Abstract
With the eruption of the obesity pandemic over the past few decades, much research has been devoted to understanding the molecular mechanisms by which the human body regulates energy balance. These studies have revealed several mediators, including gut/pancreatic/adipose hormones and neuropeptides that control both short- and long-term energy balance by regulating appetite and/or metabolism. These endogenous mediators of energy balance have been the focus of many anti-obesity drug-development programs aimed at either amplifying endogenous anorexigenic/lipolytic signaling or blocking endogenous orexigenic/lipogenic signaling. Here, we discuss the efficacy and safety of targeting these pathways for the pharmacologic treatment of obesity.
Keywords: appetite, energy balance, gut–neural axis, obesity, pharmacotherapeutics, satiety
Scope of the obesity problem
Obesity rates have escalated into a global pandemic during the past few decades [1]. It is estimated that over 1 billion adults worldwide are overweight (BMI >25 kg/m2), 300 million of whom are clinically obese (BMI >30 kg/m2) [2]. Within the USA, 65% of adults are overweight, with 32.2% of this population being classified as obese, a figure that has doubled over the past 20 years [3]. Of even greater concern are the growing worldwide rates of childhood obesity, which have reached epidemic values in developed countries [4,5]. Obesity is associated with an array of comorbidities, including Type II diabetes, hypertension, coronary artery disease, stroke, obstructive sleep apnea, cancer, osteoarthritis and liver/biliary disease, which together cause a significant decline in life expectancy for individuals with chronic obesity [6]. In fact, the negative consequences of chronic obesity outweigh those of either smoking or alcohol abuse [7]. Currently, the national healthcare costs attributable to obesity are estimated at US$70–100 billion, and it has been forecast that, if the current obesity trends continue, within the next 15 years, 20% of healthcare costs in the USA will be attributed to the care of chronic diseases associated with obesity [8].
Current treatment of obese patients
Until recently, medical care of obese patients involved individually treating the complications of obesity (i.e., diabetes, hypertension, hyperlipidemia) without focusing on treatment of the underlying pathology. Only after the obesity pandemic erupted has research demonstrated the value of weight loss and lifestyle changes in the prevention and treatment of several chronic diseases, including diabetes and cardiovascular disease. In fact, modest reductions in body mass (<10%) produce significant improvements in glycemic control, blood pressure and cholesterol levels [9,10]. Clinical intervention and enrollment in lifestyle-modification programs have proven successful in the short-term, eliciting a loss of approximately 10% of the initial weight within 6 months [11]. However, patients enrolled in lifestyle-modification programs usually regain 30–35% of their lost weight within 1 year following treatment, and more than 50% of patients return to their baseline weight in 5 years or less [12,13].
Currently, the most effective treatment to induce rapid and sustained weight reduction is bariatric surgery (gastric bypass, gastric banding and sleeve gastrectomy), which has proven effective in accomplishing long-term weight loss, improving patient lifestyle and reducing the risk of several obesity-related pathologies [14,15]. However, this procedure is associated with several serious adverse effects and, therefore, is reserved only for morbidly obese patients (BMI >40 kg/m2) or patients suffering from significant comorbidities. Thus, bariatric surgery is neither a viable, nor a desirable, option for treating the millions of obese patients worldwide [14,16].
The solution to the apparent inability to safely achieve long-term weight reduction in overweight and obese patients may be the introduction of anti-obesity pharmacotherapeutics into mainstream clinical practice. Only two drugs, sibutramine and orlistat, are currently US FDA approved for clinical use as long-term anti-obesity therapeutics, and clinical trials have demonstrated their utility in achieving more sustained (2-year) weight loss [17–19]. However, the history of anti-obesity pharmacotherapy is filled with disappointments and cases of unsafe practice, abuse and drugs with limited efficacy and serious adverse effects, and the current FDA-approved anti-obesity drugs are indeed accompanied by some of these same issues [20–24].
In order to develop new safe and effective anti-obesity therapies, much research has focused on understanding how the body regulates appetite and energy balance. Neuronal monoamine neurotransmission and endocannabinoid signaling are involved in the regulation of these processes, but many centrally acting drugs targeting these pathways induce serious cardiovascular and/or psychological side effects. However, the identification of endogenous hormonal regulators of energy balance, including gut/pancreatic/adipose hormones and neuropeptides, has led to the initiation of several drug-development programs targeting these hormonal signaling pathways. It is expected that engineering drugs that target these pathways will specifically reduce appetite and induce weight loss without causing central or peripheral side effects. Here we will discuss the efficacy and safety of these anti-obesity pharmacotherapeutics (Table 1).
Table 1.
Anti-obesity drugs targeting endogenous appetite-regulating hormone signaling.
| Target | Drug | Mechanism of action |
Reported adverse effects |
Company | Development status |
|---|---|---|---|---|---|
| AgRP | TTP-435 | AgRP inhibitor | N/A | TransTech Pharma (NC, USA) | Phase II |
| Amylin | Pramlintide | Synthetic amilin analog | Nausea, headache | Amylin Pharmaceuticals (CA, USA) | Approved for diabetes |
| CCK | GI 181771X | Selective CCK-A agonist | Nausea, vomiting, headache | GlaxoSmithKline | Terminated |
| Ghrelin | NOX-B11 | Ghrelin inactivator | N/A | NOXXON Pharma Ag. (Berlin, Germany)/Pfizer | Preclinical |
| Oral ghrelin antagonist | Ghrelin antagonist | N/A | Elixir Pharmaceuticals (MA, USA) | Preclinical | |
| GLP-1 | Vildagliptin | DDP-IV inhibitor | Headache, nasopharyngitis, cough, constipation, dizziness, increased sweating | Novartis | Phase III |
| Sitagliptin | DDP-IV inhibitor | Diarrhea, headache, nausea, nasopharyngitis | Merck | Approved for diabetes | |
| Liraglutide | Proteolysis-resistant GLP-1 analog | Mild-to-moderate hypoglycemia, nausea, diarrhea, headache | Novo Nordisk | Approved for diabetes | |
| Exenatide | Proteolysis-resistant GLP-1 analog | Mild-to-moderate hypoglycemia, nausea, vomiting, diarrhea, headache, altered kidney function | Amylin Pharmaceuticals (CA, USA)/Eli Lilly (Hampshire, UK) | Approved for diabetes | |
| Exenatide-LAR | Long-acting proteolysis-resistant GLP-1 analog | Mild-to-moderate hypoglycemia, nausea, gastroenteritis | Amylin/Eli Lilly | FDA regulatory review | |
| NN9924 | Long-acting oral GLP-1 analog | N/A | Novo Nordisk | Phase I | |
| Leptin | Metreleptin/pramlintide | Leptin/amylin analogs | Pruritus/pain at injection site, nausea | Amylin Pharmaceuticals | Phase III |
| MCH | BMS-830216 | MCH-1 receptor antagonist | N/A | Bristol-Myers Squibb | Phase I/II |
| Oleoyl estrone | Oral oleoyl estrone | Unknown | N/A | Manhattan Pharmaceuticals (NY, USA) | Terminated |
| Oxyntomodulin | TKS1225 | Long-acting oxyntomodulin analog | N/A | Pfizer | Preclinical |
| NPY | MK-0557 | Y5 receptor antagonist | Headache | Merck | Phase II |
| S-2367 | Y5 receptor antagonist | N/A | Shionogi USA, Inc. (NJ, USA) | Phase II | |
| Pancreatic polypeptide | Obinepitide | Y2/Y4 receptor agonist | N/A | 7TM Pharma (Hørsholm, Denmark) | Phase I/II |
| TM30339 | Selective Y4 receptor agonist | N/A | 7TM Pharma | Phase I/II | |
| PYY | PYY (3–36) | Intranasal active PYY | Nausea, vomiting | Nastech/Merck | Phase I/II |
AgRP: Agouti-related peptide; CCK: Cholecystokinin; DDP: Dipeptidyl peptidase; GLP: Glucagon-like peptide; LAR: Long-acting release; MCH: Melanin-concentrating hormone; N/A: Not applicable; NPY: Neuropeptide Y; PYY: Peptide tyrosine tyrosine.
Neuronal regulation of appetite
Overview of neuronal pathways
The integration of neurohormonal signals from gut and adipose tissues primarily occurs in the hypothalamus. Historically, discrete nuclei within the hypothalamus, including the paraventricular nucleus (PVN), ventromedial nucleus (VMN), dorsomedial nucleus (DMN) and lateral nucleus (LN), were regarded as control centers for appetite. Lesions to specific areas, in particular the VMN, generated a hyperphagic phenotype, while lesions to the lateral hypothalamus generated an anorexic phenotype in animal models, leading to the concept of satiety and hunger ‘centers’ [25–29]. However, current research has shown that appetite regulation is controlled by the integration of orexigenic and anorexigenic signals from a variety of hypothalamic nuclei and extra-hypothalamic tissues (Figure 1).
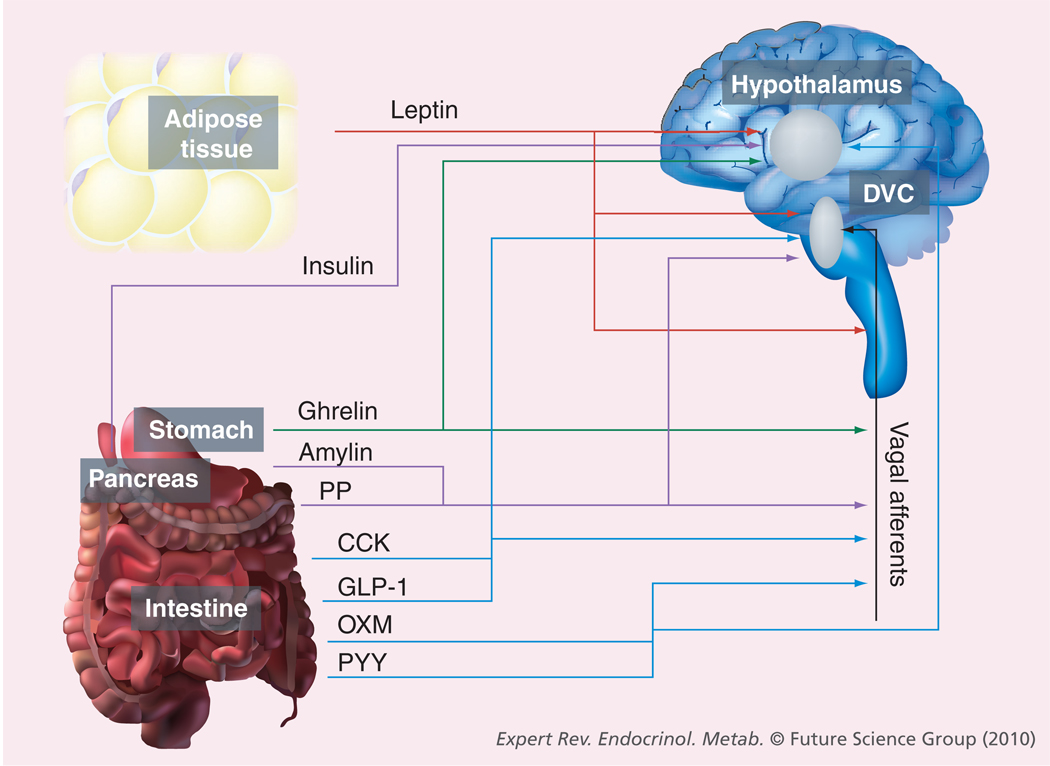
Figure 1. Endogenous appetite-regulating hormones.
Representation of the tissue of origin and target site(s) of circulating hormones that affect appetite and energy balance. Blue arrows represent intestinally derived hormones, the green arrow shows stomach-derived hormones, purple arrows show pancreas-derived hormones and the red arrow depicts adipose tissue-derived hormones.
CCK: Cholecystokinin; DVC: Dorsal vagal complex; GLP-1: Glucagon-like peptide-1; OXM: Oxyntomodulin; PP: Pancreatic polypeptide; PYY: Peptide tyrosine tyrosine.
The arcuate nucleus (ARC) is the primary neural signaling site for peripheral appetite-regulating hormones, as it lies at the base of the hypothalamus and is located outside the blood–brain barrier [30]. Indeed, the ARC is surrounded by a diffusible barrier [31], allowing the passage of circulating hormones. Two main populations of appetite-regulating neurons compose the ARC: those expressing the neuropeptides pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART), and those expressing the neuropeptides agouti-related peptide (AgRP) and neuropeptide Y (NPY) [30,32,33]. POMC/CART-expressing neurons inhibit food intake, while NPY/AgRP neurons stimulate food intake. The balance between signals from these neuronal circuits controls energy homeostasis.
Melanocortins, including the neural signaling peptide α-melanocyte stimulating hormone (α-MSH), are the cleavage products of the pro-peptide, POMC. Melanocortins regulate energy balance via activation of the melanocortin 3 receptor (MC3R) and melanocortin 4 receptor (MC4R) on second-order neurons. Intra-cerebroventricular (ICV) administration of MC3R and MC4R agonists reduces food intake, while antagonist administration produces hyperphagia in rodents [34]. Furthermore, targeted disruption of MC4R produces a murine phenotype of hyperphagia and obesity [35], and polymorphisms of this receptor have been implicated in human late-onset obesity [36].
