Abstract
Clinicians may defer antiretroviral treatment for patients with suboptimal adherence. We used a validated computer simulation of HIV disease progression to compare alternative treatment thresholds for patients with suboptimal adherence. Earlier treatment increased life expectancy across a wide adherence range (50%–100% of doses taken). Delaying treatment for patients with suboptimal adherence may not always be appropriate.
As electronic medical records make pharmacy refill histories more accessible, clinicians may defer antiretroviral (ARV) therapy initiation for patients at high risk of nonadherence because of earlier nonadherence or risk factors, such as alcohol abuse [1, 2], drug abuse [2], or mental illness [3]. Deferring treatment may reflect the concern that nonadherence accelerates the development of ARV resistance and the exhaustion of future drug options [4]. However, evidence is emerging that earlier treatment initiation may benefit increasing numbers of patients who have chronic HIV infection, and some of this benefit may be extended to individuals with suboptimal adherence [5]. We used our validated computer simulation of HIV disease progression [6–8] to address whether the benefits of earlier initiation of ARV therapy would outweigh harms for individuals expected to have suboptimal adherence.
Methods
We have developed a computer simulation of HIV progression (Appendix; online only) that is distinctive because it can weigh the benefits of earlier ARV therapy initiation (e.g., reduction in HIV-related mortality) against its harms (e.g., accrual of drug resistance mutations, reduction of future drug options, and increased exposure to toxicity from therapy) [6]. This simulation advances earlier work by explicitly modeling the biological processes that may decrease ARV effectiveness (e.g., genotypic resistance accumulation and nonadherence) and using them to estimate time to treatment failure. It has been validated extensively [6–8], closely reproducing Kaplan-Meier curves of time-to-treatment-failure and survival among 3545 ARV-naive patients [6], yielding 3-year mortality estimates similar to a 12,574 patient cohort distinct from the derivation cohort [6] and replicating clinically observed heterogeneity in the relationship of ARV therapy adherence to resistance mutation accumulation [7].
The simulation can assign otherwise similar patient cohorts to different treatment decisions (e.g., initiation of ARV therapy at a CD4+ cell count of 350 cells/µL vs. 500 cells/µL) and compare the impact of this decision on designated outcomes. Because the simulation is probabilistic, it is able to represent much of the heterogeneity of actual patient populations. Because of its long time horizon, the simulation captures the aggregate impact of long-term ARV exposure by using the analytic machinery of the computer to “sum” this exposure over time. That is, a person starting therapy earlier may experience reduced effectiveness of later regimens because of accumulated drug resistance, and this delayed harm is reflected in lower life expectancy.
Our current analyses build on recently published analyses of risks and benefits associated with earlier treatment initiation [5]. However, rather than grouping all hypothetical patients together regardless of their adherence, we stratified patients by level of ARV therapy adherence, ranging from taking 50% of prescribed ARV doses as directed (which is a typical adherence level among poorly adherent cohorts [8]) to taking 100% of doses as directed.
We assumed the probability of nonadherence would remain stable over time. However, in sensitivity analyses, we considered a scenario that assumed adherence could wane steadily and substantially over a 5-year period (e.g., someone who is initially 100% adherent could become 80% adherent by year 5).
We analyzed separate cohorts of individuals with newly diagnosed chronic HIV infection and CD4+ cell counts of 500 cells/µL, stratified by age (30 years, 40, years, and 50 years of age).We used our simulation to estimate patient life expectancy, quality-adjusted life-years (QALYs), CD4+ cell count trajectories, rate of drug resistance mutation accumulation, and rate of ARV treatment failure, comparing ARV initiation thresholds of 500, 350, and 200 cells/µL. QALY estimates reflect the favorable impact on utility from ARV-induced improvements in CD4+ cell count, together with the unfavorable impact on utility from ARV-induced adverse effects (Appendix; online only). (Utility is a unidimensional, preference-based quality-of-life measure). Resistance mutations were defined as any mutation that may confer resistance to ≥1 ARV, regardless of class, and were based on the International AIDS Society–USA panel list of the most common HIV-1 mutations conferring drug resistance [9]. New regimens were defined as any change in ≥2 ARVs.
