Abstract
Physical activity improves learning and hippocampal neurogenesis. It is unknown whether compounds that increase endurance in muscle also enhance cognition. We investigated the effects of endurance factors, peroxisome proliferator-activated receptor δ agonist GW501516 and AICAR, activator of AMP-activated protein kinase on memory and neurogenesis. Mice were injected with GW for 7 d or AICAR for 7 or 14 d. Two weeks thereafter mice were tested in the Morris water maze. AICAR (7 d) and GW improved spatial memory. Moreover, AICAR significantly, and GW modestly, elevated dentate gyrus neurogenesis. Thus, pharmacological activation of skeletal muscle may mediate cognitive effects.
Physical activity has many benefits for brain function ranging from memory to mood (Hillman et al. 2008). There is a strong positive correlation between running and performance in hippocampus-dependent spatial memory tasks (van Praag 2008). Research into mechanisms underlying effects of running on the brain has mainly focused on changes in neurotransmitters, neurotrophins, spine density, and hippocampal neurogenesis (Cotman et al. 2007; Gomez-Pinilla et al. 2008; Hillman et al. 2008; van Praag 2008). Conversely, the peripheral triggers of the cellular and molecular cascades in the brain that lead to improved cognition have remained unclear. It has been suggested that serum insulin-like growth factor-1 (IGF) may play a role as the peripheral blockade abolished the running-induced enhancement of hippocampal neurogenesis (Trejo et al. 2001). Similar observations were made following systemic blockade of vascular endothelial growth factor (VEGF) (Fabel et al. 2003). However, the possibility that skeletal muscle activation as a result of exercise or pharmacological agents underlies cognitive effects of aerobic activity has not been explored.
Recently, transcriptional factors regulating muscle fiber contractile and metabolic genes have been identified (Wang et al. 2004). The peroxisome proliferator activated receptor δ (PPARδ) is a transcription factor that regulates fast-twitch muscle fiber contraction and metabolism. Overexpression of this factor increased oxidative muscle fiber number. In addition, administration of the selective agonist GW501516 increased exercise stamina when combined with training (Narkar et al. 2008). PPARδ is controlled by the AMP-activated protein kinase (AMPK), a master metabolic regulator important for glucose homeostasis, appetite, and exercise physiology (Hardie 2004). Treatment with AMPK agonist AICAR enhanced running endurance by 45% in sedentary mice (Narkar et al. 2008). It has not been determined whether the effects of these compounds extend from the periphery to brain function, and may influence hippocampal neurogenesis and spatial memory. Here we show that systemic pretreatment with AICAR, and more modestly, GW501516, 2 wk prior to behavioral testing enhances spatial learning and hippocampal neurogenesis.
In the present study, female C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) 2-mo-old, were housed under standard conditions, three mice per cage, with food and water ad libitum. Mice were injected intraperitoneally (i.p.) with GW501516 (GW, 5 mg/kg/day; for 7 d; Enzo Life Sciences) dissolved in oil vehicle (Premium MCT Gold, Ultimate Nutrition Inc.) or an equal volume of oil; or 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR, Toronto Research Chemicals Inc.) dissolved in saline, 500 mg/kg/day or saline for 7 (ACR7) or 14 d (ACR14) (Table 1). The mice concurrently received bromodeoxyuridine (BrdU) injections (50 mg/kg; on days 1–7 [ACR7], days 1, 3, 5, 7, 9, 11, 13 [ACR14], and days 1, 3, 5, 7 [GW] of treatment; Table 1). Mice were tested in the water maze starting 2 wk after the last drug injection. After completion of water-maze testing, animals treated with GW and ACR7 were evaluated in the open field and accelerating rotarod. For histological analysis of ACR7 a separate group of mice that had not been behaviorally tested was evaluated for BrdU labeling. These mice were sacrificed either 1 d after the end of the injections to evaluate new cell proliferation, or 1 mo after to evaluate cell survival. All animal procedures were approved by the National Institute of Health Animal Care and Use Committee, protocol 396-LNS-2011.
Table 1.
