Summary
In six of twelve orthotopic liver recipients nephrotoxicity was noted after 13–22 days of treatment with 16.3±2.9 (SEM) mg/kg per day of cyclosporin A (CyA). With a decrease in the daily CyA dose to 9.2±2.3 (SEM) mg/kg kidney function returned to normal. No hepatic rejections occurred on this lowered CyA dose. In 4 out of 66 kidney recipients a switch from a CyA dose of 5.2–10.7 mg/kg daily to azathioprine was done 4–8 months after transplant because of unsatisfactory kidney function, suspected to be due to nephrotoxicity. In three patients, this resulted in an improved graft function. A fourth transplant was lost to an irreversible rejection 13 days later. Thus CyA is nephrotoxic but this toxicity is easily reversed, even after many months of treatment, and the ease with which this complication can be managed suggests that nephrotoxicity should not diminish the high expectations that transplant surgeons have for CyA.
Introduction
Cyclosporin A (CyA), a metabolite from the fungi Cylindrocarpon lucidum and Trichoderma polysporum, was discovered by Dreyfuss et al.1 Its immunosuppressive properties were first reported by Borel et al.2 The earliest clinical studies of CyA on kidney transplant patients were reported in 1978, when the possibility of nephrotoxic side effects was raised.3,4 Calne et al.3,5 reported a putative acute nephrotoxic syndrome leading to anuria or oliguria in 11 out of 32 patients treated with CyA. They thought that this problem was ameliorated by inducing forced diuresis with fluids and mannitol. They recommended delaying the administration of the first dose of CyA until post-transplantation diuresis was established. In our own experience, this early kidney failure was usually caused by acute rejection rather than by nephrotoxicity of CyA and could be prevented or treated with steroids.6,7 Thus, we have started treatment with CyA preoperatively.
To clarify the question of CyA nephrotoxicity further, we have studied renal function in liver transplant recipients who were treated with this drug. In addition, observations relevant to nephrotoxicity were made in four kidney graft recipients whose therapy was changed from CyA to azathioprine 4–8 months after transplantation.
Patients
After liver transplantation twelve patients were treated with CyA and low doses of prednisone. All twelve were given one dose of CyA, 15–20 mg/kg, before operation and this daily dose was continued afterwards intramuscularly or orally. Before operation and after wards renal function was monitored with daily serum creatinine (table I) and blood urea nitrogen (BUN) determinations, plus one to two times weekly creatinine clearances. Postoperatively, renal scans were done with 131I-hippuran and 99mTc-diethylenetriamine-pentaacetic acid (DTPA),8 and in six patients the results could be compared with similar preoperative studies. One patient died on the 19th postoperative day from a complete hepatic artery thrombosis plus a partial portal vein thrombosis. With one exception, the eleven surviving recipients now have normal liver function. The exceptional patient has chronic rejection with a bilirubin of 113 μmol/l, but he has not shown any signs of nephrotoxicity with a CyA dose of 9.5 mg/kg.
TABLE I. Nephrotoxicity* from Cyclosporin A Found in Liver Transplant Patients.
| Patient | Time of max. toxicity after transplant (days) | Max. serum creatinine (μmol/l) | CyA dose (mg/kg) | Non-toxic CyA dose (mg/kg) |
|---|---|---|---|---|
| 1 | 22 | 221 | 13.0 | 11.0 |
| 4 | 22 | 548 | 17.5 | 8.2 |
| 5 | 17 | 248 | 17.5 | 14.0 |
| 6 | 13 | 424 | 17.5 | 7.2 |
| 7 | 15 | 221 | 17.5 | 8.7 |
| 9 (a) | 13 | 813 | 20.0 | 7.5 |
| 9 (b) | 41 | 274 | 11.0 | 7.5 |
| Mean±SE | 17±3.8† | 393±206 | 16.3±2.9 | 9.2±2.3 |
Nephrotoxicity defined as a serum creatinine>175 μmol/l.
Excluding 9 (b).
From December, 1979, to September, 1980, 66 patients given 67 cadaveric kidney transplantations have received CyA and prednisone.6,7 In 4 of those 66 patients the immunosuppression was changed after 4–8 months from CyA and prednisone to azathioprine and prednisone. The prednisone dose was kept constant. Indications and timing of the switch are listed in table II.
TABLE II. Nephrotoxicity in Renal Transplanted Patients from Cyclosporin A Recorded as Kidney Function Just Before Discontinuing Cyclosporin A.
| Patient | Time of change to azathioprine alter transplant (mo) | Serum-creatinine on CyA (μmol/l) | CyA dose (mg/kg) | Serum creatinine on azathioprine (μmol/l)* | Indications for change of immunosuppression |
|---|---|---|---|---|---|
| 3 | 8 | 274 | 10.7 | 133 | Suspected nephrotoxicity plus hepatoxicity (bilirubin 50 μmol/l)† |
| 9 | 6 | 318 | 7.0 | 159 | Suspected nephrotoxicity |
| 12 | 4 | 407 | 7.0 | 230 | Suspected nephrotoxicity |
| 21 | 5 | 318 | 5.2 | Kidney loss | Suspected nephrotoxicity |
Kidney function for patients on azathioprine recorded when the function had reached a steady state. Steroid treatment remained unchanged during this period (prednisone 10–20 mg/day).
Patient 3 had a normal liver function within 2 weeks.
