Abstract
Through protein interactions mediated by their cytoplasmic C termini the GluN2A and GluN2B subunits of NMDA receptors (NMDARs) have a key role in the formation of NMDAR signaling complexes at excitatory synapses. Although these signaling complexes are thought to have a crucial role in NMDAR-dependent forms of synaptic plasticity such as long-term potentiation (LTP), the role of the C terminus of GluN2A in coupling NMDARs to LTP enhancing and/or suppressing signaling pathways is unclear. To address this issue we examined the induction of LTP in the hippocampal CA1 region in mice lacking the C terminus of endogenous GluN2A subunits (GluN2AΔC/ΔC). Our results show that truncation of GluN2A subunits produces robust, but highly frequency-dependent, deficits in LTP and a reduction in basal levels of extracellular signal regulated kinase 2 (ERK2) activation and phosphorylation of AMPA receptor GluA1 subunits at a protein kinase A site (serine 845). Consistent with the notion that these signaling deficits contribute to the deficits in LTP in GluN2AΔC/ΔC mice, activating ERK2 and increasing GluA1 S845 phosphorylation through activation of β-adrenergic receptors rescued the induction of LTP in these mutants. Together, our results indicate that the capacity of excitatory synapses to undergo plasticity in response to different patterns of activity is dependent on the coupling of specific signaling pathways to the intracellular domains of the NMDARs and that abnormal plasticity resulting from mutations in NMDARs can be reduced by activation of key neuromodulatory transmitter receptors that engage converging signaling pathways.
N-methyl-d-aspartate receptors (NMDARs) are heteromeric ligand-gated ion channels comprised of an obligatory GluN1 subunit along with different combinations of GluN2 or GluN3 subunits (Cull-Candy and Leszkiewicz 2004). In the adult brain, most NMDARs exist as heteromeric combinations of two GluN1 subunits along with different members of the GluN2 family of subunits (GluN2A, -2B, -2C and -2D). Importantly, the presence of different types of GluN2 subunits determines a number of functional characteristics of NMDARs, including agonist affinity, channel kinetics, and membrane localization (for review, see Cull-Candy et al. 2001, Cull-Candy and Leszkiewicz 2004). The cytoplasmic C-terminal tails of the different GluN2 subunits also have a crucial role in coupling NMDARs to various adaptor/scaffolding proteins, such as the membrane associated guanylate kinases or MAGUKs (Kornau et al. 1995; Kim and Sheng 2004). The MAGUKs, which include proteins such as PSD-95, PSD-93, and SAP102, in turn couple NMDARs to a host of postsynaptic signaling pathways, including the Ras GTPase-activating protein SynGAP (Chen et al. 1998; Kim et al. 1998), the A kinase anchoring protein AKAP79/150 (Colledge et al. 2000), and neuronal nitric oxide synthase (nNOS) (Brenman et al. 1996). Moreover, the C termini of GluN2A and GluN2B also directly bind key components of additional signaling pathways, such as α-calcium/calmodulin-activated protein kinase II (CamKII) (Leonard et al. 1999; Bayer et al. 2001) and phospholipase Cδ (Gurd and Bissoon 1997). Thus, GluN2 subunits not only regulate the biophysical properties of NMDARs but also have an essential role in the formation of NMDAR signaling complexes that organize signaling downstream from NMDAR activation. Indeed, disruption of these complexes is associated with pronounced alterations in NMDAR-dependent forms of synaptic plasticity such as long-term potentiation (LTP) and long-term depression (LTD) (Migaud et al. 1998; Sprengel et al. 1998; Barria and Malinow 2005; Cuthbert et al. 2007; Zhou et al. 2007; Carlisle et al. 2008; Xu et al. 2008; Foster et al. 2010).
Although proteomic approaches have revealed much about the molecular composition of NMDAR signaling complexes (Husi et al. 2000; Collins et al. 2006), the role of these complexes in regulating LTP and LTD induction are still poorly understood. For example, previous studies using genetically modified mice where the endogenous GluN2A subunits lack the cytoplasmic C terminus (GluN2AΔC) have found that these mutants exhibit deficits in both LTP (Sprengel et al. 1998; Köhr et al. 2003) and some forms of hippocampus-dependent learning (Sprengel et al. 1998; Bannerman et al. 2008). This suggests that the C terminus of GluN2A subunits has an important role in recruiting signaling molecules that facilitate LTP induction to NMDAR signaling complexes. In contrast to these studies where the endogenous GluN2A subunit gene was modified, other studies have overexpressed cDNAs encoding truncated GluN2A. For example, in hippocampal slice cultures overexpression of truncated GluN2A subunits can restore LTP in cells where LTP induction is disrupted by knockdown of GluN2B subunits while overexpression of full-length, wild-type GluN2A subunits cannot (Foster et al. 2010). While these data support the conclusion that the C terminus of GluN2A is involved in signaling, it remains unclear if both LTP inhibiting or enhancing pathways act via the C terminus and to what extent these pathways might be recruited by different patterns of synaptic activity.
Here we have reexamined the role of the C terminus of NMDAR GluN2A subunits in LTP using a variety of different LTP induction protocols and biochemical approaches to examine NMDAR signaling in hippocampal slices from wild-type and homozygous mutant mice expressing truncated GluN2A subunits lacking the cytoplasmic C terminus (GluN2AΔC/ΔC) (Sprengel et al. 1998). We find that while high-frequency stimulation (HFS) induced LTP is normal, the induction of LTP by theta-frequency patterns of synaptic stimulation is strongly disrupted in GluN2AΔC/ΔC mice. This suggests that the C terminus of GluN2A subunits couples NMDARs to downstream signaling pathways that normally facilitate the induction of LTP by low frequency patterns of synaptic activity. Western blot analysis of hippocampal homogenates revealed that basal levels of extracellular signal regulated kinase 2 (ERK2) activation and phosphorylation of AMPA receptor (AMPAR) GluA1 subunits at serine 845 (S845) are reduced in GluN2AΔC/ΔC mice. Consistent with the notion that these signaling deficits contribute to the disruption of LTP in these mutants, enhancing ERK activation and increasing GluA1 phosphorylation at S845 by β-adrenergic receptor activation reduced, and for some stimulation protocols completely rescued, the theta frequency stimulation-induced LTP deficits in GluN2AΔC/ΔC mice.
Results
Basal synaptic transmission is normal in GluN2AΔC/ΔC mutant mice
To examine whether truncation of GluN2A subunits alters basal synaptic transmission, we recorded AMPAR-mediated spontaneous miniature excitatory postsynaptic currents (mEPSCs) in CA1 pyramidal cells from wild-type and GluN2AΔC/ΔC mice. Consistent with previous findings suggesting that AMPAR-mediated synaptic transmission is not altered in GluN2A mutant mice (Sprengel et al. 1998; Steigerwald et al. 2000), both the amplitude and frequency of mEPSCs were normal in GluN2AΔC/ΔC mice (Fig. 1A–C). Evoked EPSCs recorded at postsynaptic membrane potentials of −80 mV and +40 mV were also similar in wild-type and GluN2AΔC/ΔC mice (Fig. 1D) and the ratio of NMDAR to AMPAR-mediated currents measured at both membrane potentials in GluN2AΔC/ΔC mice was not significantly different from that seen in wild-type cells (Fig. 1E). Although this suggests that that the absence of the C terminus of GluN2A subunits does not impair the synaptic localization of NMDARs, the decay of the EPSCs recorded at +40 mV tended to be slower in GluN2A mutant cells compared to wild type (Fig. 1F). While this difference did not reach statistical significance (P = 0.08), it is consistent with previous findings indicating that GluN2AΔC-containing NMDARs may be less tightly localized with respect to presynaptic release sites (Steigerwald et al. 2000).