We performed sensitivity analyses to weigh the possible impact of ARV-related toxicity, assuming a worst-case scenario (the maximum toxicity plausibly consistent with current data) [5]. We also performed additional sensitivity analyses that varied the pretreatment viral load.
Results
Starting treatment at a CD4+ cell count of 500 cells/ µL improved life-years and QALYs, compared with starting treatment at CD4+ cell counts of 350 cells/µL or 200 cells/µL, regardless of adherence. Earlier treatment conferred as much as 3.7 additional life-years (increased from 29.8 life-years to 33.5 life-years) and 3.3 additional QALYs (increased from 26.2 QALYs to 29.5 QALYs) with 100% adherence, and it conferred as much as 7.4 additional life-years (increased from 19.3 years to 26.7 years) and 6.7 additional QALYs (increased from 16.4 QALYs to 23.1 QALYs) with 50% adherence (table 1).
Table 1.
Life expectancy expressed as life-years and quality-adjusted life-years (QALYs) with different antiretroviral therapy initiation thresholds, stratified by proportion of antiretroviral doses taken as directed, assuming minimal toxicity from antiretroviral therapy and with upper-bound estimate for antiretroviral toxicity.
| Toxicity assumptions, adherence, age |
Life-years, by CD4+ cell count at therapy initiation |
QALYs, by CD4+ cell count at therapy initiation |
||||
|---|---|---|---|---|---|---|
| 200 cells/µL | 350 cells/µL | 500 cells/µL | 200 cells/µL | 350 cells/µL | 500 cells/µL | |
| Minimal toxicity assumptions | ||||||
| 50% | ||||||
| 30 years | 19.3 | 23.3 | 26.7 | 16.4 | 20.0 | 23.1 |
| 40 years | 15.7 | 18.8 | 21.4 | 13.4 | 16.2 | 18.5 |
| 50 years | 13.1 | 15.4 | 17.2 | 11.3 | 13.3 | 14.9 |
| 60% | ||||||
| 30 years | 20.1 | 23.7 | 27.2 | 17.0 | 20.3 | 23.5 |
| 40 years | 16.5 | 19.4 | 21.9 | 14.2 | 16.7 | 19.0 |
| 50 years | 13.8 | 15.9 | 17.6 | 11.9 | 13.8 | 15.3 |
| 70% | ||||||
| 30 years | 21.1 | 24.7 | 27.7 | 18.0 | 21.3 | 24.0 |
| 40 years | 17.6 | 20.3 | 22.6 | 15.1 | 17.6 | 19.6 |
| 50 years | 14.7 | 16.6 | 18.1 | 12.7 | 14.4 | 15.8 |
| 80% | ||||||
| 30 years | 22.8 | 26.0 | 28.7 | 19.5 | 22.5 | 25.0 |
| 40 years | 19.0 | 21.6 | 23.5 | 16.4 | 18.8 | 20.5 |
| 50 years | 15.6 | 17.5 | 18.8 | 13.6 | 15.3 | 16.5 |
| 90% | ||||||
