Selective plasmapheresis is a technique whereby a gamma globulin rich fraction is electrophoretically separated from heparinized whole blood maintained in extracorporeal circulation(1–3). The general procedure is similar to that employed with artificial kidneys and, more specifically, with blood electrodialysis(4). It employs the forced-flow electrophoretic technique(1,2,5,6), which has been recently employed for the preparation of anti-lymphocytic globulins from horses in vivo(7) and in vitro(8,9). In the present series of experiments it has been applied to the experimental modification of the rejection reaction of pig kidney's xenografts in dogs.
These experiments have been prompted by the urgent need for a method for removal of antibodies in previously immunized patients to allow kidney transplantation. While the central role of small lymphocytes in chronic rejection has been known a long time, the more dramatic effect of preformed circulating antibodies in causing hyperacute rejection has been recognized only recently. Kissmeyer-Nielsen, et al. (10) described hyperacute rejection mediated by IgG, IgA, and IgM antibodies to renal allografts in 2 patients. The microscopic picture of these grafts revealed cortical necrosis caused by microthrombi in the glomeruli and small arterioles similar to the picture produced by the Schwartzman reaction.
Since that report, clinical and experimental evidence of antibody mediated rejection of renal allografts has been described by Williams, Starzl, Clark, Najarian and others(11–17). Patients exhibiting this response are believed to have become immunized during multiple pregnancies, by means of repeated blood transfusions while awaiting renal transplantation, or by previous renal transplants. The reaction appears to be complement dependent. It is possible to detect these antibodies by means of a crossmatch with donor leukocytes, thus patients subject to hyperacute rejection can be identified.
A similar mechanism is believed to be responsible for the rapid rejection observed in xenografts(15–17). The pig kidney-dog recipient model chosen in the present work is an example of the most acute type of rejection known, the transplanted kidney failing without exception within 6–15 min.(18–20). In 2 dogs treated by selective plasmapheresis, the pig kidney was found to survive approximately 2 hr. with moderate urine output. A third dog retained the pig's kidney for 3 hr., with copious urine output. This significant delay in the rejection is probably attributable to the depletion of circulating antibodies. If so, it would mark the first modification of an immune reaction by the removal of circulating antibodies by purely physical means.
Other approaches to the problem of removal of preformed antibodies(21,22) include adsorption of circulating antibodies from the host by means of donor tissue, depleting the host of complement, and depleting the host of globulin. Adsorption techniques have been accomplished both in experimental and in clinical renal transplants in immunized recipients with some success. However, present adsorption techniques deplete the host of platelets and result in a bleeding diathesis all too frequently.
TECHNIQUE
Selective plasmapheresis apparatus
Forced-flow electrophoresis (FFE) was first conceived as a large scale electrophoretic technique for rapid fractionation of biological materials, permitting the purification of isoelectric protein components(1, 5). In fact it proved to be a more versatile tool, suitable for the study of a variety of electrokinetic membrane phenomena, such as electrofiltration(23) and its application to water purification(24), electroadsorption of bacteriophages(25), blood electrodialysis(4), and the electroosmotic concentration and/or desalting of protein solutions(26). Because of these various modes of its use, it is of some importance to specify the parameters employed in the present work.
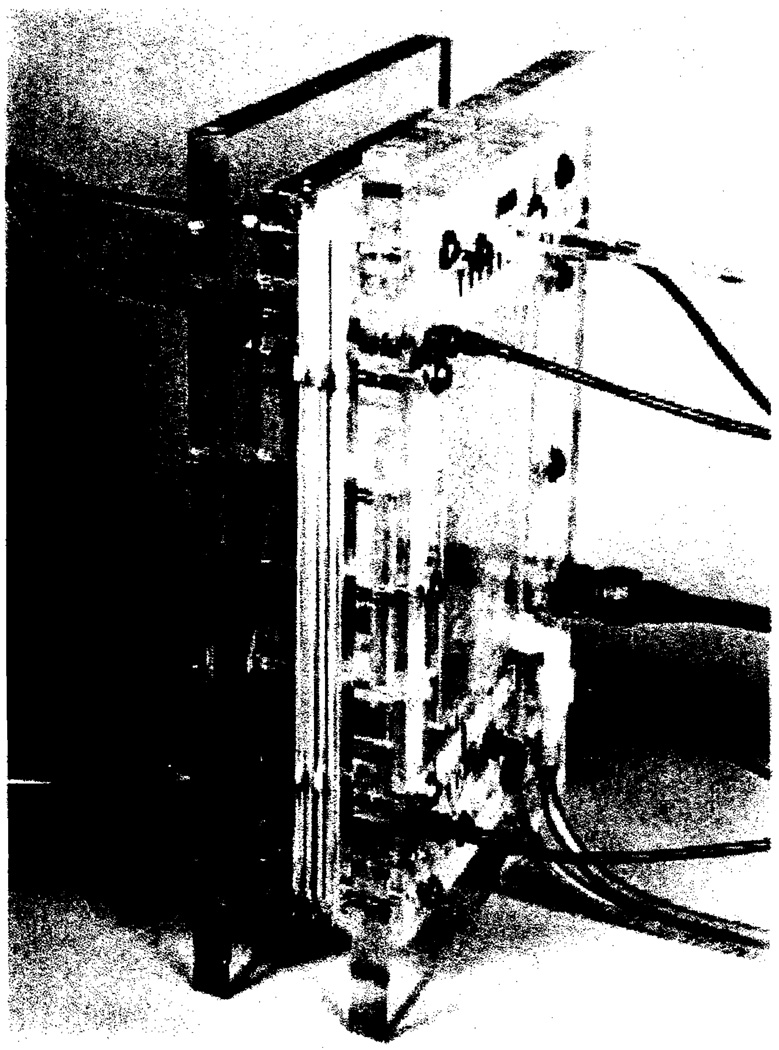
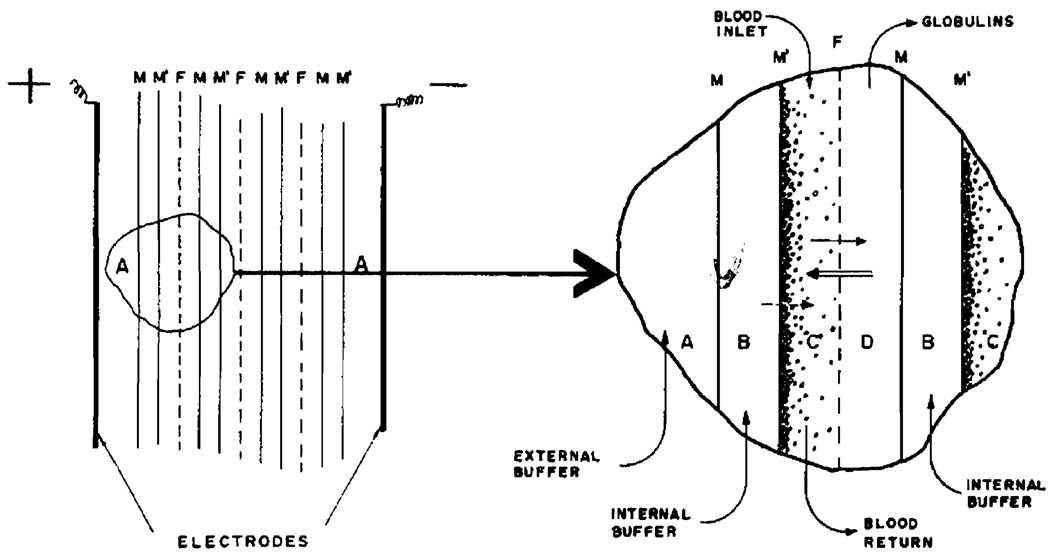
