staged screening process consisting of a variety of biologic and behavioral assessments is undertaken over a series of three clinic visits as shown in Table 3. The behavioral screening focuses on factors related to safety and the potential for CR adherence and retention over the 2 years. Food allergies and other special diet concerns that could affect participation in the study (eg, alcohol consumption) are evaluated by a nutritionist. A 14-day food record is completed to assess the participant’s ability to provide complete and detailed information over a significant interval of time. At the end of the screening process, a multidisciplinary team of behavioral experts, nutritionists, and other clinical staff scrutinizes the candidate’s entire safety and behavioral profile and determines if he or she is suitable for inclusion in CALERIE.
Table 3.
Procedures Carried Out During the Staged Screening Process
| Screening Visit No. 1 | Screening Visit No. 2 | Screening Visit No. 3 |
| Review information video | Blood chemistry, hematology, and urinalysis | Blood chemistry, hematology, and urinalysis review; repeat blood tests if necessary and review at a follow-up screening visit |
| Verify BMI | ||
| Demographic information | Serum pregnancy test and contraception use (for women) | |
| General health questionnaire | ||
| Beck Depression Inventory (32) | Physical examination | |
| 7-d Stanford PAR (33) | Medical and medications history | 14-d food record review; repeat if incomplete and review at a follow-up screening visit |
| Eating Inventory (34) | Standard 12-lead electrocardiogram | |
| Multiaxial Assessment of Eating Disorder Symptoms (35) | Barriers to participation interview | |
| Structured Clinical Interview for Diagnosis of DSM-IV Personality Disorders (36) | Body morph assessment (37) | |
| Dietitian interview | 14-d food record |
Note: BMI = body mass index; PAR = Physical Activity Recall.
Randomization and Blinding
Participants are assigned at random to the AL or CR treatment arms. Randomization is stratified by sex and BMI within each clinical site, with BMI dichotomized into normal weight, that is, 22.0 ≤ BMI < 25.0 kg/m2, versus overweight, that is, 25.0 ≤ BMI < 28.0 kg/m2. Within each stratum, individuals are allocated in a 2:1 ratio in favor of the 25% CR intervention. Randomization sequences within each stratum were generated a priori by the coordinating center using a permuted block randomization technique (38). Actual treatment assignment is carried out centrally using a telephone-based, interactive voice-response system (39). Given the nature of the CR intervention and control, it is not possible to blind participants or staff to the treatment assignments. Nevertheless, intervention staff have been distinguished from evaluation staff, and cross-communication between the two is circumscribed.
CR Intervention
A complete description of the CR intervention, with specific details concerning the participant materials, subject monitoring, and staff interventions to maintain adherence, is provided in a separate manuscript, and a summary is provided here.
The intervention is a 25% caloric reduction from baseline AL EI. As described in the following, total energy expenditure (TEE) is determined by DLW to assess baseline EI (40). This is carried out specifically for each participant, and the 25% energy reduction prescription for that participant is based on this calculation. Moreover, CALERIE is not prescribing a specific, “one-size-fits-all” dietary composition. Instead, participants make dietary selections (with the advice of intervention staff) that best allow them achieve the CR goal. Participants are allowed to vary their dietary choices as needed or desired over the course of the intervention provided that nutritional adequacy is maintained.
Thus, the overall intervention strategy uses an intensive behavioral approach coupled with dietary modifications anticipated to enhance adherence to CR. Intervention staff include psychologists and nutritionists (hereafter referred to as “counselors”) who supervise the delivery of the CR intervention in a structured and consistent manner. Behavioral strategies known to be effective in long-term weight loss studies (41,42) in tandem with dietary composition changes known to enhance satiety and reduce hunger (43) are applied.
Individual counseling is the primary mode for delivering the intervention; group counseling provides social support and an important secondary source of education. During individual sessions, the counselor provides customized information to assist with reaching and sustaining the CR goal. Group sessions are provided using an “open enrollment” process, which allows participants to begin with modules that cover information appropriate for their length of study participation. Written manuals were created specifically for CALERIE to guide participants and their counselors.
A number of intervention enhancements are provided. First, for the first 28 days of the intervention, complete CR meals are provided accompanied by detailed daily menus. Thus, by direct example, participants receive guidance on achieving their individualized CR goal (44,45). During this period, they also receive detailed training on food portion size estimation, learn to keep a food diary, and are provided a personal data assistant device with diet analysis software, all designed to prepare them for self-selecting their own diets for the duration of the study. Meals provided during the first 28 days are fully adequate in all essential nutrients. When CR diets are self-selected, participants are guided to consume macronutrients in the Acceptable Macronutrient Distribution Ranges of the Dietary Reference Intakes set by the Institute of Medicine’s Food and Nutrition Board (ie, 45%–65%, 20%–35%, and 10%–35% for carbohydrate, fat, and protein, respectively). Nutrient intakes are continually monitored by registered dietitians throughout the trial via daily self-monitoring reports and weighed 6-day food records completed every 6 months. Moderate alcohol consumption is permitted; however, participants are advised of the poor nutritional value of these calories.