Compound treatment and neurogenesis
| Group | N | BrdU injections | BrdU+ cell NR | Percent BrdU/NeuN |
|---|---|---|---|---|
| SAL7 | 7 | 7, daily | 1879 ± 126 | 80.37 ± 2.88% |
| ACR7 | 10 | 7, daily | 2595 ± 121** | 89.62 ± 1.88%* |
| SAL14 | 8 | 7, every other day | 1350 ± 87 | n.d |
| ACR14 | 7 | 7, every other day | 1272 ± 82 | n.d. |
| VEH | 10 | 4, every other day | 562 ± 70 | 85% ± 2.5% |
| GW | 8 | 4, every other day | 746 ± 72# | 90% ± 2.2% |
Female C57Bl/6 mice were injected with saline (SAL) or AICAR (500 mg/kg) daily for 7 (ACR7) or 14 d (ACR14), and with GW501516 (5 mg/kg) or vehicle (VEH) daily for 7 d. BrdU (50 mg/kg) was injected concurrently. One month after the last injection, BrdU+ cell number and differentiation (BrdU/NeuN) were quantified. New cell survival (**P < 0.0012) and neurogenesis (*P < 0.018) were significantly increased in ACR7 as compared with SAL7 controls. In the GW-treated mice a trend toward increased cell survival (#P = 0.08) as compared with VEH was observed.
Mice were trained in the Morris water maze (Morris et al. 1982) to find a platform hidden 5 mm below the surface of a pool (1.40-m diameter) filled with water made opaque with white nontoxic paint. Starting points were changed daily for each trial. A trial lasted either until the mouse had found the platform or for a maximum of 60 sec. Mice rested on the platform for 10 sec after each trial. ACR7 mice were trained with two trials per day over 11 d and, subsequently, to a new platform location with four trials per day over 5 d. ACR14 and GW mice were trained with four trials per day over 7 and 8 d, respectively. Upon completion of training, the platform was removed for 60-sec probe trials. Platform latency was recorded (Anymaze, Stoelting Co.).
In the open field test, mice were placed in the center of the open field arena and allowed to move freely for 30 min while being tracked by an automated tracking system (Activity Monitor Version 4, MED Associates). Mice were also tested on an accelerating rotarod (Med-Associates), 2.5–25 RPM in 300 sec. The latency to the first fall was recorded three consecutive times.
For histological analysis, animals were deeply anesthetized with isofluorane and perfused transcardially with 0.9% NaCl solution, followed by 4% (wt/vol) paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4). Brains were postfixed in 4% PFA for 3 d, followed by equilibration in 30% (wt/vol) sucrose. Tissue was sectioned coronally (40 µm) on a freezing microtome (Thermo- Fisher) and stored at −20°C in cryoprotectant solution. For the GW experiment a 1:6 series of sections was used for immunostaining, using double-cortin (DCX) anti-goat, 1:500 (Santa Cruz Biotechnology) in combination with the secondary antibody donkey anti-goat IgG (Jackson) conjugated with CY5 (1:250), or the secondary antibody donkey anti-goat biotin (1:250, Jackson), followed by ABC reagent (Vector laboratories) and peroxidase detection for 5 min using diaminobenzedine (DAB) as chromogen (0.25 mg/mL DAB, 0.01% H2O2). DCX-DAB-labeled cells were counted in the dentate gyrus in four equidistant sections (240 µm apart), from rostral to caudal, through a 40x objective (Olympus BX51).