Figure 1.
Basal synaptic transmission is normal in GluN2AΔC/ΔC mice. (A) Traces show example mEPSCs recorded in CA1 pyramidal cells from wild-type (GluN2A+/+) and GluN2AΔC/ΔC mice. (B,C) Both mEPSC amplitude and frequency are normal in cells from GluN2AΔC/ΔC mice. Results are from 13 cells from three wild-type mice and 11 cells from three GluN2AΔC/ΔC mice. (D) Traces show examples of evoked EPSCs recorded at holding potentials of −80 mV and +40 mV in wild-type (left) and GluN2AΔC/ΔC cells (right). (E) NMDAR to AMPAR ratios at both −80 mV and +40 mV are similar in cells from wild-type (n = 14 cells from three mice) and GluN2AΔC/ΔC mice (n = 12 cells from three mice). (F) Weighted decay time constants (τW) of EPSCs recorded at +40 mV were 84 ± 2 msec in wild-type cells and 101 ± 7 msec in GluN2AΔC/ΔC cells (P = 0.08 compared to wild type).
GluN2AΔC/ΔC exhibit theta frequency-specific deficits in LTP
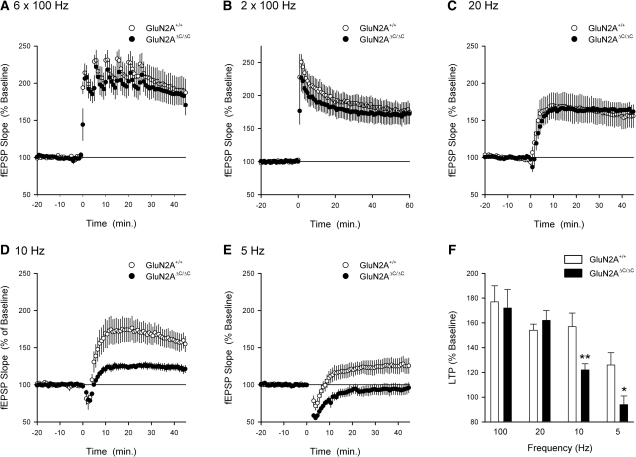
To examine whether truncation of the C terminus GluN2A subunits alters the induction of LTP by different patterns of presynaptic fiber stimulation we first examined the effects of multiple trains of HFS (100 Hz) on synaptic strength in GluN2AΔC/ΔC and wild-type mice. As shown in Figure 2A, the induction of LTP by six trains of 100 Hz in slices from GluN2AΔC/ΔC was similar to that seen in wild-type slices (fEPSPs potentiated to 190 ± 18% of baseline in wild-type slices and to 183 ± 13% of baseline in slices from GluN2AΔC/ΔC mutants, P = 0.75 compared to wild type). A weaker HFS protocol (two trains of 100 Hz stimulation) also induced robust LTP in slices from GluN2AΔC/ΔC that was not significantly different from that seen in slices from wild-type mice (fEPSPs potentiated to 177 ± 13% of baseline in wild-type slices and to 172 ± 15% of baseline in slices from GluN2AΔC/ΔC, P = 0.82 compared to wild type; Fig. 2B). Together, these findings are consistent with previous reports showing that the induction of LTP by multiple trains of high-frequency stimulation is normal in GluN2AΔC/ΔC mutants (Köhr et al. 2003).
Figure 2.
GluN2AΔC/ΔC mutants exhibit frequency-dependent deficits in LTP. (A) LTP induced by six trains of 100 Hz stimulation (starting at time = 0, intertrain interval = 5 min) in slices from GluN2AΔC/ΔC mice (n = 8 slices from five mice) is not significantly different from that seen in wild-type slices (n = 4 slices from four mice, P = 0.75). (B) Two trains of 100 Hz stimulation (delivered at time = 0, intertrain interval = 10 sec) induces similar levels of LTP in slices from wild-type (n = 6 slices from four mice) and GluN2AΔC/ΔC mice (n = 5 slices from five mice). (C) The induction of LTP by a train of 20 Hz stimulation (900 pulses, delivered at time = 0) in slices from GluN2AΔC/ΔC (n = 9 slices from seven mice) is not significantly different from that seen in wild-type slices (n = 4 slices from four mice, P = 0.63). (D) Ten Hz stimulation (900 pulses delivered at time = 0) induces significantly less LTP in GluN2AΔC/ΔC mutants (n = 14 slices from seven mice) than in wild-type slices (n = 9 slices from five mice, P < 0.01). (E) Five Hz stimulation (900 pulses delivered at time = 0) induced a small amount of potentiation in slices from wild-type mice (n = 8 slices from six mice, P < 0.05 compared to baseline) but had no lasting effect on synaptic strength in slices from GluN2AΔC/ΔC mutants (n = 6 slices from six mice, P < 0.05 compared to wild type). (F) Summary of the effects of different frequencies of presynaptic fiber stimulation on the induction of LTP in wild-type and GluN2AΔC/ΔC mice (**P < 0.01; *P < 0.05). Results for 100 Hz stimulation are from experiments shown in B.
Although our results indicate that HFS-induced LTP is normal in GluN2AΔC/ΔC mice, significant LTP deficits were observed in experiments where we examined the induction of LTP by lower frequency patterns of presynaptic fiber stimulation. As shown in Figure 3A, a long train (900 pulses) of 20 Hz stimulation induced wild-type levels of LTP in slices from GluN2AΔC/ΔC mice (Fig. 2C). However, the same number of presynaptic fiber stimulation pulses delivered at 10 Hz, a frequency that falls within the upper range of the hippocampal theta rhythm (Buzsáki 2002), induced significantly less LTP in slices from GluN2AΔC/ΔC mice (fEPSPs potentiated to 157 ± 11% of baseline in wild-type slices and to 122 ± 5% of baseline in slices from GluN2AΔC/ΔC mice, P < 0.01 compared to wild type) (Fig. 2D). Similarly, 900 pulses of presynaptic fiber stimulation at the lower range of the theta rhythm (5 Hz) induced a small (126 ± 10% of baseline), yet significant potentiation of synaptic transmission in slices from wild-type mice (P < 0.05 compared to baseline) but had no lasting effect on synaptic strength in slices from GluN2AΔC/ΔC mutant mice (fEPSPs were 94 ± 7% of baseline, P < 0.05 compared to wild type; Fig. 2E). Thus, as summarized in Figure 2F, truncation of the C terminus of GluN2A subunits strongly disrupts the induction of LTP by trains of synaptic stimulation delivered at the theta frequency (5–10 Hz) but has no effect on the induction of LTP by higher frequencies of synaptic stimulation (20 and 100 Hz).
Figure 3.
Basal levels of ERK2 phosphorylation are significantly reduced in GluN2AΔC/ΔC mice. (A) Levels of phospho-ERK2 in untreated hippocampal slices from wild-type (open bars, n = 5) and GluN2AΔC/ΔC mice (filled bars, n = 7). Total ERK1/2 levels in GluN2AΔC/ΔC mice were not different from wild type (P = 0.91). (B) A 3 min bath application of 20 µM NMDA (N) induced similar increases in ERK2 phosphorylation in slices from wild-type (open bars, n = 5) and GluN2AΔC/ΔC mice (filled bars, n = 7). *P < 0.01 compared to untreated (UT) control slices.