As participants transition to self-selected CR, a proactive and comprehensive plan provides an array of supporting services using a “toolbox” approach. Toolbox options focus on psychological, nutritional, and behavioral strategies to improve CR adherence when it begins to falter. The assignment of toolbox options is guided by an algorithmic approach that is iterative and continually updated until adherence is restored.
A Web-based computer tracking system was developed at the coordinating center to provide a standardized approach to implementing the adherence algorithms. It is accessed by the counselors, and tracks the assignment and implementation of toolbox options. It includes expected weight loss trajectories to track the participant's weight against his/her individualized goal. Ratings of hunger, satiety, and desire to eat, as well as self-reported calories from the food diaries are entered providing direct feedback to the participant and counselor. The computer tracking system prompts the counselor when a priori criteria for adherence are not being met, whereupon preprogrammed algorithms suggest toolbox strategies to promote better adherence.
Longer duration of contact with participants has been found to promote health behavior change and favor adherence to diet interventions (46). Intensive participant contact is maintained throughout the study using both individual and group counseling sessions. Moreover, in weekly meetings of each site’s intervention team, computer tracking system reports for each participant are reviewed. Adherence problems and potential barriers are assessed, and toolbox options are selected according to algorithm guidelines.
AL Control Group
Participants assigned to the control group continue on their current diets on a completely AL basis and receive no specific dietary intervention or counseling. They have quarterly contact with study investigators and undertake the outcome assessments at roughly the same schedule as CR participants. Thus, the control group represents the natural history of this participant population as it ages over the 2 years.
Outcome Determinations
Consistent with the overall aims of the study, a comprehensive array of outcome assessments is carried out on CALERIE participants, and a schematic of the schedule of evaluations is provided in Table 4. Some evaluations are only carried out on CR participants, for example, DLW at months 6 and 18. This provides more complete information on energy expenditure so that adherence in the CR group can be determined more precisely during the intervening months. Some procedures, for example, RMR, dual-energy x-ray absorptiometry, nutrient intake, and the Stanford 7-day Physical Activity Recall (33), are carried out concurrently with the DLW studies so that results are coincident with the DLW results.
Table 4.
Schedule of Outcome Evaluations
| Evaluations and Procedures | Baseline | Follow-up Mo |
||||||
| 1 | 3 | 6 | 9 | 12 | 18 | 24 | ||
| Informed consent and HIPAA authorization | X | |||||||
| Randomization | X | |||||||
| Energy metabolism | ||||||||
| TEE by DLW, RMR | XX | X* | X | X* | X | |||
| Core body temperature | X | X | X | X | ||||
| Lipids, markers of inflammation | X | X | X | |||||
| Serum insulin, glucose, C-peptide, OGTT | X | X | X | |||||
| Endocrine response/growth factors | X | X | X | |||||
| Immune function | ||||||||
| DTH | X | X | X | |||||
| Antibody response to vaccines | X | Mo 17, 18, 23, 24 | ||||||
| QoL, psychological, cognitive function | X | X | X | X | ||||
| Physical activity measurements | ||||||||
| Stanford 7-d PAR | XX | X* | X | X* | X | |||
| VO2max, muscular strength and endurance | X | X | X | |||||
| Body composition | ||||||||
| Clinic height | X | |||||||
| Clinic weight, waist circumference | X | X | X | X | X | X | X | X |
| DXA, including BMD and BMC | XX | XX* | X | X* | X | |||
| 6-d food records | X | X* | X | X* | X | |||
| Repository materials | ||||||||
| Blood | X | X | X | X | X | X | ||
| Urine, muscle and abdominal fat biopsies | X | X | X | |||||
| Safety monitoring | X | X | X | X | X | X | X | X |
Notes: BMC = bone mineral content; BMD = bone mineral density; DLW = doubly labeled water; DTH = delayed-type hypersensitivity; DXA = dual-energy x-ray absorptiometry; OGTT = oral glucose tolerance test; PAR = Physical Activity Recall; QoL = quality of life; RMR = resting metabolic rate; TEE = total energy expenditure. XX signifies that the procedure is carried out twice at this time point.
Carried out with caloric restriction participants only.
Primary outcomes are RMR measured via indirect calorimetry over a period of 30 minutes using Vista-MX metabolic measurement system (VacuMed, Ventura, CA) and core temperature recorded every minute over a 24-hour interval. Participants swallow a 8.7 × 23–mm Jonah radiocapsule (Mini Mitter Co., Inc., Bend, OR), and are fitted with the VitalSense Monitor to record the minute-by-minute temperature readings. Temperatures are averaged over specific intervals of interest, for example, 24 hours, daytime, nighttime, and so on. The secondary outcomes, thyroid-stimulating hormone and serum triiodothyronine, are measured using chemiluminescent immunoassays (ADVIA Centaur; Siemens Healthcare Diagnostics, Tarrytown, NY). Tumor necrosis factor-α is measured by multiplex immunoassay (Human Serum Adipokine Panel B; Millipore, Billerica, MA). These assays are carried out at the central biochemistry laboratory. Supplementary data provides a complete summary of all scientific methodologies for the outcome measures in CALERIE.