Immunohistochemistry for BrdU and immunofluorescent double-labeling for BrdU and the Neuronal marker NeuN were performed on a one-in-six series of equidistant (240 µm between sections) free-floating 40-µm coronal sections. The antibodies used were rat anti-BrdU (1:100, Accurate; Harlan Sera-Lab) and mouse anti-NeuN (1:100, Millipore). The fluorescent secondary antibodies used were donkey anti-mouse Cy3 (Jackson ImmunoResearch) and donkey anti-rat Alexa-Fluor 488 (Invitrogen) at a concentration of 4 µg/mL. To determine the number of surviving BrdU-labeled cells in the GW, ACR7, and ACR14 experiments, staining for BrdU with the peroxidase method was used as described (Creer et al. 2010) and the total number of BrdU-positive cells was counted in a 1:6 series of equidistant (240 µm apart) rostral-caudal sections. For cell proliferation, BrdU-labeled cells were quantified using the fractionator software; the area of the dentate gyrus was traced, and a counting frame (0.35 × 0.35 µm) was used to estimate the number of new cells using a 20X objective (StereoInvestigator system, MicroBrightfield Inc, VT; Olympus BX51).
Sections from GW and ACR7-treated animals surviving 4 wk after the last injection of BrdU were double-labeled for BrdU and NeuN as described above, analyzed by confocal microscopy (Olympus IX81 spinning disk confocal), and were quantified using imaging software (Slidebook; Intelligent Imaging Innovations, Inc.). Fifty BrdU-positive cells per animal were analyzed for coexpression of BrdU and NeuN for neuronal phenotype. Ratios of BrdU-positive cells colabeling with NeuN were determined.
ANOVA (with repeated measures over days) was applied to the water-maze training data and open-field distance traveled, and ANOVA (factorial) was used for the probe trial analysis. Histological data was analyzed using Student's t-tests.
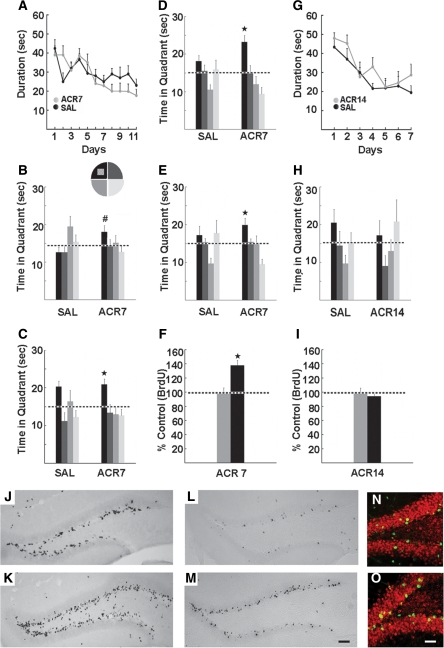
As illustrated in Figure 1, memory function was enhanced following administration of AMPK agonist AICAR (500 mg/kg) for 1 wk (ACR7), but not 2 wk (ACR14). The ACR7 mice (saline, n = 12; drug, n = 11) were trained in the Morris water maze (Morris et al. 1982) with two trials per day over 11 d, a challenging training paradigm used previously in exercise and learning studies (van Praag et al. 1999). Latency did not differ between the groups (F(1,21) = 0.60, P > 0.45; Fig. 1A). However, ACR7 mice performed significantly better than saline-treated mice (SAL7) in the probe trials. Four hours after testing on day 8, ACR 7 mice spent more time in the target quadrant than SAL7 mice (t(21) = 2.17, P < 0.05; Fig. 1B). Moreover, at 4 h after the last training trial on day 11, ACR7 mice spent more time in the target quadrant as compared with all other quadrants, (F(3,40) = 4.22, P < 0.01; one-way ANOVA over quadrants), whereas SAL7 mice did not, P > 0.05 (Fig. 1C). To further assess enhancement of memory function, the same groups of mice were trained with four trials per day over 5 d to a new platform location. Latency did not differ between groups (F(1,21) = 1.18, P > 0.32) but task retention was enhanced, as evidenced by significantly more time spent in the target quadrant as compared with all other quadrants in only the ACR7 mice at 4 h (P < 0.006) and 24 h (P < 0.04) after the last acquisition session on day 5 (Fig. 1D,E). Since the retention of the task was more apparent upon training the mice with four trials per day, we continued using this paradigm in the next experiments (ACR14 and GW). Open-field total distance (SAL7, 3541 ± 199 cm; ACR7, 3010 ± 762 cm; [F(1,15) = 2.61, P > 0.13]) and the average latency to first fall from the rotarod (SAL7, 175.5 ± 35.7 sec; ACR7, 220.3 ± 23.2 sec; [F(1,15) = 1.15, P > 0.30]) were unchanged.
