Abstract
Transcription of genes required for long-term memory not only involves transcription factors, but also enzymatic protein complexes that modify chromatin structure. Chromatin-modifying enzymes, such as the histone acetyltransferase (HAT) CREB (cyclic-AMP response element binding) binding protein (CBP), are pivotal for the transcriptional regulation required for long-term memory. Several studies have shown that CBP and histone acetylation are necessary for hippocampus-dependent long-term memory and hippocampal long-term potentiation (LTP). Importantly, every genetically modified Cbp mutant mouse exhibits long-term memory impairments in object recognition. However, the role of the hippocampus in object recognition is controversial. To better understand how chromatin-modifying enzymes modulate long-term memory for object recognition, we first examined the role of the hippocampus in retrieval of long-term memory for object recognition or object location. Muscimol inactivation of the dorsal hippocampus prior to retrieval had no effect on long-term memory for object recognition, but completely blocked long-term memory for object location. This was consistent with experiments showing that muscimol inactivation of the hippocampus had no effect on long-term memory for the object itself, supporting the idea that the hippocampus encodes spatial information about an object (such as location or context), whereas cortical areas (such as the perirhinal or insular cortex) encode information about the object itself. Using location-dependent object recognition tasks that engage the hippocampus, we demonstrate that CBP is essential for the modulation of long-term memory via HDAC inhibition. Together, these results indicate that HDAC inhibition modulates memory in the hippocampus via CBP and that different brain regions utilize different chromatin-modifying enzymes to regulate learning and memory.
Long-term memory requires the coordinated effort of transcription factors and numerous enzymes and coregulators that modify and remodel chromatin structure (for review, see Barrett and Wood 2008). One mechanism by which chromatin structure can be regulated is via the addition of functional groups to histone proteins, referred to as histone modifications, which serve two main purposes: first to provide recruitment signals for proteins involved in transcriptional activation and silencing (Kouzarides 2007; Taverna et al. 2007) and second to regulate chromatin structure by disrupting contacts between histone tails and genomic DNA, as well as between nucleosomes (Kouzarides 2007). The best-studied histone modification in learning and memory is histone acetylation and the enzymes that are associated with it, histone deacetylases (HDACs) and histone acetyltransferases (HATs).
A well known HAT involved in learning and memory is the CREB (cAMP response element binding protein) binding protein (CBP). Cbp mutant mice exhibit specific forms of impaired long-term potentiation and long-term memory (Bourtchouladze et al. 2003; Alarcon et al. 2004; Korzus et al. 2004; Wood et al. 2005, 2006; Vecsey et al. 2007). Interestingly, all five types of genetically modified Cbp mutant mice exhibit deficits in long-term memory for object recognition (Bourtchouladze et al. 2003; Alarcon et al. 2004; Korzus et al. 2004; Wood et al. 2006; Oliveira et al. 2007; Barrett and Wood 2008; Stefanko et al. 2009). This evidence suggests that the brain regions required for object recognition memory may be particularly sensitive to alterations in histone acetylation and CBP activity. Thus, the object recognition task provides a particularly useful behavioral paradigm for studying the role of histone-modifying enzymes in long-term memory processes.
In contrast to the genetic studies examining the role of CBP in memory, the majority of the studies examining HDACs in memory have been carried out using a pharmacological approach (for review, see Barrett and Wood 2008). HDAC inhibition experiments have shown that HDACs are critical negative regulators of long-term memory formation (Levenson et al. 2004; Vecsey et al. 2007; Stefanko et al. 2009) and a study examining individual HDACs has revealed that HDAC2, but not HDAC1, to be a key HDAC in regulating memory formation (Guan et al. 2009). More recently, a study has shown that HDAC3 is also a critical negative regulator of memory formation (McQuown et al. 2011). However, the underlying mechanism by which HDAC inhibition modulates long-term memory formation remains unclear.
A study by Vecsey et al. (2007) demonstrated that hippocampal long-term potentiation could be significantly enhanced by HDAC inhibition and that this effect was entirely dependent on CBP and its interaction with CREB. However, the same did not appear to be true at the level of behavior when examining long-term memory. Stefanko et al. (2009) found that HDAC inhibition could facilitate long-term memory for object recognition in CBP mutant mice. This suggested that HDAC inhibition could facilitate long-term memory independently of CBP. In the discussion of Stefanko et al. (2009), the investigators suggest that the object recognition task used may not have engaged the hippocampus, which in turn would not engage CBP-dependent mechanisms in the hippocampus. Thus, the prediction is that in an object recognition task that does engage the hippocampus, HDAC inhibition will modulate long-term memory in a CBP-dependent manner.
To test this prediction and to better understand the histone-modifying mechanisms regulating long-term memory formation in the hippocampus, we first examined the role of the hippocampus during retrieval of long-term memory for object recognition and object location by utilizing muscimol as the inhibitor of neuronal activity. We then used similar forms of training paradigms in CBP-mutant mice, as well as HDAC inhibitor treatment, to study the basic underlying mechanisms of HDAC inhibition-induced memory enhancement. The results have important implications for the basic understanding of the role of CBP and histone acetylation in different brain regions for long-term memory formation as well as the role of the hippocampus in the retrieval of long-term memory for object recognition vs. object location.
Results
Muscimol inactivation of the dorsal hippocampus in consolidation and retrieval of long-term memory for object recognition
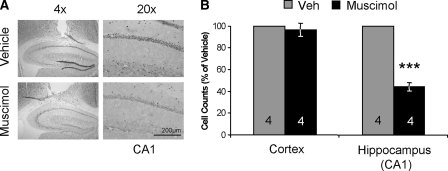
To examine the role of the hippocampus in consolidation of long-term memory for object recognition, we delivered the GABA agonist muscimol directly to the dorsal hippocampus immediately after training. First, we indirectly examined the spread of muscimol using c-fos immunohistochemistry. Mice were cannulated with hippocampal cannulae, handled for 2 min a day for five consecutive days, and then habituated to the chamber for 5 min a day for four consecutive days. Mice then received dorsal hippocampal infusions of muscimol or vehicle 1 h prior to a 10-min training session, and brains were collected 90 min following training. Figure 1 shows that muscimol infusion results in a significant decrease in hippocampal c-fos expression (44 ± 4.0% of vehicle; t-test – t(6) = 8.027; P < 0.001). There was no significant difference in the number of c-fos-positive cells in the cortex surrounding the cannula tract in muscimol-infused mice as compared to vehicle (t-test − t(6) = 0.331; P = 0.752), which demonstrates that this reduction in c-fos immunoreactivity is confined to the hippocampus.