The FFE cell pack is shown in Figure 1. Plastic end-plates hold together a series of spacers, and have means for blood and buffer circulation, as well as connectors for the direct current power supply. Its function is best illustrated in the schematic drawing of the side view of the cell pack, presented in Figure 2. Parallel membranes M and M', and filters F, held in place by means of spacers not shown, form a series of narrow channels, across which the electrical field is established. Four types of compartments can be recognized: the 2 recesses A in the end-plates house the platinum electrodes and serve for external buffer circulation; channels B serve for the circulation of internal buffer; channels C contain the flowing blood and are separated from the globulin output channels D by means of the filters F. The membranes employed are the Visking regenerated cellulose membranes, analogous in properties to the cuprophane membranes employed in artificial kidney work, but mechanically more resistant. Millipore filters of 3.0 and 0.45 mµ. porosity were employed for the fractionation.
Figure 1.
Forced-flow electrophoresis cell assembly. Overall dimensions 21 × 13".
Figure 2.
Schematic presentation of forced-flow electrophoresis. M, M' - membranes, F - filters, A - external buffer compartments, B - internal buffer channels, C - blood channel, D - globulin output channel. Solid arrows mark the direction of liquid flow, double arrow shows the direction of electrophoretic migration, broken arrow shows the direction of the electroosmotic water flux.
The solid arrows mark the direction of liquid flow, including the direction of liquid flow through the filter. The double arrow indicates the electrophoretic transport of all negatively charged blood components, in a direction counter-current to that of liquid flow. It is the balance of these 2 vectors which determine the quality of fractionation. The accumulation of negatively charged colloids in the vicinity of the anodic membranes results in polarization of cell content. The latter causes local desalting along membranes and a water influx across these membranes. The direction of water and ion flux across these membranes is indicated by the broken arrow. These 2 factors have been discussed previously(4).
The purity of the fraction removed depends largely on the rate of filtration of the globulin fraction and the voltage applied. Usually, fastest possible removal of gamma globulins is desired, and, as a result, contamination with other plasma proteins is obtained. Further purification can be achieved either through forced flow electrophoresis(7–9), or, of course, through any other standard methods of protein fractionation. If no voltage is applied, the Millipore filter is immediately clogged by erythrocytes and no filtration of plasma proteins is achievable. The applied current not only fractionates the proteins but electrophoretically removes the erythrocytes from the filter surface, permitting liquid flow. The maximum filtration rate obtainable is therefore given by the rate of clearance of the erythrocytes. The electrophoretic mobility of erythrocytes is species dependent(27), the dog having the highest mobility among the common animals, man included. As a result, faster processing is achievable in dogs, with poorer quality of fractionation.
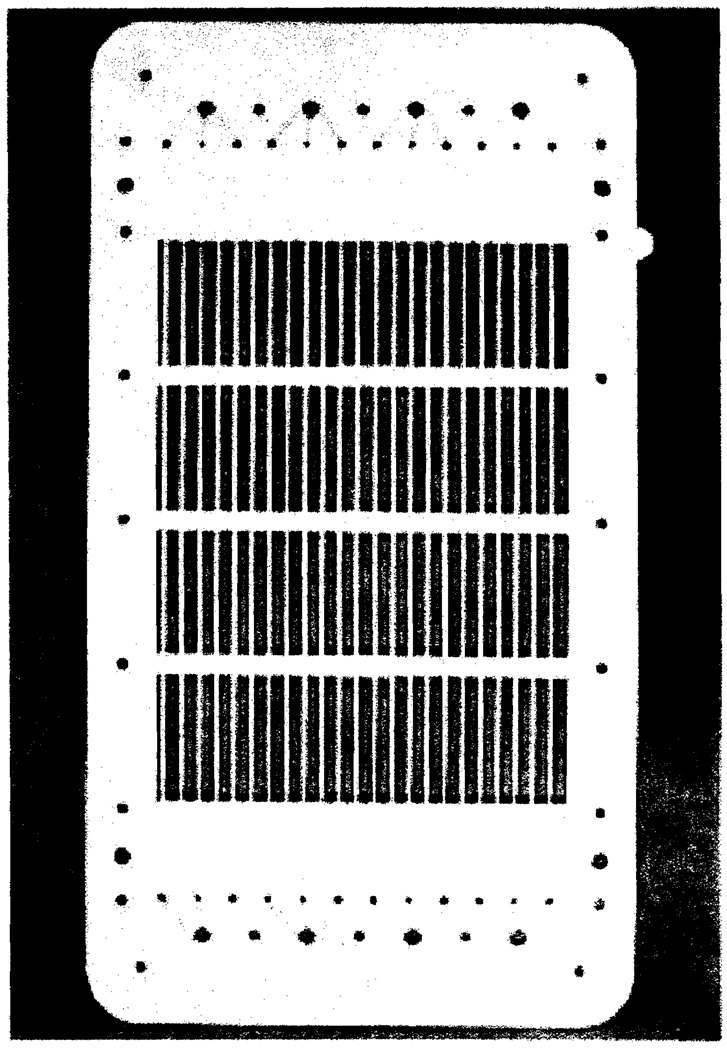
In all present experiments a cell assembly with 4 blood spacers was employed, the flow being in parallel. Each spacer has an effective filter area of 500 cm.2, 5 times larger than the apparatus used previously in blood electrodialysis(4). The internal buffer spacers (Figure 3) were injection molded with a protruding rib structure which compressed the blood input spacers to an average blood film thickness of approximately 0.030" thereby limiting the total blood volume of the apparatus to about 200–250 ml., connecting tube included. Vexar screening, similar to that employed in the disposable dialyzing membrane envelope(28), prevented the collapse of the membranes in the globulin output compartments. The pressure in the blood compartments had to be higher than that in any of the other channels. A voltage gradient of 6–8 volts/cm. was applied, resulting in a current density of 0.02–0.04 amps/cm.2. Blood flow as kept at 15–30 ml./spacer while internal buffer flow was about 5 times larger. The rate of gamma globulin withdrawal varied between 1.5 and 2.5 ml./spacer.