Figure 1.
Water maze performance and neurogenesis in mice treated with saline (SAL) or AICAR, (500 mg/kg) for 7 (ACR7) or 14 d (ACR14). (A) ACR7 mice were trained for 11 d with two trials per day in the Morris water maze, 2 wk after injections. Latency to the platform did not differ between the groups. (B,C) However, the ACR7 mice performed better than SAL7 mice in probe trials performed 4 h after the last training session on days 8 and 11. (B) In the day 8 probe ACR7 mice showed a significant preference for the target area as compared with SAL7 mice (#P < 0.05). (C) In the day 11 probe only the ACR7 mice preferred the platform quadrant in comparison to the other quadrants (*P < 0.02). (D,E) Upon training to a new platform location with four trials per day over 5 d, significant retention of platform location was observed (D) 4 h (*P < 0.006) and (E) 24 h (*P < 0.04) after the last training session in ACR7 mice. (G,H) Longer treatment with AICAR over 14 d had no effect on water-maze performance. ACR14 mice were trained with four trials per day for 7 d. (G) There was no difference in acquisition between the groups. (H) ACR14 mice showed no retention of spatial memory in the 4-h probe trial. (I) In addition, there was no effect of ACR14 on BrdU+ cell number, whereas (F) ACR7 significantly enhanced new cell survival. (J–O) Photomicrographs of BrdU+ cells 1 d (J,K) and 4 wk (L,M) after the last of a daily series of seven BrdU (50 mg/kg) and AICAR (500 mg/kg) injections in SAL7 (J,L) and ACR7 mice (K,M). Scale bar, 50 µm. (N,O) Confocal images of BrdU-positive cells in SAL7 (N) and ACR7 mice (O) 4 wk after the last injection. Sections were immunofluorescent double-labeled for BrdU (green) and NeuN, indicating neuronal phenotype (red). Scale bar, 20 µm. Error bars, SEM.
Interestingly, longer administration of the compound for 2 wk (ACR14) prior to testing did not benefit learning (SAL14, n = 8; ACR14, n = 7). Indeed, even though the total number of training trials (28 trials) for ACR14 was greater than in the initial ACR7 experiment (22 trials), the mice did not learn the task at all. There was no difference in latency between the ACR14 and SAL14 mice following training with four trials per day over 7 d (F(1,13) = 0.65, P > 0.68; Fig. 1G) and no retention of platform location in the 4-h probe trial on day 7 (Fig. 1H), suggesting that duration of drug treatment is important.
In both ACR7 and ACR14 experiments, BrdU labeling was examined (Table 1; Fig. 1F,I–M). In the ACR7 group that was not behaviorally tested, cell proliferation (1 d after the last of seven BrdU injections, n = 6 per group) was increased (SAL7, 11,750 ± 512; ACR7, 13,612 ± 561 BrdU+ cells; [t(10) = 2.24, P < 0.05]; Fig. 1J,K). In addition, new cell survival at 4 wk after the last BrdU injection (SAL7, n = 7; ACR7, n = 10) was enhanced (t(15) = 3.99, P < 0.0012; Table 1; Fig. 1L,M). Furthermore, the percentage of double-labeled BrdU/NeuN cells was higher (t(15) = 2.69, P < 0.018; Fig. 1N,O). ACR14, however, had no neurogenic effect (t(13) = 1.36, P > 0.19; SAL14, n = 8; ACR14, n = 7), correlating with the absence of spatial memory enhancement (Table 1; Fig. 1I).