Cocaine- and amphetamine-regulated transcript is co-expressed with α-MSH and has similar anorexigenic properties. Food-deprived rats show a significant decrease in CART expression, and ICV administration of recombinant CART peptide inhibits both normal and fasting-induced feeding, while ICV administration of CART antiserum increases food intake [37]. However, other studies have shown that CART has some orexigenic properties when injected into discrete hypothalamic nuclei [38], indicating that CART has different effects dependent upon the site of signaling.
Agouti-related peptide is expressed predominantly in the ARC [39] and is upregulated during fasting, while its expression is reduced following food intake [40]. ICV administration of AgRP increases food intake [41,42], and transgenic mice overexpressing AgRP exhibit a hyperphagic and obese phenotype, mainly because AgRP acts as a potent selective antagonist of MC3R and MC4R [43].
Neuropeptide Y is a potent orexigenic neuropeptide and its expression is regulated by nutritional status, with mRNA levels increasing with fasting and declining following food intake [40,44]. NPY is part of the pancreatic polypeptide family of proteins that bind to the seven-transmembrane G-protein-coupled receptors (GPCRs) Y1–Y6 [45]. While NPY signaling is still being completely defined, Y1- and Y5-receptor signaling has been shown to stimulate appetite [46], while Y2- and Y4-receptor signaling produces an anorexic phenotype due to presynaptic inhibition of NPY release [47].
Pro-opiomelanocortin/CART and NPY/AgRP neurons project to several hypothalamic nuclei, including the VMN, DMN, PVN and lateral hypothalamic area (LHA), each containing secondary neurons that process and integrate energy homeostasis signals. Lesions to these hypothalamic areas produce either hyperphagia (PVN, DMN, VMN) or hypophagia (LH), showing the importance of these second-order neurons in generating hunger and satiety responses [25–29].
These second-order neurons express signaling molecules involved in energy homeostasis which are modulated by signals from the ARC as well as the periphery. The PVN expresses the anorexigenic peptides corticotropin-releasing hormone and thyrotropin-releasing hormone, which are downregulated by NPY/AgRP signaling and upregulated by α-MSH signaling [48–50]. The LH and prefornical areas contain neurons expressing melanin-concentrating hormone (MCH). MCH is a potent appetite-stimulating protein that exhibits increased expression during fasting and induces food intake following ICV administration [51]. Two additional proteins, orexin A and orexin B, are specifically expressed in the LH and prefornical areas and have been implicated as orexigenic signaling proteins [30]. Brain-derived neurotrophic factor (BDNF) is highly expressed in the VMN and is important in the regulation of energy balance. Central BDNF administration induces a reduction in appetite and weight in rodents [52], while a mutation in the BDNF receptor, TrkB, produces a phenotype of hyperphagia and obesity in humans [53]. BDNF expression and signaling is upregulated by activation of MC4R [54], indicating that the amplification of downstream BDNF effector mechanisms may be an important strategy by which central melanocortins suppress appetite. Finally, besides modulating neuronal activity in the ARC, several circulating appetite-regulating hormones also directly signal within these secondary hypothalamic nuclei (Figure 2).
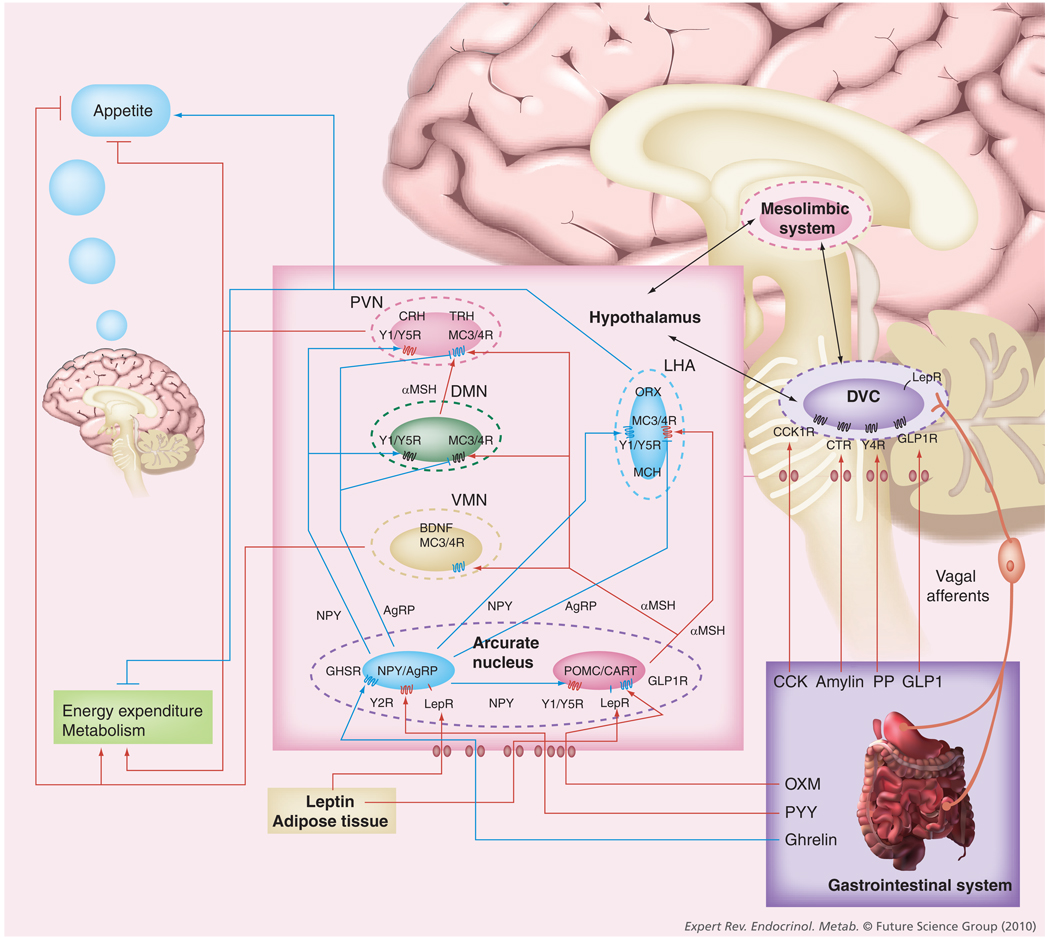
Figure 2. Central appetite-regulating neuronal circuits.
The hypothalamus is the major site of integration of anorexigenic and orexigenic signaling. Satiety hormones primarily signal directly within the hypothalamus, particularly within the arcuate nucleus or within the dorsal vagal complex of the brainstem, which communicates with the hypothalamus. Within the arcuate nucleus there exist two main populations of neurons: NPY/AgRP-expressing neurons and anorexigenic POMC/CART-expressing neurons. Several satiety hormones induce their anorexic effects by either inhibiting the activity of NPY/AgRP neurons or activating POMC/CART neurons. Projections from these neurons of the arcuate nucleus signal to second-order neurons within other hypothalamic nuclei, including the VMN, DMN, PVN and lateral hypothalamic area (LHA). These second-order hypothalamic neurons express anorexigenic neuropeptides (CRH, TRH and BDNF) and orexigenic neuropeptides (ORX and MCH), which affect appetite and energy homeostasis. Furthermore, there is an integration of signaling from the hypothalamus, brainstem and reward pathways of the mesolimbic system involved in the regulation of appetite and energy balance. Blue receptors have stimulating activity; red receptors have inhibiting activity; the blue arrows represent appetite-stimulating signals; the red arrows represent appetite-suppressing signals.
AgRP: Agouti-related peptide; BDNF: Brain-derived neurotrophic factor; CART: Cocaine- and amphetamine-regulated transcript; CCK: Cholecystokinin; CCK1R: Cholecystokinin receptor-1; CTR: Calcitonin receptor; CRH: Corticotropin-releasing hormone; DMN: Dorsomedial nucleus; DVC: Dorsal vagal complex; GHSR: Growth hormone secretagogue receptor; GLP-1: Glucagon-like peptide-1; GLP1R: Glucagon-like peptide-1 receptor; LepR: Leptin receptor; LHA: Lateral hypothalamic area; MC3/4R: Melanocortin 3/melanocortin 4 receptor; MCH: Melanin-concentrating hormone; α-MSH: α-melanocyte-stimulating hormone; NPY: Neuropeptide Y; ORX: Orexin; POMC: Pro-opiomelanocortin; PP: Pancreatic polypeptide; PVN: Paraventricular nucleus; TRH: Thyrotropin-releasing hormone; VMN: Ventromedial nucleus; Y1/Y5R: Y1/Y5 receptor; Y2R: Y2 receptor; Y4R: Y4 receptor.
There are reciprocal connections between the hypothalamus and the brainstem, in particular, the dorsal vagal complex [55,56], which receives signals from peripheral satiety hormones and vagal afferents from the GI tract. Furthermore, reward pathways have been implicated in feeding behavior. Dopamine plays an important role in the rewarding nature of feeding, and dopamine-deficient mice are hypoactive and hypophagic, a phenotype that is eliminated by dopamine replacement in the caudate putamen and nucleus accumbens [57]. Opioid signaling has also been implicated in feeding behavior, and β-endorphin- and enkephalin-deficient mice exhibit reduced food-seeking behavior at baseline [58]. In addition, the endocannabinoid system, particularly through CB1 receptor signaling, is important in inducing food intake and promoting lipogenesis through signaling in the hypothalamus, the mesolimbic system and the periphery [59].
Neuropeptide signaling modulators
Neuropeptide Y receptor antagonists
Inhibition of NPY signaling through pharmacologic blockade or genetic deletion of the Y1- and Y5-receptors reduces food intake and weight in mice, with Y1-receptor signaling appearing to be the major mediator of the orexigenic effects of NPY [60,61]. Thus, the efficacy of NPY receptor antagonists for inducing weight loss is currently being tested in humans.
Efficacy
Antagonists of NPY signaling are in preclinical and early clinical testing. A randomized clinical trial (RCT) investigated the ability of the selective, orally-active Y5-receptor antagonist (MK-0557; Merck) to induce weight loss in overweight and obese individuals during a 52-week period. The results of this study showed that the drug induced very modest weight loss over the initial 12-week period, with the degree of weight loss achieving a plateau with a dose of MK-0557 1 mg/day. Following 52 weeks of therapy, MK-0557 (1 mg/day) produced significant, but not clinically meaningful, weight loss [62]. A more recent trial testing another selective Y5-receptor antagonist (S-2367; Shionogi USA, Inc., NJ, USA) showed more positive results, with subjects receiving S-2367 (800 mg/day) losing 3.9% of their baseline weight over 54 weeks of therapy compared with placebo-treated patients who lost only 0.9% of their baseline weight (p < 0.0001). Additionally, significantly more S-2367-treated patients lost 5% or more baseline weight (35%) compared with placebo-treated patients (12%; p < 0.0001) [63]. The limited efficacy of these drugs may not be surprising, considering the modest effectiveness of Y5-receptor blockade in inducing weight loss in rodents. However, newer Y1-receptor and Y5-receptor antagonists may prove effective, particularly if used in combination therapy.
Adverse effects
No significant adverse effects were reported with the use of these agents [62,63].
Other neuropeptide modulators
Currently, a specific AgRP inhibitor (TTP-435; TransTech Pharma, NC, USA), which blocks the activity of AgRP without inhibiting signaling of the melanocortin receptors, is in Phase II clinical trials [201]. Pharmacological antagonists of the signaling of MCH also are undergoing clinical trials. As described previously, MCH is a potent appetite-stimulating peptide expressed in neurons of the LHA. Administration of a selective MCH-1 receptor (MCH-1R) antagonist to rats reduced food intake, lowered weight gain and produced effects similar to those of antidepressants and anxiolytics in rodent behavioral tests [64]. Currently, a selective MCH-1R antagonist (BMS-830216; Bristol-Myers Squibb) is in Phase I/II clinical testing [202].
The MC4R has recently been identified as the major mediator of melanocortin-induced appetite suppression. However, MC4R agonists are not currently in clinical development, reflecting an absence of tractable nonpeptide small-molecule agonists and significant adverse effects with global activation of this receptor, including penile erections, flushing, blood pressure changes, a reduction of thermogenic responses to pyrogens and suppression of pain sensation [63,65].
Neuromedin U (NMU) is another neuropeptide that has recently been implicated in regulating energy homeostasis, as transgenic NMU-deficient mice are hyperphagic and obese, ICV administration of NMU reduces the food intake and weight of rats, and direct NMU injection into the PVN reduces food intake and increases the energy expenditure of rats. The effect of NMU on appetite has been proposed to be due to stimulation of the release of the anorexigenic hormone, corticotropin-releasing hormone, by neurons of the PVN [66]. However, it has been observed that chronic administration of NMU produces no reduction in food intake or weight [67], and that diet-induced obese rats are less sensitive to the effects of NMU administration compared with lean rats [68], making the development of a NMU-targeted drug less attractive.