Figure 3.
The internal buffer spacer has a molded grid work which protrudes into the adjacent compartments, thereby limiting the blood volume.
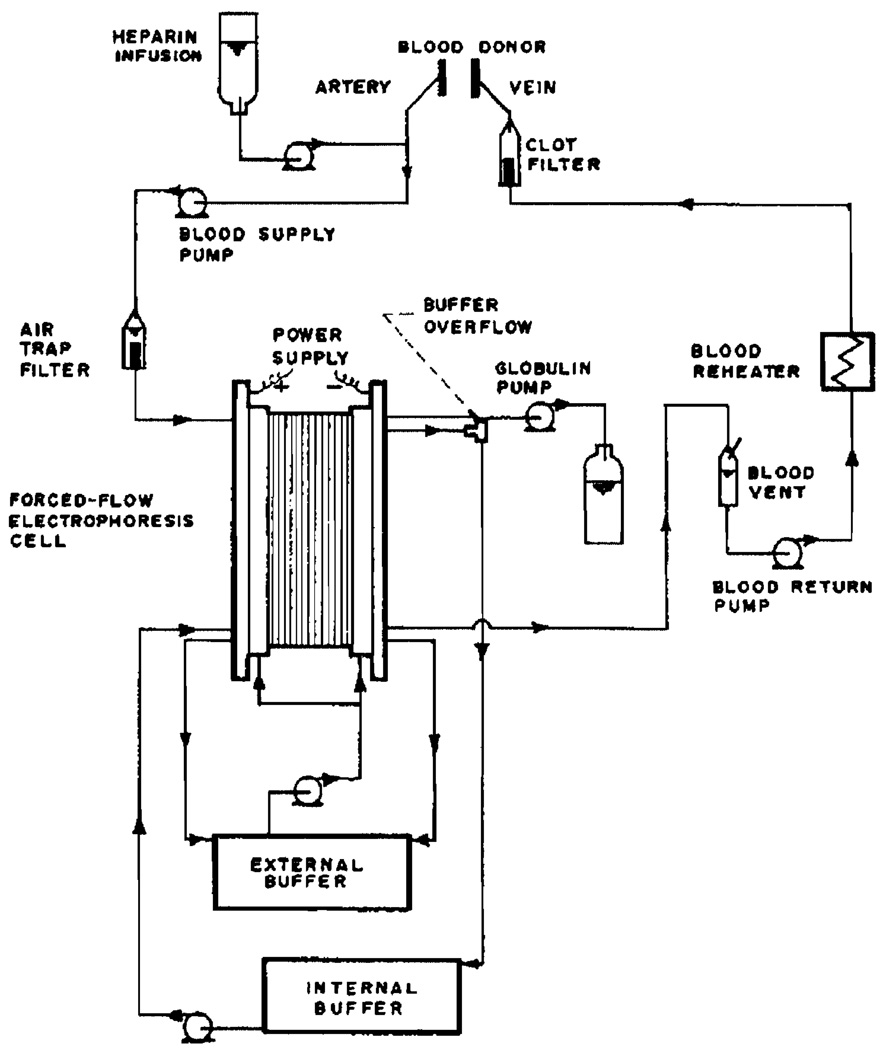
The overall blood and buffer flow is schematically presented in Figure 4. To facilitate vascular access, shunts were placed in the neck of the dogs between the external carotid artery and jugular vein. These shunts were patterned after the Quinton shunts used in artificial kidney work. While in prior work with sheep these shunts were found to remain patent for up to a month, in dogs they seem to last for about a week only. Either total body heparinization or regional heparin infusion can be employed. The apparatus itself offers a low resistance to blood flow and several plasmapheresis runs have been carried out without any blood pumps. The arteriovenous pressure differential is more than sufficient for adequate blood flow. Better fractionation, however, is obtained if the cell is isolated from periodic blood pressure variations and from the pulsatile flow resulting from the dog's heart beat. This can be achieved in a number of ways, the simplest being to use 2 non-pulsatile pumps for blood supply and return as indicated in the diagram. The globulin fraction is usually pumped. Two additional circulations of external and internal buffer are maintained by separate pumps. Both of these buffers are refrigerated to about 0–5°C. and serve to remove the heat generated by the electric current. A heat exchanger may be employed as the final element in the blood return line.
Figure 4.
Overall presentation of flow patterns in selective plasmapheresis.