Figure 1.
Intrahippocampal muscimol injection spread indirectly examined by c-fos immunoreactivity. (A) images are 4X magnification on the right and 20X magnification on the left. Histograms depict quantification of cell counts as a percent of vehicle. (A) Representative images showing c-fos immunoreactivity in sections of vehicle (top row) and muscimol-infused mice (bottom row). (B) Quantification shows that c-fos-immunoreactive cells are not changed in the cortex surrounding the cannula, but it is significantly decreased by 56% in the dorsal hippocampus. ***, P < 0.001. Numbers inside bars indicate sample size (n).
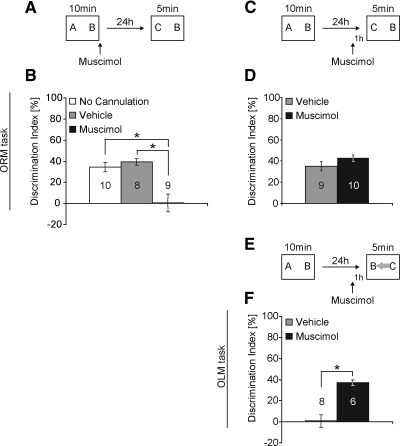
To examine the effect of muscimol in the dorsal hippocampus on consolidation, mice were cannulated with hippocampal cannulae, handled for 2 min a day for five consecutive days, and then habituated to the chamber for 5 min a day for four consecutive days. Mice received a 10-min training period (Fig. 2A), which we have shown in previous studies results in long-term memory for the familiar object (Stefanko et al. 2009). Neither the total time exploring the objects (one-way ANOVA − F(2,22) = 2.148; P > 0.05), nor the preference between the different objects (discrimination index) during training differs significantly between groups (Kruskal-Wallis one-way ANOVA − H(2) = 1.055; P > 0.05). Exploration times are presented in Supplemental Table S1. Immediately after training, mice received bilateral hippocampal delivery of 0.5 µL of muscimol (1 µg/µL in PBS) or vehicle (PBS). A Kruskal-Wallis one-way ANOVA revealed a significant difference between groups (Fig. 2B) (H(2) = 10.789; P = 0.005). A pairwise multiple comparison revealed animals receiving muscimol (n = 9) exhibited no preference for the novel object as compared to control animals receiving vehicle (n = 8; Q = 3.014, P < 0.05; Dunn's Method) or noncannulated animals (n = 10; Q = 2.632; P < 0.05). Vehicle and noncannulated animals were not significantly different from each other, showing that the surgery had no negative impact on performance. The total time spent exploring the objects during testing did not differ between groups (one-way ANOVA – F(2,24) = 0.094; P > 0.05; see Supplemental Table S1). This experiment demonstrates that the hippocampus is necessary during consolidation to form long-term memory for the familiar object.
Figure 2.
The hippocampus is engaged during object location memory retrieval. (A,C,E) Schematic diagrams for object recognition tasks. Letters (A, B, and C) in the boxes indicate objects. Gray arrow indicates a moved familiar object compared to the training. In each experiment, mice were fitted with bilateral hippocampal cannulae, allowed to recover from surgery, handled, and habituated to the context prior to a 10-min training. Animals received a bilateral injection (0.5 µL at 15 µL/h) of 1 µg/µL muscimol dissolved in PBS or PBS as a control (vehicle). Noncannulated animal did not undergo surgery or injection. (B) During a 24-h retention test, mice that received muscimol immediately after the training displayed no preference for the novel object in contrast to vehicle or noncannulated mice. (D) During a 24-h retention test, mice that received muscimol 1 h prior to the retention test displayed similar preference for the novel object compared with vehicle-treated mice. (F) During a 24-h retention test, mice that received muscimol 1 h prior to the retention test in the OLM task (moved familiar object—gray arrow) displayed a significant preference for the novel object compared to vehicle-treated mice. *, P < 0.05. Numbers inside bars indicate sample size (n).
To examine the role of the hippocampus in retrieval of long-term memory for object recognition, we delivered the GABA agonist muscimol directly to the dorsal hippocampus 1 h before the retention test (Fig. 2C,D). Neither the total time exploring the objects (t-test − t(17) = 0.148; P > 0.05), nor the preference between the different objects (discrimination index) during training differs significantly between groups (t-test − t(17) = 0.520; P > 0.05; for times see Supplemental Table S1). As shown in Figure 2D, animals receiving muscimol (n = 10) exhibited similar long-term memory for the familiar object as compared to control animals receiving vehicle (n = 9; t-test − t(17) = 1.352; P = 0.194). The total time spent exploring the objects during testing did not differ between groups (t-test – t(17) = −1.851; P > 0.05; see Supplemental Table S1). This experiment demonstrates that the hippocampus is not necessary for the retrieval of long-term memory for the familiar object.
Inactivation of dorsal hippocampus reveals intact long-term memory for object recognition when familiar object has been moved to a novel location
We hypothesized that if a familiar object changes location between training and testing it would impart novelty to the familiar object during the retention test. Thus, in a retention test in which a familiar object (see Fig. 2E, object B) has been moved to a novel location and a novel object (see Fig. 2E, object C) is introduced, a mouse is predicted to spend equal time exploring both objects, resulting in a discrimination index of zero. Because object location is thought to be encoded by the hippocampus, we predicted that muscimol inactivation of the hippocampus during the retention test would prevent processing of object location information, resulting in a mouse spending more time exploring the novel object (object C) as compared to the familiar object (object B). As shown in the previous experiment (Fig. 2D) a mouse can distinguish between a novel and familiar object without an active hippocampus.