Hormonal regulation of appetite & energy balance
Gut hormones
Cholecystokinin
Cholecystokinin (CCK) is a hormone that is found in both the brain, where it seems to work in some regions as a neurotransmitter, and in the gut, where it is synthesized and secreted primarily by I-cells in the duodenum and jejunum [69]. It is secreted by the gut in response to the intake of foods with a high fat and protein content and has a variety of effects aiding in digestion [70]. CCK signals through the CCK receptor, a seven-transmembrane GPCR, of which there are the CCK-A (CCK1) and CCK-B (CCK2) isoforms. The CCK-A receptor, which is expressed in the area of the pyloric sphincter and in vagal afferents, appears to mediate the effect of CCK on satiety. CCK-A agonists suppress food intake while blockade of the receptor inhibits the satiating effects of CCK in rodents [71,72]. In addition, rats deficient in CCK-A receptors are hyperphagic and obese [73], and humans treated with the CCK-A receptor antagonist, loxiglumide, exhibited increased caloric intake [74]. On the other hand, specific antagonism of the CCK-B receptor appears to have no effect on CCK-induced appetite reduction [75].
Efficacy
In animals, tolerance to CCK occurs rapidly (within hours) with chronic administration [76], and CCK was observed to have no effect on reducing bodyweight [77]. Additionally, whereas persistent intermittent CCK administration reduces meal sizes in rodents, it also increases feeding frequency, leading to no change in 24-h food intake and bodyweight [78]. GlaxoSmithKline terminated development of a selective CCK-A agonist (GI 181771X) after the drug failed to induce meaningful weight loss in Phase II clinical trials [79]. Interestingly, CCK potentiates the appetite- and weight-reducing effects of leptin in rodents [80], so it may prove useful in combination therapy.
Adverse effects
Chronic administration of CCK-8 to rodents induced pancreatitis [81,82], making this a concern in the chronic treatment of obesity in humans.
Glucagon-like peptide 1: exenatide, liraglutide
Glucagon-like peptide (GLP)-1 is a product of the processing of the preproglucagon gene by prohormone convertase-1 in the enteroendocrine L-cells of the ileum and proximal colon [83]. It is secreted in response to the ingestion of a meal, particularly one rich in fat and carbohydrates [84], both by direct nutrient stimulation as well as by stimulation by neural and endocrine factors [85]. The GLP-1 receptor (GLP-1R) is a GPCR expressed in pancreatic islets, the heart, lung, kidney and regions of the CNS and PNS, including the nucleus tractus solitarius and the ARC [83]. GLP-1 is an incretin, which induces the secretion of insulin by pancreatic β-cells and also stimulates insulin transcription/biosynthesis and β-cell differentiation and proliferation [85]. In addition to its actions in the pancreas, GLP-1 functions as a central satiety peptide, and its administration induces satiety and weight loss in rats and humans [86–89]. Lesions in the ARC, produced by monosodium glutamate treatment, abolish the anorectic effect of GLP-1 in rats [90]. In addition, the effect of GLP-1 on satiety is eliminated by subdiaphragmatic vagotomy or transecting brainstem–hypothalamic connections [91], indicating that hypothalamic and vagal signaling are essential to the satiating effects of GLP-1. Moreover, GLP-1 antagonizes the orexigenic effects of NPY [92].
Efficacy
The half-life of bioactive GLP-1 in the circulation is only 2 min, owing to its rapid proteolysis by dipeptidyl peptidase IV (DPP-IV) [93]. However, this pharmacokinetic issue has been circumvented by the development of the orally active DPP-IV inhibitors, vildagliptin (Novartis) and sitagliptin (Merck), and the GLP-1 analogs, which are resistant to proteolysis by DPP-IV, liraglutide (Novo Nordisk) and exenatide (Amylin Pharmaceuticals, CA, USA/Eli Lilly, Hampshire, UK), which is composed of exendin-4, a peptide extracted from the salivary gland of the Gila monster.
Exenatide is approved for the treatment of Type II diabetic patients who are not responsive to traditional therapies. A recent meta-analysis of 29 clinical trials investigating proteolysis-resistant GLP-1 analogs (exenatide and liraglutide) and DDP-IV inhibitor outcomes in patients with Type 2 diabetes, revealed that, overall, GLP-1 analogs induced an average placebo-adjusted weight loss of 2.37 kg (CI: 0.78–3.95 kg). When compared with patients receiving insulin therapy, patients receiving GLP-1 analogs lost an average of 4.76 kg more weight (CI: 3.49–6.03) [94]. Furthermore, the weight loss achieved with GLP-1 analogs was dose dependent, progressive and without plateau by 30 weeks of therapy, with more pronounced weight loss observed in patients receiving exenatide compared with liraglutide [94]. These numbers are striking, considering that these studies focused on improving glycemic control rather than inducing weight loss, so the majority of patients lost weight without the inclusion of lifestyle modifications. DPP-IV inhibitors (vildagliptin and sitagliptin) were not as effective in inducing weight loss, with subjects showing slight weight increases with DPP-IV inhibitor treatment compared with placebo [94].
While it was not as effective in producing weight loss in diabetic patients, liraglutide was reported by Novo Nordisk to induce a mean placebo-adjusted weight loss of 5.5–6.0 kg in nondiabetic obese individuals in a 1-year trial, with 75% of the subjects treated with the highest dose achieving a reduction of 5% or more of their baseline weight, and more than 35% of the subjects achieving a reduction of 10% or less of their baseline weight [203]. Liraglutide also induced significantly greater weight loss than orlistat during a 20-week RCT of nondiabetic obese individuals [95]. In January 2010, liraglutide received FDA approval as adjunct therapy for diabetes.
These GLP-1 analogs appear well suited for the treatment of obese patients with poor glycemic control, as other diabetic medications (insulin and sulfonylureas) induce weight gain. Metformin, however, is the first-line treatment for Type II diabetes and has been demonstrated to induce modest weight loss. Metformin improves insulin resistance by increasing glucose uptake and utilization and reducing gluconeogenesis and glucose output, specifically by increasing the binding of insulin to its receptors, enhancing intracellular insulin signaling, inducing the translocation of glucose transporters to the membranes of several different cell types and decreasing intestinal glucose absorption [96]. However, in a meta-analysis of metformin trials enrolling diabetic patients, subjects receiving metformin lost an average of 1.09 kg (CI: −2.29–0.11 kg) [97], an effect that is smaller than the weight loss observed with GLP-1 analogs.
Recently, a long-acting release formulation of exenatide (exenatide-LAR), injected once weekly, was demonstrated to improve glycemic control and reduce hemoglobin A51c (HbA1c) levels and bodyweight during a 15-week trial period [98]. Following 2 years of weekly treatment, exenatide-LAR induced average reductions in HbA1c (−1.7%), fasting plasma glucose (−40 mg/dl) and bodyweight (−5.8 lbs) [204]. Nasal and transdermal formulations of exenatide are also in early clinical development [205]. Moreover, Emisphere (NJ, USA) has developed oral formulations of both GLP-1 and PYY using a sodium N-[8-(2-hydroxybenzoyl) amino] caprylate (SNAC) carrier – a hydrophobic delivery agent that binds noncovalently to the peptides and allows them to be absorbed, unaltered, by the intestinal epithelium. Oral administration of these drugs causes a dose-dependent rapid rise in plasma levels, and the circulating peptides are functional [99]. In January 2010, Novo Nordisk announced the initiation of Phase I clinical testing of a long-acting oral GLP-1 analog (NN9924), which utilizes Emisphere’s carrier technology [206].
Adverse effects
Severe hypoglycemia was a rare incident in clinical trials of GLP-1 analogs, occurring in five out of 2781 patients treated with GLP-1 analogs, but mild-to-moderate hypoglycemia was more than twice as common in patients receiving GLP-1 analogs compared with those receiving placebo, peaking during the initiation of therapy [94]. In patients receiving exenatide, the most common adverse effects were nausea and vomiting. The nausea was mild to moderate and peaked by 8 weeks of treatment, declining thereafter [94]. Mild-to-moderate nausea also was the most common adverse effect reported with weekly treatment of exenatide-LAR [98]. Furthermore, in 2007, the FDA notified healthcare professionals of reports of acute pancreatitis in patients receiving exenatide. However, a claims-based active drug safety surveillance system using data from 27,996 exenatide initiators could not provide evidence supporting an increased risk of pancreatitis with exenatide therapy compared with other diabetic medications [100,101]. In November 2009, the FDA released another report and label change indicating that exenatide should not be used in patients with severe renal insufficiency or end-stage renal disease, and that kidney function should be monitored carefully in patients receiving this medication [101]. Pancreatitis occurring with liraglutide use has also been reported, and, furthermore, rodent studies have revealed the ability of high doses of liraglutide to induce medullary thyroid cancer. Post-marketing surveillance of patients receiving liraglutide will reveal if an increased risk of either of these serious adverse events exists [207].
Oxyntomodulin
Oxyntomodulin (OXM) is another product of the processing of the preproglucagon gene, and it is secreted post-prandially, along with GLP-1, by the L-cells of the lower intestine. It has less function as an incretin and greater activity in appetite suppression. Rats receiving OXM (ICV or intraperitoneal) exhibited reduced feeding and weight gain [102,103], and humans receiving intravenous infusion of OXM exhibited reduced appetites and feeding with no significant changes in circulating insulin levels [104]. Although its impact on feeding behavior continues to be defined, OXM activation of central GLP-1Rs is believed to be involved. The anorectic effects of OXM are abolished in Glp-1R knockout mice [105], and the reduction in food intake observed with ICV OXM administration or OXM injection into the PVN is eliminated with co-administration of the GLP-1R antagonist, exendin (9–39) [102]. However, compared with GLP-1, OXM binds to the GLP-1R with 100-fold-lower affinity, but it elicits the same degree of anorexia as GLP-1 at equimolar concentrations, suggesting that another OXM receptor may exist [103]. In addition, whereas intra-arcuate administration of exendin (9–39) blocks the anorectic effects of OXM, it does not affect the activity of GLP-1 [103], indicating that OXM may directly activate neurons in the ARC while GLP-1 indirectly stimulates ARC neurons, presumably through connections with the brainstem. The function of OXM in the ARC may be the stimulation of POMC neurons, as the incubation of POMC neurons with OXM ex vivo stimulates α-MSH release [103].
Efficacy
Subjects receiving a continuous intravenous infusion of OXM (3.0 pmol/kg/min) displayed a 19.3 ± 5.6% reduction in food intake compared with saline-infused subjects at a buffet meal. OXM infusion also significantly reduced cumulative 12-h food intake by 11.3 ± 6.2% but did not significantly alter cumulative 24-h food consumption [104]. In a subsequent trial, subjects injected subcutaneously with OXM (three-times daily, 30 min before each meal for 4 weeks) showed significantly reduced food intake during study meals at the beginning and end of the 4-week trial, with test subjects exhibiting an average weight loss of 2.3 ± 0.4 kg, compared with 0.5 ± 0.5 kg in the control group (p = 0.0106) [106]. In a short trial of 15 healthy overweight and obese individuals, OXM administered preprandially, three-times daily, increased activity-related energy expenditure by 26.2 ± 9.9% (p = 0.0221) and total energy expenditure by 9.5 ± 4.6% (p = 0.0495), in addition to reducing caloric intake [107].
The requirement of multiple daily injections of OXM to induce weight loss is a major barrier to therapy. TKS1225, a long-acting OXM analog, was developed by Thiakis, Ltd (London, UK), and was acquired by Wyeth Pharmaceuticals (Berkshire, UK) in 2008 [208]. The drug was then acquired by Pfizer following its 2009 merger with Wyeth.
Adverse effects
Mild nausea was reported in some subjects (<3%) [106,107]. Although OXM appears to be safe in short-term studies, the efficacy and safety of long-term OXM therapy has not yet been evaluated.
Peptide YY
Similar to NPY, Peptide YY (PYY) is a member of the pancreatic polypeptide family of proteins, which bind to the GPCRs Y1–Y6 [45]. PYY is expressed throughout the small intestine, with the highest concentration found in L-cells of the terminal ileum and colon, which secrete the peptide in response to a meal [108]. In the GI tract, PYY increases fluid and electrolyte absorption [109], reduces gastric and pancreatic secretions and delays gastric emptying [110]. Administration of PYY to rodents induces a dose-dependent decrease in food intake [111–113], and PYY-deficient mice display hyperphagia and obesity [114]. Moreover, obese humans and rodents have been observed to have reduced post-prandial circulating PYY levels compared with lean controls [115], with obese subjects displaying a progressive rise back to normal plasma PYY levels following bariatric surgery. This phenomenon has been implicated in the effectiveness of bariatric surgery in eliciting long-term weight loss. Thus, obese individuals develop a PYY deficiency, rather than developing resistance, which occurs with leptin, making PYY-replacement therapy an attractive option in combating obesity.