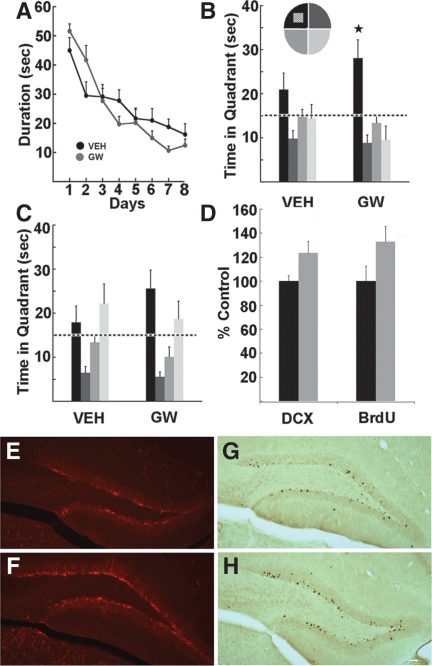
Memory function was also enhanced following administration of the PPARδ agonist, GW501516 (5 mg/kg/day for 7 d, vehicle, n = 10; GW, n = 8). Two weeks after the last injection mice were trained to find a hidden platform in the Morris water maze (Morris et al. 1982) over 8 d, with four trials per day. ANOVA with repeated measures (Days) revealed a significant interaction for latency (F(7,16) = 2.65, P < 0.02), albeit no main effect (F(1,16) = 0.12, P > 0.73; Fig. 2A). The probe trial 4 h after the last training trial on day 8 showed that only compound-treated mice spent significantly more time in the platform quadrant than in all of the other quadrants (P < 0.024; Fig. 2B), whereas the vehicle group did not show a preference. At 48 h after training, a trend toward a preference for the target quadrant was observed in GW-treated mice (Fig. 2C). Motor behavior and body weight (F(1,16) = 0.73, P > 0.41) were not altered. In the open-field distance traveled over 30 min (vehicle, 3260 ± 208.1 cm; GW, 2625 ± 298.15 cm; F(1,16) = 2.87, P > 0.11) and average latency on the accelerating rotarod to the first fall (vehicle, 176.51 ± 20.85 sec; GW, 177.28 ± 19.5 sec; F(1,16) = 0.76, P > 0.39) did not differ between the groups. To assess hippocampal cell genesis, double-cortin (DCX) and BrdU labeling were examined. A marginal increase in DCX labeling was observed (vehicle, 2180 ± 102 DCX+ cells; GW, 2690 ± 208 DCX+ cells; [t(8) = 2.20, P = 0.06]; Fig. 2D–F). Furthermore, trends were observed in GW-treated mice toward increased BrdU-positive cell survival (t(15) = 1.82, P = 0.08) and differentiation (t(15) = 1.89, P = 0.07; Table 1; Fig. 2D,G,H).
Figure 2.
The PPARδ agonist GW enhances Morris water-maze performance and new cell genesis. Mice were treated with vehicle (VEH) or GW (5 mg/kg) for 7 d. Two weeks after treatment, mice were trained for 8 d with four trials per day to find the hidden platform. (A) A significant interaction for latency over days was observed between the groups (P < 0.02). Probe trials were carried out 4 h (B) and 48 h (C) after the last training trial. (B) At 4 h, only GW-injected mice showed a significant preference for the target zone as compared with all other quadrants of the pool (P < 0.024). (C) At 48 h, GW mice showed a trend toward a preference for the target area. (D) A trend toward enhanced new cell genesis was observed in GW mice based on both DCX (P = 0.06) and BrdU (P = 0.08) labeling. (E,F) Images of DCX-positive cells in (E) vehicle and (F) GW mice. (G,H) Photomicrographs of BrdU-positive cells 4 wk after the last injection in (G) vehicle and (H) GW mice. Scale bar, 20 μm. Error bars, SEM.
