Abstract
The risks associated with early age at initiation for alcohol, cigarette, and cannabis use are well documented, yet the timing of first use has rarely been studied in genetically informative frameworks, leaving the relative contributions of genetic and environmental factors to age at initiation largely unknown. The current study assessed overlap in heritable and environmental influences on the timing of initiation across these three substances in African-American women, using a sample of 462 female twins (100 monozygotic and 131 dizygotic pairs) from the Missouri Adolescent Female Twin Study. Mean age at the time of interview was 25.1 years. Ages at first use of alcohol, cigarettes, and cannabis were gathered in diagnostic interviews administered over the telephone. Standard genetic analyses were conducted with substance use initiation variables categorized as never, late, and early onset. Variance in the timing of first use was attributable in large part to genetic sources: 44% for alcohol, 62% for cigarettes, and 77% for cannabis. Genetic correlations across substances ranged from 0.25 to 0.70. Shared environmental influences were modest for alcohol (10%) and absent for cigarettes and cannabis. Findings contrast with reports from earlier studies based on primarily Caucasian samples, which have suggested a substantial role for shared environment on substance use initiation when measured as lifetime use. By characterizing onset as timing of first use, we may be tapping a separate construct. Differences in findings may also reflect a distinct etiological pathway for substance use initiation in African-American women that could not be detected in previous studies.
Keywords: Early initiation, Alcohol, Tobacco, Cannabis, Twins
1. Introduction
1.1. Early substance use
The initiation of substance use is a key subject of investigation in prevention-related research, as the earliest indicators of potential substance-related problems manifest during this first stage of use. For both licit and illicit drugs, early age at first use is a marker of elevated risk for developing substance use disorders (SUDs). The relationship between early onset and later problem use has been demonstrated for alcohol (Chou and Pickering, 1992; Grant and Dawson, 1997; Hingson et al., 2006; McGue et al., 2001; Prescott and Kendler, 1999), cigarettes (Everett et al., 1999; Hu et al., 2006), and other drugs (Anthony and Petronis, 1995; Grant and Dawson, 1998; King and Chassin, 2007). Elevation in risk for SUDs among early users has also been found across substances (Early onset cigarette smoking, for example, has been tied to both alcohol and drug use disorders [Grant, 1998; Grucza and Bierut, 2006; Hanna and Grant, 1999]), suggesting that early initiation reflects more global risk of pathological outcomes.
The increased likelihood of co-occurring substance use among early users of a given substance of abuse is well documented. Early age at first drink has been tied to cigarette smoking and illicit drug use in several studies (Callas et al., 2004; Ellickson et al., 2003; Schmid et al., 2007; Vieira et al., 2007). Early onset cigarette smoking has similarly been associated with both alcohol and cannabis use (Korhonen et al., 2008; Kokkevi et al., 2006; Sartor et al., 2008). Prior use of alcohol or cigarettes has, in turn, been found to predict experimentation with cannabis at a young age (Coffey et al., 2000; Ellickson et al., 2004b). Consistency across substances in the course of use has been documented as well. Individuals who begin smoking cigarettes at a young age are also likely to start drinking and using cannabis at a young age (Agrawal et al., 2006). The significant overlap across licit and illicit drugs in patterns of initiation and their associated outcomes suggests the possible role of common vulnerability factors and the utility of examining initiation of use for alcohol, cigarettes, and cannabis in combination.
1.2. Genetic influences on ‘ever use’
Genetically informative research designs, which have been used extensively to study the onset of use for a single licit or illicit drug, offer a powerful means of identifying the source(s) of cross-substance vulnerability to initiation of substance use. Findings across twin studies (based on dichotomous indicators of lifetime use) indicate that the relative contributions of heritable and environmental factors vary somewhat by substance (Hopfer et al., 2003). Heritability estimates are generally lowest for initiation of alcohol use. Genetic factors have been found to account for between 0 and 39% of the variance in alcohol initiation in most studies (Fowler et al., 2007; Han et al., 1999; Rhee et al., 2003). One notable exception is Maes et al.’s (1999) estimate of 72% heritability when use without permission was specified. McGue et al. (2001) also reported that initiation of alcohol use was attributable to a substantial degree to genetics in males (55%) but this finding did not generalize across gender (for females, heritability was estimated at 11%). Genetic contributions to the onset of cigarette smoking appear to be somewhat higher, with most studies producing heritability estimates around 40% (Fowler et al., 2007; Koopmans et al., 1997; McGue et al., 2000; Rhee et al., 2003), although both considerably lower (11%, Han et al., 1999) and higher (84%, Maes et al., 1999) estimates have been reported. Approximately 30% of the variance in the onset of cannabis use is attributable to genetic factors, but as in the case of both alcohol use and cigarette smoking, heritability estimates cover a wide range (0–72%) (Fowler et al., 2007; Han et al., 1999; Kendler and Prescott, 1998; Maes et al., 1999; McGue et al., 2000; Rhee et al., 2003; Tsuang et al., 1999).
Only rarely has alcohol, cigarette, and cannabis use been examined in combination in genetically informative samples. Among the few to do so was Han et al.’s (1999) investigation with adolescent twins, which produced evidence of a common vulnerability factor that was explained largely by environmental factors shared by twins (63%, compared with 23% accounted for by genetic factors). Hopfer and colleagues (2001) reported similar findings in their study of alcohol use and cigarette smoking in an older adult cohort of female twins. Shared environmental influences, which accounted for a substantial proportion of variance in lifetime (ever use) measures of both alcohol and cigarettes, were highly correlated across substances. Findings from Koopmans et al.’s study (1999) using twins ranging in age from 12 to 25 years also indicated that, among the 12 to 16 year-olds, initiation of alcohol use and cigarette smoking were influenced in large part by the same shared environmental factors. In contrast, the association between the onset of alcohol use and cigarette smoking among 17 to 25 year-olds was attributable to common genetic factors. The relatively limited research in this area (in which initiation is measured as “lifetime use”) has produced mixed findings. The degree to which onset of alcohol, cigarette and cannabis use is attributable to common genetic or environmental factors is unclear.