The major circulating form of PYY (PYY 3–36) shows high affinity for the Y2 receptor, and some affinity for the Y1 and Y5 receptors [45]. Peripheral administration of PYY induces appetite reduction, which is related to the activation of Y2 receptors in the ARC. However, ICV administration increases food intake, which is believed to be due to PYY activation of orexigenic Y1 and Y5 receptors in second-order neurons of the PVN [116]. Therefore, PYY is believed to induce appetite suppression by activating presynaptic Y2 receptors, which inhibit the activity of NPY/AgRP neurons. Vagal afferent activity is also involved, as bilateral subdiaphragmatic vagotomy or transecting brainstem–hypothalamic connections attenuate the anorectic effects of PYY [91].
Efficacy
Continuous infusion of PYY (3–36) in healthy nonobese subjects (0.8 pmol/kg/min) for 90 min reduced hunger scores and reduced caloric intake by 36 ± 7.4% during a buffet meal 2 h after infusion (p < 0.0001) [113]. Similar observations were obtained in obese patients, demonstrating their sensitivity to the anorectic effects of PYY [117]. Furthermore, infusion of PYY inhibited feeding in a dose-dependent manner (maximal inhibition: 35%; p < 0.001 vs control) [118]. Since continuous infusion of PYY is not a useful therapeutic strategy, recent studies have investigated the efficacy of intranasal delivery of PYY (3–36) (Nastech, WA, USA/Merck) in producing weight loss. In a RCT of 84 obese individuals following a 25% calorie-deficit diet and a suggested exercise routine, subjects were administered placebo, 200 or 600 µg of PYY (3–36) by 0.1-ml intranasal spray three-times daily, preprandially for 12 weeks. However, neither dose of PYY was able to induce significant placebo-adjusted weight loss. Furthermore, more than 50% of subjects receiving PYY (3–36) 600 µg withdrew from the study owing to nausea and vomiting [119].
Adverse effects
The most common adverse effects observed with PYY therapy were nausea, abdominal discomfort and vomiting, which all occurred in a dose-dependent manner. Adverse effects occurred spontaneously within a few minutes following administration of the agent [118,119]. The high percentage of subjects experiencing nausea coupled with the frequent dosing of PYY (3–36) required to suppress appetite casts a pessimistic view on the future of the current formulation. However, an extended-release formulation of PYY may have potential utility and induce fewer adverse effects, as this would better mimic the physiological release of PYY than intravenous injection or intranasal delivery of a large bolus of PYY into the circulation.
Ghrelin
Unlike other appetite-regulating hormones, ghrelin is the only known circulating orexigenic hormone. Ghrelin is a 28 amino acid peptide cleaved from preproghrelin and is predominantly found within the stomach. It has been reported to stimulate the release of growth hormone (GH) through the activation of the GH secretagogue receptor (GHS-R) [120]. Although the physiological relevance of ghrelin in GH release has been debated, as ghrelin-deficient mice do not display defective growth [121], ghrelin appears to play a role in energy balance.
Intracerebroventricular, as well as peripheral, administration of ghrelin induces a dose-dependent increase in food intake and bodyweight in rodents [122,123]. In addition, ghrelin has been implicated in long-term energy balance, as obese individuals display reduced circulating ghrelin levels, and anorexic patients display exaggerated circulating ghrelin levels, with weight gain correlating with a decline in ghrelin [124–126]. Expression of GHS-R1a has been found throughout the CNS, particularly within specific hypothalamic nuclei, the pituitary gland and the hippocampus, with lower levels in the thyroid gland, pancreas, spleen, heart and adrenals [127,128]. Ghrelin is believed to induce hunger and feeding through hypothalamic signaling and activation of NPY/AgRP neurons in the ARC. However, vagal stimulation by ghrelin is also important, and ghrelin administration to rats in which vagal signaling has been mechanically or chemically disrupted does not increase feeding or the activation of NPY-expressing neurons [129]. Furthermore, injection of ghrelin into other sites in the CNS expressing GHS-R, including segments of the mesolimbic reward pathway, the hippocampus and the dorsal raphe nucleus, induces food intake [130,131], indicating that there are several signaling sites important to ghrelin’s orexigenic function.
Efficacy
While ghrelin administration may prove useful in stimulating feeding in patients with anorexia nervosa or cachexia due to cancer or chronic disease, the utility of blocking ghrelin’s action as a therapeutic strategy for combating obesity is also being explored. In 2006, clinical trials of an experimental ghrelin vaccine, CYT009-GhrQb (Cytos Biotechnology, Switzerland), were discontinued, as vaccination did not induce more weight loss than placebo in obese individuals after 6 months, even though all patients showed a strong ghrelin antibody response, which was boosted with subsequent vaccinations [209]. A new vaccination of ghrelin conjugated to the hapten, keyhole limpet hemocyanin (KLH), has been developed and, when administered to rats, elicited decreased feeding behavior, adiposity and bodyweight, which was proportional to the specific immune response elicited against active ghrelin [132]. A ghrelin-neutralizing RNA spiegelmer, NOX-B11, which is an aptamer that binds to and inactivates ghrelin, was developed by NOXXON Pharma Ag. Berlin, Germany). It blocked the orexigenic activity of exogenous ghrelin administration but did not alter food intake in rats [133]. In 2006, Pfizer was granted an exclusive worldwide license to NOX-B11 [210]. Furthermore, specific ghrelin antagonists (Elixir Pharmaceuticals, MA, USA) are in preclinical testing [211], and a new therapeutic target, ghrelin O-acyltransferase (GOAT), has been identified. GOAT is a membrane-bound enzyme that adds an eight-carbon octanoate to a serine residue in ghrelin, which is required for receptor binding. Thus, pharmacological inhibition of GOAT may be an effective means of inhibiting ghrelin activity, particularly since ghrelin is the only peptide known to be octanoylated [134]. However, in the context of the reduced circulating ghrelin levels observed in obese patients, antagonizing this system may not be an effective strategy in combating obesity.
Adverse effects
The original ghrelin vaccine was well tolerated by all individuals, but that development program has been discontinued. The side- effect profile of antagonizing this system is presently unknown.
Pancreatic hormones
Pancreatic polypeptide
Pancreatic polypeptide (PP) is structurally similar to PYY and may have originated as a duplication of the Pyy gene [135]. Most PP is expressed in the pancreas, with a small amount expressed throughout the GI tract, and its circulating levels rise rapidly following meal ingestion, rising proportionally to caloric intake and remaining elevated for up to 6 h [136]. PP release is induced by vagal stimulation and by other peripheral hormones, including ghrelin, motilin and secretin [137]. Hyperphagic and obese ob/ob mice exhibit reduced food intake following peripheral PP administration and exhibit a reduced amount of PP-producing pancreatic cells, an observation that has been implicated in the development of their hyperphagia and obesity [138]. Repeated administration of PP to ob/ob mice leads to reduced weight gain [139]. In addition, normal lean mice exhibit decreased feeding, as well as delayed gastric emptying, following injection of PP [139]. Furthermore, transgenic mice overexpressing PP exhibit hypophagia, slower gastric emptying and reduced weight gain [140]. Finally, there are lower fasting and meal-stimulated PP levels in obese patients [141] and exaggerated PP responses in patients with anorexia nervosa [142], indicating that PP may be involved in long-term energy balance. Similar to PYY, weight loss by obese patients normalizes PP levels [143].
Pancreatic polypeptide binds with highest affinity to the Y4 and Y5 receptors [45]. Similar to PYY, the method of PP administration affects its function in regulating appetite. ICV administration of PP to rats leads to increased feeding [144], presumably owing to stimulation of orexigenic Y5 receptors [61]. Peripheral administration of radiolabeled PP shows significant accumulation of PP in the region of the area postrema, where Y4 receptors are expressed [145,146], and the anorectic effects of PP are abolished in vagotomized mice [139]. Peripheral administration of PP increases vagal activity and leads to changes in the levels of hypothalamic neuropeptides, decreasing the orexigenic peptides, NPY and orexin, and increasing the anorexigenic peptide, urocortin [139], indicating that PP may alter feeding behavior through direct vagal stimulation, which produces downstream effects in the hypothalamus via brainstem–hypothalamic interconnections.
Efficacy
In a RCT of healthy nonobese individuals, intravenous infusion of PP (10 pmol/kg/min) reduced appetite and caloric intake by 21.8 ± 5.7% (p < 0.01) at a buffet meal 2 h following infusion. Inhibition of food intake by PP was sustained over a 24-h period with a reduction in food intake of 25.3 ± 5.8% [147]. Although PP has a longer half-life in the circulation than other satiety hormones, it is still very short [148], and specific and long lasting Y2-receptor or Y4-receptor agonists may prove more useful in eliciting long-term appetite suppression and weight loss. Currently, Obinepitide (7TM Pharma, Hørsholm, Denmark), a Y2/Y4-receptor agonist, and TM30339 (7TM Pharma), a selective Y4-receptor agonist, are in Phase I/II clinical trials [212,213]. It has been reported that Obinepitide significantly reduced food intake for up to 9 h following dosing.
Adverse effects
No adverse effects have been reported regarding PP. However, no long-term trials of PP use in humans have been performed.
Amylin: pramlintide
Amylin, or islet amyloid polypeptide (IAPP), is synthesized and secreted with insulin by pancreatic β-cells [149], and patients with Type I diabetes exhibit a deficiency of both hormones. Similar to insulin, fasting plasma levels of amylin are low, and rise dramatically in response to a meal [150,151]. Amylin works synergistically with insulin in regulating postprandial glucose levels. In addition to its role in glucose homeostasis, amylin has anorectic properties, with ICV administration reducing food intake in rats and constant infusion over 10 days reducing both feeding and adiposity [152]. Additionally, pharmacologic amylin antagonism increases rodent feeding and fat deposition [153], and transgenic amylin-deficient mice display excess weight gain [154,155].
Amylin is structurally similar to calcitonin gene-related peptide (CGRP), calcitonin and adrenomedullin [156,157], and amylin-binding receptors appear when calcitonin receptors are co-expressed with receptor activity-modifying proteins (RAMPs) [158,159]. Amylin-specific receptors have been localized to specific areas of the brain, including the area postrema [160]. The effects of amylin on gastric emptying and appetite are attenuated by vagotomy or lesioning of the area postrema/nucleus tractus solitarius, indicating that vagal signaling is essential in the satiating effects of amylin [161–163].
Efficacy
Pramlintide, a synthetic amylin analog, was developed by Amylin Pharmaceuticals (CA, USA) owing to amylin’s poor solubility and propensity to aggregate [164]. Pramlintide has a similar pharmacokinetic and pharmacodynamic profile to endogenous amylin [165]. Pramlintide is currently approved for the treatment of diabetes, and, unlike traditional diabetic medications, elicits weight loss in diabetic patients.
In a 52-week RCT, 538 insulin-treated Type II diabetic patients were administered placebo, 30-, 75- or 150-µg doses of pramlintide, subcutaneously injected three-times daily with meals. Besides reductions in HbA1c levels, all three pramlintide groups experienced significant reductions in bodyweight [166]. In another 52-week trial, 656 Type II diabetic patients on insulin therapy were administered preprandial subcutaneous injections of pramlintide (60, 90 or 120 µg). Subjects receiving 90 or 120 µg of pramlintide experienced weight losses of approximately 0.5 and 1.5 kg, respectively, which persisted until the end of the trial [167]. A RCT of 480 Type I diabetic patients receiving preprandial injections of placebo or pramlintide (30–60 µg) reported that subjects receiving pramlintide exhibited approximately a 0.5 kg reduction in weight by 52 weeks [168]. Finally, a pooled post-hoc analysis of weight loss in pramlintide-treated Type II diabetic subjects showed that, overall, pramlintide therapy (120 µg twice daily or 150 µg three-times daily) induced an average weight loss of 2.6 kg (p < 0.0001) over 52 weeks of therapy [169]. Although these weight loss figures appear modest, they are more significant when considering that these subjects did not receive lifestyle modification therapy and that traditional diabetic pharmacotherapy induces weight gain.
Adverse effects
The only adverse effects associated with pramlintide were a two-fold greater incidence of nausea and headache, which were of mild-to-moderate intensity and were limited to the first 4 weeks of therapy [166–169].
Adipose tissue hormones
Leptin
Leptin, an adipose tissue-derived hormone, was heralded as the ‘obese gene’ (ob) upon its discovery, as mice with a mutation of this gene developed morbid obesity [170]. The leptin gene was cloned in 1994 by Zhang et al. [171] and was soon discovered circulating in the plasma of normal mice and humans and was absent in leptin-deficient ob/ob mice [172]. Importantly, administration of leptin to ob/ob mice led to decreased food intake, increased energy expenditure and a 30% reduction in bodyweight following 2 weeks of treatment, displaying the importance of this circulating hormone in bodyweight regulation [172,173]. Furthermore, human congenital leptin deficiency is associated with early-onset obesity, which is effectively treated with leptin-replacement therapy [174,175].