In the present study we show that the PPARδ agonist GW501516, and more profoundly so, the AMPK activator AICAR, enhance neurogenesis and spatial memory function in sedentary mice. GW enhanced retention of spatial memory, but to a lesser extent than AICAR, concurrent with a marginal increase in dentate gyrus neurogenesis. In previous work, GW treatment did not increase running endurance unless it was paired with training (Narkar et al. 2008). Our GW results appear to reflect its modest “exercise” phenotype. The AMPK agonist AICAR had more robust effects on spatial memory function, cell proliferation, and neurogenesis, consistent with its endurance phenotype. AMPK is activated by running in rodents (Winder and Hardie 1996; Steinberg and Kemp 2009) and humans (Wojtaszewski et al. 2000), and may interact with additional transcriptional regulators such as PGC1α (Bronner et al. 2004; Jäger et al. 2007) to mediate endurance (Narkar et al. 2008). However, while the effects of both compounds were similar to voluntary exercise, overall, the results are more modest than those observed with running. Running improves learning and memory, and causes a two to fourfold increase in dentate gyrus neurogenesis (van Praag 2008). It remains to be determined whether combined administration of GW and AICAR would result in further cognitive and neurogenic improvements.
In initial research, demonstrating effects on exercise stamina compound administration was 4 wk, at the same dose as used here (Narkar et al. 2008), which activates AMPK-α2 receptors in muscle (Pold et al. 2005). However, 10 d of AICAR treatment mimicked the effect of 10 d of training on skeletal muscle AMPK activation (McConell et al. 2008). In addition, 6 d of GW501516 or AICAR treatment significantly changed muscle metabolic gene expression (Narkar et al. 2008). In our study, the PPARδ agonist was given at a 5 mg/kg dose for 1 wk to prevent hepatic toxicity (Tanaka et al. 2003). Furthermore, previous research using AMPK activation typically applied a brief exposure or a single dose (Higashida et al. 2008). In the present experiments, the effects of AICAR were clearly dependent on the duration of administration, as 7 d of treatment enhanced adult neurogenesis and performance in the water maze, but 14 d did not. It is likely that AMPK activation may have bidirectional effects on cognition similar to differential effects of short-term and chronic treatment in other systems (Fan et al. 2009). The difference between the 7 and 14 d of treatment may be explained by the recent finding that short-term AICAR treatment promoted sirtuin 1 protein expression in skeletal muscle, whereas 14 d of treatment did not (Suwa et al. 2010). Furthermore, exercise itself, when performed extensively, as in marathon racing and other forms of prolonged, heavy exertion, may increase susceptibility to inflammatory processes. We hypothesize that long-term injection of AICAR may have similar effects (Akerstrom and Pedersen 2007).
Previous investigations into potential cognitive effects of these compounds yielded equivocal results. A PPARδ agonist ameliorated maze learning deficits in a mouse model of diabetes, but has not been tested in normal controls (de la Monte et al. 2006). Systemic activation of AMPK improved maze learning in calorie-restricted mice (Dagon et al. 2005), but this could be attributed to the dietary regimen (Fontán-Lozano et al. 2007). Intracerebral infusion of AICAR impaired memory function (Dash et al. 2006), and in vitro application reduced long-term potentiation in hippocampal slices (Potter et al. 2010). These mixed results may reflect the route of drug administration. Indeed, although AMPK and PPARδ are expressed in neural cells (Hall et al. 2008; Dasgupta and Milbrandt 2009) and agonists are generally considered neuroprotective when applied in culture (Culmsee et al. 2001; Ayasolla et al. 2005; Iwashita et al. 2006; Dasgupta and Milbrandt 2007), the compounds used have a very low ability to cross the blood brain barrier, estimated at <1% for AICAR (Marangos et al. 1990). Thus, only when administered peripherally, AICAR and GW may lead to a release of factors from muscle into circulation and so enhance hippocampal synaptic plasticity and cell genesis.
Support for the cognitive effects of the compounds comes from the modest increase in double-cortin (Brown et al. 2003) and BrdU labeling following treatment with GW, as well as from the significant increase in neurogenesis with AICAR. Indeed, enhancement of adult neurogenesis has been associated with improved learning and memory (van Praag 2008; Creer et al. 2010). It is likely that these neurogenic effects are also mediated by indirect activation of peripheral factors that can cross the blood brain barrier. However, it is of interest to note that PPARs play a role in neural stem cell proliferation, migration, and differentiation (Cimini and Cerù 2008). In addition, AMPK is considered important for brain development and the maintenance of neural stem cells (Lee et al. 2007; Dasgupta and Milbrandt 2009). Mutant mice lacking the regulatory AMPK β1 subunit show atrophy of the dentate gyrus (Dasgupta and Milbrandt 2009). Interestingly, similar to hippocampal neurogenesis (Kuhn et al. 1996) and synaptic plasticity (Scheff and Price 2006), AMPK activity declines with aging in rat skeletal muscle (Reznick et al. 2007), raising the possibility that muscle AMPK levels may be a biomarker for hippocampal plasticity.
