Abstract
TtpC is a fourth required protein in the TonB2 energy transduction system in Vibrio anguillarum. TtpC is necessary for iron transport mediated by the TonB2 system and is highly conserved in all pathogenic vibrio species studied to date as well as several marine organisms. We show here that the TtpC proteins from selected pathogenic vibrio species can function with the TonB2 system of V. anguillarum to allow iron transport mediated by a chimeric TonB2 system where the native ExbB2, ExbD2 and TonB2 function with an episomally expressed TtpC in trans from a different species. The discovery that inter-species complementation occurs can be used to identify the functional regions of the TtpC proteins and will lead to an investigation of the mechanism of interaction between the TtpC protein and other members of the TonB2 system.
Keywords: TtpC, Vibrio, Iron transport
Introduction
Pathogenic bacteria possess virulence factors that allow them to invade a vertebrate host and establish colonization of the host while evading the innate and adaptive immune systems. One of these virulence factors is the ability to compete with the host cells for iron. Iron is an essential element for nearly all species and vertebrate hosts have developed mechanisms to sequester free iron in efforts to deter the growth of pathogenic bacteria. In biological fluids, iron is always bound in a complex with iron-binding proteins such as transferrin, lactoferrin or as part of the oxygen-carrying heme group in red blood cells. Thus, bacterial pathogens scavenging for iron must also possess iron-binding proteins and mechanisms to internalize bound iron in order to establish an infection. Some species express outer-membrane receptors specific for iron-binding host proteins like transferrin or heme. Alternatively, bacteria can synthesize siderophores that are used to scavenge iron from eukaryotic iron-binding proteins. Siderophores are low molecular weight compounds that have an extremely high affinity for iron, in some cases more so than host iron-binding proteins, and are secreted by the bacterium to scavenge iron from the surrounding environment. In Gram-negative species, the iron-siderophore complexes are bound on the surface of the outer membrane by receptor proteins. Most bacterial species express several outer-membrane receptors, each one specific for a different ferric iron-siderophore complex.
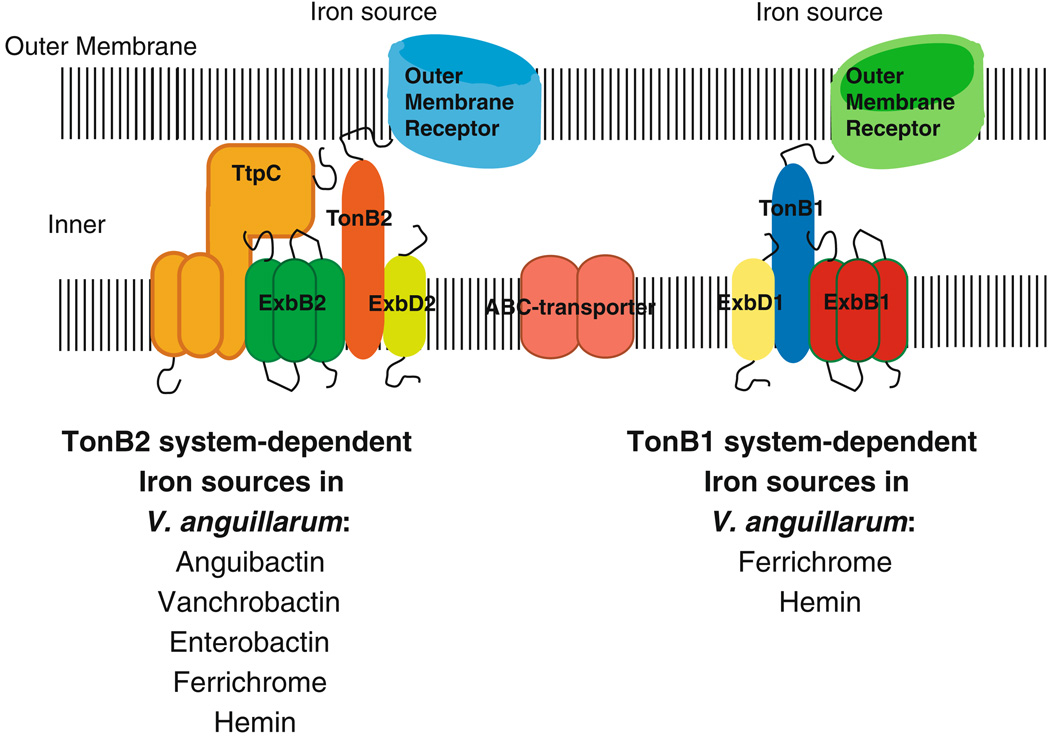
Depending on the outer membrane transport protein and inner membrane permeases being used, the ferric iron or the iron-siderophore complex is internalized into the periplasmic space and then transported through the inner membrane to the cytosol where it can be used by the cell as a cofactor in oxidation–reduction reactions and as a transcriptional co-repressor with the master-regulator Fur (Ernst et al. 1978). The energy required to power all of these steps of iron uptake is derived from the proton motive force in the inner membrane of the Gram-negative cell. This energy is harvested and transduced to the iron-siderophore complex-binding proteins in the outer membrane via a complex consisting of a TonB-family protein, the transducer, and related energy harvesting proteins, ExbB and ExbD (Brinkman and Larsen 2007). ExbB is necessary to stabilize TonB (Fischer et al. 1989; Skare and Postle 1991; Postle and Kadner 2003). The TonB protein interacts with a “TonB box” region on the periplasmic domain of the outer membrane receptor (Crosa et al. 2004). Much of the elucidation of energy transduction between the cellular membranes of Gram-negative bacteria has been determined in Escherichia coli. E. coli has one classical TonB system whereas the pathogenic vibrio species possess at least two, and in some species, three TonB systems that appear to be specific for the transport of various iron sources (Fig. 1) (Stork et al. 2004).