1.3. Goals of the current study
The above studies have established a foundation for examining common vulnerability to the onset of licit and illicit drug use. Two key issues that remain relatively under-explored are addressed in the current study. First, we examine genetic and environmental contributions to the timing of alcohol, cigarette, and cannabis use onset. Despite the well-known risks associated with early initiation, very few genetically informative studies have addressed age at initiation; even fewer have simultaneously examined timing of first use for more than one substance. Second, we focus on African-American females, who are under-represented in genetically informative studies of substance use initiation in general and virtually absent from the limited literature on cross-substance risk for onset of use. The lower prevalence of cigarette and alcohol use (Ellickson et al., 2004a; Grucza et al., 2008; Heath et al., 1999; Scarinci et al., 2002; Vega et al., 2007) and differences in sequence of licit and illicit substance initiation (Guerra et al., 2000) in African-Americans compared with Caucasians hint at distinctions in pathways of risk that cannot easily be detected in studies where African-Americans comprise only a small proportion of participants. In this study, we use data from a young adult African-American female twin sample to estimate the relative contributions of genetic and environmental factors to the timing of first use for alcohol, cigarettes, and cannabis and to assess the degree to which these influences on initiation are shared across the three substances.
2. Methods
2.1. Participants
The sample consisted of African-American female twins who had participated in the Missouri Adolescent Female Twin Study (MOAFTS), a longitudinal study of alcohol-related problems and associated psychopathology in female adolescents and young adults. Female twin pairs born in Missouri between 1 July 1975 and 30 June 1985 to Missouri-resident parents were initially identified through birth records and recruited between 1995 and 1999 for the first wave of data collection. Recruitment was conducted using a cohort-sequential design, with cohorts of 13, 15, 17, and 19 year-old female twin pairs and their families ascertained in the first 2 years and new cohorts of 13 year-old twins and their families added in the subsequent 2 years. (For further details on ascertainment, see Heath et al., 2002). A special effort was made to ensure that the ethnic composition of the sample accurately reflected the population of Missouri, where approximately 14% of adolescents and young adults identify as African-American. In 2002–2005, all female twins from the target cohort (excluding those who had previously withdrawn from the study or whose parents had refused permission for the family to be re-contacted) were approached for follow-up (Wave 4) interviews. All women who completed Wave 4 assessments were subsequently invited to participate in the fifth wave of data collection, which began in 2005.
The sample for the current study was comprised of 100 monozygotic (MZ) and 131 dizygotic (DZ) twin pairs, all of whom were 21 years of age or older at the time of last interview. (Age 21 was chosen as the cut-off based on Kaplan–Meier estimations of survival curves for age at onset of each of the three substances.) Of the 255 African-American twin pairs who met age eligibility criteria, 90.6% had complete data for both twins on initiation of use for all substances, resulting in a sample size of 462 twins (231 pairs) for the current study. In the majority of cases (89.4%), data were drawn from the fifth wave of data collection. For the remaining participants, who did not participate in Wave 5 interviews but were 21 years of age or older at the time of Wave 4 data collection, data were drawn from Wave 4 interviews. Mean age at the time of last interview was 25.1 (range = 21–31) years.
2.2. Procedure
All data were collected over the telephone by trained interviewers. An initial screening to determine zygosity of the twin pair was conducted in Wave 1 with one of the twin’s parents. Diagnostic interviews were then scheduled with parents who agreed to take part in the study. Interviews with the twins were scheduled after obtaining verbal consent (and, for those under the age of 18, the consent of parents). In Waves 4 and 5, verbal consent was also obtained from all participants prior to the start of interviews, consistent with procedures approved by the Human Research Protection Office at Washington University.
2.3. Assessment battery
The Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994; Hesselbrock et al., 1999) was adapted for interview via telephone and used to collect lifetime diagnostic data from parents and twins at Wave 1 and from twins at Wave 4. Detailed histories of alcohol, cigarette, and cannabis use and related problems were obtained from twins in Waves 1 and 4. Wave 5 data collection was also conducted with SSAGA-based telephone interviews, but differed from earlier assessments in its focus on obtaining updated rather than lifetime psychiatric and substance use histories. (The majority of questions referred to experiences from the previous 24 months, the approximate period of time between Wave 4 and Wave 5 assessments.)
2.4. Operationalizing age at onset
Alcohol
Age at initiation of alcohol use was defined as the age at which the participant first consumed a full alcoholic drink, i.e. a standard can or bottle of beer, a glass of wine or a shot of liquor. Consumption of one or more alcoholic drinks (lifetime) was reported by 87.5% of participants.
Cigarettes
Age at first cigarette use was defined as the age at which the participant tried her first cigarette. A total of 67.8% of the sample reported having tried at least one cigarette.
Cannabis
Age at first cannabis use was defined as the participant’s age the first time she used cannabis. Cannabis use (one or more times over the lifetime) was reported by 58.2% of the sample.
Age at first cannabis use was defined as the participant’s age the first time she used cannabis. Cannabis use (one or more times over the lifetime) was reported by 58.2% of the sample. Age at first use of a substance was derived from the wave of assessment in which use of that substance was first reported, toward the end of minimizing potential retrospective reporting bias. In Wave 5, respondents were not specifically asked the age at which they first drank alcohol or the age at which they first smoked a cigarette, but they were asked about age at last use. In the small number of cases in which first use of alcohol or cigarettes was reported for the first time at Wave 5 (8.7% and 1.3%, respectively, of those who ever used), age at first use was estimated in the following way: For those individuals who reported in Wave 5 that they had used alcohol or cigarettes in the previous 24 months but explicitly stated in Wave 4 interviews that they had never used that substance, first use was estimated as 2 years before age at Wave 5, the earliest age at which initiation could have occurred. Age at first cannabis use was queried in all waves of data collection. (Reliability of self-reported age at first use could not be estimated in our sample, but moderate test–retest reliability for initiation of alcohol, cigarette and illicit drug use has been reported in large-scale surveys [Johnson and Mott, 2001].)