Buffers in selective plasmapheresis
The in vivo nature of the electrophoretic fractionation obviously requires that it be conducted under physiologically acceptable conditions of pH and electrolyte composition. Because of various electrokinetic phenomena, the requirements for the buffer are not the same as those for the dialysate employed in hemodialysis. Mainly, the buffer should not only be isotonic, but also isoconductive with whole blood. Whole blood is less conductive than plasma or isotonic saline because of the mass or corpuscular elements which do not contribute to electrical conductivity. In electrodialysis of sheep's blood(4), a buffer was employed which consisted of a mixture of 60% of an isotonic balanced salt solution with 40% of a concentrated glucose solution, the final glucose concentration being 5%. This excess glucose prevented hemolysis which otherwise occurs along membranes as a result of local hypotonicity, induced by cell content polarization. In this regard, shifting from sheep to dog created a difficulty. Canine erythrocytes are highly permeable to glucose, and isotonic or even hypertonic glucose is not sufficient to create a hemolysis-preventing concentration gradient. The buffer had to be modified, therefore, by replacing glucose with sodium chloride. The best buffer so far employed consisted of an 80% balanced salt solution, with additional 2 Gm./L. sodium chloride. Its composition was: sodium chloride (7.0 Gm./L.); potassium chloride (0.3 Gm./L.); sodium bicarbonate (14.0 Gm./L.); sodium phosphate, dibasic (7H2O) (0.33 Gm./L.); calcium lactate (5H2O) (0.17 Gm./L.); magnesium chloride (6H2O) (0.16 Gm./L.); glucose (10 Gm./L.); ascorbic acid (0.025 Gm./L.). The ascorbic acid serves to reduce any free chlorine formed at the electrodes into chloride ions. The pH was kept at all times between 7.40 and 7.50, and no noticeable change in pH of effluent blood was observed. A total of 40 L. of buffer are prepared, 6 being used for the external buffer, the rest for the internal. These 2 buffers are circulated separately, to prevent contamination of blood by toxic products of electrolysis, formed at the electrodes only. The external buffer serves only to establish electrical contact between the electrodes and the actual cell pack. The internal buffer serves a variety of purposes; (a) it isolates the blood from above toxic products; (b) it provides for internal cooling of the cell pack; (c) it allows for better pH and electrolyte control of effluent blood; and (d) moreover, it provides for replacement of the fluid aliquot removed with the globulin fraction. This last point is purely fortuitous, as the electroosmotic water flux indicated by the broken arrow in Figure 1 happens to be of the same order as the flow of the globulin fraction through the filter, indicated by the solid arrow. Blood concentration remains therefore relatively constant, even though the volume of globulins removed exceeds the initial total blood volume of the animal.
Protocol
The use of dogs instead of sheep, and the switching to the larger cell necessitated some experimentation with optimum conditions for plasmapheresis. Operational parameters during each run, therefore, may have varied, in minor details. A number of runs were carried out without transplantation. In the first few experiments the dogs were kept anesthetized, or heavily sedated. This proved to be unnecessary and affected the hemodynamic picture. In most of the later experiments the dogs were completely unmedicated, and were only partially restrained in their movement during plasmapheresis. They were free to eat and drink ad libitum. They seemed to be in no discomfort, and urinated freely.
Dogs subjected to transplantation were first treated by selective plasmapheresis on 2 consecutive days. The arbitrary aim was to remove at least 2 L. of the globulin fraction in each plasmapheresis, requiring about a 4 hr. treatment. Toward the end of the second day's plasmapheresis, they were anesthetized and prepared for the kidney transplant by exposing the femoral artery and vein. One unit of osmotrol was administered to increase urine production. At the same time, the donor pig was also prepared.
The transplant was carried out by a standard technique. The renal artery was anastomosed end-to-end to the common femoral artery of the dog and the renal vein was anastomosed to the femoral vein in an end-to-side manner. The kidney was left external to the dog for purposes of observation and the dog was kept anesthetized. The ureter was amputated 6 cm. from the pelvis and urine was collected by placing the end of the ureter in 10 ml. test tubes throughout the transplant period. Biopsies were taken at the onset of evidence of rejection, which was determined by color change (motling) of the transplanted pig kidney, even though urine flow continued. Biopsies were also taken at cessation of urine flow, which signaled the end point of the experiment.
Analytical procedures
Plasma proteins and globulin fractions were analyzed electrophoretically by means of the Millipore Corp. PhoroScope system. Unfortunately, because of the presence of fibrinogen and traces of hemoglobin, electrophoretic analysis yielded exaggerated "beta globulin" values. Hemoglobin, due to its inherent color, causes greater adsorption on electrophoretic analysis than warranted by its protein content. It was, therefore, impossible to correctly estimate low levels of gamma globulins in the presence of these excessive amounts of proteins in the beta region, and the values tended to be too high. For this reason, chemical analysis was also employed. Fibrinogen(29) and gamma globulin(30) were separated by salt fractionation and determined by the biuret method, together with total proteins(31). Electrophoresis and salt fractionation do not necessarily give identical results(30), but at least 2 separate sets of data were obtained.
RESULTS
A total of 10 dogs have been treated by selective plasmapheresis. Six of these were employed for selective plasmapheresis only, and have received no kidney grafts. Four others have been submitted to selective plasmapheresis on 2 consecutive days, receiving the porcine kidney xenograft on the end of the second plasmapheresis. In 3 of these significant prolongation of xenograft survival has been obtained. The fourth dog has been treated less exhaustively, and rejected the xenograft within the usual 10 min. Significantly, with this last dog, about 2 hr. had passed between the end of the second plasmapheresis and beginning of transplantation. In the other 3 cases, plasmapheresis has been continued during the first hour of xenograft functioning. Because of the limited time and space allowed, we thought it best to present data on the 3 dogs only in which significant effects were noted. The data presented will serve amply to document the effects obtainable by plasmapheresis alone. It should be noted that slight variations in the conditions of plasmapheresis can affect the results considerably, the most important factors being the composition of the buffer, voltage applied, flow rate of the globulin fraction, and pulsatile vs. non-pulsatile flow.
In Table I are presented the summary data on the electrophoretic analysis of plasma proteins of the dogs before and after plasmapheresis. Samples were taken from the dogs just before plasmapheresis (marked initial), and after termination of treatment (marked final). For second day treatments, the final samples were taken just before beginning of blood circulation through the grafted kidney. The last 2 lines list the composition of the pooled globulin fractions from day 1 and 2, of the third dog. The percent gamma globulin reduction refers to decrease in final globulin levels, with respect to the initial globulin content on the first day of treatment. It can be seen that there is a rebound in gamma globulin levels between final values of day 1, and initial values of day 2. As noted before, electrophoretic determination of low levels of gamma globulins in the presence of high concentrations of beta proteins is not too dependable and leads to results which are probably too high.
TABLE I.