To test this prediction, mice were treated and handled as in the previous experiment except that during the test the familiar object (object B) was moved to a novel location (as indicated by the gray arrow in Fig. 2E) and the novel object (object C) was introduced in the former spot of the familiar object. Neither the total time exploring the objects (Mann–Whitney U = 16.000; n(muscimol) = 6; n(vehicle) = 8; P > 0.05), nor the preference between the different objects (discrimination index) during training differs significantly between groups (t-test – t(12) = 0.029; P > 0.05; for times see Supplemental Table S1). As shown in Figure 2F, animals receiving muscimol (n = 6) 1 h prior to the retention test exhibited significant preference for the novel object (object C) as compared to control animals receiving vehicle (n = 8; t-test – t(12) = 4869; P < 0.001). The total time spent exploring the objects during testing did not differ between groups (t-test – t(12) = 0.212; P > 0.05) (see Supplemental Table S1). Thus, vehicle-treated mice explored both objects similarly, resulting in a near zero discrimination index. However, muscimol-treated mice preferentially explored the novel object (object C), resulting in a high discrimination index, which demonstrates a significant long-term memory for the familiar object but no memory of object location. These results suggest that the hippocampus becomes engaged during retrieval of long-term memory for a familiar object when the location of that object is different between training and testing. These data also suggest that if the hippocampus is engaged during retrieval, then behavior based on the existing long-term memory for the familiar object is masked by the competing memory for object location.
Inactivation of dorsal hippocampus reveals intact long-term memory for object recognition when familiar object is placed in a novel context
To demonstrate that the effects of muscimol on the hippocampus during memory retrieval are not specific to object location, we also examined the effect of changing the context between training and testing. Similar to the results shown in Figure 2D, if the context is not changed between training and testing (see schematic in Supplemental Fig. S1A), delivery of muscimol to the dorsal hippocampus has no effect on long-term memory for the familiar object (Supplemental Fig. S1B). As shown in Supplemental Figure S1B, vehicle-treated (n = 10) as well as muscimol-treated animals (n = 10) both spent more time exploring the novel object (no statistical significant differences between groups; t-test – t(18) = 1.533; P > 0.05). In contrast, if the context is switched between training and testing (see schematic in Supplemental Fig. S1C), vehicle-treated animals (n = 7) showed no preference for the novel object, whereas muscimol-treated animals (n = 7) exhibited a strong preference for the novel object (t-test – t(12) = 6.432; P < 0.001) (Supplemental Fig. S1D). There was no statistical significant difference in exploratory behavior neither during training (Supplemental Fig. S1B: t-test – t(18) = 0.475; P > 0.05; Supplemental Fig. S1D: t-test – t(12) = 1.863; P > 0.05), nor during testing (Supplemental Fig. S1B: t-test – t(18) = 0.716; P > 0.05; Supplemental Fig. S1D: t-test – t(12) = 0.414; P > 0.05), as well as no preference for either object (discrimination index) during the training (Supplemental Fig. S1B: t-test – t(18) = 0.455; P > 0.05; Supplemental Fig. S1D: t-test – t(12) = 0.189; P > 0.05). These results are very similar to those in Figure 2, suggesting that if either object location or contextual information changes between training and testing, then the hippocampus becomes engaged, resulting in masking of long-term memory for the object itself.
Enhanced long-term memory for object recognition via HDAC inhibition is masked by engaging the hippocampus during retrieval
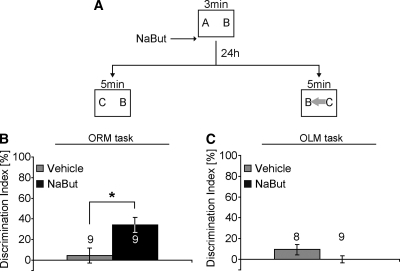
In the next experiment, we examined whether histone deacetylase (HDAC) inhibition can enhance long-term memory for object recognition, even when a familiar object has been moved to a novel location. One group of mice were given a 3-min training period (see schematic in Fig. 3A), which we have previously shown is not sufficient for a mouse to form an observable long-term memory for the familiar object 24 h after training (Stefanko et al. 2009). Immediately after training, one-half of the mice received a systemic i.p. injection of NaBut (1.2 g/kg) and the other half vehicle. These two treatment groups then were split in half to test them in different paradigms. The first set of mice (vehicle and NaBut treated) was given a retention test in which the familiar object (object B) remained in the familiar location (object recognition memory [ORM]; see left-hand side of schematic in Fig. 3A). The other set was given a retention test in which the familiar object (object B) was moved to a novel location (object location memory [OLM]; see right-hand side of schematic in Figure 3A [gray arrow indicates a moved object B]). Neither the total time exploring the objects (two-way ANOVA – experiment type [ORM, OLM]: F(1,31) = 1.511; P > 0.05; Treatment [Vehicle, NaBut]: F(1,31) = 0.132; P > 0.05; experiment type × treatment: F(1,31) = 0.094; P > 0.05), nor the preference between the different objects (discrimination index) during training differs significantly between groups (two-way ANOVA – experiment type [ORM, OLM]: F(1,31) = 0.800; P > 0.05; Treatment [Vehicle, NaBut]: F(1,31) = 3.007; P > 0.05; experiment type × treatment: F(1,31) = 0.0175; P > 0.05; see Supplemental Table S1). Immediately after training, mice received a systemic i.p. injection of NaBut (1.2 g/kg) (see Stefanko et al. 2009) or vehicle. As shown in Figure 3B, mice receiving NaBut (n = 9) exhibited significantly greater preference for the novel object than vehicle controls (n = 9; t-test – t(16) = 2.866; P = 0.011). The total time spent exploring the objects during testing did not differ between groups (t-test – t(16) = 0.760; P > 0.05; for times see Supplemental Table S1). These results are similar to our previous findings and support the conclusion that HDAC inhibition can transform a learning event that would not normally result in long-term memory (3-min training period) into an event that does result in significant long-term memory (Stefanko et al. 2009).
Figure 3.
HDAC inhibition enhances preference for the novel object in the ORM task, but does not affect performance in the object location-dependent OLM task. (A) Schematic for ORM task and OLM task. Letters (A, B, C) in the boxes indicate objects. Gray arrow indicates a moved familiar object compared to the training. (B) Mice administered NaBut immediately after training exhibit significant long-term memory for the familiar object in its familiar location. (C) In contrast, in the OLM task where the familiar object is placed in a different location, both vehicle- and NaBut-treated mice exhibit similar preference for both objects during the retention test, resulting in negligible discrimination. *, P < 0.05. Numbers inside bars indicate sample size (n).