In conclusion, we show that muscle endurance enhancing compounds improve spatial memory in sedentary mice. The behavioral enhancement may be due at least in part to increased dentate gyrus neurogenesis (Gobeske et al. 2009; Pieper et al. 2010). These findings may lead to development of therapeutic agents that confer the benefits of exercise in disease or environmental conditions where activity is compromised.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging (NIA). We thank Donna Tignor, James Hopkins, Mike Still, and Derrick Haire for animal care and technical assistance. We thank Dr. Jaroslaw Aronowski for insightful comments on the manuscript.
References
- Akerström TC, Pedersen BK 2007. Strategies to enhance immune function for marathon runners: What can be done? Sports Med 37: 416–419 [DOI] [PubMed] [Google Scholar]
- Ayasolla KR, Singh AK, Singh I 2005. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) attenuates the expression of LPS- and Aβ peptide-induced inflammatory mediators in astroglia. J Neuroinflammation 2: 21 doi: 10.1186/1742-2094-2-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner M, Hertz R, Bar-Tana J 2004. Kinase-independent transcriptional co-activation of peroxisome proliferator-activated receptor α by AMP-activated protein kinase. Biochem J 384: 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG 2003. Transient expression of double-cortin during adult neurogenesis. J Comp Neurol 467: 1–10 [DOI] [PubMed] [Google Scholar]
- Cimini A, Cerù MP 2008. Emerging roles of peroxisome proliferator-activated receptors (PPARs) in the regulation of neural stem cells proliferation and differentiation. Stem Cell Rev 4: 293–303 [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA 2007. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci 30: 464–472 [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ 2010. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci 107: 2367–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Monnig J, Kemp BE, Mattson MP 2001. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci 17: 45–58 [DOI] [PubMed] [Google Scholar]
- Dagon Y, Avraham Y, Magen I, Gertler A, Ben-Hur T, Berry EM 2005. Nutritional status, cognition, and survival: A new role for leptin and AMP kinase. J Biol Chem 280: 42142–42148 [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J 2007. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci 104: 7217–7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J 2009. AMP-activated protein kinase phosphorylates retinoblastoma protein to control mammalian brain development. Dev Cell 16: 256–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Moore AN 2006. Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-Mammalian target of rapamycin pathway. J Neurosci 26: 8048–8056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, Lester-Coll N, Plater M, Wands JR 2006. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: Relevance to Alzheimer's disease. J Alzheimers Dis 10: 89–109 [DOI] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD 2003. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci 18: 2803–2812 [DOI] [PubMed] [Google Scholar]
- Fan X, Ding Y, Brown S, Zhou L, Shaw M, Vella MC, Cheng H, McNay EC, Sherwin RS, McCrimmon RJ 2009. Hypothalamic AMP-activated protein kinase activation with AICAR amplifies counterregulatory responses to hypoglycemia in a rodent model of type 1 diabetes. Am J Physiol Regul Integr Comp Physiol 296: 1702–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontán-Lozano A, Sáez-Cassanelli JL, Inda MC, de los Santos-Arteaga M, Sierra-Domínguez SA, López-Lluch G, Delgado-García JM, Carrión AM 2007. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J Neurosci 27: 10185–10195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeske KT, Das S, Bonaguidi MA, Weiss C, Radulovic J, Disterhoft JF, Kessler JA 2009. BMP signaling mediates effects of exercise on hippocampal neurogenesis and cognition in mice. PLoS One 4: e7506 doi: 10.1371/journal.pone.0007506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S, Ying Z 2008. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci 28: 2278–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MG, Quignodon L, Desvergne B 2008. Peroxisome proliferator-Activated receptor β/δ in the brain: Facts and hypothesis. PPAR Res 2008: 780452 doi: 10.1155/2008/780452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG 2004. The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Sci 117: 5479–5487 [DOI] [PubMed] [Google Scholar]