Fig. 1.
Two TonB systems in V. anguillarum allow the bacterium to utilize different iron sources
Requirement of a fourth protein
The presence of TonB along with the accessory proteins ExbB and ExbD is conserved across many Gram-negative species. In V. anguillarum however, a fourth protein was discovered to be necessary for ferric anguibactin transport mediated by the TonB2 system (Stork et al. 2007). This protein, TtpC is encoded in the tonB2 gene cluster of V. anguillarum.
Using sucrose-density gradients, it was shown that TtpC is located in the inner membrane. Through the use of bioassays to test iron-source utilization, it was shown that TtpC is necessary for ferric-anguibactin transport mediated by the TonB2 system in the fish pathogen V. anguillarum (Table 1) (Stork et al. 2007). In addition, the TtpC homologue in the human pathogen V. cholerae is essential for enterobactin transport mediated by the V. cholerae TonB2 system. Although V. anguillarum has two TonB energy transduction systems, the TtpC protein is specific for the TonB2 system and not necessary for iron transport mediated by the TonB1 system in V. anguillarum (Table 1).
Table 1.
TtpC is necessary for iron transport mediated by the TonB2 system in V. anguillarum and V. cholerae (Stork et al. 2007)
| Strains | Iron sourcesc | ||||
|---|---|---|---|---|---|
| Ferric ammonium citrate | Anguibactin | Enterobactin | Ferrichrome | Heme | |
| V. anguillarum | |||||
| 775 Wild Type | +a | + | + | + | + |
| 775 tonB1−, ttpC− | + | − | − | − | − |
| 775 tonB1−, ttpC−/pexbB2, exbD2, tonB2(va)d | + | − | − | − | − |
| 775 tonB1−, ttpC−/pttpC, exbB2, exbD2, tonB2(va) | + | + | + | + | + |
| 775 ttpC− | + | − | − | + | + |
| V. cholerae | |||||
| CA401 Wild Type | + | NDb | + | + | + |
| CA401 exbB2−/ | + | ND | − | + | + |
| CA401 exbB2 −/pexbB2, exbD2, tonB2(va) | + | ND | − | + | + |
| CA401 exbB2 −/pttpC, exbB2, exbD2, tonB2(va) | + | ND | + | + | + |
| CA401 ttpC− | + | ND | − | + | + |
+ or − indicates growth or lack of growth around the specified iron source
ND, Not determined
5 µl of each iron source was spotted on the surface of the plates in the following concentrations; Ferric ammonium citrate: 500 µg/ml; enterobactin: 1.0 mg/ml; anguibactin: 1.0 mg/ml; ferrichrome: 1.0 mg/ml; hemin: 20 µg/ml
Mutations in chromosomally encoded genes were complemented by genes expressed from the low copy-number expression vector pACYC177
Homology of TtpC proteins in several pathogenic vibrio species
Based on the data presented in Table 1 showing that TtpC is necessary in the TonB2 system of both V. anguillarum and V. cholerae and that the TonB2 system from V. anguillarum can support iron transport of TonB2-specific iron sources in V. cholerae, we asked the question: How similar are the TtpC proteins to one another?