In contrast, when a familiar object is moved to a novel location (object B) and a novel object is introduced (object C), NaBut-treated animals (n = 9) performed similarly to vehicle-treated animals (n = 8; t-test – t(15) = 1.603; P = 0.130; Fig. 3C). Both NaBut and vehicle-treated groups explored the familiar object in a novel location (object B; see right-hand side of schematic in Fig. 3A) and the novel object (object C) to a similar extent. The total time spent exploring the objects during testing did not differ between groups (Mann-Whitney U = 35.500; P > 0.05; for times see Supplemental Table S1). NaBut-treated animals did not explore the novel object more than the familiar object even though animals in the same experiment receiving the same handling, treatment and training spend more time with the novel object in the ORM task (Fig. 3B). Together, the results from Figure 3B,C suggest that even though HDAC inhibition can enhance long-term memory for the object itself, it is not evident when the hippocampus becomes engaged during retrieval and drives behavior. Similar to Figure 2C, NaBut-treated animals have a memory for both the object location and the object itself, which results in a masking of the long-term memory for the object itself during retrieval as determined by the discrimination index. An alternative explanation is that NaBut simply failed to affect long-term memory for object location, but this is unlikely as we have shown NaBut can enhance long-term memory for object location in a previous study (Roozendaal et al. 2010), and the data shown in the next two figures.
CBPKIX/KIX homozygous knock-in mice exhibit HDAC inhibition-induced long-term memory enhancement for object recognition memory in an OLM task
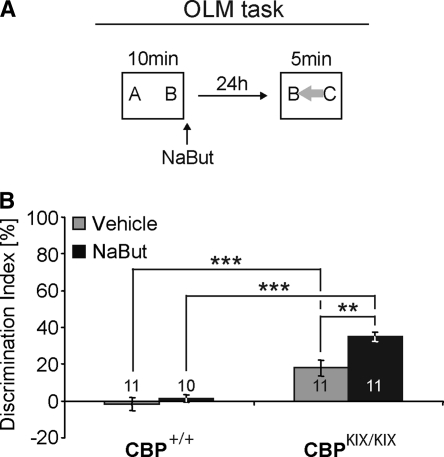
CBPKIX/KIX homozygous knock-in mice express mutant CBP protein carrying a triple point mutation in the CREB-binding (KIX) domain of CBP (Kasper et al. 2002). In a previous study we demonstrated that these mice have impaired hippocampus-dependent long-term memory for contextual fear (Wood et al. 2006). We have also shown that CBPKIX/KIX mice exhibit significantly impaired long-term memory for object recognition using a task similar to the schematic shown in Figure 2A, in which object location is not changed (Stefanko et al. 2009). Further, impaired long-term memory for object recognition in CBPKIX/KIX mice could be rescued by HDAC inhibition (Stefanko et al. 2009), suggesting that HDAC inhibition modulates long-term memory for the object itself independently of CBP.
Results from Figures 1 and 2 suggest that the hippocampal dysfunction of CBPKIX/KIX mice should allow one to observe HDAC inhibition-dependent enhancement of long-term memory for object recognition in CBPKIX/KIX mice, but not wild-type littermates. To test this, CBPKIX/KIX mice and CBP+/+ wild-type littermates were handled and trained according to the schematic in Figure 4A using a 10-min training period. Neither the total time exploring the objects (two-way ANOVA genotype: F(1,39) = 0.220; P > 0.05; treatment: F(1,39) = 0.037; P > 0.05; genotype × treatment: F(1,39) = 0.147; P > 0.05), nor the preference between the different objects (discrimination index) during training differs significantly between groups (two-way ANOVA genotype: F(1,39) = 0.632; P > 0.05; treatment: F(1,39) = 1.159; P > 0.05; genotype × treatment: F(1,39) = 2.147; P > 0.05; for times see Supplemental Table S1). Immediately after training, mice received a systemic i.p. injection of NaBut (1.2 g/kg) or vehicle. A two-way ANOVA revealed an effect of treatment (F(1,39) = 8.280; P = 0.007) and genotype (F(1,39) = 57.643; P < 0.001) with a strong trend toward an interaction (F(1,39) = 3.911; P = 0.055). Bonferroni post-hoc comparisons showed that CBPKIX/KIX mice treated with NaBut (n = 11) have significantly enhanced object memory compared to vehicle-treated CBPKIX/KIX mice (n = 11; t(20) = 3.203; P = 0.004; Fig. 3B). In contrast, wild-type mice treated with either NaBut or vehicle failed to exhibit long-term memory for the familiar object. CBPKIX/KIX mice treated with NaBut showed a significant increase in the preference for the novel object as compared to wild-type animals treated with NaBut (KIX, n = 11; WT, n = 10; t(19) = 9.236; P < 0.001). The total time spent exploring the objects during testing did not differ between groups (two-way ANOVA genotype: F(1,39) = 0.000; P > 0.05; treatment: F(1,39) = 0.857; P > 0.05; genotype × treatment: F(1,39) = 0.504; P > 0.05; for times see Table S1). The data from CBP+/+ wild-type littermate mice, showing that both vehicle- and NaBut-treated groups failed to exhibit long-term memory for the familiar object, replicate the results shown in Figure 3C. In contrast, CBPKIX/KIX mice treated with NaBut exhibited significant long-term memory for the familiar object, presumably due to impaired hippocampus-dependent memory formation in these mice. This finding will be carefully addressed in the Discussion.
Figure 4.
HDAC inhibition enhances memory for the object itself in CBPKIX/KIX homozygous knock-in mice. (A) Schematic of OLM task. Letters (A, B, C) in the boxes indicate objects. Gray arrow indicates a moved object compared to the training. (B) CBPKIX/KIX mice and wild-type littermates received 10-min training period followed immediately by i.p. injection of either NaBut (1.2 g/kg in water) or vehicle (water). During the retention test, in which the familiar object is in a different location (gray arrow), wild-type mice exhibited no preference for the novel object regardless of treatment. In contrast, CBPKIX/KIX mice displayed a poor preference for the novel object, which was significantly enhanced by NaBut treatment. **, P < 0.01; ***, P < 0.001. Numbers inside bars indicate sample size (n).
HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner
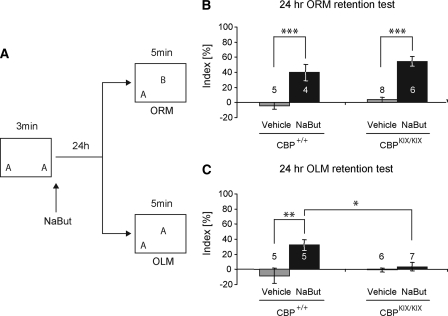
To directly examine whether HDAC inhibition modulates long-term memory for object location in a CBP-dependent manner, we used a different version of the object recognition task. In this version, all animals are trained in the same manner, but then groups are divided so that long-term memory for either object recognition or object location can be assessed (see schematic in Fig. 5A). For this task, we used a subthreshold 3-min training period, which we have shown does not lead to long-term memory for object recognition (Stefanko et al. 2009) or object location (Roozendaal et al. 2010). We predicted that HDAC inhibition would enhance long-term memory for object recognition in wild-type and CBPKIX/KIX mice (similar to results published in Stefanko et al. [2009] and similar to results shown in Fig. 4 of this study), but would fail to enhance long-term memory for object location in CBPKIX/KIX mice.
Figure 5.
HDAC inhibition fails to enhance object location memory in CBPKIX/KIX homozygous knock-in mice. (A) Schematic of a combined ORM and OLM task. Letters (A, B) in the boxes indicate objects. CBPKIX/KIX mice and wild-type littermates received a subthreshold 3 min training period followed immediately by i.p. injection of either NaBut (1.2 g/kg in water) or vehicle (water). (B) ORM task: Wild-type as well as CBPKIX/KIX mice exhibited a preference for the novel object if they were treated with NaBut. (C) OLM task: Here only the wild-type mice treated with NaBut exhibited a preference for the moved object. In CBPKIX/KIX mice a NaBut treatment does not enhance object location memory. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Numbers over bars indicate sample size (n).
In this special ORM task where a novel object is placed in a novel position (see schematic in Fig. 5A; ORM is top right), it is crucial to compensate for a possible position effect so the novel location was counterbalanced. CBPKIX/KIX mice and wild-type littermates were handled and habituated to the training chamber. Neither the total time exploring the objects (three-way ANOVA revealed no significant differences in task type, genotype, and treatment nor any interactions; see Supplemental Table S1), nor the preference between the different objects (discrimination index) during training differs significantly between groups (three-way ANOVA revealed neither significant differences in task type, genotype, and treatment nor any interactions; for discrimination indices see Supplemental Table S1). Immediately after training, mice received a systemic i.p. injection of NaBut (1.2 g/kg) or vehicle. A two-way ANOVA revealed an effect of treatment (F(1,19) = 49.949; P < 0.001), but there were neither differences between genotypes (F(1,19) = 2.914; P > 0.05), nor a statistically significant interaction between genotype and treatment (F(1,19) = 0.235; P > 0.05) in this ORM task (Fig. 5B). The total time spent exploring the objects during testing did not differ between groups (two-way ANOVA genotype: F(1,19) = 1.548; P > 0.05; treatment: F(1,19) = 2.805; P > 0.05; genotype × treatment: F(1,19) = 0.231; P > 0.05; see Supplemental Table S1). The results in Figure 5B show that a systemic delivery of an HDAC inhibitor enhanced exploration of the novel object in this ORM task in CBPKIX/KIX mice (Bonferroni t-test – t = 4.225; P < 0.001) as well as in wild-type animals (Bonferroni t-test – t = 6.022; P < 0.001).
In a separate subset of animals, a familiar object was moved to a novel location during the retention test (see schematic in Fig. 5A [OLM is bottom right]). A two-way ANOVA revealed no significant effect of genotype (F(1,19) = 2.115; P > 0.05) but an effect of treatment (F(1,19) = 9.565; P = 0.006) and a significant difference of the interaction between genotype and treatment (F(1,19) = 6.312; P = 0.021). The total time spent exploring the objects during testing did not differ between groups (two-way ANOVA genotype: F(1,19) = 0.068; P > 0.05; treatment: F(1,19) = 0.433; P > 0.05; genotype × treatment: F(1,19) = 1.476; P > 0.05; see Supplemental Table S1). The results in Figure 5C show that a systemic delivery of an HDAC inhibitor enhanced the exploration of the moved object in a 3-min subthreshold training in the OLM task only in the wild-type animals (Bonferroni t-test – t = 3.733; P = 0.001), but not in the CBPKIX/KIX mice (Bonferroni t-test – t = 0.439; P = 0.665). Within the NaBut-treated animals there is a significant difference between genotypes (KIX vs. WT – Bonferroni t-test – t = 2.853; P = 0.010). These results indicate that HDAC inhibition enhances long-term memory for object location in a CBP-dependent manner. Together, results from Figure 5 suggest that HDAC inhibition impacts long-term memory for object recognition and object location, which are retrieved by different brain structures, via different mechanisms.
Discussion
In this study we examined the role of the hippocampus in the retrieval of long-term memory for object recognition and also object location. We found that if a familiar object is moved to a novel location for the retention test, then that familiar object is treated as novel and subsequently explored to a similar extent as a completely novel object. This resulted in a discrimination index of nearly zero, which is interpreted as the animal having no significant long-term memory for object recognition. However, it seemed unlikely that the animal lost its long-term memory for the familiar object after only 24 h. We hypothesized that because the familiar object had moved to a novel location that the hippocampus was now engaged during retrieval, resulting in the animal treating the familiar object as if it were novel and exploring it to a similar extent as a novel object. Therefore, if we inactivated the hippocampus during retrieval, then the novel location of the familiar object would not be processed by the animal, resulting in a preference for the completely novel object. Indeed, this is what was observed (Fig. 2F). This was not specific to object location, as changing the context also engaged the hippocampus during retrieval and led to the same masking of long-term memory for the familiar object (Supplemental Fig. S1). Together, these results suggest that the behavior of the animal during the retention test is driven by whether the hippocampus is engaged or not.
The role of the hippocampus in long-term memory for a familiar object is still not entirely clear in the rodent literature (for review, see Winters et al. 2008; Clark and Squire 2010). Many studies have found that hippocampal damage impairs memory (Clark et al. 2000; Rampon et al. 2000; Baker and Kim 2002; Gould et al. 2002; Gaskin et al. 2003; Broadbent et al. 2004; Hammond et al. 2004; Prusky et al. 2004; Ainge et al. 2006; de Lima et al. 2006; Rossato et al. 2007), whereas other studies have found that hippocampal manipulations have no significant effect on memory for a familiar object (Mumby et al. 2002, 2005; Stupien et al. 2003; Winters et al. 2004; Forwood et al. 2005; O'Brien et al. 2006; Balderas et al. 2008). The different results obtained in these studies may be due to several factors including: the extent of hippocampal lesion size (see Broadbent et al. 2004); delay between training and testing (see Hammond et al. 2004); and design of task (e.g., duration of habituation to context) (see Stefanko et al. 2009; Oliveira et al. 2010). In our study, we found that delivering muscimol to the dorsal hippocampus 1 h prior to the retention test had no effect on retrieval of long-term memory for the familiar object (Fig. 2D). This could potentially be explained by the limited spread of muscimol in the dorsal hippocampus, however, the same manipulation significantly impaired long-term memory for the familiar object when muscimol was delivered immediately after training (Fig. 2B), demonstrating that it was sufficient to impair consolidation of long-term memory. This is similar to the results of de Lima et al. (2006), in which the same dose and injection volume of muscimol were used.