TEE Measured by DLW
DLW is used to estimate TEE in CALERIE participants. It is noninvasive and nonrestrictive, and has been shown to provide a valid measure of actual TEE under free-living conditions (47–50). The DLW method involves enrichment of body water with the stable (natural and nonradioactive) isotopes, deuterium (2H) and oxygen-18 (18O), followed by the determination of their monoexponential washout kinetics in urine. It is based on the principle that the disappearance rate of 2H reflects water turnover rate, whereas the disappearance rate of 18O reflects both water and CO2 turnover rates. The difference between the two disappearance rates, therefore, represents the rate of CO2 production. Knowing the respiratory quotient or food quotient, TEE can be calculated from the CO2 production rate. Validation studies (51–53) using calorimetry have shown that DLW method provides an accurate assessment of the CO2 production rate and hence TEE.
Two consecutive 14-day DLW studies are conducted with each participant at baseline with the average used to determine AL TEE; from this, the 25% CR prescription for that participant is derived. Urine samples are collected before dosing as well as on days 0, 7, and 14 following dosing, and shipped on dry ice to the central DLW laboratory. There, the samples are prepared for hydrogen and oxygen isotope enrichments measured by gas isotope ratio mass spectrometry using validated procedures (54,55).
Participant Rights and Safety
CALERIE is being conducted according to the ethical principles in the Declaration of Helsinki. The study is registered on ClinicalTrials.gov Website (identifier: NCT00427193). All participants review and sign the informed consent document and Health Insurance Portability and Accountability Act authorization before undertaking any study procedures. Oversight is provided by a data and safety monitoring board. The study protocol (including any revisions) is reviewed and approved by the data and safety monitoring board and institutional review boards at the participating institutions. Periodic reports summarizing participant safety are presented to the data and safety monitoring board for review. Remedial action including modifying study procedures is taken as appropriate.
Protection of participants from risks related to the intervention is of paramount concern to the CALERIE investigators. Meals and meal plans were carefully designed to maintain an adequate and balanced diet in terms of micro- and macronutrient content. Macronutrient recommendations, within ranges of intakes from the National Academy of Sciences Dietary Reference Intake guidelines (56), are followed. A complete daily vitamin and mineral supplement is provided to ensure that participants in both treatment arms meet the current recommendations for these nutrients. A daily calcium supplement of 1,000 mg is also provided. All CALERIE participants are advised of current health recommendations from the surgeon general (Centers for Disease Control and Prevention) for a minimum of 30 min/d of moderate physical activity for a minimum of 5 d/wk.
Clinical laboratory tests including hematology, serum chemistry, and urinalysis are carried out at screening and baseline, and at months 1, 3, 6, 9, 12, and 24. Vital signs are also recorded. A complete physical examination is carried out at screening, baseline, and at months 12 and 24. A heightened surveillance protocol for CR participants is applied for the following medical conditions: anemia, elevations in low-density lipoprotein cholesterol, decreases in bone mineral density, electrocardiogram abnormalities, depression and other mental health disorders, excessive weight loss, and nutritional inadequacy/eating disorders. Detailed and specific definitions for each condition are provided, and criteria for temporarily discontinuing the CR intervention, resuming it, or permanently discontinuing it are described in the CALERIE protocol.
For example, depression is monitored using the BDI (32). A score of 30 or greater (suggestive of a severe depression) at any point during the intervention triggers an interview with the site psychologist to determine whether the CR intervention needs to be permanently discontinued. If 20 ≤ BDI < 30 (suggestive of moderate depression), the BDI is readministered within 1 week. If this score is still suggestive of a moderate depression, the CR intervention is temporarily discontinued and the participant is advised to seek medical help. The CR intervention is only restarted if the BDI decreases to below 20 or a qualified mental health professional indicates that it is safe to resume. If BDI score is still 20 or greater after 1 month of treatment, the CR intervention is permanently discontinued.
Power Calculations
Based on our experience in the phase 1 studies, it was considered feasible with the resources at the three clinical sites to enroll 225 participants. A dropout rate of around 10% per year was observed in the phase 1 studies, so that a sample of approximately 180 participants is expected to complete the study. Standard deviations for the primary and secondary outcome variables were derived from the phase 1 studies. Between-group differences anticipated at the end of the study were informed by the phase 1 studies, the literature and expert opinion. Standard sample size procedures (57,58) were applied with the treatment allocation set to 2:1 in favor of the CR intervention and the type I error rate (two-tailed) set to α = .05.
The results are summarized in Table 5 and indicate that the study enjoys power in excess of 90% for both primary outcomes. Similar calculations were carried out for the secondary outcomes and a number of the exploratory outcomes (not shown). Power in excess of 80% is anticipated for many (but not all) of the these variables including serum triiodothyronine, high-density lipoprotein cholesterol, insulin and glucose, and fat mass and fat-free mass. We note that the main analytic strategy is a repeated measures analysis. It includes all the intermediate time points as well as partial information provided by participants before they drop out. All things being equal, there should be greater power from this analysis, so that these calculations are somewhat conservative.