Categories of ‘early’ and ‘late’ age at first use were created by dividing distributions of age at initiation into two approximately equal groups. For alcohol, early first use was defined as 16 years or younger, late as 17 years or older (using the lower of the two possible cut-offs to create amore conservative definition of early initiation). For cigarettes, early first use was defined as 14 years or younger, late as 15 years or older. Definitions of early vs. late age at first use of cannabis were the same as those for alcohol: 16 years or younger for early and 17 years or older for late. As described in Section 2.5.2, never-users were also included in the models.
2.5. Data analysis
2.5.1. Twin modeling
Data from monozygotic (MZ) and dizygotic (DZ) twins can be utilized to parse out the relative contributions of additive genetic (A), shared environmental (C) and non-shared environmental (E) influences on population variation in behaviors of interest (in this case, onset of alcohol, cigarette, and cannabis use). Non-additive genetic influences (including dominance, and thus denoted as D) can be estimated in place of shared environment when the correlation between members of DZ twin pairs is less than half the correlation between their MZ counterparts for a given behavior, but C and D cannot be jointly estimated when data from twins alone are used. In our sample, the DZ correlations for age at initiation were greater than half the MZ correlations for all three substances, indicating that a model incorporating C rather D (i.e., an ACE model) would be most appropriate for these data.
2.5.2. Univariate twin models
Univariate twin models were fit to raw categorical data on initiation of alcohol, cigarette, and cannabis use. Categories were created for each substance to represent never used (‘0’) and late- and early-age at first use (‘1’ and ‘2’, respectively) in order to account for the potential skewness in the continuous forms of these variables. Inclusion of non-users in the models ensured that we did not lose information from twin pairs discordant for ever using a given substance. (Among MZ pairs, discordance rates were 11.0%, 24.0%, and 27.0% for alcohol, cigarettes, and cannabis, respectively. Among DZ pairs they were 20.6%, 38.9%, and 39.7%.) In preliminary analyses, univariate models using dichotomous indicators of ever use and 2-level variables of early vs. late initiation (users only) were compared with those using the three-level variables that include non-users. Similar underlying variance structures were observed in all three modeling approaches, but models using the never/late/early categorical scheme were the most stable, providing further support for using the three-level variables. Tests of multivariate normality were conducted and assumptions were met for all three phenotypes.
Genetic models were fitted by the method of maximum likelihood, using the structural equation modeling program Mx (Neale et al., 2003). For each phenotype, the thresholds (assessed as z-scores on the underlying standard normal distribution), were adjusted for lag time between age at first use and age at the time of report (see Section 2.5.4). A series of sub-models examining the statistical significance of A, C and E were compared to the full model to derive the best-fitting univariate models. Sub-models were tested by calculating the difference between the −2 log likelihood fit of the full model and nested sub-model, which is distributed as chi-square for the given degrees of freedom.
2.5.3. Trivariate twin model
In order to assess the extent to which genetic, shared environmental and non-shared environmental influences on the onset of use overlap across substances, a trivariate triangular decomposition model (also known as a Cholesky decomposition model) was fitted. The proportions of variance attributable to common vs. substance-specific sources of influence on initiation of alcohol, cigarette, and cannabis use were estimated under this model. Models were constructed based on the best-fitting univariate models and fitted in Mx using raw categorical data. Thresholds for all three initiation variables were simultaneously adjusted for all three lag times between age at first use and age at time of report. The final model was derived by testing sub-models against the full model, as described in Section 2.5.2.
2.5.4. Adjustments for age at report
In an effort to reduce potential retrospective reporting bias (i.e., the trend toward reports of age at first use to increase slightly with respondent age [Grucza et al., 2008]), adjustments were made for the length of time between age at first use and age at the time of report. Since an individual’s reports of first alcohol, cigarette, and cannabis use could be derived from different waves of data collection, separate adjustments for time between first use and report of first use were made for each substance. A total of 10 dummy variables per substance, representing 0, 1, 2, up through 10 or more years of lag time, were included in analyses. (Dummy variables were deemed a better fit than continuous variables, which rely heavily on assumptions of linearity that are not met in our measures of lag times.) Lag times for those women who never reported any use of a given substance were coded as 0 for that substance since assignment to the ‘never-used’ category was based on reports from the most recent interview.
3. Results
3.1. Initiation of use: cross-substance distributions
Cross-substance distributions of initiation status (never, late, or early) for alcohol, cigarettes, and cannabis are shown in Table 1. Significant associations were found between initiation of alcohol and cigarettes (χ2(4) = 59.70; p < .001; r = 0.40), alcohol and cannabis (χ2(4) = 83.86; p < .001; r = 0.47), and cigarettes and cannabis (χ2(4) = 94.74; p < .001; r = 0.51). Patterns of cross-substance use initiation were in the expected direction, with those who had never used a given substance being the least likely to use other substances. Early users, by contrast, were the most likely to report co-occurring substance use, frequently reporting that they began using other substances at an early age as well. For example, 79.3% of women who had abstained from ever using alcohol never tried cannabis, whereas 46.0% of those who began drinking at 16 years of age or younger reported first using cannabis at or before age 16.
Table 1.