Two recent studies have demonstrated a double dissociation between the hippocampus and the peri-postrhinal cortex (Winters et al. 2004; Balderas et al. 2008), as well as between the hippocampus and the insular cortex (Balderas et al. 2008). In the former study, bilateral excitotoxic lesions of the hippocampus resulted in rats exhibiting impaired spatial memory as measured by a radial maze, but normal object recognition memory. In contrast, lesions of the peri-postrhinal cortex resulted in rats exhibiting normal spatial memory as measured using a radial arm maze, but impaired object recognition memory. Balderas et al. (2008) demonstrated that protein synthesis inhibition via anisomycin delivered site-specifically to the hippocampus after training had no effect on long-term memory for a familiar object, but significantly impaired object-in-context recognition memory. In contrast, protein synthesis inhibition in the insular cortex or perirhinal cortex significantly impaired long-term memory for a familiar object, but had no effect on object-in-context recognition memory. These results also agree with our recent results showing that HDAC inhibition in the insular cortex enhanced long-term memory for a familiar object, but not object location memory (Roozendaal et al. 2010). In contrast, HDAC inhibition in the dorsal hippocampus enhanced object location memory, but had no effect on long-term memory for a familiar object (Roozendaal et al. 2010). Together, these studies support differential roles of these temporal lobe regions in recognition memory.
One prediction made by our muscimol inactivation experiments (see Fig. 2) was that a mouse with hippocampal long-term memory impairments would exhibit only behavior driven by memory for the familiar object, without showing the hippocampal-induced masking of that memory in the object location-dependent task (see discussion above). We were able to test this prediction using the CBPKIX/KIX homozygous knock-in mice, which have hippocampal memory deficits (Wood et al. 2006). Using the object location-dependent task (Fig. 4), we found that CBPKIX/KIX homozygous knock-in mice exhibited a modest memory for the familiar object (DI = 20%). This agrees with a previous study showing that CBPKIX/KIX mice exhibited a modest, yet significantly impaired, long-term memory for a familiar object (Wood et al. 2006), but is different than a recent study in which CBPKIX/KIX mice exhibited no observable long-term memory for a familiar object (Stefanko et al. 2009). This may be due to the different methods used, especially in this current study in which we use an unorthodox version of an object location-dependent task. Regardless, HDAC inhibition in the CBPKIX/KIX mice significantly enhanced long-term memory for the familiar object. In contrast, wild-type littermates with functional hippocampi exhibited no significant discrimination between novel object and the familiar object in a novel location, and HDAC inhibition had no effect on the performance of wild-type animals (Figs. 3, 4). These results further support the idea that behavior is primarily driven by the hippocampus when engaged during retrieval, but not when the hippocampus is inactivated or impaired.
A mechanism by which HDAC inhibition modulates long-term memory formation in the hippocampus
One approach to understanding the role of HAT and HDAC enzymes in regulating transcription required for long-lasting synaptic plasticity and long-term memory processes has been the use of HDAC inhibitors to modulate memory formation. The modulation of memory by HDAC inhibition gives insight into the molecular mechanisms that may be pivotal for memory formation and they may also provide insight into developing novel therapeutic approaches for the treatment of human disorders associated with learning and memory impairments. One key question that has emerged is what are the molecular mechanisms by which HDAC inhibitors modulate memory? To begin to answer this question, we have used genetically modified CBP mutant mice to determine whether HDAC inhibition can modulate synaptic plasticity and memory in the absence of functional CBP. In our first study (Vecsey et al. 2007), we demonstrated that HDAC inhibition facilitates synaptic plasticity in an entirely CBP-dependent manner. In the hippocampus, HDAC inhibition could transform a transient transcription and translation independent form of long-term potentiation (LTP) into a transcription-dependent long-lasting form of LTP, which also requires CBP. These results suggested that at least with regard to synaptic plasticity, HDAC inhibition modulated hippocampal LTP in a way that was entirely dependent on CBP.
However, this appeared to not hold true at the behavioral level. In a subsequent study (Stefanko et al. 2009), we examined the ability of HDAC inhibition to modulate memory for object recognition and whether the modulation of memory by HDAC inhibition was dependent on CBP. In contrast to what we observed in the Vecsey et al. (2007) study, we found that HDAC inhibition could modulate memory for object recognition independently of CBP (similar to our findings in this study, shown in Fig. 3). Several possibilities existed for the discrepancy between the Vecsey et al. (2007) study and the Stefanko et al. (2009) study, which are presented in the discussion of the Stefanko et al. (2009) paper. The hypothesis we favored is that the form of object recognition task used in the Stefanko et al. (2009) study did not require the hippocampus and that if a hippocampus-dependent object recognition task is used, then HDAC inhibition would modulate memory in a CBP-dependent manner.
In this study, we tested this hypothesis using a task in which all animals are trained in exactly the same manner, but split into two different groups to test long-term memory for the object itself vs. object location (see schematic in Fig. 5A). Similar to our previous findings in the Stefanko et al. (2009) study, we observed that HDAC inhibition enhances long-term memory for the familiar object in both wild-type and CBPKIX/KIX mice. In contrast, HDAC inhibition failed to enhance long-term memory for the object location in CBPKIX/KIX. Together, these results suggest that HDAC inhibition modulates long-term memory in the hippocampus via a CBP-dependent mechanism. Thus, both at the level of synaptic plasticity (Vecsey et al. 2007) and behavior (this study), HDAC inhibition modulated memory in a CBP-dependent manner in the hippocampus. This has important implications for the use of HDAC inhibition as a therapeutic approach, but also begins to define the molecular mechanisms by which HDAC inhibition modulates memory processes. The mechanisms in the hippocampus are different than those in other brain regions.
