Abstract
Studying the organization and conservation of the TonB systems across the genus Vibrio, we can tease out trends in gene arrangement and function that lead to clues about the evolution and necessity of the proteins in multiple TonB systems. The TonB2 systems, with additional TtpC proteins, are in general more promiscuous regarding their interactions with many different TonB-dependent transporters in the outer membrane. Studies show that the TtpC protein spans the periplasmic space, suggesting that it can be the connection between the energy from the proton motive force and the outer membrane protein receptors, which the shorter TonB2 cannot provide. As an earlier system, the combination of the TtpC protein and a TonB2 system must have been necessary for the function of the smaller TonB2 protein and to transduce energy in a medium that can have osmotic challenges.
Keywords: iron transport, outer membrane protein receptor, pathogenic Vibrio, proton motive force, siderophore, TonB protein, virulence
Iron is a precious resource for most bacterial species. Iron ions are essential for cellular processes, acting as electron donors and acceptors in redox reactions and are also used as co-factors for a number of enzymes [1–3]. The availability of free, unbound iron is low in most environments, especially in the mammalian host where it is chelated by heme groups, transferrin and other iron-binding proteins. In order to survive in low-iron environments, the expression of siderophores and iron-uptake machinery in microorganisms is increased through derepression by the master regulator, Fur [4].
Small-molecular-weight compounds called siderophores have a high affinity for iron, more so than many host iron-binding proteins and are secreted by the bacteria to scavenge iron from the surrounding environment. Iron bound by siderophore complexes is internalized via an energy-dependent process. In Gram-negative species, the iron–siderophore complex is bound on the surface of the outer membrane by receptor proteins, internalized into the periplasmic space and then transported through the inner membrane to the cytosol. The active transport of the iron–siderophore compounds across the outer membrane requires energy that is transduced to the outer membrane via a complex of proteins called the TonB energy-transduction system [5–7]. The process of iron transport in Escherichia coli has been studied and is quite well understood. However, these processes in organisms belonging to the family Vibrionaceae, as well as other aquatic bacteria, still pose unanswered questions.
In Gram-negative organisms, the cytoplasm of the cell is separated from the environment by two lipid bilayers – the inner and outer membranes. The inner membrane is the site of cellular energy production, where the cell actively pumps protons generated during respiration into the periplasmic space between the inner and outer membranes. This proton pumping results in the generation of a proton gradient across the inner membrane with the majority of the protons in the periplasm. The potential energy created by the proton gradient across the inner membrane is known as the proton motive force. This potential energy is converted to the energy currency of the cell, ATP, by inner-membrane proteins termed ATP synthases. The outer membrane contains many membrane-bound proteins, some of which can sense changes in the surrounding environment or transport substrates into and out of the cell. However, there is no energy production in the outer membrane. If proteins in the outer membrane require energy, it must be transferred from the inner membrane proton motive force by a system of proteins that, in E. coli, consist of a TonB and its accessory proteins [5–7].
In this bacterium, in addition to the energy-transducing protein TonB, the TonB system includes two accessory proteins, ExbB and ExbD. This system has been well studied in the contexts of iron and vitamin B12 transport [8,9]. ExbB is necessary to stabilize the TonB protein in the inner membrane as TonB interacts with a ‘TonB box’ region on the periplasmic domain of the TonB-dependent transporter in the outer membrane [10]. TonB and ExbD pass through the inner membrane once with their carboxy-terminal domains (CTDs) in the periplasmic space, while ExbB has three transmembrane (TM) domains with its carboxy terminus in the cytoplasm [11–15]. Interaction between TM domains has been proven for TonB and ExbD in E. coli via formaldehyde crosslinking and western blot analysis [16].
Two models for the mechanism of TonB-mediated energy transduction in E. coli have been proposed. In the ‘shuttle’ model, the energized form of the TonB protein leaves the cytoplasmic membrane and traverses the periplasmic space to interact with the TonB box region of the outer membrane transporter [17,18]. In the ‘pulling’ model, the TonB protein remains imbedded in the inner membrane through interaction with ExbB and ExbD, but spans the periplasm to interact with the outer membrane transporter, pulling the plug domain of the TonB-dependent transporter resulting in a conformational change of the plug domain or displacement of the plug from the barrel of the transporter [19–24]. How these proteins convert the potential energy of the proton motive force into conformational changes is still unknown.
TonB proteins in Vibrio species
Vibrio spp. are Gram-negative, oxidase-positive organisms characterized by a requirement for 1–3% salt in defined growth media. Morphologically, they are short, curved rods with polar and, in some species, lateral flagella.
Vibrio species are abundant in the marine environment and are common pathogens for several marine organisms including oysters, eels and fish. Vibrio species can also cause significant morbidity and mortality when they become opportunistic pathogens of humans by ingestion of contaminated seafood or drinking water, or through direct contact with open wounds. A collection of virulence factors aid in colonization of the host including toxin production, biofilm formation and the ability to bind and actively transport iron across the cell membranes [25–30].
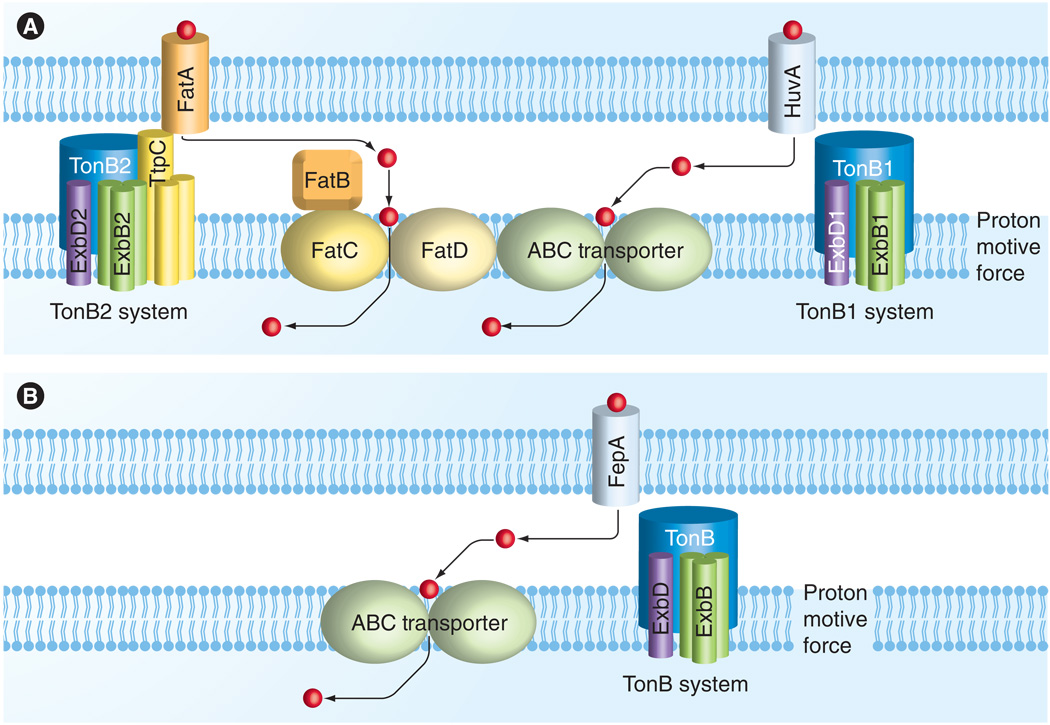
Vibrio species usually possess two chromosomes and unlike E. coli with its single TonB-ExbB–ExbD complex, they have multiple TonB systems in their genomes. These TonB systems and iron uptake proteins are depicted in Figure 1. Occhino et al. first identified the existence of multiple TonB systems in a single organism, Vibrio cholerae, in 1998 [31]. In subsequent work, Seliger et al. reported that the two TonB systems of V. cholerae are not completely redundant in regard to facilitating uptake of several different iron sources including ferrichrome, hemin, vibriobacin, enterobactin and schizokinen [32]. Multiple TonB systems have been characterized in other Vibrio species, including Vibrio anguillarum, Vibrio vulnificus, Vibrio alginolyticus [27,33,34] and Vibrio parahaemolyticus [Kuehl C, Crosa J, Unpublished Data].