- Higashida K, Higuchi M, Terada S 2008. Potential role of lipin-1 in exercise-induced mitochondrial biogenesis. Biochem Biophys Res Commun 374: 587–591 [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF 2008. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat Rev Neurosci 9: 58–65 [DOI] [PubMed] [Google Scholar]
- Iwashita A, Muramatsu Y, Yamazaki T, Muramoto M, Kita Y, Yamazaki S, Mihara K, Moriguchi A, Matsuoka N 2006. Neuroprotective efficacy of the peroxisome proliferator-activated receptor δ-selective agonists in vitro and in vivo. J Pharmacol Exp Ther 320: 1087–1096 [DOI] [PubMed] [Google Scholar]
- Jäger S, Handschin C, St-Pierre J, Spiegelman BM 2007. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci 104: 12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH 1996. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci 15: 2027–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, et al. 2007. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 447: 1017–1020 [DOI] [PubMed] [Google Scholar]
- Marangos PJ, Loftus T, Wiesner J, Lowe T, Rossi E, Browne CE, Gruber HE 1990. Adenosinergic modulation of homocysteine-induced seizures in mice. Epilepsia 31: 239–246 [DOI] [PubMed] [Google Scholar]
- McConell GK, Manimmanakorn A, Lee-Young RS, Kemp BE, Linden KC, Wadley GD 2008. Differential attenuation of AMPK activation during acute exercise following exercise training or AICAR treatment. J Appl Physiol 105: 1422–1427 [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J 1982. Place navigation impaired in rats with hippocampal lesions. Nature 24: 681–683 [DOI] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, et al. 2008. AMPK and PPARδ agonists are exercise mimetics. Cell 134: 405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM, Becker GL, Huntington P, Goldman SE, Shen CH, et al. 2010. Discovery of a proneurogenic, neuroprotective chemical. Cell 142: 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pold R, Jensen LS, Jessen N, Buhl ES, Schmitz O, Flyvbjerg A, Fujii N, Goodyear LJ, Gotfredsen CF, Brand CL, et al. 2005. Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes 54: 928–934 [DOI] [PubMed] [Google Scholar]
- Potter WB, O'Riordan KJ, Barnett D, Osting SM, Wagoner M, Burger C, Roopra A 2010. Metabolic regulation of neuronal plasticity by the energy sensor AMPK. PLoS One 5: 8996 doi: 10.1371/journal.pone.0008996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, et al. 2007. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab 5: 151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA 2006. Alzheimer's disease-related alterations in synaptic density: Neocortex and hippocampus. J Alzheimers Dis 9: 101–115 [DOI] [PubMed] [Google Scholar]
- Steinberg GR, Kemp BE 2009. AMPK in Health and Disease. Physiol Rev 89: 1025–1078 [DOI] [PubMed] [Google Scholar]
- Suwa M, Nakano H, Radak Z, Kumagai S 2010. Short-term adenosine monophosphate-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside treatment increases the sirtuin 1 protein expression in skeletal muscle. Metabolism. doi: 10.1016/j.metabol.2010.03.003 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, Watanabe M, Magoori K, Ioka RX, Tachibana K, et al. 2003. Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci 100: 15924–15929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I 2001. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci 21: 1628–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H 2008. Neurogenesis and exercise: Past and future directions. Neuromolecular Med 10: 128–140 [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH 1999. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci 96: 13427–13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM 2004. Regulation of muscle fiber type and running endurance by PPARδ. PLoS Biol 2: e294 doi: 10.1371/journal.pbio.0020294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder WW, Hardie DG 1996. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol Endocrinol Metab 270: E299–E304 [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens BJ 2000. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol 528: 221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]