The field of pancreatic transplantation sustained an important loss with the premature passing of Dr. Robert J. Corry in February 2002. He was a brilliant surgeon, a gifted clinician, and an incredibly generous individual. His legacy of humanity and clinical excellence has served as an inspiration, and this chapter is dedicated to his memory.
Traditionally, single center contributions to Clinical Transplants have tended to focus on a given center's cumulative outcomes over an extended time frame. In this chapter, however, we will juxtapose 2 very different and relatively novel experiences. The first comprises traditional pancreatic transplantation in Type I diabetics, utilizing a conceptually different approach to immunosuppression, and also describing the implementation of technical refinements designed to improve outcomes. The second includes pancreatic transplantation in the context of multivisceral transplantation. Both experiences offer new insights that may prove to be of interest to the transplant community.
Pancreatic Transplantation Under a Regimen of Campath-1H ®
Preconditioning and Low-Dose Tacrolimus Monotherapy
Dr. Corry's passing came just after the initiation of a new approach to immunosuppression after organ transplantation that was based upon 2 principles-recipient preconditioning and minimal post-transplant immunosuppression. The initial experience with pancreatic transplantation under Thymoglobulin preconditioning has been previously published (1). This chapter will focus on a more recent experience, utilizing Campath-1H® preconditioning, that has been utilized for simultaneous pancreas-kidney (SPK), pancreas after kidney (PAK), and pancreas transplantation alone (PTA). The early results with this regimen have been gratifying.
Campath-1H® is a humanized anti-CD52 monoclonal antibody. A single dose (30 mg) depletes greater than 99% of T-cells, as well as B-cells and monocytes for an extended period of time, and has allowed post-transplant maintenance immunosuppression with tacrolimus monotherapy (2,3). Two doses only of intravenous corticosteroids have been given prior to and during Campath-1H® administration, to prevent cytokine release. This immunosuppressive regimen has resulted in lower rates of rejection and complications in the early post-transplant period, without an increase in infectious complications, and with excellent patient satisfaction.
This section of the chapter will also describe the refinement of the technical aspects of pancreatic transplantation, which have evolved since the re-initiation of whole organ pancreas transplantation in Pittsburgh over 20 years ago (4), and with further modifications by Dr. Corry and others (5). These developments have led to a substantially decreased rate of thrombosis and other technical complications after pancreatic transplantation.
Rationale and Patient Characteristics
The combination of antibody preconditioning and minimal post-transplant immunosuppression is based upon 2 principles: 1) T-cell depletion creates the optimal conditions for the promotion of tolerance, and 2) excessive early post-transplant immunosuppression is potentially antitolerogenic. The induction of donor-specific tolerance towards an allograft by T-cell depletion has been demonstrated in a number of animal models (6), and the abrogation of tolerance by calcineurin inhibitors and steroids is also well described (7,8).
Thirty-seven consecutive pancreas transplants (20 SPK, 10 PAK, and 7 PTA) were performed utilizing Campath-1H® preconditioning between July 2003-August 2004. Campath-1H® was given intra-operatively. Two grams of intravenous methylprednisolone were administered, one prior to starting the Campath-1H®, and another at reperfusion. Twice daily oral tacrolimus was started at 12 hours after transplantation, with target 12-hour troughs between 10-15 ng/ml (Table1). Follow up of these patients ranges from 3-16 months with mean follow up time of 7 months.
Table 1. Immunosuppression.
| Steroids (methylprednisolone) |
| 1 gm premedication 30 min prior to Campath -1H® infusion |
| 1 gm at reperfusion of allograft (500 mg each with SPKs) |
| Campath -1H® |
| 30 mg IV over 2 hrs intraoperatively |
| Tacrolimus |
| Target 12-hour trough: 10-15 ng/ml |
| First dose approx. 12 hrs postoperatively |
Type I diabetics (c-peptide level <0.50) with end-stage renal disease, on dialysis or with a CrCl <20 mg/ml either received a simultaneous kidney-pancreas transplant (SPK) or staged pancreas-after-living-donor-kidney transplantation. Isolated PTA was performed in patients with hypoglycemic unawareness, keto-acidosis, or difficult-to-control blood glucoses. Other than the conventional contraindications of malignancy and infection, there were no absolute contraindications to transplantation, although the most common cause of nonlisting was significant cardiac disease. Pretransplant clearance by dobutamine echo or cardiac catheterization was performed on all recipients. Mean recipient age (Table 2) was 43 ± 7.9 years (range 19-59) with 19% of recipients being over 50 years of age. Mean donor age was 30.3 ± 13 years (range 12-61), with some 11% of donors over age 50. One patient underwent combined kidney-pancreas transplantation from a non-heart beating donor. Donor and recipient selection closely matches guidelines described by Dr. Corry and those published in the literature (9,10). The most important determinants of pancreas allograft use in Pittsburgh are surgeon visualization, cold ischemia time (goal <24 hrs), and donor lipase levels (peak <500 IU/dl).