| 30 years | 25.4 | 28.4 | 30.6 | 21.9 | 24.7 | 26.7 |
| 40 years | 21.0 | 23.3 | 25.0 | 18.3 | 20.4 | 21.9 |
| 50 years | 16.9 | 18.6 | 19.8 | 14.8 | 16.4 | 17.4 |
| 100% | ||||||
| 30 years | 29.8 | 32.2 | 33.5 | 26.2 | 28.4 | 29.5 |
| 40 years | 24.0 | 25.8 | 26.6 | 21.2 | 22.8 | 23.5 |
| 50 years | 18.6 | 19.8 | 20.6 | 16.5 | 17.6 | 18.2 |
| Upper-bound toxicity assumptions | ||||||
| 50% | ||||||
| 30 years | 14.5 | 15.5 | 16.4 | 12.4 | 13.6 | 14.2 |
| 40 years | 11.3 | 11.7 | 11.9 | 9.8 | 10.1 | 10.4 |
| 50 years | 8.9 | 8.9 | 8.8 | 7.8 | 7.8 | 7.7 |
| 60% | ||||||
| 30 years | 15.0 | 15.9 | 16.7 | 12.9 | 13.7 | 14.5 |
| 40 years | 11.7 | 12.0 | 12.2 | 10.2 | 10.4 | 10.6 |
| 50 years | 9.1 | 9.0 | 8.9 | 8.0 | 7.9 | 7.8 |
| 70% | ||||||
| 30 years | 15.6 | 16.4 | 17.0 | 13.4 | 14.3 | 14.8 |
| 40 years | 12.1 | 12.3 | 12.4 | 10.6 | 10.8 | 10.8 |
| 50 years | 9.3 | 9.3 | 9.0 | 8.3 | 8.2 | 8.0 |
| 80% | ||||||
| 30 years | 16.3 | 17.0 | 17.4 | 14.1 | 14.9 | 15.2 |
| 40 years | 12.5 | 12.8 | 12.7 | 11.0 | 11.3 | 11.1 |
| 50 years | 9.6 | 9.4 | 9.1 | 8.5 | 8.4 | 8.1 |
| 90% | ||||||
| 30 years | 17.1 | 17.6 | 18.0 | 15.0 | 15.5 | 15.9 |
| 40 years | 13.1 | 13.1 | 13.0 | 11.6 | 11.6 | 11.5 |
| 50 years | 9.8 | 9.6 | 9.3 | 8.8 | 8.5 | 8.2 |
| 100% | ||||||
| 30 years | 18.1 | 18.5 | 18.6 | 16.1 | 16.4 | 16.5 |
| 40 years | 13.5 | 13.5 | 13.2 | 12.1 | 12.0 | 11.7 |
| 50 years | 10.0 | 9.7 | 9.4 | 9.0 | 8.7 | 8.3 |
NOTE. The upper-bound estimate for antiretroviral toxicity confers a strong bias against earlier treatment initiation. Results shown are for viral loads of 100,000 copies/mL; lower viral loads modestly reduced the amount of benefit from earlier treatment initiation. Boldface type indicates the most favored initiation strategy.
In sensitivity analyses that varied assumptions regarding pretreatment viral load and whether adherence waned over time, earlier treatment remained the preferred strategy. In sensitivity analyses that assumed a worst-case scenario for the amount of toxicity attributable to ARV therapy, earlier treatment was no longer always preferred. However, there was no scenario in which lower adherence made earlier treatment more unfavorable (table 1), even though this scenario conferred a strong bias against earlier treatment initiation.