Basal levels of ERK2 and GluA1 S845 phosphorylation are altered in GluN2AΔC/ΔC mutant mice
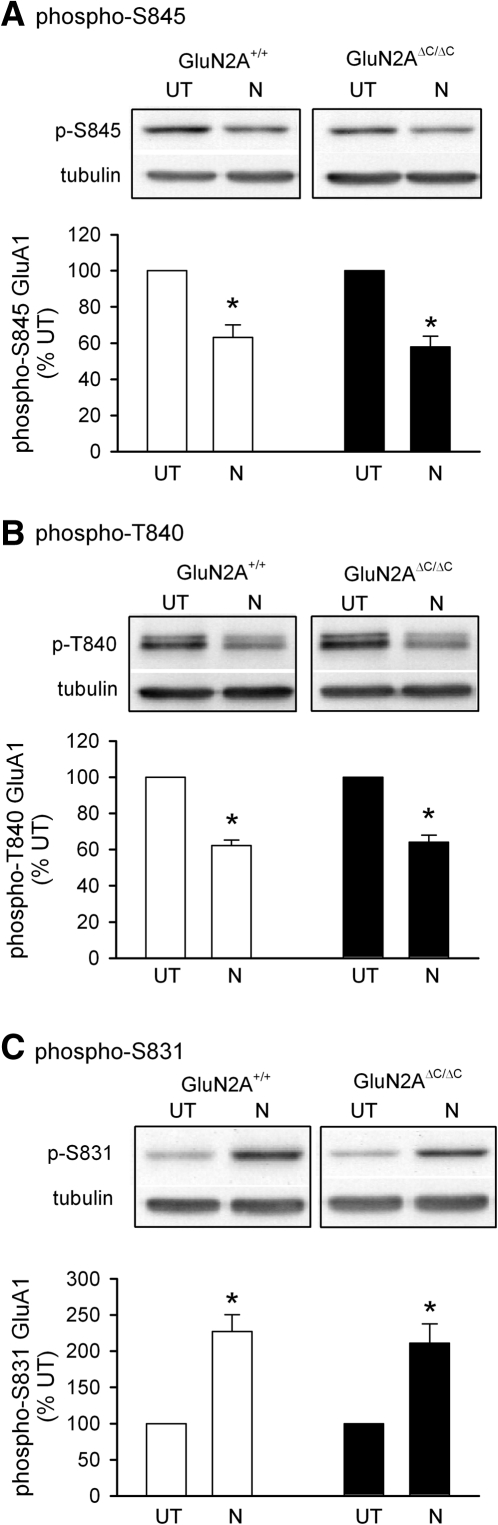
Unlike HFS-induced LTP, the induction of LTP by theta-frequency stimulation protocols is dependent on ERK activation (Winder et al. 1999; Watabe et al. 2000; Opazo et al. 2003) and strongly inhibited by protein phosphatase activity (Thomas et al. 1996; Brown et al. 2000). We thus examined ERK activation and phosphorylation of AMPAR GluA1 subunits at sites dephosphorylated by protein phosphatases activated downstream from NMDARs to determine whether truncation of GluN2A subunits might lead to deficits in theta-frequency stimulation-induced LTP through alterations in these signaling pathways. As shown in Figure 3A, immunoblotting of homogenates prepared from wild-type and GluN2AΔC/ΔC slices revealed a modest, but significant, reduction in basal levels of the phosphorylated, active form of ERK2 in slices from GluN2AΔC/ΔC mice (levels were reduced to 65 ± 9% of wild-type levels, P < 0.05) with no difference in total ERK2 levels. However, the relative increase in ERK2 phosphorylation induced by bath application of NMDA (20 µM for 3 min) was similar in slices from wild-type and GluN2AΔC/ΔC mice (phospho-ERK2 levels were increased to 400 ± 38% of untreated controls in slices from wild type mice, n = 5 compared to 457 ± 21% of untreated controls in slices from GluN2AΔC/ΔC mice, n = 7, P = 0.19; Fig. 3B). Thus, the C terminus of GluN2A is required for both the theta-frequency stimulation-induced LTP and the basal level of ERK2 phosphorylation, but not for the activation of ERK2 phosphorylation.
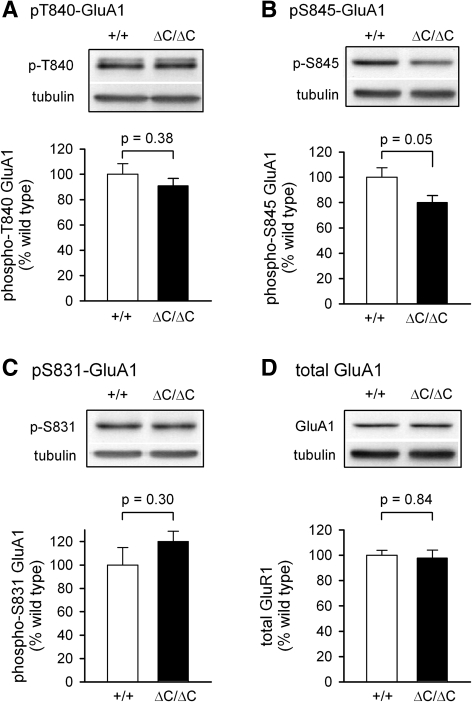
Signaling pathways downstream from the NMDA receptor also lead to changes in phosphorylation of AMPAR (Lee 2006). In particular, three phosphorylation sites on the AMPAR GluA1 (threonine 840, T840; serine 831, S831; serine 845, S845) are known to be regulated by various protein kinases and protein phosphatases downstream from NMDAR activation (Kameyama et al. 1998; Lee et al. 1998; Vanhoose and Winder 2003; Delgado et al. 2007; Coba et al. 2009). We therefore asked if the GluN2A C terminus was required for the regulation of any of these sites.
Basal levels of GluA1 phosphorylation at T840 were not altered in slices from GluN2AΔC/ΔC (levels were 91 ± 6% of wild-type levels, P = 0.38) while basal levels of GluA1 phosphorylation at S845 were lower in slices from GluN2AΔC/ΔC compared to wild-type slices (levels were 80 ± 5% of wild-type levels, P = 0.05) (Fig. 4A,B). However, dephosphorylation of AMPAR GluA1 subunits at S845 and T840 induced by transient NMDA receptor activation (bath application of 20 µM NMDA for 3 min) was not altered in slices from GluN2AΔC/ΔC mice (Fig. 5A,B). Although NMDAR activation triggers dephosphorylation of AMPAR GluA1 subunits at T840 and S845, phosphorylation of another site in GluA1, S831, is increased (Vanhoose and Winder 2003; Delgado et al. 2007). Thus, as an additional test for potential changes in protein kinase and/or protein phosphatase signaling in GluN2AΔC/ΔC mice we also compared phospho-S831 GluA1 levels in untreated and NMDA-treated slices from wild-type and GluN2AΔC/ΔC mice. As shown in Figures 4C and 5C, both basal levels of GluA1 phosphorylation at S831 and the increase in S831 phosphorylation induced by NMDAR activation were normal in slices from GluN2AΔC/ΔC mice. Together, these findings suggest that truncation of the C terminus of GluN2A subunits does not produce significant deficits in the ability of bath applied NMDA to activate the ERK pathway, protein kinases that regulate S831 phosphorylation (such as PKC and CaMKII), or protein phosphatases that dephosphorylate T840 and S845. It is, however, associated with modest, but significant, decreases in basal levels of both ERK2 activation and GluA1 S845 phosphorylation.
Figure 4.
Basal levels of GluA1 phosphorylation at S845 are reduced in hippocampal slices from GluN2AΔC/ΔC mice. Basal levels of GluA1 phosphorylation at T840 (A), S845 (B), and S831 (C) in hippocampal slices from wild-type (open bars, n = 5 in A and B, n = 3 in C) and GluN2AΔC/ΔC mice (filled bars, n = 7 in A and B, n = 3 in C). Total GluA1 levels (D) in GluN2AΔC/ΔC slices (n = 7) are not different from wild type (n = 5).