Summary of Electrophoretic Data on Transplanted Dogs
| DOG | DAY | SAMPLE | ALBUMIN | ALPHA GLOBULIN |
BETA GLOBULIN |
GAMMA GLOBULIN |
% GAMMA REDUCTION |
|---|---|---|---|---|---|---|---|
| 1 - T | 1st | initial | 48.5% | 15.2% | 18.1% | 18.1% | |
| 2nd | initial | 51.7 | 16.5 | 19.7 | 11.8 | ||
| final | 55.9 | 14.7 | 22.5 | 6.9 | 61.9 | ||
| 2 - T | 1st | initial | 51.6 | 17.2 | 22.0 | 9.1 | |
| final | 55.7 | 17.3 | 22.1 | 4.8 | 47.3 | ||
| 2nd | initial | 55.8 | 16.7 | 20.5 | 6.5 | ||
| final | 49.5 | 16.5 | 31.0 | 2.9 | 68.1 | ||
| 3 - T | 1st | initial | 45.4 | 11.8 | 28.4 | 14.2 | |
| final | 50.4 | 16.3 | 24.0 | 9.1 | 35.9 | ||
| 2nd | initial | 46.6 | 13.4 | 28.8 | 11.0 | ||
| final | 52.4 | 15.1 | 24.6 | 7.5 | 47.2 | ||
| 1st | glob. fract. | 9.9 | 12.9 | 45.5 | 31.7 | ||
| 2nd | glob. fract. | 15.7 | 13.7 | 46.0 | 24.7 |
It is for this reason that the plasma samples were also analyzed by salt fractionation methods. The corresponding data on dogs 1 and 2 are reported in Table II. A significant decrease in fibrinogen, gamma globulin, and total proteins can be noted. While the electrophoretic data show relative changes in plasma composition, these data show the absolute levels. The percent reduction of gamma globulin, expressed in these terms, is more significant than when based on electrophoretic data.
TABLE II.
Changes in Plasma Composition of Transplanted Dogs 1 and 2
| DOG | DAY | SAMPLE | TOTAL PROTEIN |
GAMMA GLOBULIN |
FIBRINOGEN | GAMMA GL. REDUCTION |
|---|---|---|---|---|---|---|
| (Gm.%) | (mg. %) | (mg. %) | (%) | |||
| 1 | 1st | initial | 7.0 | 1,300 | 200 | |
| 2nd | initial | 4.9 | 570 | 217 | ||
| final | 3.3 | 220 | 90 | 83 | ||
| 2 | 1st | initial | 6.3 | 570 | 217 | |
| final | 3.2 | 155 | 73 | |||
| 2nd | initial | 3.7 | 240 | 220 | ||
| final | 2.7 | 80 | 148 | 86 |
In Table III are presented more detailed data for dog 3. These data show not only the plasma composition before and after plasmapheresis, but also at various times during plasmapheresis. The most significant change in all proteins is seen within the first 15 min. This is due to the dilution of the dog's blood with the priming saline of the apparatus (about 250 ml.). The total blood flow through the cell is high in relation to the flow rate of the globulin fraction, and, therefore, there is no significant difference between samples taken from the blood lines before the FFE cell (B. C.) and after the cell (A. C.). During the course of plasmapheresis, large amounts of protein, mainly gamma and beta globulins, fibrinogen included, are withdrawn. This is not reflected in the values for total protein, which stay rather constant. This is probably due to a readjustment of hemoconcentration by the dog. To obtain an accurate material balance throughout plasmapheresis, repeated plasma volume determinations would be necessary. This has not been attempted.
TABLE III.
Changes in Plasma Composition of Transplant Dog #3
| DAY | SAMPLE1 | TOTAL PROTEIN |
FIBRINOGEN | PLASMA HEMOGLOBIN |
GAMMA GLOBULIN |
GAMMA GL. REDUCTION |
|---|---|---|---|---|---|---|
| (Gm.%) | (mg.%) | (mg.%) | (mg.%) | (%) | ||
| 1st | initial | 7.17 | 682 | 0 | 1,220 | |
| 15' PL-B.C. | 5.19 | 416 | 0 | 980 | ||
| 15' PL-A.C. | 4.78 | 424 | 0 | 836 | ||
| 240' PL-B.C. | 4.28 | 296 | 156 | 564 | ||
| 240' PL-A. C. | 4.18 | 255 | 161 | 544 | ||
| final | 3.96 | 270 | 135 | 534 | 56 | |
| 2nd | initial | 5.58 | 550 | 54 | 793 | |
| 15' PL-B.C. | 4.26 | 406 | 42 | 612 | ||
| 15' PL-A.C. | 3.75 | 365 | 41 | 461 | ||
| 180' PL-B.C. | 3.69 | 307 | 95 | 396 | ||
| 180' PL-A.C. | 3.73 | 274 | 102 | 459 | ||
| 270' PL-B.C. | 3.98 | 346 | 124 | 280 | 77 |
Samples were taken at indicated times during plasmapheresis (PL), from blood lines before the cell (B.C.) and after the cell (A.C.).
Column 5 of the same Table lists the free plasma hemoglobin. The problem of avoiding hemolysis in dogs has been mentioned in the section on buffers, and is more difficult to control than in sheep, horses, or man, glucose being ineffective as an osmotic agent. Nevertheless, the amount of hemolysis encountered seems to be of the same order as obtained in heart-lung machines, and is tolerated by the animals. Dogs erythrocytes are notoriously fragile, and the pumping arrangement also contributes to hemolysis.
The data on the composition of the globulin fractions removed from the 3 dogs are listed in Table IV. Initial plasma volumes have been estimated from hematocrit values, assuming a blood volume of 7.6% of body weight(32). It can be noted that each dog lost gamma globulins in an amount roughly equivalent to the total gamma globulins available in his circulation at the beginning of the experiment. As the gamma globulin blood levels were not reduced to zero, there was obviously significant mobilization of extravascular reserves or de novo synthesis of gamma globulins. Similar results have been obtained in prior work with sheep. It should also be noted that the total globulin volume withdrawn is greatly in excess of the initial plasma volume of the dog. The plasmapheresis method, as practiced, maintains reasonably good volume balance of the treated blood, even though relying on electroosmotic effects alone to achieve it.
TABLE IV.
Analysis of Globulin Fractions Removed from Transplanted Dogs
| DOG | DAY | PLASMA VOLUME1 |
VOLUME COLLECTED |
TOTAL PROTEIN |
GAMMA GLOBULIN |
TOTAL GAMMA COLLECTED |
% GAMMA COLLECTED2 |
|---|---|---|---|---|---|---|---|
| (ml.) | (ml.) | (Gm.%) | (mg.%) | (Gm.) | |||
| 1 - T | 1st | 1,050 | 1,790 | 1.61 | 460 | 8.23 | 62 |
| 2nd | 2,500 | 1.28 | 230 | 5.75 | 44 | ||
| 2 - T | 1st | 900 | 1,500 | 1.95 | 190 | 2.85 | 57 |
| 2nd | 1,990 | 1.12 | 116 | 2.30 | 45 | ||
| 3 - T | 1st | 1,100 | 1,985 | 1.25 | .379 | 7.52 | 56 |
| 2nd | 2,545 | 1.06 | .204 | 5.19 | 39 |
Estimated initial plasma volume of the dog, based on an assumed blood volume of 7.6% of body weight.