To compare the sequences we used the T-coffee server to perform a multiple sequence alignment of the amino acid sequences of the TtpC protein from five pathogenic vibrio species. The Clustal W formatted alignment and the score are shown in Fig. 2. The multiple sequence alignment produced an overall score of 66 (Poirot et al. 2003). Individual alignments of the V. anguillarum TtpC sequence to the TtpC sequence of other pathogenic vibrios by BLASTp reveal amino acid sequence similarity between 73 and 80% (Altschul et al. 1990).
Fig. 2.
Clustal W format alignment of the TtpC amino acid sequence from five pathogenic vibrio species. Species names are listed to the left of the sequence. All parameters were set to default settings for the Clustal W alignment. Consensus symbols: “*” residues in that column are identical in all sequences in the alignment, “:” conserved substitutions have been observed, “.” semi-conserved substitutions are observed
Interspecies complementation
After determining the very high similarity between the amino acid sequence of the TtpC proteins in the pathogenic vibrio species, we asked: Are the TtpC proteins from V. cholerae and V. vulnificus similar enough to the TtpC protein from V. anguillarum that they would be able to complement a chromosomal deletion in the V. anguillarum ttpC? We observed that V. cholerae ttpC can complement a ΔttpC mutation in V. anguillarum for the uptake of ferric-anguibactin, but V. vulnificus ttpC cannot. We also observed that the V. anguillarum ttpC was the only complementing gene that restored growth on all tested iron sources (Table 2). The lack of growth of the V. anguillarum ΔttpC, ΔangA/pttpCvc strain around enterobactin as an iron source indicates that while the TtpC proteins from the different species are highly similar, slight differences may impede apparent involvement with certain outer membrane receptors in V. anguillarum.
Table 2.
V. cholerae ttpC complements ΔttpC mutation in V. anguillarum for growth around 2,3-DHBA
| Strainsc | Iron sourcesb | ||||
|---|---|---|---|---|---|
| Ferric ammonium citrate | Enterobactin | 2,3-DHBA | Vanchrobacin | Hemin | |
| V. anguillarum | |||||
| 775/pMMB208 | +a | + | + | + | + |
| 775 ΔttpC, ΔangA | + | − | − | − | + |
| 775 ΔttpC, ΔangA/pttpCva | + | + | + | + | + |
| 775 ΔttpC, ΔangA/pttpCvc | + | − | + | − | + |
| 775 ΔttpC, ΔangA/pttpCvv | + | − | − | − | + |
+ or − indicates growth or lack of growth around the specified iron source
1 µl of each iron source was spotted on the surface of the plates in the following concentrations; ferric ammonium citrate: 10 mg/ml; enterobactin: 1.0 mg/ml; 2,3-DHBA: 3.0 mg/ml; vanchrobactin: 1.0 mg/ml; hemin: 20 µg/ml
Mutations in chromosomally encoded genes were complemented by genes expressed from the low copy-number expression vector pMMB208. Expression was induced with 1 mM IPTG
Requirement of TtpC dependent on TonB2
We observed that TtpC is necessary for growth of V. anguillarum on iron sources that utilize only the TonB2 system for transport through the outer membrane. Next, we set out to determine if TtpC was necessary for transport of iron sources that utilize both TonB systems in V. anguillarum. We performed bioassays using ferrichrome as an iron source capable of being taken up by both TonB systems and forced transport to occur through the TonB2 system by using tonB1 deletion mutant strains as shown in Table 3. We observed that V. anguillarum and V. cholerae TtpC proteins can complement a ΔttpC mutation in V. anguillarum for the uptake of ferrichrome mediated by the TonB2 system, but V. vulnificus TtpC cannot. From these results, we conclude that outer membrane receptors that can receive energy from the TonB1 system where TtpC is not required for transport must have a TtpC protein present to receive energy from the TonB2 system (Table 3).
Table 3.