Importantly, these mechanisms in the hippocampus are not only relevant to HDAC inhibition-dependent modulation of memory. In a very recent study, we examined the mechanism by which glucocorticoids enhance consolidation of hippocampus-dependent and hippocampus-independent object recognition memory (Roozendaal et al. 2010). We found that glucocorticoids enhanced memory consolidation via histone acetylation. More specifically, glucocorticoids enhanced memory for normal object recognition memory in a CBP-independent manner, whereas glucocorticoids enhanced memory for hippocampus-dependent object location memory in a CBP-dependent manner. These results parallel what we observed in this study.
In summary, this study demonstrates that CBP has a specific function in memory formation in the hippocampus as revealed by HDAC inhibition experiments. This suggests that although HDAC inhibition may be nonspecific with respect to relaxing chromatin structure, the ultimate effects on transcriptional regulation, cellular function (synaptic plasticity), and behavior still depends on transcriptional mechanisms normally involved in these processes (Vecsey et al. 2007; Roozendaal et al. 2010). In future studies it will be important to understand the contribution of individual HDACs (e.g., Guan et al. 2009; McQuown et al. 2011) and HATs, both genetically and with selective inhibitors, in different brain regions involved in different types of long-term memory processes.
Materials and Methods
Subjects
Male C57BL/6J mice obtained from The Jackson Laboratory were used in most experiments. The CBPKIX/KIX homozygous knock-in mice were generated as described by Kasper et al. (2002). Briefly, the targeting vector for CBP contained the point mutations Tyr650Ala, Ala654Gln, and Tyr658Ala. The three mutations were introduced into the CBP locus of 129P2/OlaHsd-derived E14 embryonic stem cells by homologous recombination. Mice carrying the mutant allele of the KIX domain of CBP (designated CBPKIX/KIX for homozygous knock-in mice) have been bred and backcrossed in a heterozygous state on a C57BL/6 genetic background for 14 generations. Mice for experiments were generated from heterozygous matings, and wild-type littermates were used as controls. Mice were 8–10 wk of age at the time of the experiment and had free access to food and water in their home cages. Lights were maintained on a 12 h light/12 h dark cycle, with all behavioral testing carried out during the light portion of the cycle. All experiments were conducted according to National Institutes of Health guidelines for animal care and use and were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine. The investigator was blind to the genotype of the mice during behavioral testing.
Object recognition
The object recognition tasks consisted of a training phase and a testing phase. Before training, all mice were handled 2 min daily for 5 d and were habituated to the experimental apparatus 5 min daily for 4 d in the absence of objects. The experimental apparatus was a white rectangular open field (30 × 23 × 21.5 cm) bedded with sawdust (plain context; represented in the schematic diagrams in each figure as a box with solid lines). A checkered context (only used in experiment shown in Supplemental Fig. S1) was created using a 0.5-cm-thick carton was molded in a hexagonal shape that fit into the plain context (represented in the schematic diagrams in each figure as a box with dashed lines). Cob bedding was used in this context. During the training phase, mice were placed in the experimental apparatus with two objects (100-mL beakers, 1-in. circumference × 1.5-in. height; large blue Lego blocks, 1 × 1 × 2 in.) and were allowed to explore for either 3 or 10 min as previously described in Stefanko et al. (2009). Some experiments required a third object (a small white light bulb, 1-in. circumference × 1.5-in. height). The objects were cleaned with ethanol between trials to make sure no olfactory cues were present. Long-term memory was tested 24 h after training. During these retention tests, mice explored the experimental apparatus for 5 min in the presence of one familiar and one novel object. For detailed information of the experimental setup, a schematic is shown in each figure. The novel object and object location were counterbalanced in all experiments. When assessing object recognition memory (ORM), the task is referred to as an ORM task in the figure. When assessing object location memory (OLM), the task is referred to as an OLM task in the figure. Training and testing trials were videotaped and analyzed by individuals blind to the treatment condition and the genotype of subjects. Videos were used to time the exploration of the novel and familiar objects. A mouse was scored as exploring an object when its head was oriented toward the object within a distance of 1 cm or when the nose was touching the object. The relative exploration time was recorded and expressed by a discrimination index (D.I. = [t-novel – t-familiar]/[t-novel + t-familiar] × 100). Mean exploration times were calculated and the discrimination indexes between treatment groups were compared. A different set of mice was used in each experiment.
Surgery
As previously described (Lattal et al. 2007), mice were anesthetized with isoflurane while immobilized on a Just for Mice stereotax (Stoelting). Bilateral 22 gauge guide cannulae (Plastics One Inc.) were implanted to target the dorsal hippocampus (AP −1.7 mm; ML ±1.2 mm; DV −1.5 mm). Injection needles (28 gauge) extended an additional 0.5 mm below the guide cannulae (total depth 2.0 mm). Cannulae placement was visually verified in cryocut sections.
Drug delivery
Sodium butyrate (NaBut; Upstate) was delivered via i.p. injections at a concentration of 1.2 g/kg dissolved in distilled water (Stefanko et al. 2009). Vehicle controls received only distilled water (vehicle; 10 mL/kg to match injection volume of NaBut-treated animals) immediately after training. Muscimol was dissolved in 0.1 M PBS (1.0 µg/µL) and was injected bilaterally in the dorsal hippocampus (0.5 µL/side) of cannulated mice. Control animals received bilateral injections of PBS.
Fos immunohistochemistry
Mice were anesthetized deeply with sodium pentobarbital (100 mg/kg, i.p.) and perfused transcardially with ice-cold PBS, pH 7.4, followed by ice-cold 4% paraformaldehyde in PBS, pH 7.4, using a peristaltic perfusion pump (Fisher Scientific). The brains were removed, post-fixed overnight at 4°C, and then transferred to 30% sucrose for 48 h at 4°C. Brains were frozen and cryocut to 20 µm coronal slices, and sections were stored in 0.1 M PBS. Floating sections were rinsed in 0.1 M PBS (pH 7.4). Endogenous peroxidase activity was inhibited by treating the tissue in 0.5% H2O2 in 70% methanol for 30 min, rinsed in PBS, and blocked for 1 h with 5% dry milk in PBS containing 0.2% Triton X-100. Sections were then incubated with a rabbit anti-Fos polyclonal primary antibody (1:8500; Santa Cruz Biotechnology) in 1% dry milk in PBS with 0.1% Triton X-100 for 40 h at 4°C. Sections were rinsed in PBS, and then incubated in a solution containing biotinylated goat anti-rabbit IgG (1:200; Vector Laboratories) in 1% dry milk in PBS for 2 h at room temperature. Sections were then rinsed in PBS and incubated in an avidin–biotin–peroxidase complex solution (Vector Laboratories) for 1 h. After rinsing with PBS, immunostaining was visualized by peroxidase reaction with a stabilized, nickel-enhanced diaminobenzidine (DAB) complex (Vector Laboratories) for approximately 3 min. The DAB reaction was terminated by rinsing with Tris-buffered saline and the tissue mounted onto glass slides.