Table 2. Donor-recipient demographics.
| Recipient age | 43 ± 7.9 yrs | (range 19-59) |
| Donor age | 30.3 ± 13 yrs | (range 12-61) |
| Pancreas CIT | 14 ± 3.3 hrs | (range 7-20) |
| Kidney CIT (SPK) | 15.1 ± 6 hrs | (range 6-28) |
Technical Aspects of Donor Preparation and Implantation
The standard technique for pancreas transplantation at the Thomas E. Starzl Transplantation Institute is iliac artery/vein revascularization and enteric drainage using a side-to-side duodenojejunostomy. Separate lateral incisions are made for the pancreas and/or kidney. Pancreas retransplantation is performed through a midline incision.
The use of stapling techniques has greatly simplified both the donor back-table preparation and graft implantation. On the backtable, staples can be used to perform the splenectomy, to ligate the root of the mesentery (both of which are reinforced with locking 4-0 polypropylene) and to ensure hemostasis across the peri-pancreas fat or connective tissue. A Y-graft to the superior mesenteric artery and splenic artery is routinely used, although revascularization of the gastroduodenal artery has been performed in donor organs with a potentially compromised blood supply to the head of the pancreas (11), as in the case of a donor with a replaced right hepatic artery or in a simultaneous pancreas-small bowel harvest.
The head of the pancreas allograft is implanted in a cephalad position with the tail placed in the pelvis. In this position the Y-graft and donor portal vein can be anastomosed to the external iliac vessels, and a loop of recipient jejunum can be mobilized to create a tension-free duodenoenterostomy A 15-20 degree counterclockwise rotation of the portal vein allows for optimal positioning of the pancreas. The duodenoenterostomy is performed with a circular staple inserted through the lumen of the distal duodenum, and the distal duodenum closed with an endo-GIA stapler (12). The duodeno-enterostomy is reinforced with a row of 4-0 silk Lembert sutures. This technique is equally effective on the left or right side of the recipient.
Outcomes
The overall one-year actuarial patient survival for all patients in this series was 100 % (Table 3).
Table 3. Patient and graft survival rates.
| N | Months | |||
|---|---|---|---|---|
| 3-6 | 6-12 | >12 | ||
| Total patients | 37 | 18 | 12 | 7 |
| Pt. Survival (100%) | 37/37 | 18 | 12 | 7 |
| Pancreas Survival (94%) | 35/37 | 18/18 | 11/12 | 6/7 |
| Kidney Survival (90%)* | 18/20 | 7/8 | 7/7 | 4/5 |
Only SPKs. No loss of kidney allografts in PAKs (N=11)
The actuarial pancreas survival at one year was 94% (35/37; Table 3). Two pancreata were lost, both of which were in SPK transplants. One pancreas was emergently removed 9 months after transplant because of a bleeding pseudoaneurysm, although the allograft was functioning normally. The other pancreas came from a donor with pancreatitis (lipase 900s), which promptly thrombosed on postoperative day one.
The actuarial kidney survival in the SPK group at one year was 90% (18/20; Table 3). A single kidney was lost to combined cellular and humoral rejection unresponsive to further Campath-1H® treatment and plasmapheresis with intravenous immunoglobulin. The other kidney thrombosed 6 weeks after SPK transplantation. Both pancreases are functioning well. No kidney allograft was lost in the PAK group.
Routine monitoring of amylase, lipase and creatinine were performed to monitor the pancreas and renal allografts. Suspicion of rejection was usually confirmed by an ultrasound-guided percutaneous biopsy and histological examination.
The overall rate of rejection was 22% (8/37; Table 4). Six of 7 responded to a methylprednisolone bolus (1 gm) and increased tacrolimus dosages. One kidney was lost as described above. Interestingly, all rejection episodes were preceded by tacrolimus trough levels <9.0 ng/ml for an extended period of time (5-7 days) as shown in Figure 1. Allograft rejection was not observed in pancreases or kidneys when the tacrolimus level was >10 ng/ml.