Percent of gamma globulin collected, from total available in dog's circulation, based on assumed plasma volume and initial gamma globulin level.
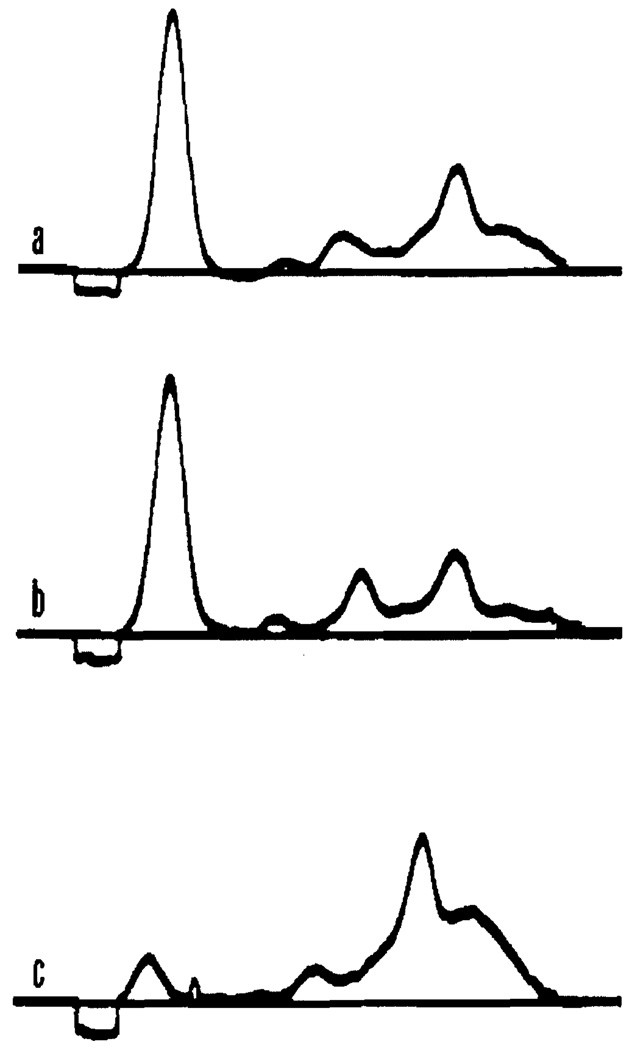
Figure 5 shows typical electrophoretic patterns. The top pattern represents the plasma of dog 3, before any plasmapheresis. The peak due to fibrinogen is clearly visible in the beta region. The center pattern is that of the dog's plasma following second plasmapheresis. Reduction in gamma globulin is clearly visible. The bottom pattern is that of the globulin fraction removed from this dog on the first day. Obviously, there are present significant amounts of all plasma proteins. By reducing the flow rate of the globulin fraction, better fractionation could be expected.
Figure 5.
Electrophoretic patterns of dog 3. Pattern a - before plasmapheresis, pattern b - after plasmapheresis, pattern c - globulin fraction removed on first day of plasmapheresis.
Blood data on dogs 1 and 2 are summarized in Table V. Data are included on initial and final samples of day 1 and initial data on day 2, followed by values obtained during the functioning of the xenograft. The zero time transplant values (0' transpl.) were obtained on samples of blood taken just before opening blood circulation to the xenograft, and represent the final values for plasmapheresis alone.
TABLE V.
Summary Blood Data on Transplant Dogs 1 and 2
| DOG | DAY | SAMPLE | HCT | WBC | PLATELETS | NEUTRO- PHILS |
LYMPHO- CYTES |
BANDS | MONO- CYTES |
EOSINO- PHILS |
|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | ||||||
| 1 | 1st | initial | 37 | 7,500 | 186,000 | |||||
| 2nd | initial | 40 | 13, 600 | 192,000 | 54 | 30 | 0 | 7 | 9 | |
| 0' transpl. | 25 | 7,270 | 65,000 | 79 | 17 | 3 | 0 | 1 | ||
| 5' transpl. | 25 | 2,768 | 69,000 | 76 | 17 | 7 | 0 | 0 | ||
| 20' transpl. | 20 | 2,855 | 54,000 | 70 | 20 | 9 | 0 | 1 | ||
| 40' transpl. | 20 | 2,810 | 57,000 | 78 | 18 | 3 | 0 | 1 | ||
| 100' transpl. | 23.5 | 10,700 | 89,000 | 75 | 18 | 6 | 1 | 0 | ||
| 2 | 1st | initial | 39 | 15,350 | 239,000 | 52 | 35 | 2 | 2 | 9 |
| final | 35 | 13,500 | 240,000 | 77 | 13 | 8 | 1 | 1 | ||
| 2nd | initial | 31 | 18,700 | 236,000 | 80 | 13 | 1 | 2 | 4 | |
| 0' transpl. | 27.5 | 11,000 | 203,000 | 78 | 15 | 2 | 1 | 4 | ||
| 5' transpl. | 29 | 5,077 | 159,000 | 70 | 22 | 2 | 0 | 6 | ||
| 20' transpl. | 30 | 7,286 | 176,000 | 80 | 15 | 0 | 1 | 4 | ||
| 40' transpl. | 27.0 | 7,041 | 174,000 | 81 | 10 | 1 | 2 | 6 | ||
| 80' transpl. | 24.5 | 7,357 | 150,000 | 81 | 13 | 4 | 2 | 0 |
Note: No basophils were found in any sample.
Corresponding data on dog 3 are reported in greater detail in Table VI. These include samples taken during plasmapheresis, before and after cell, and during xenograft functioning, from the kidney's artery and vein. As in previous cases, significant reductions in platelets can be noted. Because of the known toxic effects of heparin, as well as changes in blood viscosity during plasmapheresis, the differential counts are not as reproducible as might be desired, but no consistent change is noted.
TABLE VI.