Table 5.
Power to Detect Anticipated Differences in the Primary Outcomes With a Sample of 180 Completers
| Outcome Variable | SD* | Anticipated Difference† | Power (%) |
| RMR (kcal/d) | 110.70 | 60 | 92.6 |
| Core temperature (°C) | 0.3586 | 0.20 | 93.9 |
Notes: RMR = resting metabolic rate.
Derived from the phase 1 studies.
Between-group difference anticipated by the CALERIE (Comprehensive Assessment of the Long-term Effects of Reducing Intake of Energy) investigators in this study (see text).
DISCUSSION
The first studies of the effect of energy restriction in humans were conducted in lean men by Keys and colleagues (59) in the 1950s. In these classic experiments, lean volunteers received 50% of their habitual calorie intake. Decreased RMR when adjusted for body surface area (−31%), body weight (−20%), and cell mass (−16%) was observed. However, there were indications of malnutrition with deficiencies in many micronutrients, and for this reason, a comprehensive safety surveillance protocol is being applied in CALERIE.
Most of the other studies of the effect of CR on energy metabolism have been carried out in obese populations. In several studies, a very low calorie diet resulted in a decrease in RMR, which remained significant when normalized to body weight or fat-free mass (60–62). A meta-analysis of studies in postobese patients found a lower RMR, even after adjustment for body size and body composition (63). Comparable results in the change in RMR and sedentary 24-hour energy expenditure were also obtained among Biospherians subjected to CR for 2 years (64). Part of the adaptation seen in these different studies may be related to the cost of physical activity as elegantly shown by Weigle and Brunzell (65); however, adaptations unaccounted for by changes in body composition or physical activity are also suggested.
Thus, there is evidence for changes in energy expenditure with CR that include decreases associated with loss of weight and lean body tissue, as well as decreases that appear to be independent of body composition change, which are indicative of a metabolic adaptation to CR. Metabolic adaptation provides greater efficiency of energy utilization and is likely to have both a hormonal basis and to be linked to physical manifestations of low energy expenditure such as reduced core body temperature. Core body temperature is known to vary with metabolic rate (66) and is considered a biomarker of longevity in rodents and nonhuman primates (67,68). Preliminary data from CALERIE phase 1 indicated a significant reduction in core body temperature in the treatment groups that included CR (9).
One possible mechanism of the antiaging effects of CR is through its effects on mitochondrial reactive oxygen species production leading to a reduction in oxidative tissue damage. This is consistent with some experimental evidence in laboratory animals. If mitochondrial oxyradical production is a primary factor in aging-associated changes, it is reasonable to hypothesize that CR’s effects on oxyradical damage in mitochondrial DNA, proteins, or lipids, or all, may be more pronounced than its effects on other cellular components. In fact, effects of CR on oxidative damage to mitochondrial DNA have been reported (69). Thus, it seems reasonable to focus attention and resources on oxyradical production and mitochondrial molecular damage as important outcome measures in CALERIE. This also provides the rationale for storing skeletal muscle and adipose fat tissue in a repository for future mechanistic studies.
The CALERIE phase 2 study is the first comprehensive evaluation of the effects of CR in nonobese humans. It assesses the broad spectrum of physiological effects of CR in multiple physiological domains related to primary and secondary aging. It is informed by animals studies and our phase 1 studies, and addresses whether effects similar to those in animal models are realized in human beings. It is sufficiently large and powered to detect CR effects in multiple important physiological outcome domains.
The clear challenge in CALERIE is to obtain and maintain adherence to the 25% CR goal, and a number of processes are being implemented to meet this challenge. First, TEE derived from DLW is being used to provide an estimate of level of CR required for any participant. Second, the CR intervention is based on the well-established understanding that the decrease in total calories, rather than changes in the relative proportion of micronutrients, accounts for the effects of CR (4). Moreover, a variety of tools are provided to monitor adherence and to intervene proactively when adherence begins to falter. Intensive participant contact is maintained throughout the study using both individual and group counseling sessions.
It would certainly be desirable to have a longer-term follow-up comprising a longer portion of the human life span than the 2 years allotted to CALERIE. This would permit a more meaningful comparison between the human and nonhuman primate studies, for example. Similarly, it would be desirable to perform a long-term observational study on participants after they complete the 2-year intervention. However, CALERIE is labor intensive and entails significant expense and resources. It is also burdensome to study participants calling for a complete lifestyle change that runs counter to the usual lifestyle of community-dwelling Americans. Given these factors, the study is limited to 225 participants. However, we believe that the effort and expense will be rewarded by unprecedented insight into the effects of CR in normal and mildly overweight young- to middle-aged human subjects.
FUNDING
National Institute on Aging, National Institutes of Health (U01AG022132, U01AG020478, U01AG020487, and U01AG020480).