Figure 1. Vibrio anguillarum and Escherichia coli TonB energy transduction systems.
Heme or ferric iron–siderophore complexes are shown as red circles with arrows depicting their transport through the outer membrane TonB-dependent transporters into the periplasm and eventually into the cytoplasm where the iron can be used in cell processes. (A) The V. anguillarum TonB systems 1 and 2 are depicted as a model for Vibrio TonB systems and are compared with (B) the Escherichia coli TonB system.
It was observed that, with some exceptions, the genes of the TonB1 systems in Vibrio spp. are present in the smaller chromosome and consist of the proteins ExbB1, ExbD1 and TonB1, which are associated with heme transport genes and intervene in heme and ferrichrome transport [27,29,34–36], while the genes of the TonB2 systems, arranged as ttpC, exbB2, exbD2 and tonB2, found in the larger chromosome of V. cholerae, V. vulnificus and V. anguillarum, are more promiscuous, supplying energy for transport of all siderophore-bound iron sources such as endogenous and exogenous siderophores. In V. anguillarum, TonB2 is essential for the transport of the endogenous siderophores anguibactin and vanchrobactin, as well as of the exogenously produced sources such as enterobactin and ferrichrome. This is also the case for V. cholerae [32,37] in which the TonB2 system can also energize heme transport through the HasR outer membrane receptor [31].
In V. cholerae, strains lacking TonB1 are unable to compete with the wild-type classical strain CA401 or a mutant in tonB2 using an in vitro competition assay with heme as the sole iron source [32,38]. Conversely, the tonB2 mutant, as well as the TonB1-deficient strain, complemented with a clone expressing TonB1, did not show a growth disadvantage compared with the wild-type strain. These results suggest a preferential role for TonB1 in V. cholerae hemin uptake.
Payne’s laboratory proposed that the role of TonB1 in heme–hemoglobin uptake in medium mimicking sea water provides a physiological explanation for the presence of a second TonB. Furthermore, TonB2 could not use hemin at increased NaCl concentrations [29,32]. Seliger et al. demonstrated that the Vibrio TonB2 proteins lack the extended proline-rich sequence in the periplasmic spanning region found in Vibrio TonB1 proteins and E. coli TonB [32,39]. Considering that the periplasmic space expands when cells are grown in media with increased osmolarity [40], the authors postulated that the TonB2 protein could not interact with the heme receptor in the outer membrane under high salt conditions because of the expanded periplasmic space. This hypothesis was supported by the evidence that a strain expressing the TonB1 protein lacking 35 amino acids of the nonessential proline-rich region, as characterized by Larsen et al. in E. coli [39], was also unable to energize hemin transport at NaCl concentrations above 250 mM. The ability of V. cholerae TonB1, but not TonB2 or this TonB1 deletion to interact with the hemin receptor at high salt concentrations may be a function of the extended periplasmic domain of TonB1. However, this hypothesis needs further testing because the strains expressing TonB2 or the shortened TonB1 mutant were still able to grow in the presence of ferrichrome at high salt concentrations [32].
Receptor specificities of the TonB1 & TonB2 systems
While part of chimeric TonB proteins constructed using portions of E.coli and V. cholerae TonB proteins, the carboxy terminal one-third of either the V. cholerae TonB1 or E. coli TonB confers the receptor specificities associated with the full-length protein. Single-amino-acid substitutions near the carboxy terminus of V. cholerae TonB1 were used to determine this specificity [41]. The TonB membrane topology is conserved in TonB2s of both V. cholerae and V. anguillarum, with the CTD in the periplasm where it could theoretically interact with the periplasmic loops of TonB2-dependent transporters in the outer membrane [42]. In silico observations indicate that this is also true in the other pathogenic Vibrio spp. [Kuehl C, Crosa J, Unpublished Data] [34].
TonB-dependent receptors have conserved TonB boxes, short peptide sequences in the amino terminal and periplasmically located loops, that interact with specific TonB proteins. Mey et al. investigated this TonB specificity by switching conserved domains of TonB box peptides between E. coli and V. cholerae [41]. The TonB specificities of the receptors were not changed.
In V. cholerae, both the TonB1 and the TonB2 systems can energize the heme receptors HutA and HutR, although maximum efficiency of heme uptake through these receptors is only observed when the TonB1 system is present [43]. Mey and Payne reported that the amino acid sequences of HutA and HutR are homologous to each other and to other heme receptors [31]. They found that a double hutA–hutR mutant had a significantly decreased ability to utilize hemin when it was present as the sole iron source. A third heme receptor, HasR, was most similar to non-Vibrio heme receptors and can only be energized by the TonB2 system. HasR possesses a ‘TonB box’ unlike the TonB-binding regions found in other Vibrio heme receptors [31]. This result may be due to the role played by the TtpC proteins in all of the observed TonB2 systems [44], which is discussed in the next section.
TonB1-mediated transport of heme is a trend that continues in V. alginolyticus (Table 1). Wang et al. demonstrated that heme and hemoglobin support V. alginolyticus growth only for strains that express TonB1, in contrast to their evidence using liquid growth assays that showed the endogenous siderophore vibrioferrin uses both the TonB1 and TonB2 systems [34].
Table 1.