Table 4. Allograft rejection.
| Total | Months | |||
|---|---|---|---|---|
| 3-6 | 6-12 | >12 | ||
| Total patients | 37 | 18 | 12 | 7 |
| Rejection (22%) | 8/37 | 1/18 | 3/12 | 4/7 |
| Graft loss (3%)* | 1/37 | 0 | 0 | 0 |
Kidney allograft with humoral rejection
Figure 1. Graft rejection and FK levels.
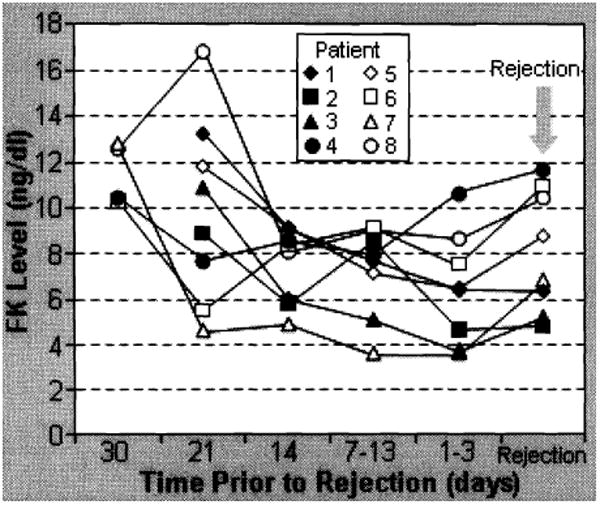
The overall complication rate in the pancreas transplants (Table 5) was 22% (8 of 37) and included thrombosis-5% (1 kidney and 1 pancreas in 2 SPK recipients); anastomotic fistula-5% (1 SPK, 1 PTA); pancreatitis—3%(1 SPK); bleeding requiring re-exploration-8% (2 SPK, 1 PAK). The thrombosis and anastomotic leak occurred prior to the implementation of the technique described above. In 40 subsequent patients (not all are included in this report because of <3 month follow-up), no thrombosis and no fistulas have been seen.
Table 5. Complications.
| Total | Months | |||
|---|---|---|---|---|
| 3-6 | 6-12 | >12 | ||
| Total patients | 37 | 18 | 12 | 7 |
| Complications (22%) | 8/37 | 3/18 | 4/12 | 1/7 |
| Thrombosis (panc) | 1 | 1 | 0 | 0 |
| Thrombosis (kid) | 1 | 0 | 0 | 1 |
| Bleeding | 3 | 2 | 1 | 0 |
| Fistula | 2 | 0 | 2 | 0 |
| Pseudoaneurysm* | 1 | 0 | 1 | 0 |
pancreas allograft
Discussion
One of the major advantages of both the immunosuppressive regimen described here and the technical refinements that have been developed is their great simplicity. This is a safe and straightforward immunosuppressive regimen, with a low rate of rejection, a low rate of infectious complications, and a low rate of technical complications. In contrast to kidney transplantation alone, weaning to spaced dosing has proceeded much more slowly, given the consequences associated with late rejection. This approach is very well tolerated. While more follow up will be required to assess the long-term outcomes, this regimen appears to have substantial potential in the care of patients undergoing pancreatic transplantation.
Pancreatic Transplantation En-Bloc with Visceral Grafts
With the recent evolution of intestinal transplantation, the pancreas has frequently been transplanted en-bloc with the intestine, liver and other abdominal viscera. In contrast to combined pancreas-kidney and solitary pancreas transplantation, the pancreas is generally included in the multivisceral graft for non-diabetic indications. The objectives of this report are to identify the indications for the procedure and its potential impact on the current United Network of Organ Sharing (UNOS) pancreatic transplant coding system and database. The immunogenicity and functional survival of the pancreas transplanted en-bloc with the other abdominal visceral organs will also be addressed.
Technical Evolution
Because of the embryonic origin of the pancreas, the gland shares its blood supply with the liver and intestine (Fig. 2) (13). Accordingly, a few technical challenges were faced with the development of intestinal, liver-intestinal, and multivisceral transplantation. The successful simultaneous recovery of intestinal, pancreatic, and hepatic grafts from the same donor for transplantation to different recipients has been described (13). In the same article, the technique of en-bloc intestinal and pancreas transplantation was described for the first time with an illustration of the back table procedure (Fig. 3). The procedure of combined liver and intestinal transplantation has evolved over the last decade to preserve the duodenum and pancreas en-bloc with the liver and intestine. The rationale of maintaining continuity of the hepatobiliary system with the gut was to avoid the potential risks of biliary reconstruction and maximize the absorptive functions of the transplanted intestine (Fig. 4). The pancreas has also been part of the modified (Fig. 5A) and full multivisceral transplantation (Fig. 5B) since the development of the procedure. However, a recent modification has been introduced to patients who have a benign disease of their foregut organs (Fig. 6) (14).