Figure 5.
Changes in GluA1 phosphorylation induced by a 3 min bath application of 20 µM NMDA. The decrease in GluA1 phosphorylation at S845 (A) and T840 (B) and the increase in GluA1 phosphorylation at S831 (C) induced by NMDA in slices from GluN2AΔC/ΔC mice (filled bars, n = 7 for A and B, n = 5 for C) was not significantly different from that seen in wild-type slices (open bars, n = 5 for A, B, and C). P < 0.01 compared to untreated (UT) control slices. NMDA had no effect on total GluA1 levels in slices from either wild-type or GluN2AΔC/ΔC mice.
β-adrenergic receptor activation rescues the induction of LTP in GluN2AΔC/ΔC mice
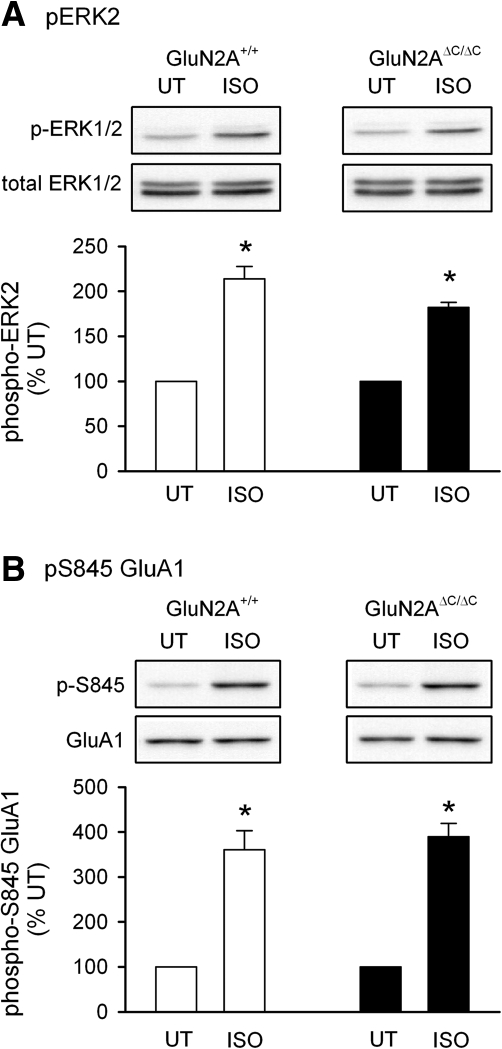
The above data shows that the GluN2A C terminus is required for the basal level of phosphorylation of synaptic proteins while leaving intact those kinase and phosphatase pathways that are induced by transient NMDA receptor activation. This led us to ask: Could reduced basal levels of ERK2 and GluA1 S845 phosphorylation contribute to the deficits in theta-frequency stimulation-induced LTP seen in GluN2AΔC/ΔC mice? To examine this we took advantage of the fact that activation of β-adrenergic receptors in the hippocampal CA1 region activates both the ERK pathway (Roberson et al. 1999; Winder et al. 1999; Opazo et al. 2003) and increases GluA1 phosphorylation at S845 (Opazo et al. 2003; Vanhoose and Winder 2003; Delgado et al. 2007; Joiner et al. 2010) and tested whether activation of β-adrenergic receptors could rescue theta-frequency stimulation-induced LTP in GluN2AΔC/ΔC mice. As shown in Figure 6, a 10 min bath application of the β-adrenergic receptor agonist isoproterenol (ISO, 1 µM) induced an approximately twofold increase in ERK2 phosphorylation and a more than threefold increase in GluA1 S845 phosphorylation in slices from both wild-type and GluN2AΔC/ΔC mice. Thus, we next examined whether β-adrenergic receptor activation can rescue theta-frequency stimulation-induced LTP in GluN2AΔC/ΔC mutant mice. Although long trains of 5 Hz stimulation induce relatively little LTP in slices from wild-type mice (Fig. 2E), shorter trains of 5 Hz stimulation (150 pulses) can induce robust LTP (Thomas et al. 1996, 1998; Carlisle et al. 2008) that is relatively insensitive to the effects of β-adrenergic receptor activation (Thomas et al. 1996). Consistent with this, short trains of 5 Hz stimulation induced robust LTP in slices from wild-type mice (fEPSPs were potentiated to 180 ± 23% of baseline; Fig. 7A) that was not significantly enhanced by bath application of ISO prior to 5 Hz stimulation (fEPSPs were potentiated to 204 ± 16% of baseline; Fig. 6B). A very different pattern of results was observed, however, in slices from GluN2AΔC/ΔC mice. First, the induction of LTP by 150 pulses of 5 Hz stimulation alone was significantly reduced compared to that seen in slices from wild-type mice (fEPSPs were 115 ± 5% of baseline, P < 0.05 compared to wild-type controls; Fig. 7A). Second, activation of β-adrenergic receptors strongly enhanced the induction of LTP by 150 pulses of 5 Hz stimulation in GluN2AΔC/ΔC mutants (fEPSPs were potentiated to 167 ± 7% of baseline, P < 0.001 compared to GluN2AΔC/ΔC control, P < 0.05 compared to wild-type 5 Hz + ISO; Fig. 7B). Together, these results indicate that β-adrenergic receptor activation can, at least partially, rescue the induction of theta-frequency stimulation-induced LTP in GluN2AΔC/ΔC mutants. Indeed, although the induction of LTP by long trains of 5 Hz stimulation is significantly compromised in slices from GluN2AΔC/ΔC mice (Fig. 2E), β-adrenergic receptor activation completely rescued the induction of LTP by this pattern of synaptic stimulation in GluN2AΔC/ΔC mice (Fig. 7C).
Figure 6.
β-adrenergic receptor stimulation-induced increases in ERK2 phosphorylation (A) and GluA1 S845 phosphorylation (B) are not altered in GluN2AΔC/ΔC mutants. Results are from four wild-type and four GluN2AΔC/ΔC mice. Activation of β-adrenergic receptors (1 µM ISO for 10 min) had no effect on either total ERK2 levels or total GluA1 levels. *P < 0.01 compared to untreated (UT) control slices.
Figure 7.
β-adrenergic receptor activation rescues theta-frequency stimulation-induced LTP in GluN2AΔC/ΔC mice. (A) A 30 sec train of 5 Hz induced robust LTP in wild-type slices (n = 10 slices from five mice) but had little effect on synaptic transmission in slices from GluN2AΔC/ΔC mutant mice (n = 10 slices from five mice, P < 0.05 compared to wild type). (B) Bath application of the β-adrenergic receptor agonist ISO (1 µM) for 10 min before 5 Hz stimulation significantly enhanced the induction of LTP in slices from GluN2AΔC/ΔC mutant mice (n = 19 slices from 12 mice, P < 0.001) compared to potentiation induced by 5 Hz stimulation alone (shown in panel A) but did not significantly enhance the induction of LTP by 5 Hz stimulation in wild-type slices (n = 9 slices from five mice, P = 0.397) compared to 5 Hz stimulation alone (shown in panel A). The amount of potentiation induced following 5 Hz stimulation in the presence of ISO in slices from GluN2AΔC/ΔC mice was, however, still significantly less than that seen in wild-type slices (P < 0.05). (C) β-adrenergic receptor activation rescues the induction of LTP by a long train of 5 Hz stimulation in GluN2AΔC/ΔC mutant mice. The potentiation induced by 900 pulses of 5 Hz stimulation delivered after a 10 min bath application of 1 µM ISO in slices from GluN2AΔC/ΔC mice (n = 10 slices from seven mice) was not significantly different from that seen in slices from wild-type mice (n = 8 slices from four mice, P = 0.945). (D) Summary of the effects of 5 Hz stimulation in the presence and absence of ISO on synaptic strength in wild-type (open bars) and GluN2AΔC/ΔC mice (filled bars).