Cross-substance distributions of the onset of alcohol, cigarette, and cannabis use.
| Alcohol | Cigarettes | Cigarettes | Cannabis | Alcohol | Cannabis | |||
|---|---|---|---|---|---|---|---|---|
| Never, n=58 | Never | 62.1% | Never, n=149 | Never | 70.5% | Never, n=58 | Never | 79.3% |
| Late (≥15 years) | 13.8% | Late (≥17 years) | 22.1% | Late (≥17 years) | 8.6% | |||
| Early (≤14 years) | 24.1% | Early (≤16 years) | 7.4% | Early (≤16 years) | 12.1% | |||
| Late (≥17 years), n=243 | Never | 35.0% | Late (≥15 years), n=149 | Never | 29.5% | Late (≥17 years), n=243 | Never | 43.2% |
| Late (≥15 years) | 38.7% | Late (≥17 years) | 45.0% | Late (≥17 years) | 40.7% | |||
| Early (≤14 years) | 26.3% | Early (≤16 years) | 25.5% | Early (≤16 years) | 16.1% | |||
| Early (≤16 years), n=161 | Never | 17.4% | Early (≤14 years), n=164 | Never | 26.8% | Early (≤16 years), n=161 | Never | 26.1% |
| Late (≥15 years) | 29.2% | Late (≥17 years) | 29.9% | Late (≥17 years) | 27.9% | |||
| Early (≤14 years) | 53.4% | Early (≤16 years) | 43.3% | Early (≤16 years) | 46.0% | |||
3.2. Univariate models
Separate genetic analyses conducted for alcohol, cigarettes, and cannabis use indicated that a sizeable proportion of variance in initiation status was attributable to additive genetic sources (A) for all three substances: 41% for alcohol, 74% for cigarettes, and 52% for cannabis. The contribution of shared environmental factors (C) to age at first use was more modest for all three substances, with point estimates of 0 for cannabis and 0.12 for alcohol and cigarettes. However, as seen in Table 2, confidence intervals for all three C estimates as well as estimated A values for alcohol and cannabis included 0. Dropping the C pathway did not result in a significant difference in model fit for alcohol (Δχ2(1) = 0.15), cigarettes (Δχ2(1) = 0.21), or cannabis (Δχ2(1) = 0.00). Dropping the A pathway also did not result in a significant difference in model fit for alcohol (Δχ2(1) = 1.39) or cannabis (Δχ2(1) = 2.57), but a deterioration in model fit was observed for cigarettes when the A pathway was dropped (Δχ2(1) = 5.66). The A and C pathways were dropped simultaneously to determine whether an E model best captured the variance in alcohol and cannabis phenotypes, but in both cases, it was a poorer fit for the data (Δχ2(1) = 20.39 and 6.81 for alcohol and cannabis, respectively). Thus, tests of model fit did not support a reduced model for either alcohol or cannabis. The wide range in confidence intervals for A and C values for cigarettes similarly indicated that a reduced model did not provide the best fit for the cigarette use initiation phenotype.
Table 2.
Magnitude of additive genetic (A), shared environmental (C) and non-shared environmental (E) influences on initiation of alcohol, cigarette, and cannabis use: univariate models and trivariate modela.
| Substance | Proportion of total variance attributable to genetic and environmental influences |
|||||
|---|---|---|---|---|---|---|
| Univariate models |
Trivariate mode |
|||||
| A | C | E | A | C | E | |
| Alcohol | 0.41 [CI: 0.00–0.71] | 0.12 [CI: 0.00–0.57] | 0.47 [CI: 0.29–0.71] | 0.44 [CI: 0.38–0.51] | 0.10 [CI: 0.07–0.13] | 0.46 [CI: 0.45–0.46] |
| Cigarettes | 0.74 [CI: 0.14–0.98] | 0.12 [CI: 0.00–0.58] | 0.14 [CI: 0.02–0.36] | 0.62 [CI: 0.59–0.63] | – | 0.38 [CI: 0.34–0.41] |
| Cannabis | 0.52 [CI: 0.00–0.80] | 0.00 [CI: 0.00–0.50] | 0.48 [CI: 0.20–0.87] | 0.77 [CI: 0.77–0.79] | – | 0.23 [CI: 0.20–0.23] |
All models adjusted for lag time between age at 1st use and age at time of report.
3.3. Trivariate model
The best-fitting trivariate model is shown with unstandardized path coefficients in Fig. 1. The proportion of total variance attributable to genetic and environmental sources is reported with 95% confidence limits in Table 2. The final model was derived by conducting a series of tests, in two stages. First, pathways whose confidence intervals in the full model included 0 (the A pathway for alcohol and the C pathways for all three phenotypes) were dropped one at a time and the resulting sub-models were compared to the full model. Of these, only the removal of the C pathway for alcohol significantly affected model fit. In the second step, starting with the pathway with the lowest Δχ2 value (C component for cannabis; Δχ2(1) =−3.85), the remaining three pathways were dropped in combination and tested against the reduced model from the previous step. No deterioration in model fit was observed when the C component for cigarettes was dropped in combination with the C component for cannabis (Δχ2(2) =−7.41), but dropping the A pathway for alcohol resulted in a poorer fit to the data (Δχ2(2) =−7.41). As seen in Table 2, variance in these three phenotypes was best represented with an ACE model for alcohol and AE models for cigarettes and cannabis.
Fig. 1.
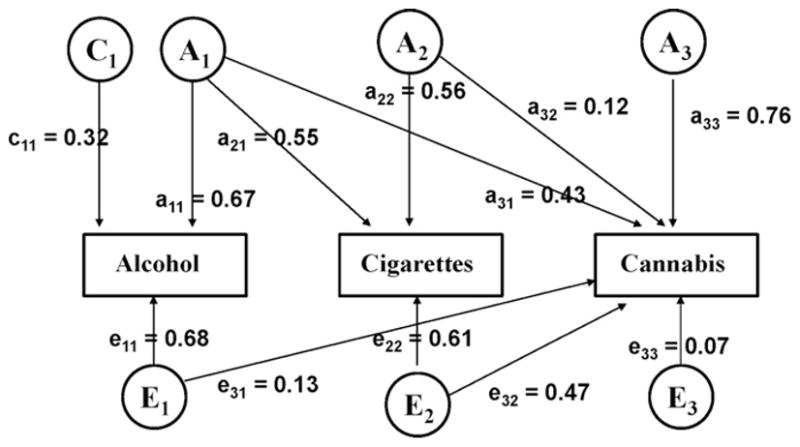
Trivariate Cholesky decomposition model: timing of first use for alcohol, cigarettes, and cannabis.