Images were acquired on an Olympus (BX51, Japan) microscope using a 4X or 20X objective, CCD camera (QImaging) and QCapture Pro 6.0 software (QImaging). All treatment groups were represented on each slide, and all images were acquired using the same exposure time. Cell counts of Fos-positive nuclei, as defined by density and size criteria, were quantified using ImageJ software (NIH) from comparable 20X images.
Data analysis
Statistics were performed using SigmaStat 3.5. For comparisons between two groups, a Students t-test was performed. One-, two-, and three-way ANOVAs were used when appropriate. Kruskal-Wallis, Dunn's Method, Mann-Whitney, and Bonferroni-corrected post-hoc analyses were performed for comparisons. (*, P < 0.05; **, P < 0.01; ***, P < 0.001.)
Acknowledgments
We thank Robert E. Clark for helpful discussion of the data. We thank Melissa Malvaez for statistical analysis. This research was supported by the National Institute of Mental Health (grant R01MH081004 to M.A.W.) and the Whitehall Foundation (M.A.W.).
Footnotes
[Supplemental material is available for this article.]
References
- Ainge JA, Heron-Maxwell C, Theofilas P, Wright P, de Hoz L, Wood ER 2006. The role of the hippocampus in object recognition in rats: Examination of the influence of task parameters and lesion size. Behav Brain Res 167: 183–195 [DOI] [PubMed] [Google Scholar]
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A 2004. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: A model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 42: 947–959 [DOI] [PubMed] [Google Scholar]
- Baker KB, Kim JJ 2002. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learn Mem 9: 58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL, Bermudez-Rattoni F 2008. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn Mem 15: 618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett RM, Wood MA 2008. Beyond transcription factors: The role of chromatin modifying enzymes in regulating transcription required for memory. Learn Mem 15: 460–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, Scott R, Tully T 2003. A mouse model of Rubinstein-Taybi syndrome: Defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci 100: 10518–10522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE 2004. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci 101: 14515–14520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Squire LR 2010. An animal model of recognition memory and medial temporal lobe amnesia: History and current issues. Neuropsychologia 48: 2234–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR 2000. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci 20: 8853–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima MN, Luft T, Roesler R, Schroder N 2006. Temporary inactivation reveals an essential role of the dorsal hippocampus in consolidation of object recognition memory. Neurosci Lett 405: 142–146 [DOI] [PubMed] [Google Scholar]
- Forwood SE, Winters BD, Bussey TJ 2005. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus 15: 347–355 [DOI] [PubMed] [Google Scholar]
- Gaskin S, Tremblay A, Mumby DG 2003. Retrograde and anterograde object recognition in rats with hippocampal lesions. Hippocampus 13: 962–969 [DOI] [PubMed] [Google Scholar]
- Gould TJ, Rowe WB, Heman KL, Mesches MH, Young DA, Rose GM, Bickford PC 2002. Effects of hippocampal lesions on patterned motor learning in the rat. Brain Res Bull 58: 581–586 [DOI] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, et al. 2009. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459: 55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW 2004. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem 82: 26–34 [DOI] [PubMed] [Google Scholar]
- Kasper LH, Boussouar F, Ney PA, Jackson CW, Rehg J, van Deursen JM, Brindle PK 2002. A transcription-factor-binding surface of coactivator p300 is required for haematopoiesis. Nature 419: 738–743 [DOI] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M 2004. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 42: 961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T 2007. Chromatin modifications and their function. Cell 128: 693–705 [DOI] [PubMed] [Google Scholar]
- Lattal KM, Barrett RM, Wood MA 2007. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav Neurosci 121: 1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD 2004. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem 279: 40545–40559 [DOI] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MA, et al. 2011. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H 2002. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learn Mem 9: 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Tremblay A, Lecluse V, Lehmann H 2005. Hippocampal damage and anterograde object-recognition in rats after long retention intervals. Hippocampus 15: 1050–1056 [DOI] [PubMed] [Google Scholar]
- O'Brien N, Lehmann H, Lecluse V, Mumby DG 2006. Enhanced context-dependency of object recognition in rats with hippocampal lesions. Behav Brain Res 170: 156–162 [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Wood MA, McDonough CB, Abel T 2007. Transgenic mice expressing an inhibitory truncated form of p300 exhibit long-term memory deficits. Learn Mem 14: 564–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AM, Hawk JD, Abel T, Havekes R 2010. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem 17: 155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM, Nelson L, Shabanpoor A, Sutherland RJ 2004. Visual memory task for rats reveals an essential role for hippocampus and perirhinal cortex. Proc Natl Acad Sci 101: 5064–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ 2000. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci 3: 238–244 [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA 2010. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci 30: 5037–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LR, Myskiw JC, Medina JH, Izquierdo I, Cammarota M 2007. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem 14: 36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA 2009. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci 106: 9447–9452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupien G, Florian C, Roullet P 2003. Involvement of the hippocampal CA3-region in acquisition and in memory consolidation of spatial but not in object information in mice. Neurobiol Learn Mem 80: 32–41 [DOI] [PubMed] [Google Scholar]
- Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ 2007. How chromatin-binding modules interpret histone modifications: Lessons from professional pocket pickers. Nat Struct Mol Biol 14: 1025–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, et al. 2007. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci 27: 6128–6140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ 2004. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: Heterogeneity of function within the temporal lobe. J Neurosci 24: 5901–5908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ 2008. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev 32: 1055–1070 [DOI] [PubMed] [Google Scholar]
- Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, Abel T 2005. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem 12: 111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Attner MA, Oliveira AM, Brindle PK, Abel T 2006. A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learn Mem 13: 609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]