Leptin is both an acute and chronic hormonal signal of energy balance, as its circulating level directly correlates with the degree of adiposity [176] but also fluctuates with feeding, as fasting reduces plasma leptin concentration [177]. Obese patients typically exhibit elevated circulating leptin levels [176], a finding also commonly seen in transgenic rodent models of obesity. The elevation of circulating leptin in obesity is primarily due to the development of leptin resistance, similar to the rise in insulin levels observed in Type II diabetic patients.
The leptin receptor is a member of the gp130 family of cytokine receptors and is highly expressed within the hypothalamus [178]. Leptin receptors activate an associated janus kinase (Jak2), inducing phosphorylation of the intracellular Tyr985 and Tyr1138 residues [179]. This leads to the activation of signal transducer and activator of transcription-3 (STAT3), which impacts the transcription of specific neuropeptides, increasing the transcription of Pomc and suppressing the transcription of Agrp [180]. The leptin receptor-associated Jak2 also phosphorylates insulin receptor substrate (IRS) proteins, particularly IRS-2, which stimulates the phosphoinositide 3-kinase (PI3K) pathway. Activation of PI3K leads to the activation of Akt, which phosphorylates and inactivates forkhead box O1 (FOXO1), a transcription factor that stimulates the expression of NPY and AgRP but inhibits POMC expression. Activation of the PI3K pathway through leptin receptor signaling has also been implicated in the depolarization and activation of POMC-expressing neurons [181]. Furthermore, 5´-AMP-activated protein kinase (AMPK), an energy-sensing protein that is active during low-energy states and stimulates feeding, is inhibited in multiple areas of the hypothalamus through leptin receptor signaling [181]. Finally, the leptin receptor activates the suppressor of cytokine signaling-3 (SOCS3) protein, which inhibits further leptin receptor signaling and has been implicated in the development of leptin resistance [179].
Signaling in the ARC appears to be central to leptin’s ability to generate its anorexigenic response, as both NPY/AgRP and POMC/CART populations of neurons express leptin receptors [182,183] and ICV leptin fails to reduce food intake in rats if the ARC is damaged [184]. Leptin inhibits NPY/AgRP signaling and downregulates the expression of these neuropeptides, while it upregulates POMC expression and stimulates POMC/CART signaling in the ARC [185–187].
Efficacy
Clinical trials exploring the utility of exogenous leptin therapy as an anti-obesity therapeutic strategy have been disappointing. The failure of leptin therapy to induce weight loss is believed to be due to the development of leptin resistance in most obese individuals. Leptin resistance appears to be mechanistically linked to the development of endoplasmic reticulum (ER) stress, which occurs within the hypothalamus in obesity. Administration of chemical chaperones (4-phenyl butyric acid [PBA] and tauroursodeoxycholic acid [TUDCA]), which resolve ER stress, leads to increased leptin sensitivity and a significantly increased ability for leptin to induce hypophagia and weight loss in mice [188]. Combination therapy with chemical chaperones that resolve central ER stress may be a solution to improving the efficacy of recombinant leptin in obese humans.
Adverse effects
As with the majority of endogenous hormones, the adverse effects of leptin are limited to the consequences of injection, including pain at the injection site and pruritus [189].
Pramlintide plus leptin combination therapy
Diet-induced obese rats are insensitive to the anorectic or weight-reducing effects of leptin administration. However, amylin is effective in reducing food intake and weight in obese rats, and its effectiveness increases when co-administered with leptin. The weight loss synergy observed with amylin was not observed when other anorexigenic peptides (e.g., PYY and GLP-1/exendin-4 analog) were co-administered with leptin, indicating that amylin specifically increases the efficacy of leptin. Moreover, obese rats pretreated with amylin display increased leptin signaling in the VMN and area postrema, indicating that amylin restores leptin sensitivity in certain brain areas [190].
Efficacy
In a 24-week RCT, 177 overweight and obese individuals were instructed to follow a 40% calorie-deficit diet and were treated with pramlintide (180 µg twice daily, then 360 µg twice daily) during a 4-week lead-in period with a target of achieving 2–8% weight loss. Following the run-in, subjects who exhibited adequate weight loss were randomized to receive pramlintide (360 µg) plus metreleptin (5 mg), pramlintide (360 µg) plus placebo, or metreleptin (5 mg) plus placebo and were instructed to follow a 20% calorie deficit diet. Each drug was administered separately by subcutaneous injection, twice daily. During this 20-week treatment period, weight loss in subjects remaining on pramlintide or who switched to metreleptin monotherapy declined, reaching stable weight loss plateaus of 8.4 ± 0.9% (7.9 ± 0.9 kg) and 8.2 ± 1.3% (7.4 ± 1.3 kg), respectively. However, subjects receiving combination therapy continued to lose weight, achieving a mean weight loss of 12.7 ± 0.9% (11.5 ± 0.9 kg), and, importantly, weight loss did not plateau during the 20-week treatment period [190]. In a subsequent 28-week Phase II trial, overweight and obese individuals (BMI: 27–35 kg/m2) treated with pramlintide (360 µg)/metreleptin (5 mg), twice daily, lost an average of 11% of their baseline weight, which was significantly greater than subjects receiving placebo (1.8%) or either agent alone (~5.0%; p < 0.01) [214]. In an extension of this 28-week trial, subjects receiving pramlintide/metreleptin combination therapy for a total of 52 weeks showed sustained weight loss, while those subjects receiving placebo regained almost all their weight [215]. Pramlintide/metreleptin combination therapy is currently being advanced into Phase III trials for the treatment of obesity.
Adverse effects
The most common adverse effect from pramlinitide plus leptin was nausea [191]. While effective, this therapy requires subcutaneous injections, which is a major barrier to most of the hormone-replacement therapies discussed previously.
Oleoyl-estrone
Oleoyl-estrone (OE) is a naturally occurring circulating hormone, which is packaged in lipoproteins derived from adipose tissue. Similar to leptin, OE levels correlate with adiposity in humans [192], but obese individuals have lower circulating OE levels than would be expected. In an investigation of estrone fatty esters, OE administered to rats intravenously for 14 days with osmotic minipumps induced dose-dependent weight loss with decreased food intake [193]. Furthermore, intravenous administration of OE to normal rats induced weight loss with a preservation of body protein and wasting of fat stores [194]. Additionally, administration of OE appears to have long-lasting effects in lean rodents, as weight loss is maintained for 26 days following 2 weeks of constant OE infusion in these animals, whereas obese rats regain weight immediately following cessation of OE infusion [195]. This finding has been linked to the deficient leptinergic system observed in obesity. Importantly, oral administration of OE by gavage or in drinking water induced a dose-related loss of adipose tissue along with a decrease in food intake with no change in metabolic rate [193,196]. Therefore, unlike peptide hormones, OE is amenable to oral administration.
Efficacy
Oral OE (150–300 µmol/day) administered without dietary restrictions to a morbidly obese (BMI: 51.9 kg/m2) individual over ten consecutive 21-day trial periods followed by 2-month recovery periods, induced a weight loss of 38.5 kg (BMI: 40.5 kg/m2) over 27 months [197]. This report, along with the animal data, prompted clinical testing of the effectiveness of oral OE in combating obesity, which was sponsored by Manhattan Pharmaceuticals (NY, USA). However, this investigation was discontinued in 2007, as RCTs investigating the drug’s utility in obese and morbidly obese patients failed to demonstrate significant placebo-adjusted weight losses [216].
Expert commentary
There are scientific, regulatory and economic challenges to the development of safe and effective anti-obesity drugs and their implementation into mainstream clinical practice. Many of the current drug formulations described previously require injection, a major barrier to patient compliance that needs to be addressed through the development of orally active drugs that target the same pathways. In addition, considering the history of morbidity/mortality associated with obesity medications, substantial evidence for the safety of these new agents will be needed for physicians to routinely prescribe these drugs, particularly when safe, effective drugs for treating the comorbidities of obesity exist. Furthermore, while there is a general consensus that obesity is a disease, most physicians prefer to pharmacologically treat the comorbidities and to address obesity through lifestyle- modification programs, using anti-obesity drug therapy only as a last resort. However, a patient may struggle unsuccessfully for months or years to lose weight, when early anti-obesity pharmacotherapy may have been of tremendous benefit in the reduction of both weight and the risk for the development of comorbidities. Therefore, educating patients and physicians about the safety and efficacy of these new drugs will be of great importance.
The FDA’s guidelines for the development of products for weight management pose another significant hurdle in the development of anti-obesity drugs. According to the FDA, weight management by a new agent should be demonstrated over the course of at least 1 year before a product can be considered effective. The FDA’s efficacy standards include stipulations that: statistically significant weight loss must occur between drug and placebo of 5% or more, the number of drug-treated subjects losing 5% or more of their baseline weight must be 35% or more and approximately double the number of placebo-treated subjects losing 5% or more baseline weight. Additionally, in order to adequately address safety concerns, no fewer than 3000 subjects must be assigned to the experimental drug, and no fewer than 1500 subjects assigned to placebo for a 1-year period [198]. Although these guidelines will result in safer, more effective drugs entering clinical practice, such requirements may also force pharmaceutical companies to limit the number of anti-obesity drugs they develop and cause the premature termination of many drug trials.
Furthermore, since the FDA does not consider the metabolic syndrome as a discrete disease entity, a drug cannot receive approval for the treatment of this syndrome without evidence of its ability to normalize or improve all components of the syndrome, independent of weight loss. In addition, in order for a drug to receive a standalone indication for the prevention or treatment of Type 2 diabetes, dyslipidemia, hypertension or any other weight-related co-morbidity, it must be shown that the product effectively prevents or treats that co-morbidity through a mechanism that is independent of weight loss [217]. Thus, the FDA perpetuates the idea that weight loss is separate from the clinical prevention of several common pathologies, including Type II diabetes and cardiovascular disease. Importantly, this will serve as a basis for insurance companies to refrain from reimbursing patients for anti-obesity medication, as they package anti-obesity drugs with cosmetic procedures in their lists of exclusions to coverage. Since obesity is currently not considered a disease unto itself, without FDA indication for another condition, patients may have to pay out-of-pocket for anti-obesity pharmacotherapeutics, which will present a major barrier to patient care, especially in low-income populations with disproportionate obesity rates. For example, a 1-month supply of orlistat or sibutramine costs approximately US$120–140, which presents a major hurdle for patients of low socioeconomic status to receive treatment.
Five-year view
In their 1999 Endocrine Reviews paper on obesity pharmacotherapeutics, George Bray and Frank Greenway pointed out the similarities between the evolution of hypertension and anti-obesity therapies. Before the introduction of the diuretic chlorothiazide in 1958, the mainstays of treatment for hypertension were: a very low-salt diet, drug treatment with reserpine, hydralazine, and ganglionic blockers, which all had limited utility reflecting significant adverse effects, or surgical sympathectomy, a drastic, yet effective, surgical procedure [199]. These treatments are analogous to the anti-obesity therapies of: a low calorie, low-carbohydrate, and/or low-fat diet, drug treatment with agents limited by their side effect profiles and gastric bypass surgery. Today, there is an array of safe and effective drugs for the treatment of hypertension, and it can be envisioned from the amount of resources going into the generation of new anti-obesity drugs that this field may follow the same pattern.
Currently, there are tremendous numbers of anti-obesity therapeutics in preclinical and clinical testing, with several showing great promise to be superior alternatives to sibutramine and orlistat. There has been a recent movement towards the development of combination therapeutics for the treatment of obesity, and based on the positive results achieved with these agents and the effectiveness of combination drug therapy in treating a variety of other pathologies, new anti-obesity drug combinations are expected to be developed. Agents that target gut/pancreatic/adipose hormone and neuropeptide signaling will be further developed, as these are endogenous regulators of appetite and energy balance and, therefore, their manipulation is expected to induce fewer adverse effects. Furthermore, new delivery methods, including oral, intranasal and transdermal formulations, will make these drugs more attractive to patients and physicians. Additionally, a better understanding of how the body regulates appetite and energy balance will likely result in the discovery of new therapeutic targets. For example, an obstacle such as obesity-related leptin resistance may be circumvented as we further define the mechanisms by which central leptin resistance develops in obesity. However, scientific, regulatory and economic hurdles still impede the rapid entry of anti-obesity pharmacotherapeutics into mainstream clinical care.
Key issues
Obesity is a global pandemic with many associated comorbidities, including cardiovascular disease and Type II diabetes. It has been estimated that the health and economic consequences of chronic obesity outweigh those of either smoking or alcohol abuse.
There is currently an unmet clinical need for safe and effective therapies to combat obesity and induce long-term weight loss. Bariatric surgery, owing to its risks, is reserved for only morbidly obese patients and those with serious comorbidities, while the two anti-obesity drugs that are US FDA approved for long-term use, sibutramine and orlistat, have limited efficacy and are associated with cardiovascular and gastrointestinal side effects, respectively, which make them unattractive options for patients and physicians.