Heritable influences explained a substantial amount of variance in initiation status for alcohol (44%), cigarettes (62%), and cannabis (77%). Shared environmental factors had a modest influence on alcohol use initiation (10%) but did not contribute significantly to the onset of cigarette or cannabis use. There was evidence for a considerable degree of overlap in genetic factors influencing initiation status for alcohol and cigarettes (rA1–A2 = 0.70; CI: 0.61–0.75) and alcohol and cannabis (rA1–A3 = 0.49; CI: 0.36–0.83), but the genetic correlation between cigarettes and cannabis was more modest (rA2–A3 = 0.25; CI: 0.13–0.26). A large degree of overlap was observed in individual-specific environmental influences on initiation of cigarette smoking and onset of cannabis use (rE2–E3 = 0.95; CI: 0.80–0.97). Unique environmental contributions to the onset of alcohol use were also correlated (to a lesser degree) with those contributing to cannabis use initiation (rE1–E3 = 0.27; CI: 0.19–0.51), but we were able to drop the E pathway from alcohol to cigarettes without a deterioration in model fit (Δχ2(1) =−2.00).
4. Discussion
The current study examined genetic and environmental influences on the onset of alcohol, cigarette, and cannabis use in young African-American women. Estimates of common vs. substance-specific sources of variance were derived using a trivariate Cholesky decomposition model that adjusted for lag time between age at time of report and age at first use for each of the three substances. Our investigation makes a unique contribution to the literature on heritability of substance use initiation through its examination of the timing of first use, its assessment of overlapping influences across substances, and its focus on an understudied population.
For all three cross-substance comparisons, initiation of use for one substance (categorized as never, late or early) was strongly associated with the timing of first use for the other, providing further support that risk for co-occurring substance use is elevated in early users of alcohol (Callas et al., 2004; Ellickson et al., 2003; Schmid et al., 2007; Vieira et al., 2007), cigarettes (Korhonen et al., 2008; Kokkevi et al., 2006; Sartor et al., 2008), and cannabis (Coffey et al., 2000). More specifically, our findings are consistent with other studies reporting that risk for early use of licit or illicit drugs is increased in early initiates of another substance of abuse (e.g., risk for early initiation of cannabis is elevated in individuals who began drinking and smoking cigarettes at a young age [Agrawal et al., 2006; Schmid et al., 2007]). Furthermore, despite the lower overall prevalence of alcohol and cigarette (but not cannabis) use among African-Americans (Ellickson et al., 2004a; Grucza et al., 2008; Heath et al., 1999; Scarinci et al., 2002; Vega et al., 2007), we found the same patterns of cross-substance use as those found in the larger literature, which is based primarily on Caucasians.
A substantial genetic contribution to the timing of initiation was found for alcohol (44%), cigarette (62%), and cannabis (77%) use. Shared environmental factors had a modest influence on the timing of first drink (10%), but we found no evidence that they play a role in shaping the onset of cigarette and cannabis use—a surprising finding given the support in the literature for the role of shared environment in substance use initiation, especially in girls (Han et al., 1999; Hopfer et al., 2001). A significant number of studies on alcohol (Fowler et al., 2007; Han et al., 1999), cigarette (Koopmans et al., 1999; Han et al., 1999), and cannabis use (Fowler et al., 2007; Maes et al., 1999; McGue et al., 2000) have reported that greater than half of the variance in initiation can be accounted for by shared environmental factors. Discrepancies between our findings and those reported elsewhere may be attributable to differences in the phenotypes under study. Whereas the current study addressed the timing of first use, the vast majority of genetically informative studies have operationalized initiation more broadly as ever vs. never use, which may not capture the risk specific to early onset. Although one of the few known studies to address age at first use found evidence for primarily environmental influences on initiation of alcohol and cigarette use (Stallings et al., 1999), the very limited literature in this area and the demographic differences between our sample and the older, all-Caucasian twin cohort used by Stallings and colleagues suggest that the nature of risk for early vs. late onset use remains an open question.
The disparity in findings between our study and prior investigations may also reflect differences across ethnic groups in factors associated with risk for drug and alcohol use, such as perceived peer use (Callas et al., 2004; D’Amico and McCarthy, 2006; Korhonen et al., 2008; Kosterman et al., 2000), accessibility of substances (Beyers et al., 2004; Komro et al., 2007), religiosity (Brook et al., 1999; Jeynes, 2006; Turner-Musa and Lipscomb, 2007; Wills et al., 2003), and parental attitudes toward substance use (Beyers et al., 2004; Callas et al., 2004). There is some evidence, for example, that lower prevalence of substance use in African-Americans is attributable in part to higher religiosity (Heath et al., 1999; Nasim et al., 2007; Wallace et al., 2003) and lower perceived peer substance use (Heath et al., 1999; Vega et al., 1993). These differences in risk factors may translate into distinctions in the relative contributions of genetic and environmental factors to substance use onset, although this hypothesis has yet to be tested empirically.
By decomposing the variance in each of the three phenotypes and estimating cross-substance correlations, we were able to test for the contribution of common vulnerability factors to the association between the timing of first use for alcohol, cigarettes, and cannabis and determine that this common vulnerability is primarily genetic in nature. The limited research examining overlap across substances in factors influencing initiation has produced mixed results. In their study using a sample of older Caucasian women, Hopfer et al. (2001) reported that lifetime (ever) use of alcohol and cigarettes was attributable to the same shared environmental factors. Young et al.’s (2006) investigation of alcohol, cigarette, and cannabis use in an adolescent sample revealed modest but significant correlations across substances in both shared environmental and genetic influences, although they defined use as repeated use, thus potentially tapping a different construct than age at first use. Perhaps the most intriguing finding in this area comes from a study of 12 to 25 year-olds by Koopmans et al. (1997), who found high correlations between shared environmental influences on initiation of alcohol and cigarette use in 12 to 16 year-olds, but concluded that among 17 to 25 year-olds, common influences on initiation were attributable to genetic sources. The trend for heritability estimates for substance use initiation to increase with age has been noted in a review of twin and adoption studies of adolescent substance use (Hopfer et al., 2003) and may help to explain the relatively high estimates of genetic contributions to onset of first use in our young adult sample.