Discussion
Theta-frequency stimulation-induced LTP is specifically disrupted by loss of the C terminus of NMDAR GluN2A subunits
Consistent with previous findings (Sprengel et al. 1998; Köhr et al. 2003), our results indicate that the C-terminal domain of GluN2A subunits has a crucial, facilitatory role in the induction of LTP in the hippocampal CA1 region. Our results indicate, however, that the LTP deficits observed in GluN2AΔC/ΔC mutants are remarkably frequency dependent and are only observed following the induction of LTP by frequencies of presynaptic fiber stimulation that fall with the theta rhythm, a 5–12 Hz oscillation of neural activity that occurs within the hippocampus during certain behaviors (Buzsáki 2002). Like high-frequency stimulation-induced LTP, the induction of LTP by theta frequency patterns of synaptic stimulation is NMDAR-dependent (Thomas et al. 1996, 1998; Winder et al. 1999; Watabe and O'Dell 2003). Thus, a reduction in NMDAR activity that increases the threshold for LTP induction could provide a simple explanation for the theta-frequency LTP deficits in GluN2AΔC/ΔC mice. We found, however, that both basal AMPAR-mediated synaptic transmission and the NMDAR-mediated component of evoked EPSCs were normal in slices from GluN2AΔC/ΔC mice (Fig. 1). This suggests that the LTP deficits observed in GluN2AΔC/ΔC mutants are not due to alterations in NMDAR currents but instead reflect alterations in signaling downstream from NMDAR activation (also see Köhr et al. 2003).
C-terminal truncation of NMDAR GluN2A subunits leads to alterations in both ERK and AMPA receptor GluA1 subunit phosphorylation
The induction of LTP by theta-frequency stimulation protocols exhibits a number of unique properties that may provide important clues regarding how disruption of signaling pathways dependent on interactions with the C terminus of GluN2A subunits compromises the induction of LTP. For example, unlike high-frequency stimulation-induced LTP, the induction of theta-frequency stimulation-induced LTP is highly dependent on ERK signaling (Winder et al. 1999; Watabe et al. 2000; Opazo et al. 2003). Thus we investigated whether alterations in NMDAR-dependent activation of the ERK pathway might contribute to the theta-frequency stimulation-induced LTP deficits in GluN2AΔC/ΔC mice. Although activation of the ERK pathway induced by bath application of NMDA was not altered, we did find a modest, but significant, decrease in basal levels of ERK phosphorylation in GluN2AΔC/ΔC mice. Some studies suggest that activation of GluN2A subunit-containing NMDARs is specifically involved in NMDAR-dependent activation of the ERK pathway through activation of the Ras guanine nucleotide exchange factor Ras-GRF2 (Li et al. 2006; Jin and Feig 2010). Although this suggests that deficits in ERK signaling in GluN2AΔC/ΔC mutants may be due to alterations in the ability of NMDARs to couple to activators of the ERK pathway, the role of the C terminus of GluN2A subunits in coupling GluN2A-containing NMDARs to Ras-GRF2 is not known. Moreover, other studies have found that activation of either GluN2A or GluN2B-containing NMDARs can produce activation of the ERK pathway in mature neurons (Kim et al. 2005). Importantly, GluN2B-containing receptors, but not GluN2A-containing NMDARs, are coupled to SynGAP (Kim et al. 2005), a synaptic Ras GTPase-activating protein that acts as a suppressor of both basal (Komiyama et al. 2002) and NMDAR-dependent increases in ERK1/2 phosphorylation (Rumbaugh et al. 2006). GluN2B-containing NMDARs also appear to be preferentially coupled to the tyrosine phosphatase striatal-enriched phosphatase (STEP), another negative regulator of ERK activation (Surojit and Connor 2010). Thus, truncation of the C terminus of GluN2A subunits may alter the balance of activators and inhibitors of the ERK pathway coupled to NMDARs in a way that favors inhibition of ERK activation.
The induction of LTP by theta-frequency stimulation is also strongly regulated by activation of protein phosphatases 1 and/or 2A that normally act to inhibit the induction of LTP by these patterns of synaptic stimulation (Thomas et al. 1996; Brown et al. 2000). Thus we also investigated whether alterations in NMDAR-dependent activation of these protein phosphatases might contribute to the theta-frequency stimulation-induced LTP deficits in GluN2AΔC/ΔC mice. Although NMDA-induced dephosphorylation of AMPAR GluA1 subunit phosphorylation at S845 and T840 was not altered, basal levels GluA1 phosphorylation at S845 were significantly reduced in hippocampal slices from GluN2AΔC/ΔC mice. Protein kinase A (PKA) mediated phosphorylation of GluA1 at S845 is thought to trigger the insertion of AMPARs into extrasynaptic sites where they are primed for insertion into synaptic sites following the induction of LTP (Esteban et al. 2003; Oh et al. 2006). Thus, lower levels of GluA1 S845 phosphorylation could contribute to the LTP deficits in GluN2AΔC/ΔC by decreasing the pool of extrasynaptic AMPARs available for incorporation into synapses during LTP induction. Recent studies suggest that ERK also has a role in AMPAR trafficking during LTP (Patterson et al. 2010) and thus alterations in AMPAR trafficking due to reduced ERK activity in GluN2AΔC/ΔC mice may also contribute to deficits in LTP in these mutants. Notably, basal levels of GluA1 phosphorylation at T840 and S831 were not altered in slices from GluN2AΔC/ΔC mice. This suggests that the lower levels of S845 phosphorylation are not due to changes in the activity of protein phosphatases 1 and 2A, which regulate GluA1 phosphorylation at all these sites. It thus seems more likely that loss of the C terminus of GluN2A subunits leads to alterations in NMDAR coupling to PKA signaling. One possibility is that truncation of the C terminus of GluN2A subunits compromises coupling of NMDARs to MAGUKs, such as PSD-95, that normally recruit PKA to NMDAR signaling complexes by interacting with A kinase anchoring proteins (Colledge et al. 2000). PSD-95 also couples GluN2A-containing NMDARs to nNOS (Brenman et al. 1996; Christopherson et al. 1999; Al-Hallaq et al. 2007) which, when activated, leads to the production of cGMP and activation of cGMP-dependent protein kinases that can also phosphorylate GluA1 subunits at S845 (Serulle et al. 2007). Thus, deletion of the C terminus of GluN2A subunits may also lead to alterations in GluA1 S845 phosphorylation via changes in NOS and cGMP signaling.
