Dear Editor,
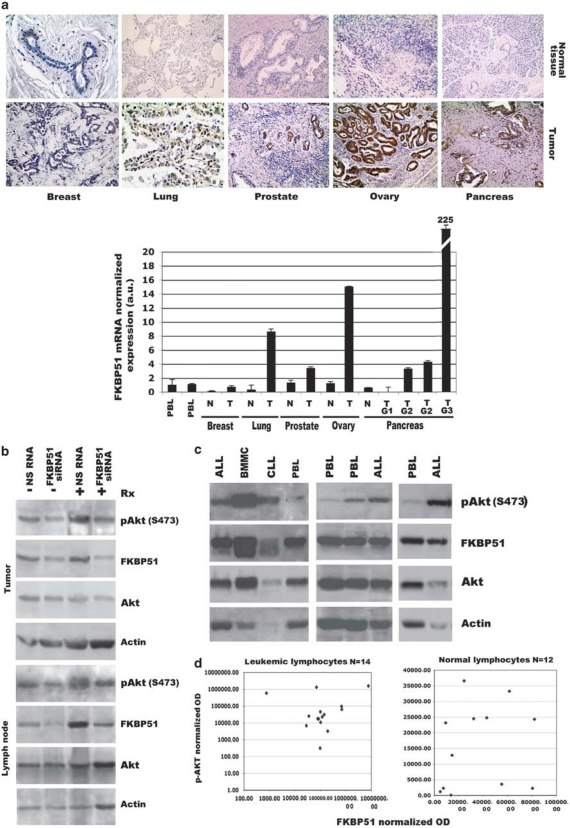
FK506-binding protein (FKBP) 51 is a cochaperone, which belongs to the immunophilin family, a group of proteins with peptidyl-prolyl isomerase activity. FKBPs regulate several biological processes through protein–protein interaction.1 In particular, FKBP51 is a component of the steroid receptor complex, with a role in steroid resistance;2 moreover, it is involved in NF-κB activation because of its isomerase activity, which is essential for the function of subunit-α in the IκB kinase complex.3 According to Baughman et al.,4 FKBP51 is abundantly expressed in lymphocytes and in several other tissues, but it is expressed at low levels in the pancreas, spleen, and stomach. There is increasing evidence of an association of FKBP51 hyperexpression with cancer6, 7, 8 and a relevant role of this protein in sustaining cell growth,5 malignancy, and resistance to therapy.6, 7, 8 An immunohistochemistry study of expression of FKBP51 in 50 tumoral samples acquired from our pathology section, including breast, lung, pancreas, ovary, and prostate (10 samples for each tumor), and a comparable number of normal tissue samples showed an intense signal in 38 out of 50 tumors analyzed, whereas normal tissues of the same histotypes showed a weak/absent immunohistochemical signal. The 12 tumor samples with low/negative immunohistochemistry were the 10 breast cancer samples and 2 out of 10 pancreatic tumors. Interestingly, these two pancreatic tumors belonged to the well-differentiated histotype (G1). Figure 1a (upper panel) shows representative immunohistochemical findings. Measurement of FKBP51 mRNA levels in deparaffinized tissues using real-time PCR confirmed the immunohistochemistry results (representative results in Figure 1a, lower panel). Taken together, these findings support the hypothesis that FKBP51 is a promising novel tumoral marker. The association of FKBP51 overexpression with cancer is in line with the rapidly emerging concept that NF-κB drives tumorigenesis in the most common genetic alterations associated with cancer.9, 10
Figure 1.
(a) (Upper panel) FKBP51 immunochemical staining of normal and neoplastic tissues. Serial sections of 4 μm from routinely formalin-fixed, paraffin-embedded blocks were cut and mounted on poly--lysine-coated glass slides. (Lower panel) Deparaffinized sections were incubated overnight at 4°C with anti-FKBP51 primary antibody (F-13, Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1 : 50. The standard streptavidin–biotin–peroxidase complex technique was performed. Hematoxylin was used for nuclear counterstaining. (Lower panel) Normalized expression rates of FKBP51 mRNA (a.u., arbitrary units) in PBL and deparaffinized tissues; each histogram is referred to a representative normal or tumoral sample. Values represent means and S.D. of arbitrary units from three different real-time experiments, each performed in triplicate. Total RNA was isolated from PBLs with Trizol (Invitrogen, Carlsbad, CA, USA) and from paraffinized tumors using the High Pure RNA Paraffin Kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's instructions. In all, 1 μg of each RNA was used for cDNA synthesis with Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV RT, Invitrogen). Gene expression was quantified by real-time PCR using the iQ SYBR Green Supermix (Bio-Rad, Foster City, CA, USA) and specific real-time-validated QuantiTect primers for FKBP51 (FW: 5′-GTGGGGAATGGTGAGGAAACGC-3′ REV: 5′-CATGGTAGCCACCCCAATGTCC-3′) and specific primers for β-actin. Relative quantitation of FKBP51 transcript across multiple samples was performed by using a coamplified β-actin internal control for sample normalization. The values of each sample were compared to PBL (expression=1) for an estimate of the relative expression change fold of FKBP51. (b) Western blot assay of pAkt (S473) and FKBP51 levels in lysates prepared from melanoma xenografts and locoregional lymph nodes obtained from athymic nu/nu mice (Charles River Laboratory, Wilmington, MA, USA). For pAkt (S473) detection, the rabbit polyclonal antibody clone D9E (Cell Signaling, Danvers, MA, USA) was used, and for FKBP51, the goat polyclonal antibody F-13 (Santa Cruz