The substantial contribution of genetic factors to the timing of first use found for alcohol, cigarettes, and cannabis indicates that although the prevalence of substance use is relatively low for African-American girls and young women compared with rates in males and other ethnic groups, early use is highly heritable. The considerable overlap between heritable factors that contribute to the timing of first alcohol use and those that influence onset of cigarette and cannabis use suggests that familial risk for problem drinking is a global marker of risk for early initiation of licit and illicit drugs. The fact that nearly all of the individual-specific environmental influences on onset of cigarette smoking and initiation of cannabis use were traceable to a common factor suggests that environmental exposures (e.g., association with deviant peers) that increase risk for starting to smoke at a young age also impact the likelihood of early onset of cannabis use. Our findings differ from those in studies based on primarily Caucasian samples that have addressed substance use initiation as a dichotomous construct (ever use), thus suggesting that efforts aimed at preventing early substance use in African-American girls may need to be based on a different etiological model than one derived from findings in the larger literature.
4.1. Limitations and future directions
The current study has some limitations that should be kept in mind when interpreting results. First, substance use history was reported retrospectively and was therefore subject to potential recall biases. However, by using the earliest reports of first use (across multiple waves of data collection) and adjusting for lag times between age at first use and age at time of report, we were able to minimize these biases while gathering data from women who had passed through the age of risk for substance use initiation. Second, although our final model of cross-substance use initiation provides clear support for the primary role of heritable factors, the wide range in confidence intervals for A and C in the univariate models suggests that evidence should be interpreted as preliminary and that further studies are needed to confirm that familial influences on substance use onset are attributable to genetic rather than shared environmental sources. Third, due to power limitations, we were unable to test the equal environment assumption (EEA), the assumption that correlations in exposure to environmental events relevant to the trait under study are equal in MZ and DZ twins. Given the support for the validity of the EEA in studies addressing substance use and other psychiatric issues (Hettema et al., 1995; Kendler et al., 1993; Xian et al., 2000), the likelihood that there were violations and that these violations significantly impacted our results is low, but further investigation of the validity of the EEA in relation to substance use initiation appears to be warranted (Kendler and Gardner, 1998).
Results from the current study suggest a number of possible directions for future work in this area. Extension of this line of research to African-American males and members of other under-represented ethnic groups will address critical questions about the generalizability of these findings and their implications for tailoring prevention efforts to the needs of various risk groups. Examination of genetic and environmental contributions to additional transitions in the course of substance use (e.g., symptom onset) will address the extent to which these influences fluctuate over time, potentially introducing the need for different intervention approaches at different stages of use. Investigation of possible mediators of the correspondence of age at onset for these three substances of abuse, such as externalizing psychopathology (attributable in part to the same genetic factors that influence substance use initiation [McGue et al., 2001]), will further our understanding of the pathway of risk that can lead to substance use disorders. Finally, our estimates of the relative contribution of heritable and environmental influences on the timing of first alcohol, cigarette, and cannabis use provide a starting point for identifying the specific genes and environmental exposures that impact initiation of these substances of abuse (Merikangas et al., 2006) and the degree to which these influences may vary across gender and ethnic groups.
Acknowledgments
Role of funding sources
Funding for this study was provided by grants AA009022, AA007728, AA017010, and AA011998 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA), grant HD049024 from the National Institute of Child Health and Human Development (NICHD), and grants DA18660 and DA023668 from the National Institute on Drug Abuse (NIDA). NIAAA, NICHD, and NIDA had no further role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors
Drs. Bucholz, Heath, and Madden designed the longitudinal study from which data were drawn and assisted in editing the manuscript. Drs. Agrawal and Lynskey collaborated with Dr. Sartor on conceptual and analytic approaches to the research questions addressed and assisted in editing the manuscript. Dr. Sartor conducted the statistical analyses and wrote the first draft of the manuscript. All authors contributed to and have approved the manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Agrawal A, Grant JD, Waldron M, Duncan AE, Scherrer JF, Lynskey MT, Madden PAF, Bucholz KK, Heath AC. Risk for initiation of substance use as a function of age of onset of cigarette, alcohol, and cannabis use: Findings in a Midwestern female twin cohort. Prev Med. 2006;43:125–128. doi: 10.1016/j.ypmed.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Beyers JM, Toumbourou JW, Catalano RF, Arthur MW, Hawkins JD. A cross-national comparison of risk and protective factors for adolescent substance use: The United States and Australia. J Adolesc Health. 2004;35:3–16. doi: 10.1016/j.jadohealth.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Brook JS, Kessler RC, Cohen P. The onset of marijuana use from preadolescence and early adolescence to young adulthood. Dev Psychopathol. 1999;11:901–914. doi: 10.1017/s0954579499002370. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report of the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Callas PW, Flynn BS, Worden JK. Potentially modifiable psychosocial factors associated with alcohol use during early adolescence. Addict Behav. 2004;29:1503–1515. doi: 10.1016/j.addbeh.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Chou SP, Pickering RP. Early onset of drinking as a risk factor for lifetime alcohol-related problems. Br J Addict. 1992;87:1199–1204. doi: 10.1111/j.1360-0443.1992.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Coffey C, Lynskey M, Wolfe R, Patton GC. Initiation and progression of cannabis use in a population-based Australian adolescent longitudinal study. Addiction. 2000;95:1679–1690. doi: 10.1046/j.1360-0443.2000.951116798.x. [DOI] [PubMed] [Google Scholar]