TonB system specificities for siderophore transport.
| Species | Siderophores produced |
Siderophores used |
TonB system used |
Ref. |
|---|---|---|---|---|
| Vibrio anguillarum | Anguibactin | Anguibactin | TonB2 | [27] |
| Vanchrobactin | Vanchrobactin | TonB2 | [58] | |
| Enterobactin | TonB2 | [27] | ||
| Ferrichrome | TonB1 and 2 | [27] | ||
| Hemin | TonB1 | [27] | ||
| Vibrio cholerae | Vibriobactin | Vibriobactin | TonB1 and 2 | [29] |
| Enterobactin | TonB2 | [29] | ||
| Agrobactin | Unknown | [59] | ||
| Fluvibactin | Unknown | [60] | ||
| Ferrichrome | TonB1 and 2 | [29] | ||
| Hemin | TonB1 and 2 | [29] | ||
| Vibrio vulnificus | Vulnibactin | Vulnibactin | TonB1 and 2 | [Alice, Unpublished Data] [61] |
| Hydroxamate type | Hydroxamate-type | TonB1 and 2 | [Alice, Unpublished Data] [62] | |
| Aerobactin | Unknown | [63] | ||
| Hemin | TonB1 and 2 | [35] | ||
| Vibrio parahaemolyticus | Vibrioferrin | Vibrioferrin | Unknown | [64] |
| Ferrichrome | Unknown | [64] | ||
| Aerobactin | Unknown | [64] | ||
| Hemin | Unknown | [36] | ||
| Vibrio alginolyticus | Vibrioferrin | Vibrioferrin | TonB1 and 2 | [34] |
| Ferrichrome | TonB1 and 2 | [34] | ||
| Hemin | TonB1 | [34] |
In the case of V. anguillarum, functional analysis of the CTD of the TonB2 protein revealed that a deletion of the final two amino acids did not change 55Fe–anguibactin transport efficiency, but that larger deletions resulted in V. anguillarum strains that were no longer capable of Fe–anguibactin transport [42]. In the same study, alanine substitution mutations at the far carboxy terminus showed that the length of the protein, as opposed to the specific amino acids involved at positions 204–206, was important for function in 55Fe–anguibactin transport. Similar mutations were made at positions 201–203, which demonstrated the essentiality of those residues for transport. These observations verified that TonB2 was essential for iron transport of some iron–siderophore complexes and that the organization of the carboxy terminus of the V. anguillarum TonB2 was significantly different from that of the E. coli TonB.
Another player in the game
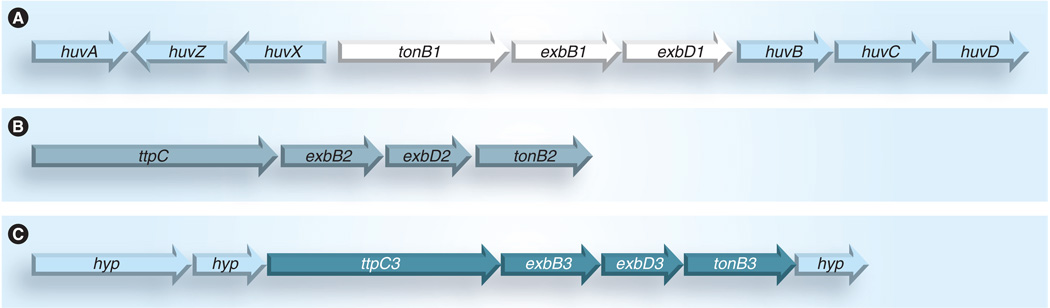
In Vibrio species and Vibrionaceae family members, a second and occasionally a third TonB system are observed. These TonB2 (and TonB3) systems (Figure 2) consist of the classic ExbB2, ExbD2 and TonB2 (or ExbB3, ExbD3 and TonB3) proteins as well as a fourth protein TtpC [37].
Figure 2. Genetic arrangement of the tonB gene clusters in Vibrio spp.
(A) The tonB1 operon and surrounding heme uptake genes of Vibrio anguillarum are shown as a representation of Vibrio tonB1 gene clusters. (B) Conserved arrangement of the Vibrio tonB2 gene cluster. (C) The tonB3 gene cluster surrounded by conserved hypothetical proteins is found in several pathogenic Vibrio spp. and other marine organisms, as noted in Table 2.
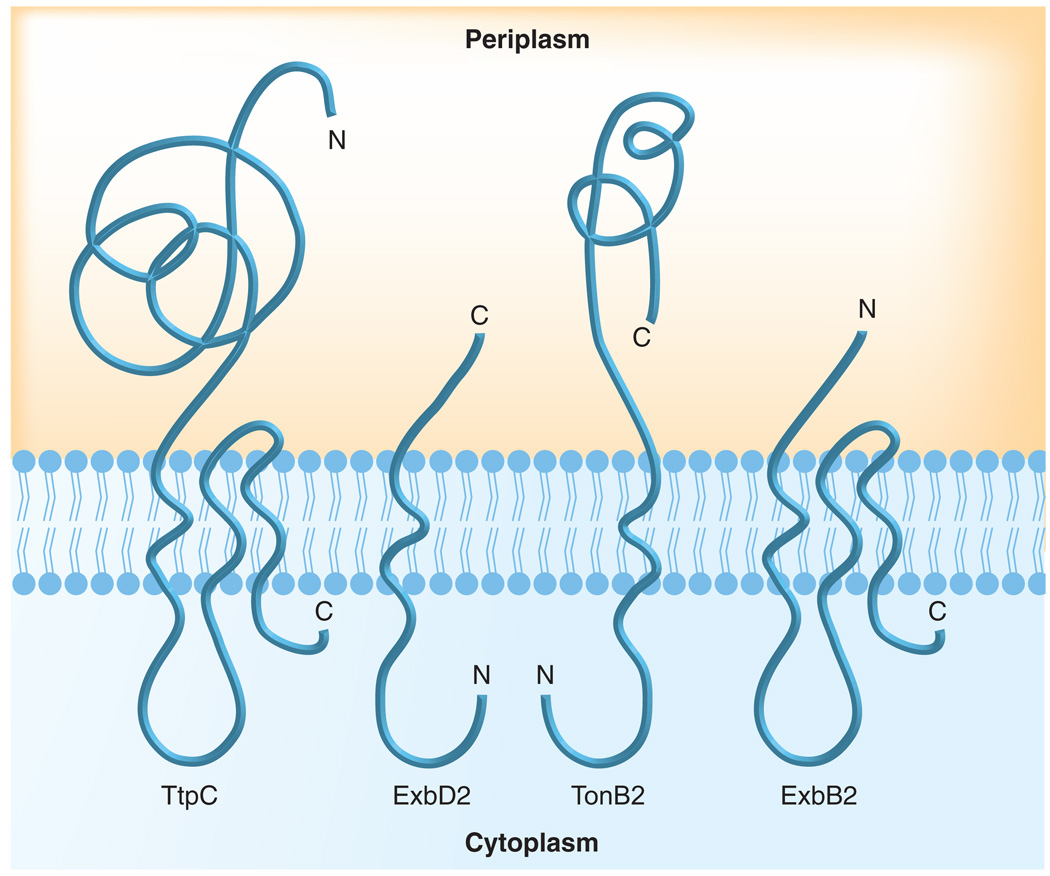
It is remarkable that the 49 kDa TtpC protein is essential for TonB2-mediated iron transport in V. anguillarum and V. cholerae and has since been identified upstream of the TonB2 and TonB3 gene clusters in all Vibrio and other aquatic species examined (Table 2). TtpC is predicted to span the membrane three times with a carboxy-terminal distribution of TM domains highly similar to the ExbB proteins, leaving the majority of the protein, including the amino terminal signal sequence, predicted to be in the periplasm (Figure 3). Work is currently underway to determine the actual membrane topology of the TtpC protein.