β-adrenergic receptor activation rescues the induction of LTP in GluN2AΔC/ΔC mice
Consistent with the notion that alterations in ERK signaling and GluA1 S845 phosphorylation contribute to LTP deficits in GluN2AΔC/ΔC mutants, activation of β-adrenergic receptors, which both activates ERK and increases GluA1 phosphorylation at S845 (Fig. 5), rescued the induction of theta-frequency stimulation-induced LTP in GluN2AΔC/ΔC mice (Fig. 6). Importantly, β-adrenergic receptor stimulation and activation of PKA can enhance LTP induction via a number of different mechanisms in addition to ERK activation and AMPAR phosphorylation (O'Dell et al. 2010). For example, β-adrenergic receptor-activation mediated inhibition of small conductance, Ca2+-activated potassium channels as well as A-type potassium channels have been implicated in the LTP enhancing effects of β-adrenergic receptor activation (Yuan et al. 2002; Faber et al. 2008; for review, see O'Dell et al. 2010). Moreover, β-adrenergic receptor activation can inhibit the activity of protein phosphatases that oppose LTP induction (Thomas et al. 1996; Brown et al. 2000) as well as enhance the translation of proteins needed for the long-term maintenance of LTP (Gelinas et al. 2007). The ability of β-adrenergic receptor activation to rescue theta-frequency stimulation-induced LTP in GluN2AΔC/ΔC mutants may thus also reflect compensation for deficits in NMDAR modulation of these processes caused by loss of the C terminus of GluN2A subunits. Our results suggest, however, that protein phosphatase signaling downstream from NMDARs is not altered in GluN2AΔC/ΔC mice. Because the deficits in theta-frequency stimulation-induced LTP in GluN2AΔC/ΔC mutants are evident at very early time points post-LTP induction (5–10 min) our results also suggest that alterations in protein synthesis are unlikely to be involved. They do not, however, rule out the possibility that deficits in basal translation rates, perhaps due to alterations in basal ERK activity, might contribute to LTP deficits in GluN2AΔC/ΔC mutants. Our results also do not rule out the possibility that deletion of the C terminus of GluN2A subunits leads to alterations in NMDAR modulation of dendritic potassium channels. Indeed, ERK activation inhibits A-type potassium channels in hippocampal dendrites (Yuan et al. 2002) and thus the lower basal levels of ERK activation in GluN2AΔC/ΔC mice may lead to changes in dendritic excitability that contribute to the LTP deficits in these mutants. Although we did not observe any obvious alterations in postsynaptic complex spike bursting during theta frequency stimulation (Thomas et al. 1998) in GluN2AΔC/ΔC mice (data not shown), future experiments that examine this possibility in more detail could provide important insights into the mechanisms underlying LTP deficits produced by truncation of the C terminus of GluN2A subunits.
Comparison of results between gene targeting and overexpression approaches
Our LTP findings are in general agreement with previous studies of GluN2AΔC/ΔC mutants generated by gene targeting of the endogenous exon encoding the GluN2A C terminus (Sprengel et al. 1998; Köhr et al. 2003). In contrast to these studies that found a reduction in LTP with one stimulation protocol we have examined a wide range of frequencies in identical experimental conditions, which uncovered frequency-specific effects. A completely different experimental approach was reported in a recent study using RNA interference approaches in hippocampal organotypic slice cultures from wild-type rats came to just the opposite conclusion—that the C terminus of GluN2A subunits recruits negative regulators of LTP induction to NMDA receptor signaling complexes (Foster et al. 2010). Their conclusion derived from experiments that combined a knockdown of GluN2B with the overexpression of a cDNA encoding a truncated GluN2A in the presence of expression of the endogenous full-length GluN2A (full-length and truncated GluN2A are coexpressed). In part, the conclusions of Foster et al. (2010) were based on the finding that deficits in pairing-induced LTP caused by knockdown of GluN2B subunits could be overcome by overexpression of GluN2A subunits lacking the C terminus but not by overexpression of full-length GluN2A subunits. There are a number of methodological differences that may contribute to this discrepancy, but there are three that we believe are potentially interesting. First, GluN2B-containing NMDARs are not altered in GluN2AΔC/ΔC mutants (Köhr et al. 2003) while the experiments of Foster et al. (2010) examined the role of the C terminus of GluN2A subunits in LTP in the context of GluN2B knockdown. The different effects of C-terminal truncation of GluN2A subunits on LTP induction thus appears to depend on the presence of GluN2B subunits. This suggests that interactions between signaling molecules recruited to NMDAR signaling complexes through the C termini of both GluN2A and GluN2B subunits may have an important role in regulating LTP induction. Second, pairing protocols induce an ERK-independent form of LTP in the mouse hippocampus (Opazo et al. 2003) while the induction of LTP by theta-frequency stimulation is highly ERK-dependent. Thus, another possibility is that the C terminus of GluN2A subunits couples NMDARs to both LTP enhancing and suppressing signaling pathways that have distinct roles in the induction of LTP by different patterns of synaptic activation. Finally, in our experiments we examined the role of the C terminus of GluN2A subunits in LTP induction in the hippocampus of adult mice while organotypic slices prepared from the hippocampus of 7-d-old rats were used in the experiments of Foster et al. (2010). Thus, there may be important changes in the molecular composition of NMDAR signaling complexes that occur during development that are dependent on the C terminus of NMDAR GluN2A subunits. Although additional experiments will be needed to address these possibilities, our results along with the findings of previous studies (Sprengel et al. 1998; Köhr et al. 2003; Foster et al. 2010) suggest that a better understanding of the role of the C terminus of GluN2A subunits in organizing NMDAR signaling complexes is likely to reveal important new insights into the mechanisms underlying the induction of NMDAR-dependent forms of LTP.
Future strategies for rescue of mutations affecting NMDA receptor complexes
Human mutations in proteins within the signaling complexes of NMDA receptor complexes (Husi et al. 2000; Collins et al. 2006; Fernandez et al. 2009) are known to be involved in many brain diseases (Grant et al. 2005). For example, recently it was reported that mutations in GluN2A and GluN2B result in epilepsy and mental retardation (Endele et al. 2010). In the current study of GluN2A mutations, we have found that the LTP deficits can be significantly reduced using β-adrenergic stimulation based on the insight that phosphorylation of signaling proteins downstream from β-adrenergic receptors are shared with those impacted by the glutamate receptor mutation. This demonstrates that mutation phenotypes affecting plasticity mechanisms are amenable to pharmacological manipulation and suggests an interesting avenue for further research that may lead to development of new therapies for brain diseases.
Materials and Methods
Slice preparation and extracellular recording
Hippocampal slices (400 µm thick) from adult (three to eight months of age) wild-type and GluN2AΔC/ΔC mice (Sprengel et al. 1998) were prepared using standard techniques approved by the UCLA Institutional Animal Care and Use Committee. Slices were allowed to recover for a least 1 h in an interface-slice type recording chamber (at 30°C) perfused with oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (ACSF) containing 124 mM NaCl, 4.4 mM KCl, 25 mM NaHCO3, 1 mM NaH2PO4, 2 mM CaCl2, 1.2 mM MgSO4, and 10 mM glucose. For extracellular recordings a bipolar, nichrome wire stimulating electrode placed in stratum radiatum of the CA1 region was used to activate Schaffer collateral/commissural fiber synapses (basal stimulation rate = 0.02 Hz) and the evoked field excitatory postsynaptic potentials (fEPSPs) were recorded using a glass microelectrode filled with ACSF (resistance = 5–10 MΩ). At the start of each experiment we first identified the maximal fEPSP amplitude that could be elicited in each slice and then adjusted the intensity of presynaptic fiber stimulation to evoke fEPSPs with amplitude approximately 50% of the maximal amplitude. LTP was elicited using either conventional HFS protocols (multiple trains of 100 Hz stimulation, each 1 sec in duration, with inter-train intervals of 10 sec or 5 min) or different numbers of single pulses of presynaptic fiber stimulation delivered at 5–20 Hz. For each protocol the average fEPSP slope measured over the last 5 min of the experiment was used for statistical comparisons (two-tailed Student t-tests). In cases where the same stimulation protocol was delivered to multiple slices from the same animal the results were averaged and the N used for statistical tests corresponds to the number of animals in each group. All results are reported as mean ± SEM.