- D’Amico EJ, McCarthy DM. Escalation and initiation of younger adolescents’ substance use: The impact of perceived peer use. J Adolesc Health. 2006;39:481–487. doi: 10.1016/j.jadohealth.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Ellickson PL, Orlando M, Tucker JS, Klein DJ. From adolescence to young adulthood: Racial/ethnic disparities in smoking. Am J Public Health. 2004a;94:293–299. doi: 10.2105/ajph.94.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellickson PL, Tucker JS, Klein DJ. Ten-year prospective study of public health problems associated with early drinking. Pediatrics. 2003;111:949–955. doi: 10.1542/peds.111.5.949. [DOI] [PubMed] [Google Scholar]
- Ellickson PL, Tucker JS, Klein DJ, Saner H. Antecedents and outcomes of marijuana use initiation during adolescence. Prev Med. 2004b;39:976–984. doi: 10.1016/j.ypmed.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Everett SA, Warren CW, Sharp D, Kann L, Husten CG, Crossett LS. Initiation of cigarette smoking and subsequent smoking behavior among U.S. high school students. Prev Med. 1999;29:327–333. doi: 10.1006/pmed.1999.0560. [DOI] [PubMed] [Google Scholar]
- Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, McBride A, van den Bree MBM. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007;101:413–422. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF. Age at smoking onset and its association with alcohol consumption and DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:59–73. doi: 10.1016/s0899-3289(99)80141-2. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the Longitudinal Alcohol Epidemiological Survey. J Adolesc Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of drug use and its association with DSMIV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiological Survey. J Subst Abuse. 1998;10:163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Bierut LJ. Cigarette smoking and the risk for alcohol use disorders among adolescent drinkers. Alcohol Clin Exp Res. 2006;30:2046–2054. doi: 10.1111/j.1530-0277.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Bucholz KK, Rice JP, Bierut LJ. Secular trends in the lifetime prevalence of alcohol dependence in the United States: A re-evaluation. Alcohol Clin Exp Res. 2008;32:763–770. doi: 10.1111/j.1530-0277.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra LM, Romano PS, Samuels SJ, Kass PH. Ethnic differences in adolescent substance initiation sequences. Arch Pediatr Adolesc Med. 2000;154:1089–1095. doi: 10.1001/archpedi.154.11.1089. [DOI] [PubMed] [Google Scholar]
- Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94:981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- Hanna EZ, Grant BF. Parallels to early onset alcohol use in the relationship of early onset smoking with drug use and DSM-IV drug and depressive disorders: Findings from the National Longitudinal Epidemiological Survey. Alcohol Clin Exp Res. 1999;23:513–522. [PubMed] [Google Scholar]
- Heath AC, Howells W, Bucholz KK, Glowinski AL, Nelson EC, Madden PA. Ascertainment of a mid-western U.S. female adolescent twin cohort for alcohol studies: Assessment of sample representativeness using birth record data. Twin Res. 2002;5:107–112. doi: 10.1375/1369052022974. [DOI] [PubMed] [Google Scholar]
- Heath AC, Madden PAF, Grant JD, McLaughlin TL, Todorov AA, Bucholz KK. Resiliency factors protecting against teenage alcohol use and smoking: influences of religion, religious involvement and values, and ethnicity in the Missouri Adolescent Female Twin Study. Twin Res. 1999;2:145–155. doi: 10.1375/136905299320566013. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA—a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. Physical similarity and the equal-environment assumption in twin studies of psychiatric disorders. Behav Genet. 1995;25:327–335. doi: 10.1007/BF02197281. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence. Arch Pediatr Adolesc Med. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Hopfer CJ, Stallings MC, Hewitt JK. Common genetic and environmental vulnerability for alcohol and tobacco use in a volunteer sample of older female twins. J Stud Alcohol. 2001;62:717–723. doi: 10.15288/jsa.2001.62.717. [DOI] [PubMed] [Google Scholar]
- Hopfer CJ, Crowley TJ, Hewitt JK. Review of twin and adoption studies of adolescent substance use. J Am Acad Child Adolesc Psychiatry. 2003;42:710–719. doi: 10.1097/01.CHI.0000046848.56865.54. [DOI] [PubMed] [Google Scholar]
- Hu MC, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Am J Public Health. 2006;96:299–308. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeynes WH. Adolescent religious commitment and their consumption of marijuana, cocaine, and alcohol. J Health Soc Policy. 2006;21:1–20. doi: 10.1300/J045v21n04_01. [DOI] [PubMed] [Google Scholar]
- Johnson TP, Mott JA. The reliability of self-reported age at onset of tobacco, alcohol and illicit drug use. Addiction. 2001;96:1187–1198. doi: 10.1046/j.1360-0443.2001.968118711.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Twin studies of adult psychiatric and substance dependence disorders: are they biased by differences in the environmental experiences of monozygotic and dizygotic twins in childhood and adolescence? Psychol Med. 1998;28:625–633. doi: 10.1017/s0033291798006643. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A test of the equal-environment assumption in twin studies of psychiatric illness. Behav Genet. 1993;23:21–27. doi: 10.1007/BF01067551. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. Am J Psychiatry. 1998;155:1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- King KM, Chassin L. A prospective study of the effects of age of initiation of alcohol and drug use on young adult substance dependence. J Stud Alcohol Drugs. 2007;68:256–265. doi: 10.15288/jsad.2007.68.256. [DOI] [PubMed] [Google Scholar]