Table 2.
Marine species with TtpC-TonB2 system homologs.
| TtpC2–TonB2 | TtpC3–TonB3 |
|---|---|
| Vibrio anguillaram | |
| Vibrio cholerae | |
| Vibrio coralliiltticus | |
| Vibrio furnissii | |
| Vibrio metschniovi | |
| Vibrio mimicus | |
| Vibrio orientalis | |
| Vibrio shilonii | |
| Vibrio splendidus | |
| Vibrio alginolyticus | V. alginolyticus |
| Vibrio angustum | V. angustum |
| Vibrio fischeri | V. fischeri |
| Vibrio harveyi | V. harveyi |
| Vibrio parahaemolyticus | V. parahaemolyticus |
| Vibrio vulnificus | V. vulnificus |
| Aliivibrio salmonicida | A. salmonicida |
| Photobacterium profundum | P. profundum |
| Teredinibacter turnerae | T. turnerae |
| Aeromonas hydrophilia | |
| Aeromonas salmonicida | |
| Photobacterium damselae | |
| Pseudomonas mendocina | |
| Pseudomonas stutzeri | |
| Shewanella halifaxenis | |
| Shewanella putrefaciens |
Figure 3. Predicted membrane topologies of the proteins in the Vibrio TonB2 energy transduction systems.
The membrane topologies of Escherichia coli ExbB, ExbD and TonB are known, as is the membrane topology of Vibrio anguillarum TonB2, which has one transmembrane domain, with its amino terminus in the cytoplasm. Based on amino acid sequence homologies and in silico transmembrane domain predictions, the predicted membrane topologies of the Vibrio TonB2 system proteins in the inner membrane are shown.
It is possible that TtpC could aid the shorter TonB2 to span the periplasmic space; however, there is another potential reason that could have motivated the evolutionary appearance of TtpC. The V. anguillarum TonB2 CTD has two significant differences in tertiary structure as compared with the solution structure of the E. coli TonB CTD [42]. General observations show that the V. anguillarum TonB2-CTD is less basic overall than the E. coli TonB-CTD. The TonB boxes of many TonB-dependent receptors contain primarily hydrophobic and acidic residues and this difference in the composition of the electrostatic surface between TonB2 and TonB may affect recruitment of TonB box regions to the TonB2 CTD. In fact, this appears to be the case since no in vitro binding was observed between the ten amino acid TonB box peptide of the E. coli enterobactin receptor FepA (EDTITVTAAP) or V. anguillarum anguibactin receptor FatA (ESITVYGEA) and the CTD of TonB2 [42]. However, binding was observed between the TonB box peptides and the E.coli TonB CTD [42].
In the V. anguillarum TonB2 protein, the loop extending from the α2 helix to the β3 strand is significantly longer than the corresponding region in E. coli TonB. The β4 strand present in the E. coli TonB CTD is absent from the V. anguillarum protein. Functional complementation between E. coli TonB and V. anguillarum TonB2 in a V. anguillarum tonB2-deletion background demonstrated that E. coli TonB is capable of substituting for V. anguillarum TonB2 in ferric-anguibactin transport through FatA; however, neither enterobactin nor vanchrobactin supported growth in bioassays with this strain. Conversely, TonB2, as well as chimeric V. anguillarum TonB2 proteins that possessed the missing β4 strand present in E. coli TonB, failed to complement enterobactin uptake in a TonB deletion mutation in E. coli, although this same chimeric TonB2-β4 was able to support Fe–anguibactin transport in V. anguillarum at wild-type levels [42]. This evidence underscores the dependence of V. anguillarum TonB2 on its accessory protein, TtpC.
The necessity of the novel TtpC protein for iron transport mediated by the TonB2 system was first identified by a Tn10 transposon mutagenesis screen in V. anguillarum [37]. The TtpC protein of V. anguillarum was originally annotated as TolR owing to its sequence homology to the 457 amino acid V. cholerae protein (also TolR) encoded upstream of V. cholerae exbB2. This V. cholerae protein was annotated as TolR because the carboxy terminal half of the protein had highest sequence homology to the much shorter TolR protein of the TolQRAB system [37]. The annotation as TolR was discovered to be misleading upon further molecular characterization of the protein. Although the carboxy terminal TM domain region, specifically the region from predicted TM domain 2 to TM domain 3, is highly similar to the MotA/TolQ/ExbB family; the amino-terminal portion of the protein has no significant homology to any characterized or predicted protein except for other TtpC proteins. Thus, it is in a class by itself.
Stork et al. identified that the TtpC protein was present in four multiprotein complexes formed when proteins in V. anguillarum cells were crosslinked with 1% formaldehyde [37]. The multiprotein complexes were absent and TtpC became unstably expressed in the membrane fraction of cells deleted for the TonB2 protein. These data suggest that TonB2 and TtpC interact with each other and that TonB2 expression stabilizes TtpC in the membrane [37]. Very-high-molecular-weight complexes of more than 150kDa were also identified to contain TtpC. The authors proposed that these complexes might contain outer membrane receptor proteins specific for ferric siderophores or other iron sources.
The TtpC proteins upstream of the tonB2 gene clusters in several pathogenic Vibrio species are highly similar with amino acid sequence similarities between 73 and 80%. The V. cholerae TtpC amino acid sequence has 66% identity to that of V. anguillarum using the align function on the National Center for Biotechnology Information’s Basic Local Alignment Search Tool (BLAST)p interface [45]. The TtpC proteins are also essential for the TonB2-mediated transport of enterobactin, vibriobactin and hemin in V. cholerae, vulnibactin in V. vulnificus and likely, vibrioferrin in V. parahaemolyticus and V. alginolyticus [Kuehl C, Crosa J, Unpublished Data] [46].
The enterobactin receptors in both V. anguillarum and V. cholerae are energized by their respective TonB2 energy transduction systems [27,32]. Stork et al. used ferric-enterobactin utilization bioassays to show that the TtpC protein from V. anguillarum was unable to complement a mutation in the TtpC protein of the V. cholerae TonB2 system and that growth around ferric-enterobactin as an iron source was only restored when the entire V. anguillarum TonB2 system was present in the complementing plasmid, indicating that slight differences at the level of amino acid sequence may abrogate physical interactions or other involvement between the TtpC–TonB2 protein complex [37].