Detailed Blood Data on Transplant Dog #3
| DAY | SAMPLE1 | HCT | WBC | PLATELETS | NEUTRO- PHILS |
LYMPHO- CYTES |
BANDS | MONO- CYTES |
EOSINO- PHILS |
|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | |||||
| 1st | initial | 43 | 26,600 | 136,000 | 72 | 25 | 0 | 1 | 2 |
| 15' PL.-B.C. | 35 | 15,600 | 97,000 | 61 | 35 | 1 | 0 | 3 | |
| 15' PL. -A.C. | 33 | 16,000 | 97,000 | 66 | 33 | 0 | 0 | 1 | |
| 240' PL.-0.C. | 33 | 15,750 | 94,000 | 81 | 14 | 5 | 0 | 0 | |
| 240' PL.-A.C. | 32 | 15,500 | 88,000 | 77 | 14 | 8 | 1 | 0 | |
| final | 32.5 | 15,000 | 108,000 | 78 | 18 | 0 | 2 | 2 | |
| 2nd | initial | 32 | 23,700 | 49,000 | 83 | 14 | 2 | 1 | 0 |
| 15' PL. -B.C. | 28 | 21,000 | 34,000 | 77 | 21 | 0 | 0 | 2 | |
| 15' PL. -A.C. | 26 | 19,750 | 32,000 | 69 | 22 | 4 | 1 | 4 | |
| 180' PL. -B.C. | 27 | 19,600 | 50,000 | 77 | 17 | 4 | 0 | 2 | |
| 180' PL. -A.C. | 26.5 | 19,950 | 43,000 | 86 | 13 | 1 | 0 | 1 | |
| 0' TR | 27.5 | 19,100 | 69,000 | 89 | 5 | 4 | 1 | 1 | |
| 5' TR-A | 25 | 8,750 | 32,000 | 75 | 13 | 11 | 0 | 1 | |
| 5' TR-V | 25.5 | 8,600 | 17,000 | 64 | 32 | 3 | 0 | 1 | |
| 10' TR-A | 25.5 | 9,250 | 31,000 | 66 | 24 | 7 | 0 | 3 | |
| 10' TR-V | 28 | 7,220 | 20,000 | 53 | 42 | 1 | 2 | 2 | |
| 30' TR-A | 26.5 | 11,200 | 54,000 | 84 | 9 | 4 | 2 | 1 | |
| 30' TR-V | 27.5 | 11,100 | 42,000 | 74 | 21 | 3 | 0 | 2 | |
| 50' TR-A | 23.0 | 16,050 | 48,000 | 85 | 11 | 3 | 0 | 1 | |
| 50' TR-V | 25.5 | 15,900 | 34,000 | 80 | 16 | 2 | 1 | 1 | |
| 70' TR-A | 30 | 17,250 | 48,000 | 87 | 11 | 2 | 0 | 0 | |
| 70' TR-V | 32 | 17,400 | 43,000 | 76 | 17 | 4 | 3 | 0 | |
| 120' TR-A | 27 | 22,650 | 52,000 | 90 | 6 | 2 | 1 | 1 | |
| 180' TR-A | 33 | 23,100 | 46,000 | 85 | 7 | 8 | 0 | 0 |
Samples were taken at the indicated times during plasmapheresis (PL.), from blood lines before the cell (B.C.) and after the cell (A. C.). Samples were also taken during transplantation (TR), from the transplanted kidney's artery (A) and vein (V). No basophils were found in any sample.
Because of the involvement of fibrinogen in the rejection reaction, the variations in fibrinogen values during the xenograft functioning are reproduced in Table VII. Significant loss of fibrinogen is observed.
TABLE VII.
Fibrinogen Changes During Pig'S Kidney Transplantation
| TIME | DOG 1 | DOG 2 | DOG 3 | DOG 3 |
|---|---|---|---|---|
| (min.) | (arterial) | (arterial) | (arterial) | (venous) |
| 0 | 102 | 148 | 346 | - |
| 5 | 104 | 165 | 303 | 293 |
| 10 | 75 | 162 | 288 | 270 |
| 20 | 52 | 164 | 251 | 255 |
| 40 | 52 | 95 | - | - |
| 60 | - | 92 | 157 | 241 |
| 80 | - | 80 | 305 | 253 |
| 120 | - | 98 | 270 | - |
| 180 | - | - | 278 | - |
The most important result is, of course, the prolongation of the xenograft function, and the urine production was as follows:
| dog 1: | 32 ml. during first 110 min. |
| dog 2: | 18 ml. during the first 100 min. |
| dog 3: | 197 ml. during the first 60 min. |
| 67 ml. during the second 60 min. | |
| 9 ml. during the third 60 min. |
Examination of biopsy samples taken at various times will be published at a later date.
DISCUSSION
Selective plasmapheresis is a method whereby substantial quantities of circulating antibodies can be withdrawn. These are partially replaced by extravascular reserves or de novo synthesis. In prior experiments with sheep(33), hyperimmunized against various antigens, it was noted that antibody titers after plasmapheresis did not necessarily return to pretreatment values, even though electrophoretically measured gamma globulin did return to its normal level. Some antibody titers remained substantially lowered, while in other instances, there was an increase in antibody levels, mimicking an anamnestic response. The response to selective plasmapheresis may well depend on the antigen employed, or on other circumstances.
In this series of experiments it has been shown that plasmapheresis, carried out immediately before xenograft transplantation, can significantly delay the rejection reaction. While porcine kidneys are normally rejected within 6–15 min., 3 out of 4 dogs have had survival in excess of 100 min., and one dog had urine production for 180 min. It is open to speculation whether more exhaustive plasmapheresis, or combination of plasmapheresis with immunosuppressive therapy, would result in further prolongation.
While it is not excluded that this prolongation was due in part to lowering of platelets, complement titer, fibrinogen, or other factors, we believe that the mechanism was depletion of antibodies. These results represent the first modification of an acute immune response through the use of an artificial-kidney-like device.
SUMMARY
Porcine kidney xenografts on dogs are normally rejected within 6–15 min. Selective plasmapheresis permits substantial depletion of circulating antibodies and results in a significant delay of the rejection reaction.
ACKNOWLEDGMENT
We wish to thank Drs. Charles Zukoski and Scott Clark, Miss Joyce Trembath, Mr. Paul Taylor, Mr. Yosh Arai, Mrs. Isabelle Snow, and Mrs. Ruth Gibbs, for their assistance.