Understanding the endogenous regulation of short- and long-term energy balance has led to the initiation of several anti-obesity drug-development programs targeting these pathways.
Exogenous supplementation of particular hormonal regulators of appetite and energy balance (GLP-1, PYY, OXM, PP, amylin) has proven effective in reducing appetite and inducing weight loss. However, a major limitation to the use of these peptides is that their delivery currently requires injection.
The development of new delivery methods of these peptide hormones, including oral, intranasal and transdermal formulations, will make these drugs more attractive to patients and physicians. Furthermore, orally active drugs targeting hormone and neuropeptide receptors are in early development.
There still remain several scientific, regulatory and economic barriers to the development and mainstream clinical use of anti-obesity pharmacotherapeutics.
Acknowledgments
Financial & competing interests disclosure
Support was provided by NIH grants R01 CA75123, R01 CA95026, RC1 CA75123 and P30 CA56036, and from Targeted Diagnostic and Therapeutics, Inc (SAW). Michael A Valentino is the recipient of a predoctoral fellowship from the Pharmaceutical Research and Manufacturers of America (PhRMA) Foundation. Francheska Colon-Gonzalez is the recipient of a post-doctoral fellowship from the PhRMA Foundation. Scott A Waldman is the Samuel MV Hamilton Endowed Professor and is a consultant to Merck and CombiMab, Inc., the Chair of the Data Safety Monitoring Board for the C-Cure TrialÔ sponsored by Cardio3 Biosciences and the Chair (uncompensated) of the Scientific Advisory Board to Targeted Diagnostics and Therapeutics, Inc.
No writing assistance was utilized in the production of this manuscript.
Footnotes
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.James WP. The epidemiology of obesity: the size of the problem. J. Intern. Med. 2008;263(4):336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Reducing Risks, Promoting Healthy Life. Geneva, Switzerland: World Health Organisation; The World Health Report 2002. 2002
- 3.Daniels J. Obesity: America’s epidemic. Am. J. Nurs. 2006;106(1):40–49. doi: 10.1097/00000446-200601000-00028. [DOI] [PubMed] [Google Scholar]
- 4.Dehghan M, Akhtar-Danesh N, Merchant AT. Childhood obesity, prevalence and prevention. Nutr. J. 2005;4:24. doi: 10.1186/1475-2891-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obes. Rev. Suppl. 2004;5(1):4–104. doi: 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 6.Kopelman P. Health risks associated with overweight and obesity. Obes. Rev. 2007;8 Suppl. 1:13–17. doi: 10.1111/j.1467-789X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 7.Sturm R. The effects of obesity, smoking, and drinking on medical problems and costs. Health Affairs. 2002;21(2):245–253. doi: 10.1377/hlthaff.21.2.245. [DOI] [PubMed] [Google Scholar]
- 8.Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007;121(7):492–496. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller ER, 3rd, Erlinger TP, Young DR, et al. Results of the diet, exercise, and weight loss intervention trial (DEW-IT) Hypertension. 2002;40(5):612–618. doi: 10.1161/01.hyp.0000037217.96002.8e. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein DJ. Beneficial health effects of modest weight loss. Int. J. Obes. 1992;16(6):397–415. [PubMed] [Google Scholar]
- 11.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132(6):2226–2238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 12.Sarwer DB, von Sydow Green A, Vetter ML, Wadden TA. Behavior therapy for obesity: where are we now? Curr. Opin. Endocrinol. Diabetes Obes. 2009;16(5):347–352. doi: 10.1097/MED.0b013e32832f5a79. [DOI] [PubMed] [Google Scholar]
- 13.Perri M, Corsica J. Improving the maintenance of weight lost in behavioral treatment of obesity. In: Wadden T, Stunkard A, editors. Handbook of Obesity Treatment. NY, USA: Guilford Press; 2002. pp. 357–379. [Google Scholar]
- 14.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 15. Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004;351(26):2683–2693. doi: 10.1056/NEJMoa035622.. • Along with [14] provides data showing the long-term outcomes of bariatric surgery in obese patients.
- 16.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann. Intern. Med. 2005;142(7):547–559. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 17.Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Lancet. 1998;352(9123):167–172. doi: 10.1016/s0140-6736(97)11509-4. [DOI] [PubMed] [Google Scholar]
- 18.Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999;281(3):235–242. doi: 10.1001/jama.281.3.235. [DOI] [PubMed] [Google Scholar]
- 19.James WP, Astrup A, Finer N, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. Lancet. 2000;356 Suppl.:2119–2125. doi: 10.1016/s0140-6736(00)03491-7. [DOI] [PubMed] [Google Scholar]
- 20.Diets, drugs and surgery for weight loss. Treat. Guidel. Med. Lett. 2008;6(68):23–28. [PubMed] [Google Scholar]
- 21.Bays HE. Current and investigational antiobesity agents and obesity therapeutic treatment targets. Obes. Res. 2004;12(8):1197–1211. doi: 10.1038/oby.2004.151. [DOI] [PubMed] [Google Scholar]
- 22.Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007;369(9555):71–77. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- 23.Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335(7631):1194–1199. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofbauer KG, Nicholson JR, Boss O. The obesity epidemic: current and future pharmacological treatments. Annu. Rev. Pharmacol. Toxicol. 2007;47:565–592. doi: 10.1146/annurev.pharmtox.47.120505.105256. [DOI] [PubMed] [Google Scholar]
- 25.Brobeck JR. Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol. Rev. 1946;26:541–559. doi: 10.1152/physrev.1946.26.4.541. [DOI] [PubMed] [Google Scholar]
- 26.Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J. Biol. Med. 1951;24:123–140. [PMC free article] [PubMed] [Google Scholar]
- 27.Sclafani A. Neural pathways involved in the ventromedial hypothalamic lesion syndrome in the rat. J. Comp. Physiol. Psychol. 1971;77(1):70–96. doi: 10.1037/h0031574. [DOI] [PubMed] [Google Scholar]
- 28.Sclafani A, Kirchgessner A. The role of the medial hypothalamus in the control of food intake: an update. Feeding Behavior. 1986;9:27–66. [Google Scholar]
- 29.Stellar E. The physiology of motivation. Psychol. Rev. 1954;61(1):5–22. doi: 10.1037/h0060347. [DOI] [PubMed] [Google Scholar]
- 30.Wynne K, Stanley S, McGowan B, Bloom SR. Appetite control. J. Endocrinol. 2005;184(2):291–318. doi: 10.1677/joe.1.05866. [DOI] [PubMed] [Google Scholar]
- 31.Rethelyi M. Diffusional barrier around the hypothalamic arcuate nucleus in the rat. Brain Res. 1984;307(1–2):355–358. doi: 10.1016/0006-8993(84)90494-3. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 33. Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307(5717):1909–1914. doi: 10.1126/science.1109951.. •• Along with [32] provides information about the neurologic mechanisms controlling appetite and energy balance.
- 34.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortlnergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385(6612):165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 35.Huszar D, Lynch CA, Fairchild-Huntress V, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 36.Argyropoulos G, Rankinen T, Neufeld DR, et al. A polymorphism in the human agouti-related protein is associated with late-onset obesity. J. Clin. Endocrinol. Metab. 2002;87(9):4198–4202. doi: 10.1210/jc.2002-011834. [DOI] [PubMed] [Google Scholar]
- 37.Kristensen P, Judge ME, Thim L, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393(6680):72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 38.Abbott CR, Rossi M, Wren AM, et al. Evidence of an orexigenic role for cocaine- and amphetamine-regulated transcript after administration into discrete hypothalamic nuclei. Endocrinology. 2001;142(8):3457–3463. doi: 10.1210/endo.142.8.8304. [DOI] [PubMed] [Google Scholar]
- 39.Shutter JR, Graham M, Kinsey AC, Scully S, Lüthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11(5):593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 40.Swart I, Jahng JW, Overton JM, Houpt TA. Hypothalamic NPY, AGRP, and POMC mRNA responses to leptin and refeeding in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283(5):R1020–R1026. doi: 10.1152/ajpregu.00501.2001. [DOI] [PubMed] [Google Scholar]
- 41.Hagan MM, Rushing PA, Pritchard LM, et al. Long-term orexigenic effects of AgRP-83---132 involve mechanisms other than melanocortin receptor blockade. Am. J. Physiol. 2000;279(1):R47–R52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- 42.Rossi M, Kim MS, Morgan DGA, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of α-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139(10):4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 43.Ollmann MM, Wilson BD, Yang YK, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278(5335):135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 44.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1998;1(4):271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 45.Larhammar D. Structural diversity of receptors for neuropeptide Y, peptide YY and pancreatic polypeptide. Regul. Pept. 1996;65(3):165–174. doi: 10.1016/0167-0115(96)00110-3. [DOI] [PubMed] [Google Scholar]
- 46.Inui A. Neuropeptide Y feeding receptors: Are multiple subtypes involved? Trends Pharmacol. Sci. 1999;20(2):43–46. doi: 10.1016/s0165-6147(99)01303-6. [DOI] [PubMed] [Google Scholar]
- 47.King PJ, Widdowson PS, Doods HN, Williams G. Regulation of neuropeptide Y release by neuropeptide Y receptor ligands and calcium channel antagonists in hypothalamic slices. J. Neurochem. 1999;73(2):641–646. doi: 10.1046/j.1471-4159.1999.0730641.x. [DOI] [PubMed] [Google Scholar]
- 48.Fekete C, Légrádi G, Mihály E, et al. α-melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J. Neurosci. 2000;20(4):1550–1558. doi: 10.1523/JNEUROSCI.20-04-01550.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fekete C, Sarkar S, Rand WM, et al. Agouti-related protein (AGRP) has a central inhibitory action on the hypothalamic–pituitary–thyroid (HPT) axis; comparisons between the effect of AGRP and neuropeptide Y on energy homeostasis and the HPT axis. Endocrinology. 2002;143(10):3846–3853. doi: 10.1210/en.2002-220338. [DOI] [PubMed] [Google Scholar]
- 50.Sarkar S, Lechan RM. Central administration of neuropeptide Y reduces α-melanocyte-stimulating hormone-induced cyclic adenosine 5´-monophosphate response element binding protein (CREB) Endocrinology. 2003;144(1):281–291. doi: 10.1210/en.2002-220675. [DOI] [PubMed] [Google Scholar]
- 51.Qu D, Ludwig DS, Gammeltoft S, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380(6571):243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 52.Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp. Neurol. 1995;131(2):229–238. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 53.Yeo GSH, Connie Hung C-C, Rochford J, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 2004;7(11):1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 54.Xu B, Goulding EH, Zang K, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003;6(7):736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ricardo JA, Tongju Koh E. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153(1):1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- 56.Ter Horst GJ, De Boer P, Luiten PGM, Van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31(3):785–797. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- 57.Szczypka MS, Kwok K, Brot MD, et al. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30(3):819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 58.Hayward MD, Pintar JE, Low MJ. Selective reward deficit in mice lacking β-endorphin and enkephalin. J. Neurosci. 2002;22(18):8251–8258. doi: 10.1523/JNEUROSCI.22-18-08251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 2005;8(5):585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- 60.Mashiko S, Moriya R, Ishihara A, et al. Synergistic interaction between neuropeptide Y1 and Y5 receptor pathways in regulation of energy homeostasis. Eur. J. Pharmacol. 2009;615(1–3):113–117. doi: 10.1016/j.ejphar.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 61.Kanatani A, Mashiko S, Murai N, et al. Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology. 2000;141(3):1011–1016. doi: 10.1210/endo.141.3.7387. [DOI] [PubMed] [Google Scholar]