SUPPLEMENTARY MATERIAL
Supplementary material can be found at: http://biomed.gerontologyjournals.org/
Appendix 1. Investigators and Staff Participating in CALERIE (Comprehensive Assessment of the Long-term Effects of Reducing Intake of Energy)
The following is a list of the principal investigators (PIs), Coinvestigators (CIs), site intervention leaders (SILs), intervention counselors (ICs), study managers (SMs), project leaders (PLs), study coordinators (SCs), and other staff (OS) participating in the CALERIE study.
Pennington Biomedical Research Center (clinical site)—PI: Eric Ravussin, PhD; CI: Catherine Champagne, RD, PhD; Alok Gupta, MD; Corby Martin, PhD; Leanne Redman, PhD; Steven Smith, MD; Donald Williamson, PhD; SIL: Corby Martin, PhD; IC: Michelle Begnaud, RD; Barbara Cerniauskas, RD; Allison Davis, MS; Jeanne Gabrielle, PhD; Heather Walden, MS; SM: Natalie Currier, RD; Mandy Shipp, RD; SC: Sarah Masters; Melody McNicoll; OS: Shelly Prince, MS, RD; Courtney Brock, RD; Renee Puyau, RD; Conrad Earnest, PhD; Jennifer Rood, PhD; Tiffany Stewart, PhD; Lillian Levitan, PhD; Crystal Traylor, WHNP; Susan Thomas, WHNP; Valerie Toups, LPN; Karen Jones, RN; Stephanie Tatum, RN; Celeste Waguespack, RN; Kimberly Crotwell, LPN; Lisa Dalfrey, LPN; Amy Braymer, LPN; Rhonda Hilliard, LPN; Onolee Thomas, RN; Jennifer Arceneaux, RN; Stacie LaPrarie, RN; Allison Strate, RN; Jana Ihrig, RN; Susan Mancuso, RN; Christy Beard, RN; Alicia Hymel; Desti Shepard; John Correa; Denise Jarreau; Brenda Dahmer; Grace Bella; Elizabeth Soroe; Bridget Conner; Paige McCown; Stephanie Anaya; Melissa Lupo.
Tufts University (clinical site)—PI: Susan B. Roberts, PhD; CI: Sai Krupa Das, PhD; Simin Meydani, PhD; Roger Fielding, MD; Isaac Greenberg, PhD; Anastassios Pittas, MD; Edward Saltzman, MD; Tammy Scott, PhD; SIL: Cheryl Gilhooly, RD, PhD; IC: Kimberly Gerber, PhD; Isaac Greenberg, PhD; Marjory Kaplan, PhD; Christy Karabetian, MA; Russell Kennedy, PhD; Lisa Robinson, RD; OS: Assefa, Senait; Verona Bembridge; Maria Berlis; Scarlett Buer; Robert Carabello; Cherie Campbell; Lauren Collins, RN; Marybeth Doherty, RN; Alicia Freed, RD; Chervonte Hernandez; Gyna Jean-Baptiste, RN; Mary Krasinski, RN; Marie Lim-Lucas, MPH, RD; Ekaterina Maslova; Barbara Maxwell, RN; Jean McShea, RN; Ann Muchowski, RN; Margaret Mulkerrin; Kerry Murphy; Carol Nelsen, RN; Megan O’Neill; Helen Rasmussen, RD, PhD; Brenda Roche; Eneida Roman; Gregory Sproull; Marie St. Victor, RN; Susan Storer, RN; Katherine Strissel, PhD; Stephanie Valliere; Margaret Vilme, RN; Justin Wheeler; Jill Wiley, RN; Fania Yangarber.
Washington University (clinical site)—PI: John O. Holloszy, MD; CI: Luigi Fontana, MD; Sam Klein, MD; Charles Lambert, PhD; B. Selma Mohammed, MD, PhD; Susan Racette, PhD; Dennis Villareal, MD; SIL: Rick Stein, PhD; IC: Karen Cotton, Psy D; Margaret Hof, MS, RD, LD; Cherie Massmann, MA, LPC, NCC; Kathleen Obert, MS, RD, LD; Marni Pearlman, MA, PLPC; Tina M Reising, Psy D; Laura Weber, MSEd, RD, LD; SM: Mary Uhrich, MS; SC: Morgan Schram, MS; OS: Mel Meyer, RN, BSN, CRC; Chelsea Carlen, BS; Lisa Kee, DTR; Barbara Larson, DTR; Mary McFerson, BS, DTR; Rebecca Sabatino, BS; Bridgett Toennies, RRT.