Figure 2. The embryonic origin of the liver, pancreas and alimentary canal.
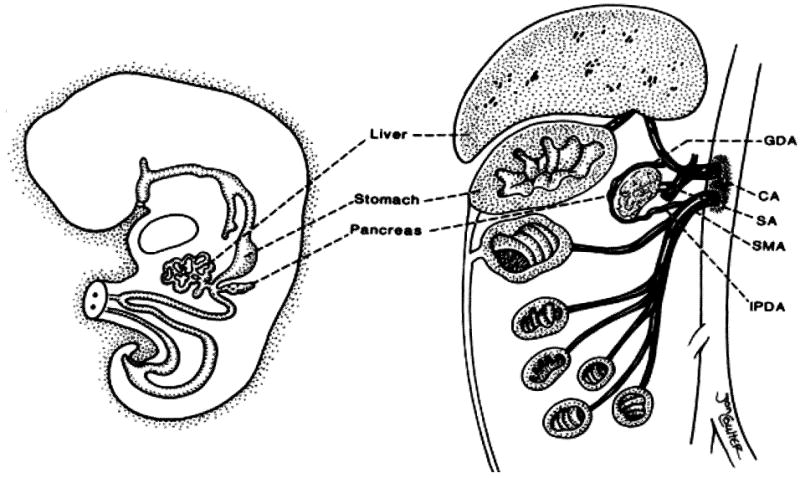
Note the shared axial blood supply and its segmental distribution, CA, celiac axis; GDA, gastroduodenal artery; IPDA, inferior pancreaticoduodenal artery; SA, splenic artery; SMA superior mesenteric artery. (Reproduced from ref 13, with permission.)
Figure 3. Back table vascular reconstruction of the composite intestinal-pancreatic allograft.
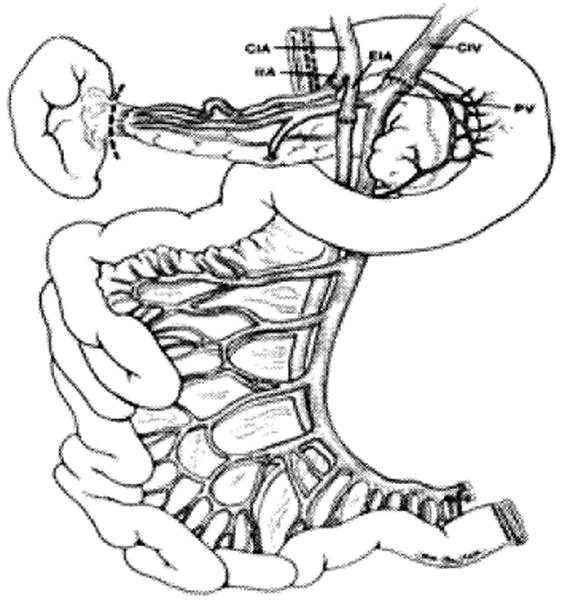
Note continuity of the pancreas, duodenum, and small intestine with intact vascular pedicle, CIA, common iliac artery; CIV common iliac vein; EIA, external iliac artery; IIA internal iliac artery; PV, portal vein. (Reproduced from ref 13, with permission).
Figure 4. Combined liver and intestine (left) without the duodenum and pancreas and (right) en-bloc with duodenum and pancreas.
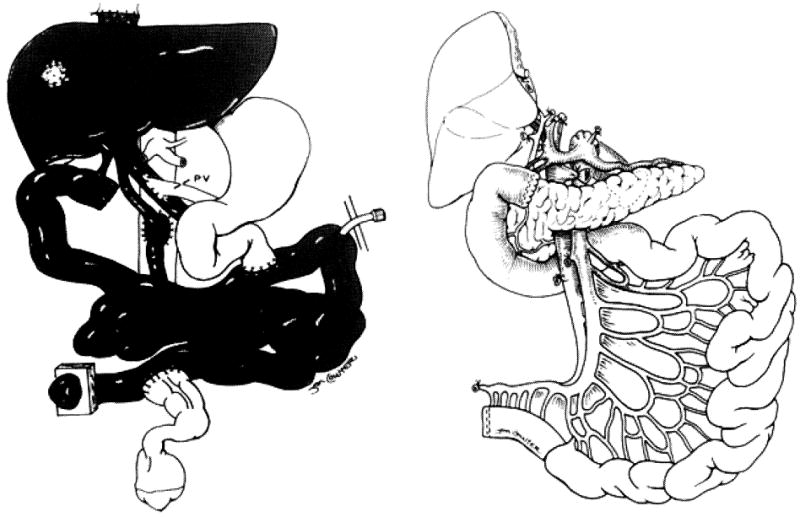
Note the continuity of the hepatobiliary system with the duodenum. Resection of the left lobe of the liver was performed because of loss of abdominal domain of the recipient (Reproduced from ref 13, with permission).