Interspecies complementation of ΔttpC deletion mutations in V. anguillarum and V. cholerae TonB2-dependent iron uptake with the TtpC proteins of other Vibrio spp. is currently being explored.
Virulence attributes of the multiple TonB systems
The role of iron transport in virulence has been studied in several Vibrio species using a variety of infection models, while in some cases the natural host–pathogen interaction can be examined. The marine fish pathogen V. anguillarum requires an active iron uptake mechanism mediated by the siderophore anguibactin to be able to cause an infection in a vertebrate fish host [27,47,48]. However, it can also acquire iron via transport of heme and siderophores secreted by other microorganisms, such as ferrichrome and enterobactin. Once bound to iron, ferric anguibactin is transported back into the cell cytosol through the specific outer membrane receptor FatA [49–51]. TonB2, but not TonB1, functions in the transport of anguibactin and enterobactin, while both TonB proteins can operate in the transport of ferrichrome and heme. tonB2 mutants are severely attenuated in virulence by more than 100-fold, while the tonB1 mutants show only a tenfold decrease in virulence [27]. Complementation of the tonB2 and tonB1–tonB2 mutants with the wild-type tonB2 gene results in restoration of virulence to a level close to that of the wild-type. These results demonstrate that a functional tonB2 system rather than tonB1 is essential for ferric-anguibactin transport and virulence of V. anguillarum in the natural vertebrate host.
In a similar vein, another fish pathogen that possesses two sets of TonB systems, Vibrio alginolyticus, can infect zebrafish [34]. However, in this case, when inoculated intraperitoneally, mutants of either of the two TonB systems demonstrate a marked attenuation in virulence, indicating that both systems are essential for the virulence of this bacterium [34]. As in V. anguillarum, the two systems were arranged as TonB1–ExbB1–ExbD1 and TtpC–ExbB2–ExbD2–TonB2, respectively. The TonB1 system specifically contributed to hemin and hemoglobin uptake and both of the TonB systems support iron uptake mediated by ferrichrome and vibrioferrin, the endogenous siderophore of V. alginolyticus [34].
Virulence of the human pathogens V. cholerae and V. vulnificus has also been studied with respect to TonB1 or TonB2 requirement, using the suckling mouse model for V. cholerae and the iron-overloaded subcutaneous infection mouse model for V. vulnificus [46]. Mouse colonization assays carried out in Payne’s laboratory using V. cholerae mutants in the TonB1 or TonB2 systems indicate a role for both TonB systems and mutations in either system resulted in reduced ability to compete with the wild-type in vivo [32]. V. vulnificus possesses three tonB systems and multiplies rapidly in host tissues under iron-overloaded conditions. The TonB1 and TonB2 systems involved in vulnibactin transport are essential for virulence in the iron-overloaded mice inoculated subcutaneously. However, these genes were induced under iron-limiting conditions, indicating that active iron transport is important in infection by this bacterium, even under high-iron conditions. Expression of the TonB3 cluster occurs only when the bacterium grows in human serum and does not show any relevance to pathogenesis [46]. TonB3 could play a role in transport processes associated with metabolic or energetic steps still unidentified.
In summary, except for the absolute necessity for the TonB2 system in V. anguillarum virulence, the TonB1 and TonB2 systems are equally responsible for virulence in all the other members of the Vibrionaceae that were examined.
Molecular machinery: comparing TonB2 cluster proteins with torque-generating MotA/B subunits of the flagellar motor
The carboxy terminal TM domain region in the TtpC proteins is most similar to members of the MotA/TolQ/ExbB family of proteins. In E. coli, turning the flagellar motor requires the conversion of H+ ion flux through a proton channel in the inner membrane into a mechanical force applied to the flagellar rotor through the cytoplasmic region of the MotA protein. Ions pass through the inner membrane via a channel composed of four MotB proteins and the TM domain of two MotA proteins [52]. This ion flux is thought to cause a conformational change in MotA, allowing it to move from one FliG subunit of the rotor to the next FliG subunit, thereby turning the rotor [53].
In V. parahaemolyticus, the flagellar motor is powered by Na+ ion flux across the inner membrane. Jaques et al. identified MotA and MotB homologs in screens for mutants that could swim in the presence of phenamil, a Na+-channel inhibitor [54]. The flagellar motor of V. alginolyticus is also powered by Na+ ion flux. Sato and Homma showed that purified V. alginolyticus PomAB complexes (MotAB homologs) reconstituted in liposomes allowed Na+ flux, demonstrating that PomA and PomB formed the Na+ channel [55]. They later demonstrated that a fused dimer of PomA along with PomB allowed motility and that a single functional PomA unit in the complex was not sufficient, as two functional PomA subunits are required to interact with PomB [56]. The functional characteristics of the closest homologs to the CTD of Vibrio TtpC proteins is intriguing and may offer some insight into the molecular mechanisms involved in TonB2-mediated energy transduction.
The high peptide sequence similarity between the TM domains of the MotA, ExbB2 and TtpC proteins point toward similar structure and function of the proteins [57]. This leads us to hypothesize that ExbB2 proteins and the TM domains of TtpC proteins in the TonB2 complex could be arranged as part of a proton channel, making them key players in the transduction of energy from the inner membrane proton motive force to the outer membrane receptors via interaction with TonB2 and, possibly, the amino-terminal region of TtpC. A similar mechanism of TM domain interaction was described using the E.coli TonB system [57] and may be similar to the arrangement of the TonB system proteins present in the Vibrio spp.
Conclusion
Studying the organization and conservation of the TonB systems across the genus Vibrio, we can tease out trends in gene arrangement and function that lead to clues about the evolution and necessity of the proteins in multiple TonB systems. The TonB2 systems with the additional TtpC proteins are, in general, more promiscuous regarding their interactions with many different TonB-dependent transporters in the outer membrane (Table 1). Often, the TonB2 system is associated with transducing energy for uptake of endogenously produced siderophores that are essential for virulence. In some species, such as V. alginolyticus, these virulence-determining siderophores are also transported with energy supplied by the TonB1 system. Studies by Crosa et al. show that the TtpC protein spans the periplasmic space, suggesting that it can be the connection between the energy from the proton motive force and the outer membrane protein receptors, which the shorter TonB2 cannot provide. However, this process requires TonB2 because in its absence the TtpC protein is unstable and degrades rapidly. This is where TtpC must play a role in bridging the problem of a short TonB2. As an earlier system, the combination of the TtpC protein and a TonB2 system must have been necessary for the function of the smaller TonB2 protein and to transduce energy in a medium that can have osmotic challenges [32]. It is an evolutionary mystery as to why the TtpC–TonB2 systems have evolved and remained as an important energy transduction system in Vibrio spp. and other aquatic bacteria, while the acquisition of the TonB1 systems appears to have occurred more recently in the evolution of these bacteria, possibly motivated by the concomitant carriage of the heme uptake genes.