REFERENCES
- 1.Bier M. Preparative electrophoresis without supporting media. In: Bier M, editor. Electrophoresis. New York, N. Y.: Academic Press; 1959. p. 263. [Google Scholar]
- 2.Bier M. Membrane Processes for Industry. Birmingham, Ala.: Southern Research Institute; 1966. Forced-flow electrophoresis and its biomedical applications; p. 218. [Google Scholar]
- 3.Bier M. Selective plasmapheresis and its effects on sheep (abstract); Sixth Intern. Congr. Biochem; New York: 1964. p. 1964. [Google Scholar]
- 4.Bier M, Bruckner GC, Roy HE. Blood electrodialysis. Trans. Amer. Soc. Artif. Int. Organs. 1967;13:227. [Google Scholar]
- 5.Bier M. New principle of preparative electrophoresis. Science. 1957;125:1084. doi: 10.1126/science.125.3257.1084. [DOI] [PubMed] [Google Scholar]
- 6.Hannig K. Preparative electrophoresis. In: Bier M, editor. Electrophoresis. Vol. II. New York: Academic Press; 1967. p. 423. [Google Scholar]
- 7.Logan EF, Stenhouse A, Watt JG, Clark AE. Recovery of immunoglobulin G from horses by combination of selective plasmapheresis and forced flow electrophoresis. (in press) [PubMed] [Google Scholar]
- 8.Moberg AW, Gewurz H, Simmons RL, Gunnarsson A, Merkel F, Najarian JS. A new efficient method for preparation of immunoelectrophoretically pure horse antihuman antilymphoblast globulin. Surg. Forum. 1969;20:261. [PubMed] [Google Scholar]
- 9.Merkel FK, Simmons RL, Moore GE, Moberg AW, Najarian JS. Studies of anti-lymphoblast globulin. Rev. Surg. (in press) [PubMed] [Google Scholar]
- 10.Kissmeyer-Nielsen F, Peterson VP, Olsen S, Fieldborg O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966;11:662. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 11.Lee HM, Weymouth RF, Harlan WR, Holden KR, Stanley GM, Millington GA, Hume DM. Studies in hyperacute and chronic renal homograft rejection in man. Surgery. 1967;62:204. [Google Scholar]
- 12.Terasaki PI, Thrasher DL, Hauber TH. Adv. in Transplantation. Baltimore, Md.: Williams & Wilkins; 1968. Serotyping for homotransplantation. XIII. Immediate kidney transplant rejection and associated performed antibodies; p. 225. [Google Scholar]
- 13.Najarian JS, Foker JE. Mechanisms of kidney allograft rejection. Transplantation Proc. 1969;1:184. [PubMed] [Google Scholar]
- 14.Milgrom F, Kano K, Klassen J. Role of humoral antibodies in rejection of renal allografts. Transplantation Proc. 1969;1:1013. [PubMed] [Google Scholar]
- 15.Clark DS, Foker JE, Gewurz H, Good RA, Varco RL. Effector mechanisms in renal graft rejection. Surgery. 1967;62:770. [PubMed] [Google Scholar]
- 16.Foker JE, Clark DS, Pickering RJ, Good RA, Varco RL. Studies on the mechanism of canine renal allograft rejection. Transplantation Proc. 1969;1:296. [PubMed] [Google Scholar]
- 17.Cochrum KC, Davis WC, Kountz SL, Fudenberg HH. Renal autograft rejection initiated by passive transfer of immune plasma. Transplantation Proc. 1969;1:301. [PubMed] [Google Scholar]
- 18.Linn BS, Jennson JA, Portal P, Snyder GB. Renal xenograft prolongation by suppression of natural antibody. J. Surg. Res. 1968;8:211. doi: 10.1016/0022-4804(68)90088-7. [DOI] [PubMed] [Google Scholar]
- 19.Linn BS, Jennson JA, Padro V, Snyder GB. Current Topics in Surgical Research. Academic Press; 1969. Ultrastructural precursors of rejection in re-implanted and X-vivo renal xenografts. [Google Scholar]
- 20.Perper RJ, Najarian JS. Experimental renal heterotransplantation. Transplantation. 1966;4:377. doi: 10.1097/00007890-196607000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Giles J, Starzl TS. Heterograft protection by antibody depletion. (To be published) [Google Scholar]
- 22.Perper RJ, Merkel FK, Najarian JS. Prolongation of renal xenograft survival. JAMA. 1968;204:531. [Google Scholar]
- 23.Moulik SP, Cooper FC, Bier M. Forced-flow electrophoretic filtration of clay suspensions. J. Colloid Interface Sci. 1967;24:427. [Google Scholar]
- 24.Bier M, Moulik SP. Water purification by large scale electrophoresis; Proc. Third Annual American Water Resources Conference; San Francisco, Calif.: American Water Resources Assoc.; 1967. p. 524. [Google Scholar]
- 25.Bier M, Bruckner GC, Cooper FC, Roy HE. Concentration of bacteriophage by electrophoresis. In: Berg G, editor. Transmission of Viruses by the Water Route. New York, N. Y.: Interscience Publishers; 1967. p. 57. [Google Scholar]
- 26.Bier M. Symposium on Electrodialysis. Boston, Mass.: Electrochemical Society; 1968. Electrophoretic membrane processes. (in press) [Google Scholar]
- 27.Brinton CC, Lauffer MA. The electrophoresis of viruses, bacteria, and cells, and the microscope method of electrophoresis. In: Bier M, editor. Electrophoresis. New York, N. Y.: Academic Press; 1959. p. 480. [Google Scholar]
- 28.Bier M. Discussion. Trans. Amer. Soc. Artif. Int. Organs. 1968;14:97. [Google Scholar]
- 29.Reiner M, Cheung HL. Fibrinogen. Stand. Meth. Clin. Chem. 1961;3:114. [Google Scholar]
- 30.Friendman HS. Gamma globulin in serum. Stand. Meth. Clin. Chem. 1958;2:40. [Google Scholar]
- 31.Reinhold JG. Total Protein, albumin, and globulin. Stand. Meth. Clin. Chem. 1953;1:88. [Google Scholar]
- 32.Schermer S. Die Blutmorphologie der Laboratoriumstiere. 2nd Ed. Verlag, Leipzig, Germany: Johann Ambrosius Barth; 1958. [Google Scholar]
- 33.Bier M. Personal Communication [Google Scholar]