Figure 5.
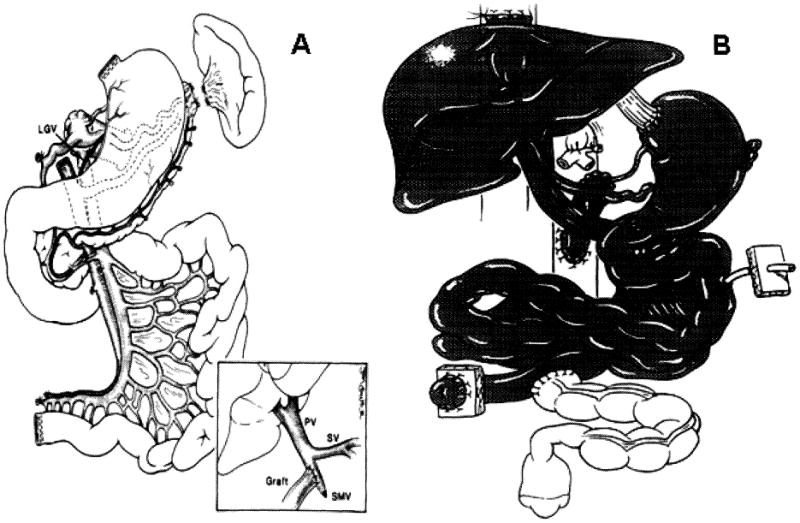
Figure 5A. Modified multivisceral graft that contains stomach, duodenum, pancreas and small intestine.
Note preservation of the gastroepiploic arcade and left gastric pedicle including the left gastric vein (LGV). Inset: venous drainage of the composite visceral graft to the side of the recipient superior mesenteric vein (SMV) stump by using the donor common iliac vein as an extension graft without compromising the recipient portal venous flow during graft implantation. PV, portal vein; SV splenic vein.
Figure 5B. Full multivisceral transplant.
Figure 6.
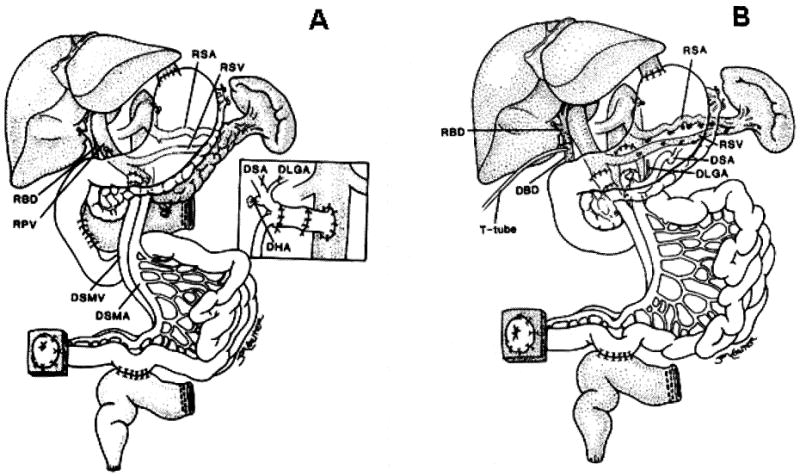
Figure 6A. Transplantation of a modified multivisceral graft (unshaded organs) containing the pancreas and all of the hollow intra-abdominal viscera in continuity from the esophagogastric junction to the terminal ileum. The native liver, spleen, pancreas, and a C-loop of duodenum have been retained. The procedure was used to treat a patient suffering from psuedo-obstruction. Biliary drainage from the native liver as well as from both pancreases was accomplished with a side-to-side host-to-graft duodenal anastomosis. The insert demonstrates preservation of the donor splenic (DSA) and left gastric (DLGA) arteries (with Carrel patch) with ligation of the donor hepatic artery (DHA) stump.