Future perspective
It appears that the challenge for the next few years will be to understand the exact mechanism by means of which the TtpC and TonB2 proteins join forces to transduce the energy of the proton motive force to the outer membrane receptor proteins. The form of energy being transfered, be it ATP or potential energy released in a conformational change of TtpC or TonB2, is yet unknown and is crucial to defining the mechanism of energy transfer.
Executive summary
Iron transport
-
▪
The process of iron transport in Escherichia coli has been studied and is quite well understood. However, these processes in organisms belonging to the Vibrionaceae family, as well as other aquatic bacteria, still pose unanswered questions.
-
▪
The inner membrane is the location of cellular energy production known as the proton motive force.
-
▪
There is no energy production in the outer membrane. If proteins in the outer membrane require energy, it must be transferred from the inner membrane proton motive force by a system of proteins that consist of TonB and its accessory proteins.
TonB proteins in Vibrio species
-
▪
The Vibrio species are abundant in the marine environment and are common pathogens for humans and several marine organisms including oysters, eels and fish.
-
▪
Vibrio species usually possess two chromosomes and, unlike E. coli with its single TonB–ExbB–ExbD complex, they have multiple TonB systems in their genomes.
-
▪
The two TonB systems of Vibrio cholerae and Vibrio anguillarum are not completely redundant in regard to facilitating uptake of several different iron sources including ferrichrome, hemin, vibriobacin, enterobactin and schizokinen.
-
▪
The TonB2 systems are more promiscuous than the TonB1 systems, supplying energy for transport of all siderophore-bound iron sources such as endogenous and exogenous siderophores.
-
▪
TonB2 shows significant differences at the carboxy-terminal end from the TonB1 proteins and TonB from E. coli. These differences might have led to the evolutionary appearance of the 49 kDa TtpC protein.
Requirement of TtpC in the TonB2 system
-
▪
The necessity of the novel TtpC protein for iron transport mediated by the TonB2 system was first identified by a Tn10 transposon mutagenesis screen in V. anguillarum.
-
▪
The TtpC protein is essential for TonB2-mediated iron transport in V. anguillarum and V. cholerae and has since been identified upstream of the TonB2 and TonB3 gene clusters in all Vibrio and other aquatic species examined.
-
▪
TonB1 is longer than TonB2. This could be the reason for the discrepancy in heme uptake between the two TonB systems in V. cholerae under high salt concentrations, similar to the marine environment. It is possible that TtpC could aid the shorter TonB2 to span the periplasmic space.
-
▪
Except for the absolute necessity for the TonB2 system in V. anguillarum virulence, the TonB1 and TonB2 systems are equally responsible for virulence in all the other members of the Vibrionaceae examined.
-
▪
The carboxy-terminal transmembrane domain region in the TtpC proteins is most similar to members of the MotA/TolQ/ExbB family of proteins, which provide the mechanical force to turn the flagellar rotor.
-
▪
ExbB2 proteins and the transmembrane domains of TtpC proteins in the TonB2 complex could be arranged as part of a proton channel, making them key players in the transduction of energy from the inner membrane proton motive force to the outer membrane receptors via interaction with TonB2 and, possibly, the amino-terminal region of TtpC.
Acknowledgments
The research from our laboratory presented in this review was funded by NIH grants 5RO1-AI 019018 and 5RO1-AI 065981. Carole J Kuehl was supported by a graduate student training grant fellowship from the NIH 5T32-AI 007472-13.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Ann. Rev. Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan A, Reichard P. Ribonucleotide reductases. Ann. Rev. Biochem. 1998;67:71–98. doi: 10.1146/annurev.biochem.67.1.71. [DOI] [PubMed] [Google Scholar]
- 3.Roux A, Payne SM, Gilmore MS. Microbial telesensing: probing the environment for friends, foes, and food. Cell Host Microbe. 2009;6(2):115–124. doi: 10.1016/j.chom.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol. Gen. Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- 5.Braun V. Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB–ExbB–ExbD-dependent receptor. Proteins FEMS Microbiol. Rev. 1995;16(4):295–307. doi: 10.1111/j.1574-6976.1995.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 6. Crosa JH, Mey AR, Payne SM. Iron Transport in Bacteria. DC, USA: ASM Press; 2004. ▪ In-depth analysis of many bacterial iron transport systems.
- 7.Postle K, Larsen RA. TonB-dependent energy transduction between outer and cytoplasmic membranes. Biometals. 2007;20(3–4):453–465. doi: 10.1007/s10534-006-9071-6. [DOI] [PubMed] [Google Scholar]
- 8.Hantke K, Braun V. Membrane receptor dependent iron transport in Escherichia coli. FEBS Lett. 1975;49(3):301–305. doi: 10.1016/0014-5793(75)80771-x. [DOI] [PubMed] [Google Scholar]
- 9.Fischer E, Gunter K, Braun V. Involvement of ExbB and TonB in transport across the outer membrane of Escherichia coli: phenotypic complementation of exb mutants by overexpressed tonB and physical stabilization of TonB by ExbB. J. Bacteriol. 1989;171(9):5127–5134. doi: 10.1128/jb.171.9.5127-5134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmer BM, Thomas MG, Larsen RA, Postle K. Characterization of the exbBD operon of Escherichia coli and the role of ExbB and ExbD in TonB function and stability. J. Bacteriol. 1995;177(16):4742–4747. doi: 10.1128/jb.177.16.4742-4747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannavy K, Barr GC, Dorman CJ, et al. TonB protein of Salmonella typhimurium. A model for signal transduction between membranes. J. Mol. Biol. 1990;216(4):897–910. doi: 10.1016/S0022-2836(99)80009-6. [DOI] [PubMed] [Google Scholar]
- 12.Jaskula JC, Letain TE, Roof SK, Skare JT, Postle K. Role of the TonB amino terminus in energy transduction between membranes. J. Bacteriol. 1994;176(8):2326–2338. doi: 10.1128/jb.176.8.2326-2338.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kampfenkel K, Braun V. Membrane topology of the Escherichia coli ExbD protein. J. Bacteriol. 1992;174(16):5485–5487. doi: 10.1128/jb.174.16.5485-5487.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kampfenkel K, Braun V. Topology of the ExbB protein in the cytoplasmic membrane of Escherichia coli. J. Biol. Chem. 1993;268(8):6050–6057. [PubMed] [Google Scholar]
- 15.Karlsson M, Hannavy K, Higgins CF. A sequence-specific function for the N-terminal signal-like sequence of the TonB protein. Mol. Microbiol. 1993;8(2):379–388. doi: 10.1111/j.1365-2958.1993.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 16. Ollis AA, Manning M, Held KG, Postle K. Cytoplasmic membrane protonmotive force energizes periplasmic interactions between ExbD and TonB. Mol. Microbiol. 2009;73(3):466–481. doi: 10.1111/j.1365-2958.2009.06785.x. ▪ Underscores the possible mechanism proton motive force energy transduction to the outer membrane proteins.