Whole-cell recordings
Low resistance (2–6 MΩ) patch electrodes filled with a solution containing 102 mM cesium gluconate, 17.5 mM CsCl, 10 mM TEA-Cl, 5 mM QX314, 4.0 mM Mg-ATP, 0.3 mM Tris-GTP, and 20 mM HEPES (pH = 7.2) were used to record excitatory postsynaptic currents (EPSCs). In these experiments slices with CA3 region removed were maintained in a submerged-slice recording chamber and bathed in a modified ACSF containing picrotoxin (100 µM), elevated concentrations of CaCl2 and MgSO4 (4 mM each) and a lower concentration of KCl (2.4 mM). To examine the AMPAR and NMDAR-mediated components of the evoked EPSCs we measured the amplitude of EPSCs recorded at postsynaptic membrane potentials (Vhold) of −80 or +40 mV. The AMPAR-mediated component of the EPSC was measured at the time after EPSC onset corresponding to the peak amplitude of the EPSC at −80 mV while the amplitude of the EPSC 50 msec after onset was used to estimate the NMDAR-mediated component. The decay time constant of NMDAR-mediated EPSCs was obtained from double exponential fits to the decay of synaptic currents measured at +40 mV and calculated as a weight mean decay time constant (Rumbaugh and Vicini 1999). The intensity of presynaptic fiber stimulation was adjusted to elicit EPSCs with a peak amplitude of 200–300 pA at Vhold = −80 mV. Miniature EPSCs (mEPSCs) were recorded at −80 mV in cells from slices bathed in a modified ACSF containing 1.0 µM tetrodotoxin and 100 µM picrotoxin. A template-based event detection routine in pClamp 10 (Molecular Devices) was used to analyze mEPSCs. Detected events smaller than 6 pA were excluded from the analysis. Statistical comparisons of evoked EPSCs were performed using two-tailed Student t-tests and Kolmogorov-Smirnov tests were used for comparisons of mEPSC amplitude and inter-event interval distributions. The results from multiple cells from the same animal were averaged and the N used for statistical tests equals the number of animals in each group.
Western immunoblotting
Hippocampal slices from wild-type and GluN2AΔC/ΔC mice were maintained in interface chambers perfused at 2 mL/min with warm (30°C), oxygenated ACSF and allowed to recover for 2–2.5 h. Slices were either left untreated or exposed to bath applied receptor agonists and then collected by rapidly transferring them into pre-frozen microcentrifuge tubes (Kontes Glass Company) kept on crushed dry ice. Treated slices were collected for analysis after a 10 min application of ISO (1 µM) or a 3 min application of NMDA (20 µM). Stock solutions of ISO (1 mM in H2O) were prepared before each experiment and diluted to 1 µM in ACSF prior to application. In all experiments three slices were pooled for each treatment condition.
The techniques used for Western blot analysis are described in detail elsewhere (Delgado et al. 2007). Briefly, slices were homogenized in an ice-cold homogenization buffer that contained both protease inhibitors (Protease Inhibitors Complete, Roche Molecular Biochemicals) and a mixture of different protein phosphatase inhibitors (Phosphatase Inhibitor Cocktails I and II, Sigma-Aldrich). 20 µg of total protein from each sample was resolved on 12% SDS-PAGE gels and then transferred onto nitrocellulose membranes. Primary antibody incubations were done overnight (at 4°C) and, after three washes in Tris-buffered saline containing 0.05% Tween-20, blots were incubated for two or more hours at room temperature in HRP-conjugated secondary antibodies. Images of immunoreactive bands visualized using enhanced chemiluminesence were acquired using a cooled CCD camera-based image acquisition and analysis system (Quantity One, Bio-Rad). All blots were re-probed with an anti-tubulin antibody and the optical density values for each band of interest was normalized to the density values obtained for tubulin in the same lane to control for potential variations in loading. Mann-Whitney tests and two-tailed Student t-tests were used to determine statistical significance.
Antibodies to dually phosphorylated ERK1/2 and total ERK1/2 were obtained from Cell Signaling Technology. Antibodies to GluA1 and phospho-T840 GluA1 were obtained from Abcam and antibodies for phospho-S845 GluA1 and the βIII isoform of tubulin were obtained from Upstate Biotechnology (Millipore). Anti-phospho-S831 antibodies were obtained from Invitrogen.
Acknowledgments
This work was supported by National Institute of Mental Health grant number MH609197 (T.J.O.) and the Wellcome Trust and Wellcome Trust Genes to Cognition Programme (S.G.N.G.). T.J.O. is a member of the UCLA Brain Research Institute. We are grateful to Rolf Sprengel and Peter Seeburg for GluN2AΔC/ΔC mice; to Maksym Kopanitsa, Tomás Ryan, and Erin Gray for helpful comments on the manuscript; and to Kathryn Elsegood, Carole Frost, and Dierk Biggs for help with animal shipments.
References
- Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ 2007. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci 27: 8334–8343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Niewoehner B, Lyon L, Romberg C, Schmitt WB, Taylor A, Sanderson DJ, Cottam J, Sprengel R, Seeburg PH, et al. 2008. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. J Neurosci 28: 3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R 2005. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CamKII. Neuron 48: 289–301 [DOI] [PubMed] [Google Scholar]
- Bayer KU, De Konninck P, Leonard AS, Hell JW, Schulman H 2001. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411: 801–805 [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, et al. 1996. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 84: 757–767 [DOI] [PubMed] [Google Scholar]
- Brown GP, Blitzer RD, Connor JH, Wong T, Sheolikar S, Iyengar R, Landau EM 2000. Long-term potentiation induced by θ frequency stimulation is regulated by a protein phosphatase-1-operated gate. J Neurosci 20: 7880–7887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G 2002. Theta oscillations in the hippocampus. Neuron 33: 325–340 [DOI] [PubMed] [Google Scholar]
- Carlisle HJ, Fink AE, Grant SGN, O'Dell TJ 2008. Opposing effects of PSD-93 and PSD-95 on long-term potentiation and spike timing-dependent plasticity. J Physiol 586: 5885–5900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Rojas-Soto M, Oguni A, Kennedy MB 1998. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron 20: 895–904 [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Hillier BJ, Lim WA, Bredt DS 1999. PSD-95 assembles a ternary complex with the N-methyl-d-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem 274: 27467–27473 [DOI] [PubMed] [Google Scholar]
- Coba MP, Pocklington AJ, Collins MO, Kopanitsa MV, Uren RT, Swamy S, Croning MDR, Choudhary JS, Grant SGN 2009. Neurotransmitters drive combinatorial multistate postsynaptic density networks. Sci Signaling 2: ra19 doi: 10.1126/scisignal.2000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD 2000. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron 27: 107–119 [DOI] [PubMed] [Google Scholar]
- Collins MO, Husi H, Yu L, Brandon JM, Anderson CN, Blackstock WP, Choudhary JS, Grant SG 2006. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J Neurochem 97: 16–23 [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN 2004. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE 255: re16. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M 2001. NMDA receptor subunits: Diversity, development and disease. Curr Opin Neurobiol 11: 327–335 [DOI] [PubMed] [Google Scholar]
- Cuthbert PC, Stanford LE, Coba MP, Ainge JA, Fink AE, Opazo P, Delgado JY, Komiyama NH, O'Dell TJ, Grant SG 2007. Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies. J Neurosci 27: 2673–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado JY, Coba M, Anderson CNG, Thompson KR, Gray EE, Heusner CL, Martin KC, Grant SGN, O'Dell TJ 2007. NMDA receptor activation dephosphorylates AMPA receptor glutamate receptor 1 subunits at threonine 840. J Neurosci 27: 13210–13221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endele S, Rosenberger G, Geider K, Popp B, Tamer C, Stefanova I, Milh M, Kortum F, Fritsch A, Pientka FK, et al. 2010. Mutations in GRIN2A and BRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat Genet 42: 1021–1026 [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R 2003. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6: 136–143 [DOI] [PubMed] [Google Scholar]