- 62.Erondu N, Gantz I, Musser B, et al. Neuropeptide Y5 receptor antagonism does not induce clinically meaningful weight loss in overweight and obese adults. Cell Metab. 2006;4(4):275–282. doi: 10.1016/j.cmet.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 63.Sargent BJ, Moore NA. New central targets for the treatment of obesity. Br. J. Clin. Pharmacol. 2009;68(6):852–860. doi: 10.1111/j.1365-2125.2009.03550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borowsky B, Durkin MM, Ogozalek K, et al. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat. Med. 2002;8(8):825–830. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- 65.Adan RA, Tiesjema B, Hillebrand JJ, Fleur SE, Kas MJ, Krom M. The MC4 receptor and control of appetite. Br. J. Pharmacol. 2006;149(7):815–827. doi: 10.1038/sj.bjp.0706929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitchell J, Maguire J, Davenport A. Emerging pharmacology and physiology of neuromedin U and the structurally related peptide neuromedin S. Br. J. Pharmacol. 2009;158(1):87–103. doi: 10.1111/j.1476-5381.2009.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson EL, Murphy KG, Todd JF, et al. Chronic administration of NMU into the paraventricular nucleus stimulates the HPA axis but does not influence food intake or bodyweight. Biochem. Biophys. Res. Commun. 2004;323(1):65–71. doi: 10.1016/j.bbrc.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 68.Novak CM, Zhang M, Levine JA. Sensitivity of the hypothalamic paraventricular nucleus to the locomotor-activating effects of neuromedin U in obesity. Brain Res. 2007;1169(1):57–68. doi: 10.1016/j.brainres.2007.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moran TH. Cholecystokinin and satiety: current perspectives. Nutrition. 2000;16(10):858–865. doi: 10.1016/s0899-9007(00)00419-6. [DOI] [PubMed] [Google Scholar]
- 70.Crawley JN. Biological actions of cholecystokinin. Peptides. 1994;15(4):731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 71.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997;272(4):41–44. doi: 10.1152/ajpregu.1997.272.4.R1245. [DOI] [PubMed] [Google Scholar]
- 72.Moran TH, Shnayder L, Hostetler AM, McHugh PR. Pylorectomy reduces the satiety action of cholecystokinin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1988;255(6) doi: 10.1152/ajpregu.1988.255.6.R1059. [DOI] [PubMed] [Google Scholar]
- 73.Moran TH, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006;361(1471):1211–1218. doi: 10.1098/rstb.2006.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beglinger C, Degen L, Matzinger D, D’Amato M, Drewe J. Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280(4):49–44. doi: 10.1152/ajpregu.2001.280.4.R1149. [DOI] [PubMed] [Google Scholar]
- 75.Asin KE, Bednarz L. Differential effects of CCK-JMV-180 on food intake in rats and mice. Pharmacol. Biochem. Behav. 1992;42(2):291–295. doi: 10.1016/0091-3057(92)90529-o. [DOI] [PubMed] [Google Scholar]
- 76.Crawley JN, Beinfeld MC. Rapid development of tolerance to the behavioural actions of cholecystokinin. Nature. 1983;302(5910):703–706. doi: 10.1038/302703a0. [DOI] [PubMed] [Google Scholar]
- 77.Lukaszewski L, Praissman M. Effect of continuous infusions of CCK-8 on food intake and body and pancreatic weights in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1988;254(1):R17–R22. doi: 10.1152/ajpregu.1988.254.1.R17. [DOI] [PubMed] [Google Scholar]
- 78.West DB, Fey D, Woods SC. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1984;15(5):R776–R787. doi: 10.1152/ajpregu.1984.246.5.R776. [DOI] [PubMed] [Google Scholar]
- 79.Fong TM. Advances in anti-obesity therapeutics. Expert Opin. Investig. Drugs. 2005;14(3):243–250. doi: 10.1517/13543784.14.3.243. [DOI] [PubMed] [Google Scholar]
- 80.Matson CA, Ritter RC. Long-term CCK-leptin synergy suggests a role for CCK in the regulation of bodyweight. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999;276(4):45–44. doi: 10.1152/ajpregu.1999.276.4.R1038. [DOI] [PubMed] [Google Scholar]
- 81.Plusczyk T, Westermann S, Rathgeb D, Feifel G. Acute pancreatitis in rats: effects of sodium taurocholate, CCK-8, and Sec on pancreatic microcirculation. Am. J. Physiol. 1997;272(2 Pt 1):G310–G320. doi: 10.1152/ajpgi.1997.272.2.G310. [DOI] [PubMed] [Google Scholar]
- 82.Pandol SJ, Periskic S, Gukovsky I, et al. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology. 1999;117(3):706–716. doi: 10.1016/s0016-5085(99)70465-8. [DOI] [PubMed] [Google Scholar]
- 83.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 84.Brubaker PL. The glucagon-like peptides: pleiotropic regulators of nutrient homeostasis. Ann. NY Acad. Sci. 2006;1070:10–26. doi: 10.1196/annals.1317.006. [DOI] [PubMed] [Google Scholar]
- 85.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 86.Meeran K, O’Shea D, Edwards CMB, et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7–36) amide or exendin-(9–39) alters bodyweight in the rat. Endocrinology. 1999;140(1):244–250. doi: 10.1210/endo.140.1.6421. [DOI] [PubMed] [Google Scholar]
- 87.Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 88.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Invest. 1998;101(3):515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Näslund E, King N, Mansten S, et al. Prandial subcutaneous injections of glucagon-like peptide-1 cause weight loss in obese human subjects. Br. J. Nutr. 2004;91(3):439–446. doi: 10.1079/BJN20031064. [DOI] [PubMed] [Google Scholar]
- 90.Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide 1(7–36) amide’s central inhibition of feeding and peripheral inhibition of are abolished by neonatal monosodium glutamate treatment. Diabetes. 1998;47(4):530–537. doi: 10.2337/diabetes.47.4.530. [DOI] [PubMed] [Google Scholar]
- 91.Abbott CR, Monteiro M, Small CJ, et al. The inhibitory effects of peripheral administration of peptide YY 3–36 and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal–brainstem–hypothalamic pathway. Brain Res. 2005;1044(1):127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 92.Furuse M, Matsumoto M, Mori R, Sugahara K, Kano K, Hasegawa S. Influence of fasting and neuropeptide Y on the suppressive food intake induced by intracerebroventricular injection of glucagon-like peptide-1 in the neonatal chick. Brain Res. 1997;764(1–2):289–292. doi: 10.1016/s0006-8993(97)00623-9. [DOI] [PubMed] [Google Scholar]
- 93.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993;214(3):829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 94. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in Type 2 diabetes: systematic review and meta--analysis. JAMA. 2007;298(2):194–206. doi: 10.1001/jama.298.2.194.. •• Meta-analysis of the effectiveness of GLP-1 analog therapy in treating diabetes and inducing weight loss.
- 95.Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374(9701):1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 96.Bailey CJ, Turner RC. Metformin. N. Engl. J. Med. 1996;334(9):574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 97.Avenell A, Brown TJ, McGee MA, et al. What interventions should we add to weight reducing diets in adults with obesity? A systematic review of randomized controlled trials of adding drug therapy, exercise, behaviour therapy or combinations of these interventions. J. Hum. Nutr. Diet. 2004;17(4):293–316. doi: 10.1111/j.1365-277X.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 98.Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and bodyweight in subjects with Type 2 diabetes. Diabetes Care. 2007;30(6):1487–1493. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- 99.Beglinger C, Poller B, Arbit E, et al. Pharmacokinetics and pharmacodynamic effects of oral GLP-1 and PYY3–36: a proof-of-concept study in healthy subjects. Clin. Pharmacol. Ther. 2008;84(4):468–474. doi: 10.1038/clpt.2008.35. [DOI] [PubMed] [Google Scholar]
- 100.Dore DD, Seeger JD, Arnold Chan K. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared with metformin or glyburide. Curr. Med. Res. Opin. 2009;25(4):1019–1027. doi: 10.1185/03007990902820519. [DOI] [PubMed] [Google Scholar]
- 101.Gallwitz B. Benefit-risk assessment of exenatide in the therapy of Type 2 diabetes mellitus. Drug Saf. 2010;33(2):87–100. doi: 10.2165/11319130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 102.Dakin CL, Gunn I, Small CJ, et al. Oxyntomodulin inhibits food intake in the rat. Endocrinology. 2001;142(10):4244–4250. doi: 10.1210/endo.142.10.8430. [DOI] [PubMed] [Google Scholar]
- 103.Dakin CL, Small CJ, Batterham RL, et al. Peripheral oxyntomodulin reduces food intake and bodyweight gain in rats. Endocrinology. 2004;145(6):2687–2695. doi: 10.1210/en.2003-1338. [DOI] [PubMed] [Google Scholar]
- 104.Cohen MA, Ellis SM, Le Roux CW, et al. Oxyntomodulin suppresses appetite and reduces food intake in humans. J. Clin. Endocrinol. Metab. 2003;88(10):4696–4701. doi: 10.1210/jc.2003-030421. [DOI] [PubMed] [Google Scholar]
- 105.Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127(2):546–558. doi: 10.1053/j.gastro.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 106.Wynne K, Park AJ, Small CJ, et al. Subcutaneous oxyntomodulin reduces bodyweight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes. 2005;54(8):2390–2395. doi: 10.2337/diabetes.54.8.2390. [DOI] [PubMed] [Google Scholar]
- 107.Wynne K, Park AJ, Small CJ, et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J. Obes. 2006;30(12):1729–1736. doi: 10.1038/sj.ijo.0803344. [DOI] [PubMed] [Google Scholar]
- 108.Adrian TE, Ferri GL, Bacarese-Hamilton AJ. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89(5):1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 109.Liu CD, Aloia T, Adrian TE, et al. Peptide YY: a potential proabsorptive hormone for the treatment of malabsorptive disorders. Am. Surg. 1996;62(3):232–236. [PubMed] [Google Scholar]
- 110.Adrian TE, Savage AP, Sagor GR. Effect of peptide YY on gastric, pancreatic, and biliary function in humans. Gastroenterology. 1985;89(3):494–499. doi: 10.1016/0016-5085(85)90442-1. [DOI] [PubMed] [Google Scholar]
- 111.Adams SH, Won WB, Schonhoff SE, Leiter AB, Paterniti JR., Jr Effects of peptide YY(3–36) on short-term food intake in mice are not affected by prevailing plasma ghrelin levels. Endocrinology. 2004;145(11):4967–4975. doi: 10.1210/en.2004-0518. [DOI] [PubMed] [Google Scholar]
- 112.Chelikani PK, Haver AC, Heidelberger RD. Intravenous infusion of peptide YY(3–36) potently inhibits food intake in rats. Endocrinology. 2005;146(2):879–888. doi: 10.1210/en.2004-1138. [DOI] [PubMed] [Google Scholar]
- 113. Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY3–36 physiologically inhibits food intake. Nature. 2002;418(6898):650–654. doi: 10.1038/nature00887.. •• Established PYY as a satiety hormone regulating appetite and bodyweight.
- 114.Batterham RL, Heffron H, Kapoor S, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4(3):223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 115.Le Roux CW, Batterham RL, Aylwin SJB, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147(1):3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 116.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132(6):2116–2130. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 117.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3–36. N. Engl. J. Med. 2003;349(10):941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 118.Degen L, Oesch S, Casanova M, et al. Effect of peptide YY3–36 on food intake in humans. Gastroenterology. 2005;129(5):1430–1436. doi: 10.1053/j.gastro.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 119.Gantz I, Erondu N, Mallick M, et al. Efficacy and safety of intranasal peptide YY3–36 for weight reduction in obese adults. J. Clin. Endocrinol. Metab. 2007;92(5):1754–1757. doi: 10.1210/jc.2006-1806. [DOI] [PubMed] [Google Scholar]
- 120.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 121.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Cell. Mol. Biol. 2003;23(22):7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. doi: 10.1038/35038090.. • Established the orexigenic activity of ghrelin.