- 17.Larsen RA, Letain TE, Postle K. In vivo evidence of TonB shuttling between the cytoplasmic and outer membrane in Escherichia coli. Mol. Microbiol. 2003;49(1):211–218. doi: 10.1046/j.1365-2958.2003.03579.x. [DOI] [PubMed] [Google Scholar]
- 18.Letain TE, Postle K. TonB protein appears to transduce energy by shuttling between the cytoplasmic membrane and the outer membrane in Escherichia coli. Mol. Microbiol. 1997;24(2):271–283. doi: 10.1046/j.1365-2958.1997.3331703.x. [DOI] [PubMed] [Google Scholar]
- 19.Chimento DP, Kadner RJ, Wiener MC. Comparative structural analysis of TonB-dependent outer membrane transporters: implications for the transport cycle. Proteins. 2005;59(2):240–251. doi: 10.1002/prot.20416. [DOI] [PubMed] [Google Scholar]
- 20.Devanathan S, Postle K. Studies on colicin B translocation: FepA is gated by TonB. Mol. Microbiol. 2007;65(2):441–453. doi: 10.1111/j.1365-2958.2007.05808.x. [DOI] [PubMed] [Google Scholar]
- 21.Gumbart J, Wiener MC, Tajkhorshid E. Mechanics of force propagation in TonB-dependent outer membrane transport. Biophys. J. 2007;93(2):496–504. doi: 10.1529/biophysj.107.104158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma L, Kaserer W, Annamalai R, et al. Evidence of ball-and-chain transport of ferric enterobactin through FepA. J. Biol. Chem. 2007;282(1):397–406. doi: 10.1074/jbc.M605333200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawelek PD, Croteau N, Ng-Thow-Hing C, et al. Structure of TonB in complex with FhuA, E. coli outer membrane receptor. Science. 2006;312(5778):1399–1402. doi: 10.1126/science.1128057. [DOI] [PubMed] [Google Scholar]
- 24.Shultis DD, Purdy MD, Banchs CN, Wiener MC. Outer membrane active transport: structure of the BtuB:TonB complex. Science. 2006;312(5778):1396–1399. doi: 10.1126/science.1127694. [DOI] [PubMed] [Google Scholar]
- 25.Kim YR, Lee SE, Kim CM, et al. Characterization and pathogenic significance of Vibrio vulnificus antigens preferentially expressed in septicemic patients. Infect. Immun. 2003;71(10):5461–5471. doi: 10.1128/IAI.71.10.5461-5471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JH, Rho JB, Park KJ, et al. Role of flagellum and motility in pathogenesis of Vibrio vulnificus. Infect. Immun. 2004;72(8):4905–4910. doi: 10.1128/IAI.72.8.4905-4910.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stork M, Di Lorenzo M, Mourino S, Osorio CR, Lemos ML, Crosa JH. Two tonB systems function in iron transport in Vibrio anguillarum, but only one is essential for virulence. Infect. Immun. 2004;72(12):7326–7329. doi: 10.1128/IAI.72.12.7326-7329.2004. ▪ Describes the identification of two TonB systems in the fish pathogen, Vibrio anguillarum, and demonstrates the necessity of the TonB2 cluster for virulence of this bacterium.
- 28.Faruque SM, Biswas K, Udden SM, et al. Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc. Natl Acad. Sci. USA. 2006;103(16):6350–6355. doi: 10.1073/pnas.0601277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wyckoff EE, Mey AR, Payne SM. Iron acquisition in Vibrio cholerae. Biometals. 2007;20(3–4):405–416. doi: 10.1007/s10534-006-9073-4. [DOI] [PubMed] [Google Scholar]
- 30.Childers BM, Klose KE. Regulation of virulence in Vibrio cholerae: the ToxR regulon. Future Microbiol. 2007;2:335–344. doi: 10.2217/17460913.2.3.335. [DOI] [PubMed] [Google Scholar]
- 31. Occhino DA, Wyckoff EE, Henderson DP, Wrona TJ, Payne SM. Vibrio cholerae iron transport: haem transport genes are linked to one of two sets of tonB, exbB, exbD genes. Mol. Microbiol. 1998;29(6):1493–1507. doi: 10.1046/j.1365-2958.1998.01034.x. ▪ First report of two TonB systems in bacteria.
- 32. Seliger SS, Mey AR, Valle AM, Payne SM. The two TonB systems of Vibrio cholerae: redundant and specific functions. Mol. Microbiol. 2001;39(3):801–812. doi: 10.1046/j.1365-2958.2001.02273.x. ▪ Identifies TonB1- and TonB2-dependent receptor specificity in the transport of ferric siderophores and heme.
- 33.O’Malley SM, Mouton SL, Occhino DA, et al. Comparison of the heme iron utilization systems of pathogenic Vibrios. J. Bacteriol. 1999;181(11):3594–3598. doi: 10.1128/jb.181.11.3594-3598.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Q, Liu Q, Cao X, Yang M, Zhang Y. Characterization of two TonB systems in marine fish pathogen Vibrio alginolyticus: their roles in iron utilization and virulence. Arch. Microbiol. 2008;190(5):595–603. doi: 10.1007/s00203-008-0407-1. ▪ Demonstrates the presence of two TonB systems in this bacterium and that both are necessary for virulence and in vitro iron uptake.
- 35.Litwin CM, Byrne BL. Cloning and characterization of an outer membrane protein of Vibrio vulnificus required for heme utilization: regulation of expression and determination of the gene sequence. Infect. Immun. 1998;66(7):3134–3141. doi: 10.1128/iai.66.7.3134-3141.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong HC, Liu CC, Yu CM, Lee YS. Utilization of iron sources and its possible roles in the pathogenesis of Vibrio parahaemolyticus. Microbiol Immunol. 1996;40(11):791–798. doi: 10.1111/j.1348-0421.1996.tb01144.x. [DOI] [PubMed] [Google Scholar]
- 37. Stork M, Otto BR, Crosa JH. A novel protein, TtpC, is a required component of the TonB2 complex for specific iron transport in the pathogens Vibrio anguillarum and Vibrio cholerae. J. Bacteriol. 2007;189(5):1803–1815. doi: 10.1128/JB.00451-06. ▪ Discovery of the TtpC protein, an essential component of TonB2-mediated iron uptake in Vibrio anguillarum and Vibrio cholerae.