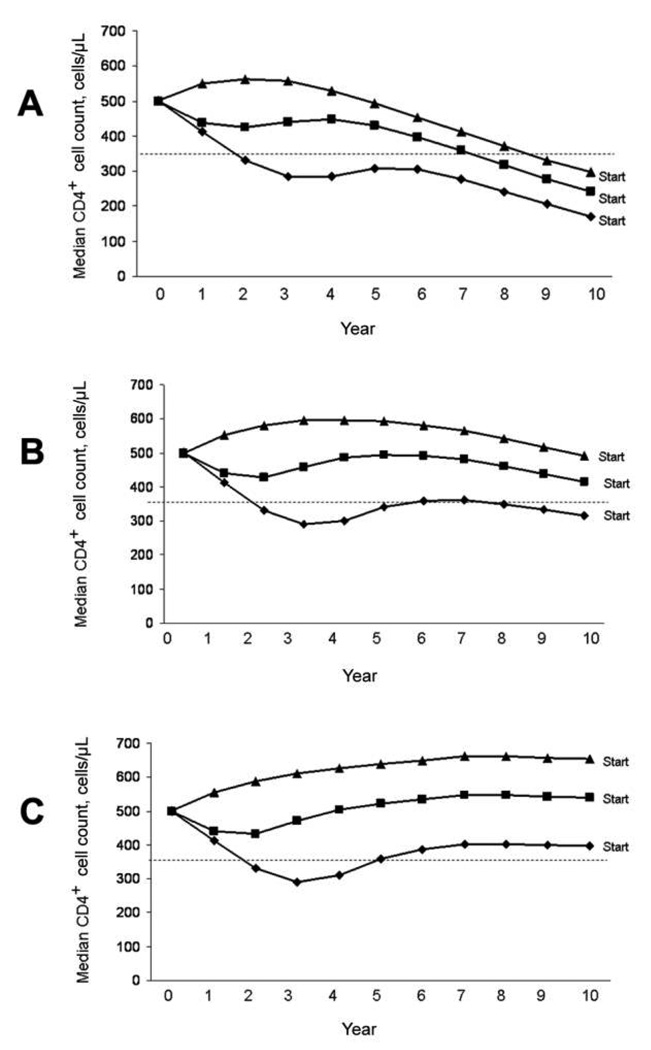
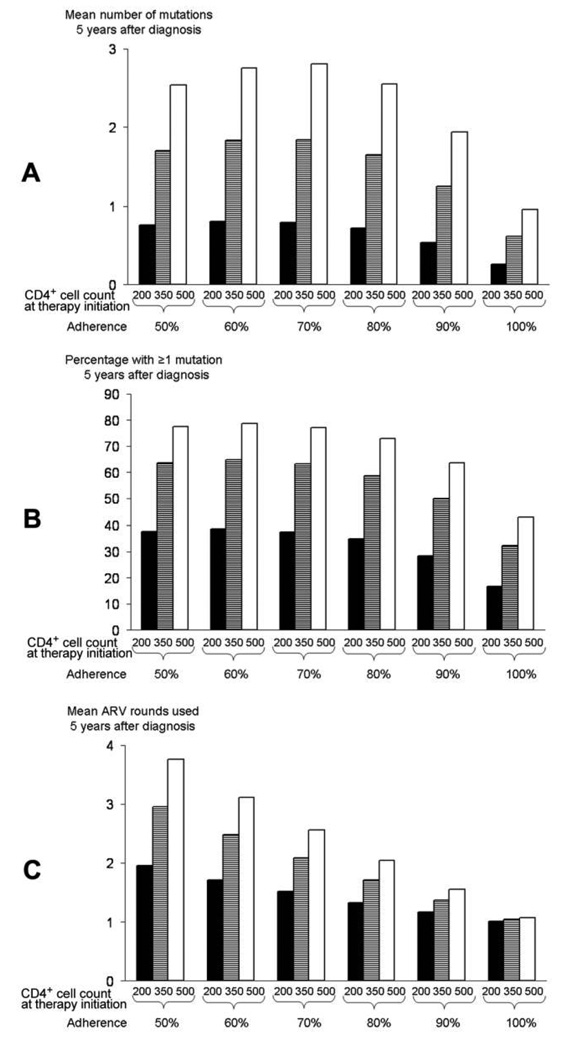
Poorly adherent patients were benefited by earlier treatment because their CD4+ cell counts were substantially more likely to remain in favorable ranges (e.g., CD4+ cell count >350 cells/ µL) if treatment was initiated early (figure 1). For poorly adherent patients, early treatment initiation was required to keep CD4+ cell count trajectories elevated, whereas for completely adherent patients, treatment substantially elevated CD4+ cell count trajectories, even when it was started late. This immunological benefit from earlier ARV therapy initiation outweighed the harms of greater resistance mutation accumulation (figures 2A and 2B) and reduced future drug options (figure 2C) in all adherence strata, even though these harms were greater in patients with poor adherence.
Figure 1.