- Kokkevi A, Gabhainn SN, Spyropoulou M Risk Behavior Group Focus Group of the HBSC. Early initiation of cannabis use: A cross-national European perspective. J Adolesc Health. 2006;39:712–719. doi: 10.1016/j.jadohealth.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Komro KA, Maldonado-Molina MM, Tobler AL, Bonds JR, Muller KE. Effects of home access and availability of alcohol on young adolescents’ alcohol use. Addiction. 2007;102:1597–1608. doi: 10.1111/j.1360-0443.2007.01941.x. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, Slutske WS, Heath AC, Neale MC, Boomsma DL. The genetics of smoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behav Genet. 1999;29:383–393. doi: 10.1023/a:1021618719735. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, van Doornen LJP, Boomsma DI. Association between alcohol use and smoking in adolescent and young adult twins: A bivariate genetic analysis. Alcohol Clin Exp Res. 1997;21:537–546. [PubMed] [Google Scholar]
- Korhonen T, Huiznak AC, Dick DM, Pulkkinen L, Rose RJ, Kaprio J. Role of individual, peer, and family factors in the use of cannabis and other illicit drugs: A longitudinal analysis among Finnish adolescent twins. Drug Alcohol Depend. 2008;97:33–43. doi: 10.1016/j.drugalcdep.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterman R, Hawkins JD, Guo J, Catalano RF, Abbott RD. The dynamics of alcohol and marijuana initiation: Patterns and predictors of first use in adolescence. Am J Public Health. 2000;90:360–366. doi: 10.2105/ajph.90.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes HH, Woodward CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, Rutter M, Simonoff E, Pickles A, Carbonneau R, Neale MC, Eaves LJ. Tobacco alcohol and drug use in eight- to sixteen-year-old twins: The Virginia Twin Study of Adolescent Behavioral Development. J Stud Alcohol. 1999;60:293–305. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. Am J Med Genet. 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;25:1156–1165. [PubMed] [Google Scholar]
- Merikangas KR, Low NCP, Hardy J. Commentary: Understanding sources of complexity in chronic diseases—The importance of integration of genetics and epidemiology. Int J Epidemiol. 2006;35:590–592. doi: 10.1093/ije/dyl007. [DOI] [PubMed] [Google Scholar]
- Nasim A, Belgrave FZ, Jagers RJ, Wilson KD, Owens K. The moderating effects of culture on peer deviance and alcohol use among high-risk African-American adolescents. J Drug Educ. 2007;37:335–363. doi: 10.2190/DE.37.3.g. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6. Department of Psychiatry, Medical College of Virginia; Richmond, VA: 2003. [Google Scholar]
- Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: A non-causal association. Alcohol Clin Exp Res. 1999;23:101–107. [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Xian H, Scherrer JF, Lynskey MT, Duncan AE, Haber JR, Grant JD, Bucholz KK, Jacob T. Psychiatric and familial predictors of transition times between smoking stages: results from an offspring-of-twins study. Addict Behav. 2008;33:235–251. doi: 10.1016/j.addbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarinci IC, Robinson LA, Alfano CM, Zbikowski SM, Kelsges RC. The relationship between socioeconomic status, ethnicity, and cigarette smoking in urban adolescents. Prev Med. 2002;34:171–178. doi: 10.1006/pmed.2001.0967. [DOI] [PubMed] [Google Scholar]
- Schmid B, Hohm E, Blomeyer D, Zimmerman US, Schmidt MH, Esser G, Maucht M. Concurrent alcohol and tobacco use during early adolescence characterizes a group at risk. Alcohol Alcohol. 2007;42:219–225. doi: 10.1093/alcalc/agm024. [DOI] [PubMed] [Google Scholar]
- Stallings MC, Hewitt JK, Beresford T, Heath AC, Eaves LJ. A twin study of drinking and smoking onset and latencies from first use to regular use. Behav Genet. 1999;29:409–421. doi: 10.1023/a:1021622820644. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Harley RM, Xian H, Eisen S, Goldberg J, True WR, Faraone SV. Genetic and environmental influences on transitions in drug use. Behav Genet. 1999;29:473–479. doi: 10.1023/a:1021635223370. [DOI] [PubMed] [Google Scholar]
- Turner-Musa J, Lipscomb L. Spirituality and social support on health behaviors of African-American undergraduates. Am J Health Behav. 2007;31:495–501. doi: 10.5555/ajhb.2007.31.5.495. [DOI] [PubMed] [Google Scholar]
- Vega WA, Zimmerman RS, Warheit GJ, Apospori E, Gil AG. Risk factors for early adolescent drug use in four ethnic and racial groups. Am J Public Health. 1993;83:185–189. doi: 10.2105/ajph.83.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega WA, Chen KW, Williams J. Smoking, drugs, and other behavioral health problems among multiethnic adolescents in the NHSDA. Addict Behav. 2007;32:1949–1956. doi: 10.1016/j.addbeh.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Vieira DL, Ribiero M, Laranjeira R. Evidence of association between early alcohol use and risk of later problems. Rev Bras Psiquaitr. 2007;29:222–227. doi: 10.1590/s1516-44462007000300006. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Brown TN, Bachman JG, Laveist TA. The influence of race and religion on abstinence from alcohol, cigarettes, and marijuana among adolescents. J Stud Alcohol. 2003;64:843–848. doi: 10.15288/jsa.2003.64.843. [DOI] [PubMed] [Google Scholar]
- Wills TA, Gibbons FX, Gerrard M, Murry VM, Brody GH. Family communication and religiosity related to substance use and sexual behavior in early adolescence: A test for pathways through self-control and prototype perceptions. Psychol Addict Behav. 2003;17:312–323. doi: 10.1037/0893-164X.17.4.312. [DOI] [PubMed] [Google Scholar]
- Xian H, Scherrer JF, Eisen SA, True WR, Heath AC, Goldberg J, Lyons MJ, Tsuang MT. Self-reported zygosity and the equal-environments assumption for psychiatric disorders in the Vietnam Era Twin Registry. Behav Genet. 2000;30:303–310. doi: 10.1023/a:1026549417364. [DOI] [PubMed] [Google Scholar]
- Young SE, Rhee SH, Stallings MC, Corley RP, Hewitt JK. Genetic and environmental vulnerabilities underlying adolescent substance use and problem use: General or specific? Behav Genet. 2006;36:603–615. doi: 10.1007/s10519-006-9066-7. [DOI] [PubMed] [Google Scholar]