Duke Clinical Research Institute (coordinating center)—PI: James Rochon, PhD; CI: Connie W. Bales, PhD; Carl F. Pieper, DrPH; William Kraus, MD; PL: Katherine M. Galan, RN; OS: Richard Adrian, BS; Eleanor Law Allen, BA; William Blasko, BS; Manjushri Bhapkar, MS; Nikka Brown, BSN; Maria Butts, RN, BSN; Elaina K. Cossin, BS; Jennifer Curry, AAS; Jamie Daniel, BS, MS; Kathleen S. Diemer, RN; Lee Greiner, BS, MS; Darryl Johnson, BS; Cassandra Jones, BSEE; Lauren Lindblad, MS; Luanne McAdams, RN, MSN; Marty Mansfield, BA, PhD; Senthil Murugesan, MS; Lucy Piner, MS, ACSM CES; Christopher Plummer, BS; Mike Revoir, BS; Pamela Smith, RN, BSN; Monica Spaulding, MPH; James Topping, MS.
Baylor College of Medicine (doubly labeled water laboratory)—PI: William W. Wong, PhD; OS: Lucinda L. Clarke, AA; Chun W. Liu, BS; J. Kennard Fraley, MPH.
University of California at San Francisco (dual-energy x-ray absorptiometry reading center)—PI: Ann V. Schwartz, PhD; CI: John Shepherd, PhD; OS: Lisa Palermo, MS; Susan Ewing, MS; Michaela Rahorst; Caroline Navy.
University of Vermont (biochemistry laboratory)—PI: Michael Lewis, MD, MBA; CI: Russell P. Tracy, PhD; OS: Rebekah Boyle, BS, MS; Elaine Cornell, BS; Patrick Daunais, BS; Dean Draayer, PhD; Melissa Floersch, BS; Nicole Gagne, BA; Florence Keating, BS; Angela Patnoad, BS.
University of Cincinnati (nutrition reading center)—PI: Marcia Schmidt, MS, RD, LD; OS: Marcia Gavin BS, RD, LD; Frida Wiener MS, RD, LD; Ashley Hughes, DTR; Laura Benken.
University of Pittsburgh (intervention counseling curriculum)—PI: Amy Otto, PhD.
Data and safety monitoring board—Jeffrey Halter, MD (chair); David M. Buchner, MD, MPH; Patricia Elmer, PhD; Mark Espeland, PhD; Steven B. Heymsfield, MD; Xavier Pi-Sunyer, MD; Thomas Prohaska, MD; Sue Shapses, PhD; John Speakman, DSc; Richard Weindruch, PhD.
National Institute on Aging (primary funding agency)—Evan C. Hadley, MD; Judy Hannah, PhD; Sergei Romashkan, MD.
National Institute of Diabetes and Digestive and Kidney Diseases (cosponsor)—Mary Evans, PhD.
References
- 1.Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–712. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCay CM, Crowel MF, Maynard LA. The effect of retarded growth upon the length of the life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 3.Walford RL, Harris SB, Weindruch R. Dietary restriction and aging: historical phases, mechanisms and current directions. J Nutr. 1987;117:1650–1654. doi: 10.1093/jn/117.10.1650. [DOI] [PubMed] [Google Scholar]
- 4.Barzilai N, Bartke A. Biological approaches to mechanistically understand the healthy life span extension achieved by calorie restriction and modulation of hormones. J Gerontol A Biol Sci Med Sci. 2009;64:187–191. doi: 10.1093/gerona/gln061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingram DK, Roth GS, Lane MA, et al. The potential for dietary restriction to increase longevity in humans: extrapolation from monkey studies. Biogerontology. 2006;7:143–148. doi: 10.1007/s10522-006-9013-2. [DOI] [PubMed] [Google Scholar]
- 6.Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J Gerontol A Biol Sci Med Sci. 2003;58:212–219. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- 7.Lane MA, Baer DJ, Tilmont EM, et al. Energy balance in rhesus monkeys (Macaca mulatta) subjected to long-term dietary restriction. J Gerontol A Biol Sci Med Sci. 1995;50:B295–B302. doi: 10.1093/gerona/50a.5.b295. [DOI] [PubMed] [Google Scholar]
- 8.Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heilbronn LK, de Jonge L, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willcox DC, Willcox BJ, He Q, Wang NC, Suzuki M. They really are that old: a validation study of centenarian prevalence in Okinawa. J Gerontol A Biol Sci Med Sci. 2008;63:338–349. doi: 10.1093/gerona/63.4.338. [DOI] [PubMed] [Google Scholar]
- 11.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 13.Austad S. Advances in vertebrate aging research 2007. Aging Cell. 2008;7:119–124. doi: 10.1111/j.1474-9726.2008.00374.x. [DOI] [PubMed] [Google Scholar]
- 14.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 15.Blanc S, Schoeller D, Kemnitz J, et al. Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J Clin Endocrinol Metab. 2003;88:16–23. doi: 10.1210/jc.2002-020405. [DOI] [PubMed] [Google Scholar]