Impact of different treatment-initiation thresholds on CD4+ cell count trajectory if diagnosis occurs at a CD4+ cell count of 500 cells/ µL. Results for adherence levels of 50% (A), 80% (B), and 100% (C) are shown. Times reflect the interval following diagnosis of HIV infection and not the interval following treatment initiation. Accordingly, at any particular interval, individuals starting antiretroviral therapy at a CD4+ cell count of 200 cells/µL had less exposure to therapy than did those starting therapy at a CD4+ cell count of 500 cells/µL. Note that median CD4+ cell count does not reach the treatment threshold before ascending, because at any particular time, patients with lower (i.e., sub-median) CD4+ cell counts are more likely to be below the treatment threshold and, therefore, to receive selective treatment initiation. Results are shown for individuals 40 years of age with baseline viral loads of 100,000 copies/mL. Results for other ages and viral loads differed only modestly. Triangles, initiation of therapy at CD4+ cell count of 500 cells/µL; squares, initiation of therapy at CD4+ cell count of 350 cells/µL; diamonds, initiation of therapy at CD4+ cell count of 200 cells/µL.
Figure 2.
Impact of different treatment initiation thresholds on (A) mean number of resistance mutations accumulated, with all antiretroviral (ARV) drug classes combined, (B) proportion of patients with ≥1 resistance mutation, and (C) number of ARV regimens used, by adherence to therapy and CD4+ cell count at ARV therapy initiation (200 cells/µL, 350 cells/µL, or 500 cells/µL). Times reflect the interval after diagnosis of HIV infection and not the interval after treatment initiation. Accordingly, 5 years after receiving a diagnosis of HIV infection, individuals starting treatment at a CD4+ cell count of 200 cells/µL will have less exposure to therapy than those starting at a CD4+ cell count of 500 cells/µL. Results are shown for individuals 40 years of age with baseline viral loads of 100,000 copies/mL; results for other ages and viral loads differed only modestly. Even when ARV adherence is 100%, factors unrelated to adherence (e.g., pharmacokinetics, drug interactions, and low-level viral replication) may occasionally result in the development of drug resistance mutations.
Discussion
Our results suggest that even patients with suboptimal adherence may benefit from earlier initiation of ARV therapy. Although health care providers may be reluctant to start ARV therapy earlier because of concerns about inducing resistance mutations and narrowing future drug options, our results suggest that these dangers are outweighed by the benefit of preventing CD4+ cell count decreases into high-risk strata. Therefore, individuals with risk factors for poor adherence, such as hazardous levels of alcohol consumption, drug abuse, or mental illness, may not warrant distinct criteria for starting ARV therapy.
This is not to say that adherence does not matter. Improving adherence substantially increases life expectancy and QALYs. Indeed, among individuals who started treatment at a CD4+ cell count of 500 cells/µL, improving adherence from 50% to 80% resulted in as much as 2.0 more life-years and 1.9 more QALYs, and improving adherence from 80% to 100% yielded as much as an additional 4.8 more life-years and 4.5 more QALYs. Therefore, individuals who are at high-risk for nonadherence are likely to benefit substantially from surveillance to detect poor adherence and interventions to improve it, either by targeting risk factors (e.g., screening and treating for hazardous levels of alcohol consumption) or by more-generic approaches (e.g., computer-generated prompts when patients have not filled their prescriptions recently).
A major limitation of our analysis is that it is not an epidemic model and, therefore, cannot evaluate how earlier treatment initiation may impact the transmission of drug-resistant virus to individuals newly infected with HIV. Although treating individuals who have suboptimal adherence earlier may improve outcomes in such patients, they may be more likely to transmit a drug-resistant HIV strain and to adversely affect the outcomes of others. However, it seems unlikely that treating patients with suboptimal adherence would increase the overall probability of transmitting drug-resistant virus. Even though a higher proportion of transmitted virus may be drug resistant (by 2-fold to 3-fold, based on our results), this risk is likely to be offset by a lower overall probability of transmitting virus (by 2-fold to 14-fold, based on applying the relationship between viral load suppression and transmission probability [10,11] to the ~1.5 log decrease in viral load with 50% adherence [12]).