Note that an interposition arterial graft was initially anastomosed to the recipient infra-renal aorta and prior to allograft implantation. RSA, recipient splenic artery; RSV, recipient splenic vein; RBD, recipient bile duct; RPV, recipient portal vein, DSMV, donor superior mesenteric vein, DSMA, donor superior mesenteric artery. (Reproduced from ref 14, with permission.)
Figure 6B. The use of a modified multivisceral graft (stomach, duodenum, pancreas, and small bowel) following abdominal visceral exenteration with preservation of the host liver and spleen (shaded organs). The portosplenic circulation is maintained intact during graft insertion and the preserved spleen protects the patient from the risk of PTLD. This modified multivisceral transplantation has been used to treat recipients with massive gastrointestinal polyposis, and extensive Crohn's disease.
Note the duct to duct biliary reconstruction. RBD, recipient bile duct; DBD, donor bile duct. (Reproduced from ref 14, with permission.)
Recipient Operation
Most patients who require intestinal and multivisceral transplantation with inclusion of the pancreas are critically ill and have multiple complex medical issues. The mean number of abdominal surgeries prior to transplantation was 4±4 with a range of 0-25. In addition, these patients can lose their abdominal domain and frequently require reconstruction of their abdominal wall.
The arterial reconstruction of the intestinal and multivisceral grafts is uniformly established through placement of an infrarenal aortic graft. The venous drainage of the combined intestinal/pancreatic and modified (without liver) multivisceral transplantation is commonly into the native portal system. However, in 2 recipients, the drainage was into the recipient vena cava. When the pancreas is part of a liver/intestinal and full multivisceral graft, continuity of the portomesenteric venous system is maintained as shown in Figures 4-6. Obviously, all pancreatic grafts were drained enterically and orthotopically.
Indications
The whole pancreas was transplanted en-bloc in 78 (32%) out of a total of 246 consecutive primary cadaveric abdominal visceral transplantations that were performed at our institution over the past 13 years. Of these, 43 were adults and 35 were children with a mean age of 40.5 ± 10.1 years, and 4.8 ± 5.8 years, respectively. Included with the pancreas was the intestine in 4 (5%) grafts, stomach and intestine in 14 (18%) grafts, liver and intestine in 28 (36%) grafts, and stomach, intestine and liver in the remaining 32 (41%) grafts. The primary indications for inclusion of the pancreas were technical in 32 (41%), vascular thrombosis in 18 (23%), gastrointestinal dysmotility in 13 (17%), gut neoplasm in 10 (13%), trauma in 3 (4%), and diabetes in the remaining 2 (2%) recipients. Insulin-dependent diabetes was also an associated disorder in another 2 patients, giving a total of 4 (5%) recipients who were diabetic prior to transplantation. Most recipients were non-diabetic, and the indications for inclusion of the pancreas were mainly technical and vascular insufficiency.
Donor Characteristics
All donors were deceased and hemodynamically stable. The mean age was 15.7±13.8 with a range of 3 days to 50 years. All but 2 allografts were ABO identical. Human leukocyte antigen (HLA) matching was random, with no cases of zero -A, -B, -DR mismatches. T- and B-lymphocytotoxic crossmatches were positive after dithiothreitol (DTT) treatment in 13 (17%) patients. At one time, because of the reported adverse effects of positive donor CMV serology on outcome, attempts were made to avoid using CMV-seropositive intestinal donors for CMV-seronegative recipients, particularly those who did not need replacement of the native liver (15); these considerations became less important with the development of newer immunosuppressive regimens. Management policies and retrieval operations have been described previously (16-18). No attempts were made to treat the donor with anti-lymphocyte preparations. However, the intestinal component of 17 (22%) grafts was irradiated ex-vivo with a single dose of 7.5 Gy. In addition, donor bone marrow cells were given intravenously to 25 (32%) recipients within the first 24 hours after allograft reperfusion at 2.4 − 9 × 108/kg BW. The University of Wisconsin (UW) solution was used for graft preservation in all but the first case, with a mean cold ischemia time (CIT) of 8.9±1.7 hours.