TtpC is necessary for usage of iron sources when transport is mediated by the TonB2 system
| Strainsc | Iron sourcesb | |||
|---|---|---|---|---|
| TonB1- and TonB2-mediated transport | TonB2-mediated transport | |||
| Ferric ammonium citrate | Ferrichrome | Enterobactin | 2,3-DHBA | |
| V. anguillarum | ||||
| 775/pMMB208 | +a | + | + | + |
| 775 ΔtonB1, ΔttpC, ΔangA | + | − | − | − |
| 775 ΔtonB1, ΔttpC, ΔangA/pttpCva | + | + | + | + |
| 775 ΔtonB1, ΔttpC, ΔangA/pttpCvc | + | + | − | + |
| 775 ΔtonB1, ΔttpC, ΔangA/pttpCvv | + | − | − | − |
+ or − indicates growth or lack of growth around the specified iron source
1 µl of each iron source was spotted on the surface of the plates in the following concentrations; ferric ammonium citrate: 10 mg/ml; enterobactin: 1.0 mg/ml; 2,3-DHBA: 3.0 mg/ml; ferrichrome: 1.0 mg/ml
Mutations in chromosomally encoded genes were complemented by genes expressed from the low copy-number expression vector pMMB208. Expression was induced with 1 mM IPTG
TtpC not required for TonBE. coli
It was observed previously that the TonB protein from E. coli could complement deletion mutations in both tonB1 and tonB2 in V. anguillarum for the utilization of ferric-anguibactin as an iron source (López et al. 2008). This strain still had a wild type TtpC protein so ttpC was also deleted in the tonB1, tonB2 double mutant and complemented with tonB from E. coli on a the low copy-number vector pMMB208 (Morales et al. 1991) to determine if the TonB from E. coli needed a functional TtpC protein to energize transport of ferric-anguibactin in V. anguillarum. Bioassays with these strains were performed and are presented in Table 4. The TonB protein from E. coli does not need a functional TtpC protein to energize the transport of ferric-anguibactin. From this, we may conclude that while the function of TonB1va or TonBec do not require TtpC, the TtpC protein is required for TonB2 function in V. anguillarum (Tables 3 and 4).
Table 4.
In V. anguillarum, the TonB protein from E. coli does not require TtpC to mediate transport of the endogenous siderophore anguibactin
| Strainsc | Iron sourcesb | ||
|---|---|---|---|
| Ferric ammonium citrate | Enterobactin | Anguibactin | |
| V. anguillarum | |||
| 775/pMMB208 | +a | + | + |
| CSL68: ΔtonB1, ΔtonB2/pMMB208 | + | − | − |
| CSL64: ΔtonB1, ΔtonB2/pton8E. coli | + | − | + |
| 775 ΔttpC/pMMB208 | + | − | − |
| 775 ΔtonB1, ΔtonB2, ΔttpC/pMMB208 | + | − | − |
| 775 ΔtonB1, ΔtonB2, ΔttpC/ptonBE. coli | + | − | + |
+ or − indicates growth or lack of growth around the specified iron source
1 µl of each iron source was spotted on the surface of the plates in the following concentrations; ferric ammonium citrate: 10 mg/ml; enterobactin: 1.0 mg/ml; anguibactin: 1.0 mg/ml
Mutations in chromosomally encoded genes were complemented by genes expressed from the low copy-number expression vector pMMB208. Expression was induced with 1 mM IPTG
Lack of anguibactin uptake or lack of anguibactin production?
When 2,3-DHBA is spotted on the surface of the V. anguillarum bioassay plates as an iron source, the ΔttpC, ΔangA mutant strains complemented with V. anguillarum and V. cholerae ttpCs are able to grow but when 2,3-DHBA is supplied to the ΔttpC, ΔangA mutant strain complemented with the V. vulnificus ttpC, no growth is observed (Table 2). Because of this observation, we asked the question: Is the lack of growth seen in the mutant complemented with V. vulnificus ttpC due to a lack of iron transport mediated by the TonB2 system or is it due to that particular strain not being able to produce anguibactin from the 2,3-DHBA? To answer this question, we used the anguibactin-indicator strains V. anguillarum CC9-8 (Tolmasky et al. 1988), which cannot produce or transport anguibactin and V. anguillarum CC9-16 which cannot produce anguibactin but is proficient in transport. 3 µl of overnight, IPTG-induced culture of the complemented ΔttpC, ΔangA mutants grown in CM9 minimal media were used as the iron sources in the bioassay (Table 5). When 2,3-DHBA was added to the bioassay plate, CC9-16 (Tolmasky et al. 1988), was able to grow around all of the supernatants tested indicating that all strains were producing anguibactin when grown on 2,3-DHBA.
Table 5.