- 16.Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004;305:1423–1426. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- 17.Das SK, Gilhooly CH, Golden JK, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85:1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 18.Racette SB, Weiss EP, Villareal DT, et al. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61:943–950. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontana L, Villareal DT, Weiss EP, et al. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293:E197–E202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 20.Larson- Meyer DE, Heilbronn LK, Redman LM, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pittas AG, Roberts SB, Das SK, et al. The effects of the dietary glycemic load on type 2 diabetes risk factors during weight loss. Obesity. 2006;14:2200–2209. doi: 10.1038/oby.2006.258. [DOI] [PubMed] [Google Scholar]
- 22.Weiss EP, Racette SB, Villareal DT, et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92:865–872. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin CK, Anton SD, Han H, et al. Examination of cognitive function during six-months calorie restriction: results of a randomized controlled trial. Rejuvenation Res. 2007;10:179–190. doi: 10.1089/rej.2006.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masoro EJ, Yu BP, Bertrand HA. Action of food restriction in delaying the aging process. Proc Natl Acad Sci USA. 1982;79:4239–4241. doi: 10.1073/pnas.79.13.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu BP, Masoro EJ, Mc Mahan CA. Nutritional influences on aging of Fischer 344 rats: physical, metabolic and longevity characteristics. J Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 27.Lambert J, Lamonthe JM, Zernicke RF, Auer RN, Reimer RA. Dietary restriction does not adversely affect bone geometry and mechanics in rapidly growing male Wistar rats. Pediatr Res. 2005;57:227–231. doi: 10.1203/01.PDR.0000148715.61869.4E. [DOI] [PubMed] [Google Scholar]
- 28.Bacon L, Stern JS, Van Loan MD, Keim NL. Size acceptance and intuitive eating improve health for obese, female chronic dieters. J Am Diet Assoc. 2005;105:929–936. doi: 10.1016/j.jada.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Cole TJ. Secular trends in growth. Proc Nutr Soc. 2000;59:317–324. doi: 10.1017/s0029665100000355. [DOI] [PubMed] [Google Scholar]
- 30.Whiting SJ, Vatanparast H, Baxter-Jones A, Faulkner RA, Mirwald R, Bailey DA. Factors that affect bone mineral accrual in the adolescent growth spurt. J Nutr. 2004;134:696S–700S. doi: 10.1093/jn/134.3.696S. [DOI] [PubMed] [Google Scholar]
- 31.Weindruch R, Sohal RS. Caloric intake and aging. N Eng J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Prob Pharmacopsych. 1974;7:151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 33.Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 34.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 35.Anderson DA, Williamson DA, Duchmann EG, Gleaves DH, Barbin JM. Development and validation of a multifactorial treatment outcome measure for eating disorders. Assessment. 1999;6:7–20. doi: 10.1177/107319119900600102. [DOI] [PubMed] [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 37.Stewart TM, Williamson DA, Smeets MAM, Greenway FL. A computerized assessment of body image: a psychometric study. Obes Res. 2000;9:43–50. doi: 10.1038/oby.2001.6. [DOI] [PubMed] [Google Scholar]
- 38.Matts MP, Lachin JM. Properties of permuted-block randomization in clinical trials. Control Clin Trials. 1988;9:327–344. doi: 10.1016/0197-2456(88)90047-5. [DOI] [PubMed] [Google Scholar]
- 39.Krischer JP, Hurley C, Pillalamarri M, et al. An automated patient registration and treatment randomization system for multicenter clinical trials. Control Clin Trials. 1991;12:367–377. doi: 10.1016/0197-2456(91)90017-g. [DOI] [PubMed] [Google Scholar]
- 40.Schoeller DA. Measurement of energy expenditure in freeliving human beings by using doubly labeled water. J Nutr. 1988;118:1278–1289. doi: 10.1093/jn/118.11.1278. [DOI] [PubMed] [Google Scholar]
- 41.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12(suppl 12):151S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 42.Williamson DA, Stewart TM. Behavior and lifestyle: approaches to treatment of obesity. J La State Med Soc. 2005;157 (Spec No 1): S50–S55. [PubMed] [Google Scholar]
- 43.Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev. 2001;59:129–139. doi: 10.1111/j.1753-4887.2001.tb07001.x. [DOI] [PubMed] [Google Scholar]
- 44.Jeffery RW, Wing RR, Thorson C, et al. Strengthening behavioral interventions for weight loss: a randomized trial of food provision and monetary incentives. J Consult Clin Psychol. 1993;61:1038–1045. doi: 10.1037//0022-006x.61.6.1038. [DOI] [PubMed] [Google Scholar]
- 45.Wing RR, Jeffery RW, Burton LR, Thorson C, Nissinoff KS, Baxter JE. Food provision vs. structured meal plans in the behavioral treatment of obesity. Int J Obes Relat Metab Disord. 1996;20:56–62. [PubMed] [Google Scholar]
- 46.Perri MG, Corsica JA. Improving the maintenance of weight lost in behavioral treatment of obesity. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York, NY: Guilford Press; 2002. pp. 57–79. [Google Scholar]