Immunosuppression
Immunosuppression has evolved since the beginning of the program because of the high risk of intestinal allograft rejection. Tacrolimus-based immunosuppression was the underpinning of intestinal and multivisceral transplantation, and was used for all 78 recipients. With the initial 14 (18%) patients, prednisone was added from the outset as a second agent. Induction therapy was utilized between 1995-2001 in 23 (29%) patients with cyclophosphamide in 3 and daclizumab in 20 cases. A tolerogenic protocol was initiated in July of 2001 with recipient pretreatment and post-transplant tacrolimus monotherapy. In 41 pretreated recipients, Thymoglobulin was used in 40 patients and Campath -1H® was used in the remaining case. Azathioprine, mycophenolate mofetil, or sirolimus were added as adjunct maintenance immunosuppression agents in selected cases at different time periods. In all but the first 8 recipients, prostaglandin E1 was infused intravenously during the early postoperative period. Episodes of rejection were initially treated with steroids and adjustments of tacrolimus dosing. OKT3, Thymoglobulin, or Campath -1H ®were used to treat steroid-resistant or severe rejection episodes. Details of the immunosuppressive protocols and drug dosage are described elsewhere (15, 19).
Survival Outcome
With a mean follow up of 30 ± 26 months (0.3 – 116) and as of August 2004, 52 pancreatic recipients with intestinal and multivisceral transplantations were alive, for an overall survival rate of 67%. The causes of death were infection (n=11), PTLD (n=5), rejection (n=4), technical (n=3), GVHD (n=1) and others (n=2). Another 5 grafts were lost due to primary non-function in 2, arterial thrombosis in 2, and mycotic pseudoaneurysm in the remaining one. The actuarial patient survival rate was 81% at one year and 77% at 5 years; the 4 diabetic recipients were insulin independent, and while functional studies of the pancreas were not performed, pancreatic graft retention rates of 76% and 62% were observed at one and 5 years, respectively. In spite of increasing patient complexity, the one-year survival rate has recently improved to 91%. The recent improvement in outlook may be related to technical innovations, early viral detection, allograft immune-modulation, and recipient pretreatment.
Pancreatic Rejection
Despite the high immunogenicity of the concomitantly transplanted intestine, high degree of HLA mismatch, and transplanting the pancreas across a positive cytotoxic crossmatch, only 6 (8%) pancreatic glands experienced mild to moderate acute rejection simultaneously with the intestine and/or liver allograft. The diagnosis was made based on clinical and biochemical data, and all episodes were successfully treated with steroids and/or anti-lymphocytic agents. Chronic rejection was diagnosed on histopathologic examination of an explanted graft or an autopsy specimen on 3 (4%) occasions. These rates of acute and chronic pancreatic rejection are significantly lower than those observed in the simultaneously transplanted intestine.
Preconditioning and Successful Weaning
With recipient pretreatment, patient and graft survival have significantly improved, with a one-year patient survival rate of 94% and a graft survival rate of 90%. There has been no significant increase in morbidity, with a low risk of opportunistic infections, including viral infections.
Attempts of weaning with spaced doses of tacrolimus monotherapy were successful in 49% (n=20) of the pretreated recipients. With a mean follow up of 19 ± 7 (range: 10 –35) months, 9 patients were on a single daily dose of tacrolimus, 3 on every other day, 2 on 3 times per week, and 6 on 2 times per week. There is no single example of graft loss to acute or chronic rejection because of weaning.
Discussion
En-bloc pancreas transplantation is common with intestinal and multivisceral transplantation. Diabetes is a rare indication for inclusion of the pancreatic gland. The lower risk of graft thrombosis is mainly related to the establishment of a large size infrarenal aortic graft and the common use of a Carrel patch during the back table arterial reconstruction. The gland is immunologically protected by the concomitantly transplanted organs, particularly the liver, despite the co-existence of high immunologic risk factors, including a high degree of HLA mismatching and pre-existing antibodies.
Summary.
Campath-1H® preconditioning with tacrolimus monotherapy is an effective immunosuppressive regimen for pancreas transplantation, with acceptable patient and graft survival rates early after transplantation. Rejection rates are low under this protocol if the tacrolimus level is kept consistently >10 ng/ml. This immunosuppressive protocol, combined with recent technical refinements, has resulted in lower rates of thrombosis and overall complications. Pancreatic transplantation en-bloc with visceral grafts has the following unique features:
Diabetes is a rare indication, and HLA matching is not required.
The gland is immunologically protected by the simultaneously transplanted visceral organs.
Disease gravity, surgical complexity and gut alloimmunity influence the overall pancreatic allograft survival.
The current UNOS listing criteria and data registry should be modified for obvious logistic and scientific reasons.
Acknowledgments
The authors would like to thank Mrs. Darlene Koritsky for her dedication and careful preparation of the manuscript.