- 38.Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl Acad. Sci. USA. 1987;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larsen RA, Wood GE, Postle K. The conserved proline-rich motif is not essential for energy transduction by Escherichia coli TonB protein. Mol. Microbiol. 1993;10(5):943–953. doi: 10.1111/j.1365-2958.1993.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 40.Stock JB, Rauch B, Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J. Biol. Chem. 1977;252(21):7850–7861. [PubMed] [Google Scholar]
- 41.Mey AR, Payne SM. Analysis of residues determining specificity of Vibrio cholerae TonB1 for its receptors. J. Bacteriol. 2003;185(4):1195–1207. doi: 10.1128/JB.185.4.1195-1207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lopez CS, Peacock RS, Crosa JH, Vogel HJ. Molecular characterization of the TonB2 protein from the fish pathogen Vibrio anguillarum. Biochem. J. 2009;418(1):49–59. doi: 10.1042/BJ20081462. ▪ Physical characterization by NMR of the carboxy terminus of the V. anguillarum TonB2 protein.
- 43. Mey AR, Payne SM. Haem utilization in Vibrio cholerae involves multiple TonB-dependent haem receptors. Mol. Microbiol. 2001;42(3):835–849. doi: 10.1046/j.1365-2958.2001.02683.x. ▪ Demonstrates that the heme receptors HutA and HutR are supplied with energy by the TonB1 system, while HasR heme transport is mediated by TonB2.
- 44.Kuehl CJ, Crosa JH. Molecular and genetic characterization of the TonB2-cluster TtpC protein in pathogenic vibrios. Biometals. 2009;22(1):109–115. doi: 10.1007/s10534-008-9194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alice AF, Naka H, Crosa JH. Global gene expression as a function of the iron status of the bacterial cell: influence of differentially expressed genes in the virulence of the human pathogen Vibrio vulnificus. Infect. Immun. 2008;76(9):4019–4037. doi: 10.1128/IAI.00208-08. ▪ First report of the existence of a third TonB cluster in a marine Vibrio and demonstration that TonB1 and TonB2 systems are equally important for virulence.
- 47.Di Lorenzo M, Poppelaars S, Stork M, Nagasawa M, Tolmasky ME, Crosa JH. A nonribosomal peptide synthetase with a novel domain organization is essential for siderophore biosynthesis in Vibrio anguillarum. J. Bacteriol. 2004;186(21):7327–7336. doi: 10.1128/JB.186.21.7327-7336.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Lorenzo M, Stork M, Naka H, Tolmasky ME, Crosa JH. Tandem heterocyclization domains in a nonribosomal peptide synthetase essential for siderophore biosynthesis in Vibrio anguillarum. Biometals. 2008;21(6):635–648. doi: 10.1007/s10534-008-9149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Actis LA, Potter SA, Crosa JH. Iron-regulated outer membrane protein OM2 of Vibrio anguillarum is encoded by virulence plasmid pJM1. J. Bacteriol. 1985;161(2):736–742. doi: 10.1128/jb.161.2.736-742.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Actis LA, Tolmasky ME, Farrell DH, Crosa JH. Genetic and molecular characterization of essential components of the Vibrio anguillarum plasmid-mediated iron-transport system. J. Biol. Chem. 1988;263(6):2853–2860. [PubMed] [Google Scholar]
- 51.Lopez CS, Crosa JH. Characterization of ferric-anguibactin transport in Vibrio anguillarum. Biometals. 2007;20(3–4):393–403. doi: 10.1007/s10534-007-9084-9. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura S, Morimoto YV, Kami-ike N, Minamino T, Namba K. Role of a conserved prolyl residue (Pro173) of MotA in the mechanochemical reaction cycle of the proton-driven flagellar motor of Salmonella. J. Mol. Biol. 2009;393(2):300–307. doi: 10.1016/j.jmb.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 53.Berg HC. Bacterial flagellar motor. Curr. Biol. 2008;18(16):R689–R691. doi: 10.1016/j.cub.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Jaques S, Kim YK, McCarter LL. Mutations conferring resistance to phenamil and amiloride, inhibitors of sodium-driven motility of Vibrio parahaemolyticus. Proc. Natl Acad. Sci. USA. 1999;96(10):5740–5745. doi: 10.1073/pnas.96.10.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato K, Homma M. Functional reconstitution of the Na+-driven polar flagellar motor component of Vibrio alginolyticus. J. Biol. Chem. 2000;275(8):5718–5722. doi: 10.1074/jbc.275.8.5718. [DOI] [PubMed] [Google Scholar]
- 56.Sato K, Homma M. Multimeric structure of PomA, a component of the Na+-driven polar flagellar motor of Vibrio alginolyticus. J. Biol. Chem. 2000;275(26):20223–20228. doi: 10.1074/jbc.M002236200. [DOI] [PubMed] [Google Scholar]
- 57. Zhai YF, Heijne W, Saier MHJ. Molecular modeling of the bacterial outer membrane receptor energizer, ExbBD/TonB, based on homology with the flagellar motor, MotAB. Biochim. Biophys. Acta. 2003;1614(2):201–210. doi: 10.1016/s0005-2736(03)00176-7. ▪ Describes the similarities between the flagellar motor components and the members of the TonB systems.
- 58.Balado M, Osorio CR, Lemos ML. FvtA is the receptor for the siderophore vanchrobactin in Vibrio anguillarum: utility as a route of entry for vanchrobactin analogues. Appl. Environ. Microbiol. 2009;75(9):2775–2783. doi: 10.1128/AEM.02897-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griffiths GL, Sigel SP, Payne SM, Neilands JB. Vibriobactin, a siderophore from Vibrio cholerae. J. Biol. Chem. 1984;259(1):383–385. [PubMed] [Google Scholar]
- 60.Yamamoto S, Okujo N, Fujita Y, Saito M, Yoshida T, Shinoda S. Structures of two polyamine-containing catecholate siderophores from Vibrio fluvialis. J. Biochem. 1993;113(5):538–544. doi: 10.1093/oxfordjournals.jbchem.a124079. [DOI] [PubMed] [Google Scholar]
- 61.Webster AC, Litwin CM. Cloning and characterization of vuuA, a gene encoding the Vibrio vulnificus ferric vulnibactin receptor. Infect. Immun. 2000;68(2):526–534. doi: 10.1128/iai.68.2.526-534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biosca EG, Fouz B, Alcaide E, Amaro C. Siderophore-mediated iron acquisition mechanisms in Vibrio vulnificus biotype 2. Appl. Environ. Microbiol. 1996;62(3):928–935. doi: 10.1128/aem.62.3.928-935.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanabe T, Naka A, Aso H, et al. A novel aerobactin utilization cluster in Vibrio vulnificus with a gene involved in the transcription regulation of the iutA homologue. Microbiol. Immunol. 2005;49(9):823–834. doi: 10.1111/j.1348-0421.2005.tb03671.x. [DOI] [PubMed] [Google Scholar]
- 64.Funahashi T, Tanabe T, Shiuchi K, Nakao H, Yamamoto S. Identification and characterization of genes required for utilization of desferri-ferrichrome and aerobactin in Vibrio parahaemolyticus. Biol. Pharm. Bull. 2009;32(3):359–365. doi: 10.1248/bpb.32.359. [DOI] [PubMed] [Google Scholar]