Other important limitations include insufficient precision to stratify results by antiretroviral sequencing strategies (for example, preferentially initiating treatment with nonnucleoside reverse-transcriptase inhibitors for patients with suboptimal adherence, because they might be more adherent to such medication [12]). The simulation does not consider the potential correlation of age with mode of HIV transmission, which may have hidden implications for disease risk. Finally, the toxicity sensitivity analysis is based on a worst-case scenario that is likely to bias results greatly in favor of later treatment initiation. Therefore, this sensitivity analysis is suggestive when it favors earlier treatment initiation, but it is difficult to interpret when it favors later treatment initiation.
However, these limitations should be interpreted in the context of the simulation’s unique strengths. This is the first computer simulation study to evaluate how nonadherence may impact ARV therapy initiation thresholds. This study’s clinical implication is that earlier treatment initiation may improve life expectancy even for individuals who are likely to have suboptimal adherence. Its public health implication is that earlier treatment initiation for patients with poor adherence to therapy may exert substantial benefit that may counteract the harm associated with increasing transmission of drug-resistant strains. These results may offer guidance to clinical care decisions until the results of alternative initiation strategies in atrisk populations are available from randomized controlled trials.
Acknowledgments
We thank Cynthia Omokaro, for her assistance with manuscript preparation, and Yale Information Technology Services, for use of its high-performance computing cluster.
Financial support. The National Institute of Alcohol Abuse and Alcoholism (K23 AA14483–01).
Footnotes
Potential conflicts of interest. M.S.R. has received an unrestricted research grant from and consults for Archimedes. M.B.G. has received an honorarium and consultancy fees from Monogram Biosciences. R.S.B. is a methodological consultant for United BioSource. All other authors: no conflicts.
References
- 1.Braithwaite RS, McGinnis K, Conigliaro J, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29:1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 2.Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003;114:573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 3.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Accessed 20 May 2008];The Panel on Clinical Practices for the Treatment of HIV Infection convened by the Department of Health and Human Services. 2008 January 29; Available at http://AIDSinfo.nih.gov.
- 5.Braithwaite RS, Roberts MS, Chang CH, et al. Influence of alternative thresholds for initiating HIV treatment on life expectancy and quality-adjusted life expectancy: a decision model. Ann Intern Med. 2008;148:178–185. doi: 10.7326/0003-4819-148-3-200802050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braithwaite RS, Justice AC, Fusco JS, et al. Estimating the proportion of patients infected with human immunodeficiency virus who will die of comorbid disease. Am J Med. 2005;118:890–898. doi: 10.1016/j.amjmed.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Braithwaite RS, Shechter S, Roberts MS, et al. Explaining variability in the relationship between antiretroviral adherence and HIV mutation accumulation. J Antimicrob Chemother. 2006;58:1036–1043. doi: 10.1093/jac/dkl386. [DOI] [PubMed] [Google Scholar]
- 8.Braithwaite RS, Chang CC, Shechter S, Schaefer A, Roberts MS. Estimating the rate of accumulating drug mutations in the HIV genome. Value in Health. 2007;10:204–213. doi: 10.1111/j.1524-4733.2007.00170.x. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch MS, Brun-Vezinet F, D’Aquila RT, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: recommendations of an International AIDS Society–USA Panel. JAMA. 2000;283:2417–2426. doi: 10.1001/jama.283.18.2417. [DOI] [PubMed] [Google Scholar]
- 10.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 11.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 12.Braithwaite RS, Kozal MJ, Chang CH, et al. Adherence, virologic and immunologic outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS. 2007;21:1579–1590. doi: 10.1097/QAD.0b013e3281532b31. [DOI] [PMC free article] [PubMed] [Google Scholar]