- Faber ESL, Delaney AJ, Power JM, Sedlak PL, Crane JW, Sah P 2008. Modulation of SK channel trafficking by beta adrenoceptors enhances excitatory synaptic transmission and plasticity in the amygdala. J Neurosci 28: 10803–10813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez E, Collins MO, Uren RT, Kopanitsa MV, Komiyama NH, Croning MD, Zografos L, Armstrong JD, Choudhary JS, Grant SG 2009. Targeted tandem affinity purification of PSD-95 recovers core postsynaptic complexes and schizophrenia susceptibility proteins. Mol Syst Biol 5: 269 doi: 10.1038/msb.2009.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KA, McLaughlin N, Edbauer D, Phillips M, Bolton A, Constantine-Paton M, Sheng M 2010. Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. J Neurosci 30: 2676–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen PV 2007. ERK and mTOR signaling couple beta-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J Biol Chem 282: 27527–27535 [DOI] [PubMed] [Google Scholar]
- Grant SG, Marshall MC, Page KL, Cumiskey MA, Armstrong JD 2005. Synapse proteomics of muliprotein comples: En route from genes to nervous system diseases. Hum Mol Genet 14: R225–R234 [DOI] [PubMed] [Google Scholar]
- Gurd JW, Bissoon N 1997. The N-methyl-d-aspartate receptor subunits NR2A and NR2B bind to the SH2 domains of phospholipase C-gamma. J Neurochem 69: 623–630 [DOI] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG 2000. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci 3: 661–669 [DOI] [PubMed] [Google Scholar]
- Jin S-X, Feig LA 2010. Long-term potentiation in the CA1 hippocampus induced by NR2A subunit-containing NMDA glutamate receptors is mediated by Ras-GRF2/Erk Map kinase singaling. PLoS ONE 5: e11732 doi:10.1371/journal.pone.0011732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner M-LA, Lisé M-F, Yuen EY, Kam AYF, Zhang M, Hall DD, Malik ZA, Qian H, Chen Y, Ulrich JD 2010. Assembly of a β2-adrenergic receptor-GluR1 signalling complex for localized cAMP signaling. EMBO Journal 29: 482–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama K, Lee HK, Bear MF, Huganir RL 1998. Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron 21: 1163–1175 [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M 2004. PDZ domain proteins of synapses. Nat Rev Neurosci 5: 771–781 [DOI] [PubMed] [Google Scholar]
- Kim JH, Liao D, Lau LF, Huganir RL 1998. SynGAP: A synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron 20: 683–691 [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Shen M 2005. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron 46: 745–760 [DOI] [PubMed] [Google Scholar]
- Köhr G, Jensen V, Koester HJ, Mihaljevic ALA, Utvik JK, Kvello A, Ottersen OP, Seeburg PH, Sprengel R, Hvalby Ø 2003. Intracellular domains of NMDA receptor subtypes are determinants for long-term potentiation induction. J Neurosci 23: 10791–10799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama NH, Watabe AM, Carlisle HJ, Porter K, Charlesworth P, Monti J, Strathdee DJC, O'Carroll CM, Martin SJ, Morris RGM, et al. 2002. SynGAP regulates ERK/MAPK signaling, synaptic Plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J Neurosci 22: 9721–9732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH 1995. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269: 1737–1740 [DOI] [PubMed] [Google Scholar]
- Lee HK 2006. Synaptic plasticity and phosphorylation. Pharmacol Ther 112: 812–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Kameyama K, Huganir RL, Bear MF 1998. NMDA induces long-term synaptic depression and dephosphorylation of GluR1 subunit of AMPA receptors in hippocampus. Neuron 21: 1151–1162 [DOI] [PubMed] [Google Scholar]
- Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW 1999. Calcium/calmodulin-dependent protein kinase II is associated with the NMDA receptor. Nature 385: 439–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Tian X, Hartley DM, Feig LA 2006. Distinct roles for Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) and Ras-GFR2 in the induction of long-term potentiation and long-term depression. J Neurosci 26: 1721–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, et al. 1998. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396: 433–439 [DOI] [PubMed] [Google Scholar]
- O'Dell TJ, Connor SA, Gelinas JN, Nguyen PV 2010. Viagra for your synapses: Enhancement of hippocampal long-term potentiation by activation of beta-adrenergic receptors. Cell Signal 22: 728–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR 2006. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem 281: 752–758 [DOI] [PubMed] [Google Scholar]
- Opazo P, Watabe AM, Grant SGN, O'Dell TJ 2003. Phosphatidylinositol 3-kinase regulates the induction of long-term potentiation through extracellular signal-related kinase-independent mechanisms. J Neurosci 23: 3679–3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MA, Szatmari EM, Yasuda R 2010. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc Natl Acad Sci 107: 15951–15956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD 1999. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci 19: 4337–4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh G, Vicini S 1999. Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule cell neurons. J Neurosci 19: 10603–10610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh G, Adams JP, Kim JH, Huganir RL 2006. SynGap regulates synaptic sthregth and mitogen-activated protein kinases in cultured neurons. Proc Natl Acad Sci 103: 4344–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff EB 2007. A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron 56: 670–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel R, Suschanek B, Amico C, Brusa R, Burnashev N, Rozov A, Hvalby Ø, Jensen V, Paulsen O, Andersen P, et al. 1998. Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell 92: 279–289 [DOI] [PubMed] [Google Scholar]
- Steigerwald P, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Köhr G 2000. C-terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci 20: 4573–4581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surojit P, Connor JA 2010. NR2B-NMDA receptor-mediated increases in intracellular Ca2+ concentration regulate the tyrosine phosphatase, STEP, and ERK MAP kinase signaling. J Neurochem 114: 1107–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Moody TD, Makhinson M, O'Dell TJ 1996. Activity-dependent β-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron 17: 475–482 [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Watabe AM, Moody TD, Makhinson M, O'Dell TJ 1998. Postsynaptic complex spike bursting enables the induction of LTP by theta frequency synaptic stimulation. J Neurosci 18: 7118–7126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoose AM, Winder DG 2003. NMDA and β-adrenergic receptors differentially signal phosphorylation of glutamate receptor type 1 in area CA1 of hippocampus. J Neurosci 23: 5827–5834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe AM, O'Dell TJ 2003. Age-related changes in theta frequency stimulation-induced long-term potentiation. Neurobiol Aging 24: 267–272 [DOI] [PubMed] [Google Scholar]
- Watabe AM, Zaki PA, O'Dell TJ 2000. Coactivation of β-adrenergic and cholinergic receptors enhances the induction of long-term potentiation and synergistically activates mitogen-activated protein kinase in the hippocampal CA1 region. J Neurosci 20: 5924–5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder DG, Martin KC, Muzzio IA, Rohrer D, Chruscinski A, Kobilka B, Kandel ER 1999. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by β-adrenergic receptors. Neuron 24: 715–726 [DOI] [PubMed] [Google Scholar]
- Xu W, Schlüter OM, Steiner P, Czervlonke BL, Sabatini B, Malenka RC 2008. Molecular dissociation of the role of PSD-95 in regulating synaptic strength and LTD. Neuron 57: 248–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L-L, Adams JP, Swank M, Sweatt JD, Johnston D 2002. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activate protein kinase pathway. J Neurosci 22: 4860–4868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Takahashi E, Li W, Halt A, Wiltgen B, Ehninger D, Li G-D, Hell JW, Kennedy MB, Silva AJ 2007. Interactions between the NR2B receptor and CaMKII modulate synaptic plasticity and spatial learning. J Neurosci 27: 13843–13853 [DOI] [PMC free article] [PubMed] [Google Scholar]