Results
Significant renal function abnormalities were noted on seven occasions in six of the twelve liver transplant recipients. The deteriorations occurred suddenly with sharp rises in the serum creatinine and BUN after 13–22 days of CyA treatment. A drop in urine output and weight gain was also noted. The CyA dose at the time ranged between 11 and 20 (mean 16.3) mg/kg (table I). The renal scans showed normal blood flow, with decreased clearance, a finding described in kidney transplants as consistent with acute tubular necrosis (ATN).8
After decreasing the CyA dose, the foregoing changes in renal function promptly disappeared. The narrow margin between an acceptable situation and clinically overt nephrotoxicity is exemplified by liver patient 9. Early pronounced nephrotoxicity was relieved by reducing CyA from 20.0 to 7.5 mg/kg per day. Later, an increase in the CyA from 7.5 to 11.0 mg/kg resulted in a second example of nephrotoxicity (see figure). A third episode was observed one month after the time shown in the figure when the dose of 11 mg/kg was temporarily reinstituted. The non-toxic CyA dose has been found to range from 7.4 to 14.0 (mean 9.2) mg/kg in patients with liver grafts. None of these patients have shown any signs of acute hepatic rejection.
Figure 1. Kidney function in liver recipient no. 9.
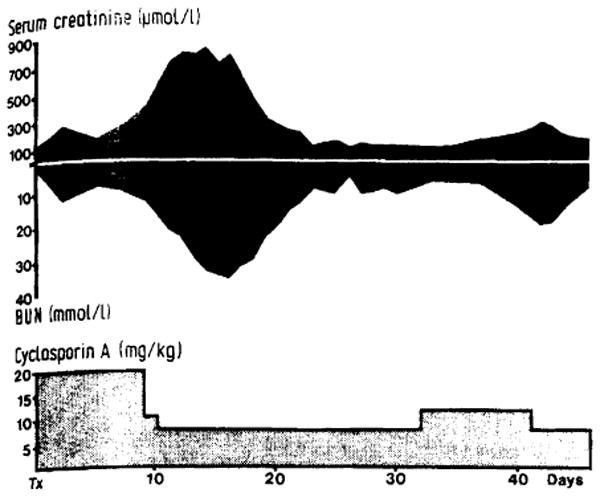
CyA dose of 20 mg/kg daily resulted in a severe episode of uraemia. Patient not dialysed. After reduction in CyA dose, kidney function returned to normal. Later increase in CyA from 7.5 to 11.0 mg/kg daily caused second deterioration in kidney function; function again normal after CyA dose adjustment.
SI CONVERSION.
Creatinine: 1 μmol/l = 0.011 mg/dl
Bilirubin: 1 μmol/l = 0.059 mg/dl
Urea nitrogen: 1 mmol/l = 1.4 mg/dl
Just before changing from CyA to azathioprine, all 4 of the renal transplant patients had good renal blood flow on scans which were interpreted as consistent with ATN. After the switch, renal function improved within 1–3 weeks in three recipients with halving of the average serum creatinine concentrations. Two of these three recipients had a normal renal function after the transplant. It took 5–7 months on a stable CyA dose of 7 to 10.7 mg/kg for the nephrotoxicity to reveal itself. The fourth patient experienced graft rejection within 13 days of changing to azathioprine.
Discussion
The opportunity to measure renal function in twelve liver transplant recipients permits conclusions to be drawn about the nephrotoxicity of CyA without the confusion inherent with such studies on kidney transplant patients. The good liver function which was achieved in eleven of these patients precludes the hepatorenal syndrome as a factor. Six patients exhibited nephrotoxicity in doses of 11.0–20.0 mg/kg daily. The complication responded to dose reduction and was completely reversible.
The evidence of nephrotoxicity is almost as convincing in the four renal recipients who were changed from CyA to azathioprine. Here, nephrotoxicity apparently occurred in doses as low as 5.2 mg/kg daily. The risk of such a therapeutic change under these circumstances was shown by the prompt rejection of one of the four kidneys despite substitution of azathioprine for CyA. In the exceptional case, just as in the other three, the renal scans before the drug switch had shown the combination of good perfusion plus impaired clearance, which we have associated with nephrotoxicity rather than rejection.6
Although nephrotoxicity was thus a feature of CyA therapy after both liver and kidney transplantation, the usefulness of the drug was little affected. Dose adjustments were easy to make and were not followed by rejection in most instances. In our view, CyA remains a major advance in clinical immunosuppression.
References
- 1.Dreyfuss M, Haerri E, Hoffman H, et al. Cyclosporin A and C: new metabolites from Trichoderma polysporum (Link ex Pers) Ritai Europ J Appl Microbiol. 1976;3:125–33. [Google Scholar]
- 2.Borel JF, Feurer C, Gubler HU, Stahelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6:468–75. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- 3.Calne RY, White DJG, Thiru S, et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978;ii:1323–27. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- 4.Powles RL, Barrett AJ, Clink H, et al. Cyclosporin A for the treatment of graft-versus-host disease in man. Lancet. 1978;ii:1327–31. doi: 10.1016/s0140-6736(78)91971-2. [DOI] [PubMed] [Google Scholar]
- 5.Calne RY, Rolles K, White DJG, et al. Cyclosporin A initially as the only immuno-suppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases and 2 livers. Lancet. 1979;ii:1033–36. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 6.Starzl TE, Weil R, Iwatsuki S, Klintmalm GBG, et al. The use of Cyclosporin A and prednisone in cadaver kidney transplantation. Surg Gynecol Obstet. 1980;151:17–26. [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Weil R, Iwatsuki S, Klintmalm GBG, et al. Liver transplantation, 1980, with particular reference to Cyclosporin A. Transplant Proc. in press. [PMC free article] [PubMed] [Google Scholar]
- 8.Stables DP, Klingensmith CW, III, Johnson ML. Renal transplantation. In: Rosenfield AT, Glickman MG, Hodson J, editors. Diagnostic imaging in renal disease. New York: Appleton-Centry-Crofts; 1979. pp. 167–213. [Google Scholar]