- 47.Prentice AM. Applications of the doubly-labelled water (2H2-18O) method in free-living adults. Proc Nutr Soc. 1988;47:259–268. doi: 10.1079/pns19880043. [DOI] [PubMed] [Google Scholar]
- 48.Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jequier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol. 1986;250:R823–R830. doi: 10.1152/ajpregu.1986.250.5.R823. [DOI] [PubMed] [Google Scholar]
- 49.Schoeller DA. Energy expenditure from doubly labeled water: some fundamental considerations in humans. Am J Clin Nutr. 1983;38:999–1005. doi: 10.1093/ajcn/38.6.999. [DOI] [PubMed] [Google Scholar]
- 50.Wong WW, Butte NF, Ellis KJ, et al. Pubertal African-American girls expended less energy at rest and during physical activity than Caucasian girls. J Clin Endocrinol Metab. 1999;84:906–911. doi: 10.1210/jcem.84.3.5517. [DOI] [PubMed] [Google Scholar]
- 51.Klein PD, James WP, Wong WW, et al. Calorimetric validation of the doubly-labelled water method for determination of energy expenditure in man. Hum Nutr Clin Nutr. 1984;38:95–106. [PubMed] [Google Scholar]
- 52.Schoeller DA, Webb P. Five-day comparison of the doubly labeled water method with respiratory gas exchange. Am J Clin Nutr. 1984;40:153–158. doi: 10.1093/ajcn/40.1.153. [DOI] [PubMed] [Google Scholar]
- 53.Seale JL, Conway JM, Canary JJ. Seven-day validation of doubly labeled water method using indirect room calorimetry. J Appl Physiol. 1993;74:402–409. doi: 10.1152/jappl.1993.74.1.402. [DOI] [PubMed] [Google Scholar]
- 54.Wong WW, Lee LS, Klein PD. Deuterium and oxygen-18 measurements on microliter samples of urine, plasma, saliva, and human milk. Am J Clin Nutr. 1987;45:905–913. doi: 10.1093/ajcn/45.5.905. [DOI] [PubMed] [Google Scholar]
- 55.Wong WW, Clarke LL, Llaurador M, Klein PD. A new zinc product for the reduction of water in physiological fluids to hydrogen gas for 2H/1H isotope ratio measurements. Eur J Clin Nutr. 1992;46:69–71. [PubMed] [Google Scholar]
- 56.Institute of Medicine. Dietary Reference Intakes: Applications in Dietary Planning. Food and Nutrition Board. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 57.Lachin JM. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials. 1981;2:93–114. doi: 10.1016/0197-2456(81)90001-5. [DOI] [PubMed] [Google Scholar]
- 58.Machin D, Campbell MJ, Fayers PM, Pinol APY. Statistical Tables for Clinical Studies. 2nd ed. Oxford: Blackwell Scientific; 1997. [Google Scholar]
- 59.Keys A, Brozek J, Henschel A, Mickelson O, Taylor H. The Biology of Human Starvation. Minneapolis: University of Minnesota Press; 1950. [Google Scholar]
- 60.Fricker J, Rozen R, Melchior JC, Apfelbaum M. Energy-metabolism adaptation in obese adults on a very-low-calorie diet. Am J Clin Nutr. 1991;53:826–830. doi: 10.1093/ajcn/53.4.826. [DOI] [PubMed] [Google Scholar]
- 61.Vansant G, Van Gaal L, Van Acker K, De Leeuw I. Short and long term effects of a very low calorie diet on resting metabolic rate and body composition. Int J Obes. 1989;13:87–89. [PubMed] [Google Scholar]
- 62.Keesey RE. Physiological regulation of body weight and the issue of obesity. Med Clin North Am. 1989;73:15–27. doi: 10.1016/s0025-7125(16)30689-7. [DOI] [PubMed] [Google Scholar]
- 63.Astrup A, Gotzsche PC, van de Werken K, et al. Meta-analysis of resting metabolic rate in formerly obese subjects. Am J Clin Nutr. 1999;69:1117–1122. doi: 10.1093/ajcn/69.6.1117. [DOI] [PubMed] [Google Scholar]
- 64.Weyer C, Walford RL, Harper IT, et al. Energy metabolism after 2 y of energy restriction: the biosphere 2 experiment. Am J Clin Nutr. 2000;72:946–953. doi: 10.1093/ajcn/72.4.946. [DOI] [PubMed] [Google Scholar]
- 65.Weigle DS, Brunzell JD. Assessment of energy expenditure in ambulatory reduced-obese subjects by the techniques of weight stabilization and exogenous weight replacement. Int J Obes. 1990;14(suppl 1):69, 77–77. discussion. [PubMed] [Google Scholar]
- 66.Rising R, Keys A, Ravussin E, Bogardus C. Concomitant interindividual variation in body temperature and metabolic rate. Am J Physiol. 1992;263:E730–E740. doi: 10.1152/ajpendo.1992.263.4.E730. [DOI] [PubMed] [Google Scholar]
- 67.Roth GS, Lane MA, Ingram DK, et al. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297(5582):811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- 68.Lane MA, Baer BJ, Rumpler WV, et al. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc Natl Acad Sci USA. 1996;93:4159–4164. doi: 10.1073/pnas.93.9.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drew B, Phaneuf S, Dirks A, et al. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am J Physiol Regul Integr Comp Physiol. 2003;284:R474–R480. doi: 10.1152/ajpregu.00455.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.