References
- 1.Starzl TE, Murase N, Abu-Almagd K, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361:1502. doi: 10.1016/s0140-6736(03)13175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcos A, Eghtesad B, Fung JJ, et al. Use of aletuzumab and tacrolimus monotherapy for cadaveric liver transplantation: with particular reference to hepatitis C virus. Transplantation. 2004;77:926. doi: 10.1097/01.tp.0000142674.78268.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro R, Tan H, Basu A, et al. Campath 1-H preconditioning and tacrolimus monotherapy with subsequent weaning in renal transplant recipients. Am J Transplant. 2004;4:405. [Google Scholar]
- 4.Starzl TE, Iwatsuki S, Shaw BW, Jr, et al. Pancreaticoduodenal transplantation in humans. Surg Gynecol Obstet. 1984;159:265. [PMC free article] [PubMed] [Google Scholar]
- 5.Nghiem DD, Corry RJ. Techniques of simultaneous pancreaticoduodenal transplantation with urinary drainage of pancreatic secretion. Am J Surg. 1987;153:405. doi: 10.1016/0002-9610(87)90588-5. [DOI] [PubMed] [Google Scholar]
- 6.Starzl T, Zinkernagel RM. Transplantation tolerance from a historical perspective. Nature Rev Immunol. 2001;1:233. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transplant Immunol. 2004;13:117. doi: 10.1016/j.trim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Smiley ST, Csizmadia V, Gao W, et al. Differential effects of cyclosporine A, methylprednisolone, mycophenolate, and rapamycin on CD154 induction and requirement of NFkB: implications for tolerance induction. Transplantation. 2000;10:415. doi: 10.1097/00007890-200008150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nature Med. 1999;4:686. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 10.Kapur S, Bonham CA, Dodson SF, et al. Strategies to expand the donor pool for pancreas transplantation. Transplantation. 1999;67:284. doi: 10.1097/00007890-199901270-00017. [DOI] [PubMed] [Google Scholar]
- 11.Krieger NR, Odorico JS, Heisey DM, et al. Underutilization of pancreas donors. Transplantation. 2003;75:1271. doi: 10.1097/01.TP.0000061603.95572.BF. [DOI] [PubMed] [Google Scholar]
- 12.Thai NL, Khan A, Tom K, et al. Revascularization of the gastroduodenal artery in a pancreas allograft from a donor with a replaced right hepatic artery. Transplantation. doi: 10.1097/01.tp.0000145056.82764.53. in press. [DOI] [PubMed] [Google Scholar]
- 13.Fridell JA, Milgrom ML, Henson S, et al. Use of the end-to-end anastomotic circular stapler for creation of the duodenoenterostomy for enteric drainage of the pancreas allograft. J Am Coll Surg. 2004;198:495. doi: 10.1016/j.jamcollsurg.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Elmagd K, Fung J, Bueno J, Martin D, Madariaga J, Mazariegos G, Bond G, Molmenti E, Corry R, Starzl TE, Reyes J. Logistics and technique for procurement of intestinal, pancreatic, and hepatic grafts from the same donor. Ann Surg. 2000;232:680. doi: 10.1097/00000658-200011000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-Elmagd K, Reyes J, Bond G, Mazariegos G, Wu T, Murase N, Sindhi R, Martin D, Colangelo J, Zak M, Janson D, Ezzelarab M, Dvorchik I, Parizhskaya M, Deutsch M, Demetria A, Fung J, Starzl T. Clinical intestinal transplantation: A decade of a single center experience. Ann Surg. 2001;234:404. doi: 10.1097/00000658-200109000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abu-Elmagd K, Reyes J, Todo S, Rao A, Lee R, Irish W, Furukawa H, Bueno J, McMichael J, Fawzy A, Murase N, Demetris J, Rakela J, Fung J, Starzl T. Clinical intestinal transplantation: New prospectives and immunologic considerations. J Am Coll Surg. 1998;186:512. doi: 10.1016/s1072-7515(98)00083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starzl TE, Todo S, Tzakis A, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335. [PMC free article] [PubMed] [Google Scholar]
- 18.Casavilla A, Selby R, Abu-Elmagd K, et al. Logistics and technique for combined hepatic-intestinal retrieval. Arch Surg. 1992;216:605. doi: 10.1097/00000658-199211000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furukawa H, Abu-Elmagd K, Reyes J, Nour B, Tzakis A, Todo S, Starzl TE. Technical aspects of intestinal transplantation. In: Braverman MH, Tawes RL, editors. Surgical Technology International II. Surgical Technology International; 1994. pp. 165–170. [PubMed] [Google Scholar]
- 20.Abu-Elmagd KM, Bond G, Reyes J, Fung J. Intestinal transplantation: A coming of age. Adv Surg. 2002;36:65. [PubMed] [Google Scholar]