Growth around anguibactin-producing V. anguillarum strains
| Indicator strains | Anguibactin-producing strainsb | |||||
|---|---|---|---|---|---|---|
| 775 Wild type |
ΔttpC | ΔttpC, ΔangA/ pttpCva |
ΔttpC, ΔangA/ pttpCvc |
ΔttpC, ΔangA/ pttpCvv |
Ferric ammonium citrate |
|
| CC9-8 | −a | − | − | − | − | + |
| CC9-16 | + | + | − | − | − | + |
| CC9-8 + DHBA | − | − | − | − | − | + |
| CC9-16 + DHBA | + | + | + | + | + | + |
+ or − indicates growth or lack of growth around the specified iron source
3 µl of each anguibactin-producing strain was spotted on the surface the plate
Future directions
The genetic complementations of the ΔttpC mutation in V. anguillarum show that the V. vulnificus TtpC is unable to complement the ΔttpC mutant to restore growth around the TonB2-specific iron sources. These results illuminate a potential mechanism by which TtpC could be working. Previous data from M. Stork shows that protein–protein interactions occur between TtpC and TonB2 in V. anguillarum (Stork et al. 2007). Based on these observations, we postulate that the interactions between the TonB2 protein and the V. vulnificus TtpC in the V. anguillarum ΔttpC, ΔangA mutant strain are either not occurring all together or a necessary interaction between the two proteins, or possibly, with the accessory proteins ExbB2 and ExbD2, is unstable and does not allow for energy transduction by the TonB2 system.
Based on the results from Tables 2, 3 and 4, the TtpC protein may be essential for interactions between the TonB2 system and the outer-membrane receptor. This theory is supported by the observation that the V. cholerae TtpC is able to complement growth around anguibactin as an iron source but is unable to complement growth around vanchrobactin or enterobactin as iron sources. We note that these potential interactions could be very specific due to the high similarity between the V. anguillarum and V cholerae TtpC protein sequences.
Abbreviations
- 2,3-DHBA
2,3-Dihydroxybenzoic acid
- IPTG
Isopropyl-beta-d-thiogalactopyranoside
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Brinkman K, Larsen R. Interactions of the energy transducer TonB with non-cognate energy harvesting complexes. J Bacteriol. 2007;190(1):421–427. doi: 10.1128/JB.01093-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa JH, Mey AR, Payne SM. Iron transport in bacteria. Washington DC: ASM Press; 2004. [Google Scholar]
- Ernst JF, Bennett RL, Rothfield LI. Constitutive expression of the iron-enterochelin and ferrichrome uptake systems in a mutant strain of Salmonella typhimurium. J Bacteriol. 1978;135(3):928–934. doi: 10.1128/jb.135.3.928-934.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E, Gunter K, Braun V. Involvement of ExbB and TonB in transport across the outer membrane of Escherichia coli: phenotypic complementation of exb mutants by overexpressed tonB and physical stabilization of TonB by ExbB. J Bacteriol. 1989;171:5127–5134. doi: 10.1128/jb.171.9.5127-5134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López CS, Peacock RS, Crosa JH, Vogel HJ. Molecular characterization of the TonB2 protein from Vibrio anguillarum. Biochem J. 2008 Oct 30; doi: 10.1042/BJ20081462. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales VM, Backman A, Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991;97:39–47. doi: 10.1016/0378-1119(91)90007-x. [DOI] [PubMed] [Google Scholar]
- Poirot O, O’Toole E, Notredame C. Tcoffee@igs: a web server for computing, evaluating and combining multiple sequence alignments. Nucleic Acids Res. 2003;31(13):3503–3506. doi: 10.1093/nar/gkg522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K, Kadner RJ. Touch and Go: tying TonB to transport. Mol Microbiol. 2003;49:869–882. doi: 10.1046/j.1365-2958.2003.03629.x. [DOI] [PubMed] [Google Scholar]
- Skare JT, Postle K. Evidence for a TonB-dependent energy transduction complex in Escherichia coli. Mol Microbiol. 1991;5:2883–2890. doi: 10.1111/j.1365-2958.1991.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Stork M, DiLorenzo M, Mouriño S, Osorio CR, Lemos ML, Crosa JH. Two tonB systems function in iron transport in Vibrio anguillarum, but only one is essential for virulence. Infect Immun. 2004;72(12):7326–7329. doi: 10.1128/IAI.72.12.7326-7329.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork M, Otto BR, Crosa JH. A novel protein, TtpC, is required component of the TonB2 complex or specific iron transport in the pathogens Vibrio anguillarum and Vibrio cholerae. J Bacteriol. 2007;189(5):1803–1815. doi: 10.1128/JB.00451-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmasky ME, Actis LA, Crosa JH. Genetic analysis of the iron uptake region of the Vibrio anguillarum plasmid pJM1: molecular cloning of genetic determinants encoding a novel trans activator of siderophore biosynthesis. J Bacteriol. 1988;160:860–866. doi: 10.1128/jb.170.4.1913-1919.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]