Biotechnology) was used. When tumors reached ∼10 mm in mean diameter, mice received a single intratumoral injection of FKBP51 siRNA (5′-ACCUAAUGCUGAGCUdAU-3′) or (nonsilencing) NS RNA. After 48 h, mice were subjected to tumor irradiation, and after a further 48 h, animals were killed and tumors and lymph nodes excised for preparation of lysates. (c) Western blot assays of pAkt (S473) and FKBP51 levels in lysates prepared from different samples of mononuclear cells. Mononuclear cells of acute lymphoblastic leukemia (ALL) were separated by bone marrow; BMMCs were bone marrow mononuclear cells separated from a noninfiltrated bone marrow sample from a lymphoma patient. Mononuclear cells of chronic lymphocytic leukemia (CLL) were separated by peripheral blood. Peripheral blood lymphocytes (PBLs) were from normal donors. (d) Scatterplot of FKBP51 OD versus pAkt (S473) OD. FKBP51 and pAkt (S473) expression levels were quantified by densitometry using ImageJ 1.42q (NIH, http://rsb.info.nih.gov/ij/) for Macintosh. Integrated ODs of pAkt were normalized to Akt, whereas integrated ODs of FKBP51 were normalized to actin
Recently, it has been found, in tumor cell lines, that FKBP51 acted as a scaffold to facilitate the interaction between Akt and PH domain leucine-rich repeat protein phosphatase, which mediates dephosphorylation of pAkt (S473).11 This raised the question whether FKBP51 may work out as a tumor suppressor by deactivating Akt. As we previously found that intratumoral injection of FKBP51 siRNA followed by irradiation produced extensive apoptosis in melanoma xenografts,8 we investigated the effect of FKBP51 downmodulation on pAkt (S473) levels in our mouse model of melanoma, in both xenografts and locoregional lymph nodes. Histological examination showed that the mouse lymphatic tissue was not metastatic. Figure 1b shows a representative outcome. On the basis of these results, pAkt (S473) levels were not enhanced with downmodulation of FKBP51 in normal or cancerous tissue.
This observation suggests that pathways upstream from Akt, which are often deregulated in cancer, control activated-Akt levels. This hypothesis is supported by the findings that leukemic lymphocytes often display higher levels of pAkt (S473) in comparison with normal lymphocytes (Figure 1c) even in the presence of similar or higher FKBP51 levels. To assess whether an inverse correlation subsisted between FKBP51 and pAkt (S473) levels, we used western blot to measure these levels in samples of primary lymphatic leukemia and normal peripheral blood lymphocytes. Expression levels were quantified by densitometry. Spearman's ρ correlation did not indicate any relationship between the two variables (Figure 1d; P=0.788, left; P=0.199, right). Taken together, these findings do not support an essential role of FKBP51 as a factor that controls the phosphorylation status of Akt inside the cell, thereby weakening the hypothesis that the protein may work out as tumor suppressor.
In conclusion, although FKBP51 function is not yet fully elucidated, however, there are clear data suggesting that this immunophilin is often hyperexpressed in tumors and has an active role in pre-neoplastic disorders5 and cancer.6, 7, 8
Acknowledgments
We thank AIRC for supporting our work.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Supplementary Material
References
- Kang CB, et al. Neurosignals. 2008. pp. 318–325. [DOI] [PubMed]
- Silverstein AM, et al. J Biol Chem. 1997. pp. 16224–16230. [DOI] [PubMed]
- Bouwmeester T, et al. Nat Cell Biol. 2004. pp. 97–105. [DOI] [PubMed]
- Baughman G, et al. Biochem Biophys Res Commun. 1997. pp. 437–443. [DOI] [PubMed]
- Giraudier S, et al. Blood. 2002. pp. 2932–2940. [DOI] [PubMed]
- Jiang W, et al. Neoplasia. 2008. pp. 235–243. [DOI] [PMC free article] [PubMed]
- Periyasamy S, et al. Oncogene. 2010. pp. 1691–1701. [DOI] [PMC free article] [PubMed]
- Romano S, et al. Cell Death Differ. 2010. pp. 145–157. [DOI] [PubMed]
- Huang W-C, et al. Molecular Cell. 2007. pp. 75–87. [DOI] [PMC free article] [PubMed]
- Meylan E, et al. Nature. 2009. pp. 104–107. [DOI] [PMC free article] [PubMed]
- Pei H, et al. Cancer Cell. 2009. pp. 259–266. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.