- 123.Wren AM, Small CJ, Abbott CR, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50(7–12):2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 124.Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 125.Otto B, Cuntz U, Fruehauf E, et al. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur. J. Endocrinol. 2001;145(5):669–673. [PubMed] [Google Scholar]
- 126.Tolle V, Kadem M, Bluet-Pajot MT, et al. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J. Clin. Endocrinol. Metab. 2003;88(1):109–116. doi: 10.1210/jc.2002-020645. [DOI] [PubMed] [Google Scholar]
- 127.Guan XM, Yu H, Palyha OC, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Mol. Brain Res. 1997;48(1):23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 128.Gnanapavan S, Kola B, Bustin SA, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 2002;87(6):2988–2991. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 129.Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123(4):1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 130.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26(11):2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 131.Carlini VP, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, De Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem. Biophys. Res. Commun. 2004;313(3):635–641. doi: 10.1016/j.bbrc.2003.11.150. [DOI] [PubMed] [Google Scholar]
- 132.Zorrilla EP, Iwasaki S, Moss JA, et al. Vaccination against weight gain. Proc. Natl Acad. Sci. USA. 2006;103(35):13226–13231. doi: 10.1073/pnas.0605376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moran TH, Dailey MJ. Gut peptides: targets for antiobesity drug development? Endocrinology. 2009;150(6):2526–2530. doi: 10.1210/en.2009-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang J, Zhao T-J, Goldstein JL, Brown MS. Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proc. Natl Acad. Sci. USA. 2008;105(31):10750–10755. doi: 10.1073/pnas.0805353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hort Y, Baker E, Sutherland GR, Shine J, Herzog H. Gene duplication of the human peptide YY gene (PYY) generated the pancreatic polypeptide gene (PPY) on chromosome 17q21.1. Genomics. 1995;26(1):77–83. doi: 10.1016/0888-7543(95)80085-z. [DOI] [PubMed] [Google Scholar]
- 136.Adrian TE, Bloom SR, Bryant MG. Distribution and release of human pancreatic polypeptide. Gut. 1976;17(12):940–944. doi: 10.1136/gut.17.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006;361(1471):1187–1209. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Malaisse-Lagae F, Carpentier JL, Patel YC. Pancreatic polypeptide: a possible role in the regulation of food intake in the mouse. Hypothesis. Experientia. 1977;33(7):915–917. doi: 10.1007/BF01951279. [DOI] [PubMed] [Google Scholar]
- 139.Asakawa A, Inui A, Yuzuriha H, et al. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology. 2003;124(5):1325–1336. doi: 10.1016/s0016-5085(03)00216-6. [DOI] [PubMed] [Google Scholar]
- 140.Ueno N, Inui A, Iwamoto M, et al. Decreased food intake and bodyweight in pancreatic polypeptide-overexpressing mice. Gastroenterology. 1999;117(6):1427–1432. doi: 10.1016/s0016-5085(99)70293-3. [DOI] [PubMed] [Google Scholar]
- 141.Reinehr T, Enriori PJ, Harz K, Cowley MA, Roth CL. Pancreatic polypeptide in obese children before and after weight loss. Int. J. Obes. 2006;30(10):1476–1481. doi: 10.1038/sj.ijo.0803393. [DOI] [PubMed] [Google Scholar]
- 142.Fujimoto S, Inui A, Kiyota N, et al. Increased cholecystokinin and pancreatic polypeptide responses to a fat-rich meal in patients with restrictive but not bulimic anorexia nervosa. Biol. Psychiatry. 1997;41(10):1068–1070. doi: 10.1016/S0006-3223(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 143.Reinehr T, Enriori PJ, Harz K, Cowley MA, Roth CL. Pancreatic polypeptide in obese children before and after weight loss. Int. J. Obes. 2006;30(10):1476–1481. doi: 10.1038/sj.ijo.0803393. [DOI] [PubMed] [Google Scholar]
- 144.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115(1):427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 145.Larsen PJ, Kristensen P. The neuropeptide Y (Y4) receptor is highly expressed in neurones of the rat dorsal vagal complex. Mol. Brain Res. 1997;48(1):1–6. doi: 10.1016/s0169-328x(97)00069-7. [DOI] [PubMed] [Google Scholar]
- 146.Whitcomb DC, Taylor IL, Vigna SR. Characterization of saturable binding sites for circulating pancreatic polypeptide in rat brain. Am. J. Physiol. Gastrointest. Liver Physiol. 1990;259(4):22–24. doi: 10.1152/ajpgi.1990.259.4.G687. [DOI] [PubMed] [Google Scholar]
- 147.Batterham RL, Le Roux CW, Cohen MA, et al. Pancreatic polypeptide reduces appetite and food intake in humans. J. Clin. Endocrinol. Metab. 2003;88(8):3989–3992. doi: 10.1210/jc.2003-030630. [DOI] [PubMed] [Google Scholar]
- 148.Adrian TE, Greenberg GR, Besterman HS, Bloom SR. Pharmacokinetics of pancreatic polypeptide in man. Gut. 1978;19(10):907–909. doi: 10.1136/gut.19.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pittner RA, Albrandt K, Beaumont K, et al. Molecular physiology of amylin. Cell. Physiol. Biochem. 1994;55(Suppl.):19–28. doi: 10.1002/jcb.240550004. [DOI] [PubMed] [Google Scholar]
- 150.Koda JE, Fineman M, Rink TJ, Dailey GE, Muchmore DB, Linarelli LG. Amylin concentrations and glucose control. Lancet. 1992;339(8802):1179–1180. doi: 10.1016/0140-6736(92)90785-2. [DOI] [PubMed] [Google Scholar]
- 151.Koda JE, Fineman MS, Kolterman OG, Caro JF. 24 hour plasma amylin profiles are elevated in IGT subjects vs. normal controls. Diabetes. 1995;44(Suppl. 1) [Google Scholar]
- 152.Rushing PA, Hagan MM, Seeley RJ, Lutz TA, Woods SC. Amylin: a novel action in the brain to reduce bodyweight. Endocrinology. 2000;141(2):850–853. doi: 10.1210/endo.141.2.7378. [DOI] [PubMed] [Google Scholar]
- 153.Rushing PA, Hagan MM, Seeley RJ, et al. Inhibition of central amylin signaling increases food intake and body adiposity in rats. Endocrinology. 2001;142(11):5035–5038. doi: 10.1210/endo.142.11.8593. [DOI] [PubMed] [Google Scholar]
- 154.Gebre-Medhin S, Mulder H, Pekny M, et al. IAPP (amylin) null mutant mice; plasma levels of insulin and glucose, bodyweight and pain responses. Diabetologia. 1997;40:29A. [Google Scholar]
- 155.Devine E, Young AA. Weight gain in male and female mice with amylin gene knockout. Diabetes. 1998;47(Suppl. 1):A317. [Google Scholar]
- 156.Cooper GJ. Amylin compared with calcitonin gene-related peptide: structure, biology, and relevance to metabolic disease. Endocr. Rev. 1994;15(2):163–201. doi: 10.1210/edrv-15-2-163. [DOI] [PubMed] [Google Scholar]
- 157.Young AA, Wang MW, Gedulin B, Rink TJ, Pittner R, Beaumont K. Diabetogenic effects of salmon calcitonin are attributable to amylin-like activity. Metab. Clin. Exp. 1995;44(12):1581–1589. doi: 10.1016/0026-0495(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 158.McLatchie LM, Fraser NJ, Main MJ, et al. RAMPS regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393(6683):333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 159.Muff R, Bühlmann N, Fischer JA, Born W. An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology. 1999;140(6):2924–2927. doi: 10.1210/endo.140.6.6930. [DOI] [PubMed] [Google Scholar]
- 160.Poyner D, Marshall I, Brain SD, et al. In: The CGRP Family: Calcitonin Gene- Related Peptide (CGRP), Amylin, and Adrenomedullin. Poyner D, editor. TX, USA: Landes Bioscience; 2000. pp. 1–258. [Google Scholar]
- 161.Jodka C, Green D, Young A, Gedulin B. Amylin modulation of gastric emptying in rats depends upon an intact vagus nerve. Diabetes. 1996;45(Suppl. 1):A235. [Google Scholar]
- 162.Edwards GL, Gedulin BR, Jodka C, Dilts RP, Miller CC, Young A. Area postrema (AP)-lesions block the regulation of gastric emptying by amylin. Neurogastroenterol. Motil. 1998;10(4):26. [Google Scholar]
- 163.Lutz TA, Mollet A, Rushing PA, Riediger T, Scharrer E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int. J. Obes. 2001;25(7):1005–1011. doi: 10.1038/sj.ijo.0801664. [DOI] [PubMed] [Google Scholar]
- 164.Edelman SV, Weyer C. Unresolved challenges with insulin therapy in Type 1 and Type 2 diabetes: potential benefit of replacing amylin, a second β-cell hormone. Diabetes Technol. Ther. 2002;4(2):175–189. doi: 10.1089/15209150260007390. [DOI] [PubMed] [Google Scholar]
- 165.Young AA, Vine W, Gedulin BR, et al. Preclinical pharmacology of pramlintide in the rat: comparisons with human and rat amylin. Drug Dev. Res. 1996;37(4):231–248. [Google Scholar]
- 166.Ratner RE, Want LL, Fineman MS, et al. Adjunctive therapy with the amylin analog pramlintide leads to a combined improvement in glycemic and weight control in insulin-treated subjects with Type 2 diabetes. Diabetes Technol. Ther. 2002;4(1):51–61. doi: 10.1089/15209150252924094. [DOI] [PubMed] [Google Scholar]
- 167.Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with Type 2 diabetes. Diabetes Care. 2003;26(3):784–790. doi: 10.2337/diacare.26.3.784. [DOI] [PubMed] [Google Scholar]
- 168.Whitehouse F, Kruger DF, Fineman M, et al. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in Type 1 diabetes. Diabetes Care. 2002;25(4):724–730. doi: 10.2337/diacare.25.4.724. [DOI] [PubMed] [Google Scholar]
- 169.Maggs D, Shen L, Strobel S, Brown D, Kolterman O, Weyer C. Effect of pramlintide on A1C and bodyweight in insulin-treated African Americans and Hispanics with Type 2 diabetes: a pooled post hoc analysis. Metabolism. 2003;52(12):1638–1642. doi: 10.1016/j.metabol.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 170.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. Obes. Res. 1996;4(1):101. doi: 10.1002/j.1550-8528.1996.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 171.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 172. Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777.. • Provides evidence for leptin’s role in bodyweight regulation.
- 173.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on bodyweight regulation in ob/ob mice. Science. 1995;269(5223):540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 174.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 175.Farooqi IS, O’Rahilly S. Monogenic obesity in humans. 2005;56:443–458. doi: 10.1146/annurev.med.56.062904.144924. [DOI] [PubMed] [Google Scholar]
- 176. Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334(5):292–295. doi: 10.1056/NEJM199602013340503.. •• Provides evidence indicating that obese individuals are hyperleptinemic owing to leptin resistance.
- 177.Ahlma RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 178.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83(7):1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 179.Münzberg H, Myers MG, Jr, et al. Molecular and anatomical determinants of central leptin resistance. Nat. Neurosci. 2005;8(5):566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 180.Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog. Horm. Res. 2004;59(1):287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 181.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am. J. Physiol. Endocrinol. Metab. 2009;297(6):E1247–E1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138(10):4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 183.Baskin DG, Breininger JF, Schwartz MW. Leptin receptor mRNA identifies a subpopulation of neuropeptide y neurons activated by fasting in rat hypothalamus. Diabetes. 1999;48(4):828–833. doi: 10.2337/diabetes.48.4.828. [DOI] [PubMed] [Google Scholar]
- 184.Tang-Christensen M, Holst JJ, Hartmann B, Vrang N. The arcuate nucleus is pivotal in mediating the anorectic effects of centrally administered leptin. NeuroReport. 1999;10(6):1183–1187. doi: 10.1097/00001756-199904260-00005. [DOI] [PubMed] [Google Scholar]
- 185.Schwartz MW, Baskin DG, Bukowski TR, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45(4):531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 186.Stephens TW, Basinski M, Bristow PK, et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377(6549):530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- 187.Schwartz MW, Seeley RJ, Woods SC, et al. Leptin increases hypothalamic proopiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46(12):2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 188.Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9(1):35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 189.Hukshorn CJ, Saris WHM, Westerterp-Plantenga MS, Farid AR, Smith FJ, Campfield LA. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J. Clin. Endocrinol. Metab. 2000;85(11):4003–4009. doi: 10.1210/jcem.85.11.6955. [DOI] [PubMed] [Google Scholar]
- 190. Roth JD, Roland BL, Cole RL, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc. Natl Acad. Sci. USA. 2008;105(20):7257–7262. doi: 10.1073/pnas.0706473105.. • Proof-of-concept study indicating the efficacy of combined pramlintide–metreleptin therapy in producing weight loss
- 191.Gadde KM, Allison DB. Combination therapy for obesity and metabolic disease. Curr. Opin. Endocrinol. Diabetes Obes. 2009;16(5):353–358. doi: 10.1097/MED.0b013e3283304f90. [DOI] [PubMed] [Google Scholar]
- 192.Fernández-Real JM, Sanchis D, Ricart W, et al. Plasma oestrone-fatty acid ester levels are correlated with body fat mass in humans. Clin. Endocrinol. 1999;50(2):253–260. doi: 10.1046/j.1365-2265.1999.00669.x. [DOI] [PubMed] [Google Scholar]
- 193.Sanchis D, Balada F, Del Mar Grasa M, et al. Oleoyl-estrone induces the loss of body fat in rats. Int. J. Obes. 1996;20(6):588–594. [PubMed] [Google Scholar]
- 194.Sanchis D, Balada F, Picó C, et al. Rats receiving the slimming agent oleoyl-estrone in liposomes (Merlin-2) decrease food intake but maintain thermogenesis. Arch. Physiol. Biochem. 1997;105(7):663–672. doi: 10.1076/apab.105.7.663.11391. [DOI] [PubMed] [Google Scholar]
- 195.Adán C, Cabot C, Vilà R, et al. Oleoylestrone treatment affects the ponderostat setting differently in lean and obese Zucker rats. Int. J. Obes. 1999;23(4):366–373. doi: 10.1038/sj.ijo.0800828. [DOI] [PubMed] [Google Scholar]
- 196.Remesar X, Guijarro P, Torregrosa C, et al. Oral oleoyl-estrone induces the rapid loss of body fat in Zucker lean rats fed a hyperlipidic diet. Int. J. Obes. 2000;24(11):1405–1412. doi: 10.1038/sj.ijo.0801393. [DOI] [PubMed] [Google Scholar]
- 197.Alemany M, Fernández-López JA, Petrobelli A, Granada M, Foz M, Remesar X. Weight loss in a patient with morbid obesity under treatment with oleoyl-estrone. Med. Clin. (Barc.) 2003;121(13):496–499. doi: 10.1016/s0025-7753(03)74000-7. [DOI] [PubMed] [Google Scholar]
- 198.Sonnenberg GE, Matfin G, Reinhardt RR. Drug treatments for obesity: where are we heading and how do we get there? Br. J. Diabetes Vasc. Dis. 2007;7(3):111–118. [Google Scholar]
- 199. Bray GA, Greenway FL. Current and potential drugs for treatment of obesity. Endocr. Rev. 1999;20(6):805–875. doi: 10.1210/edrv.20.6.0383.. •• Provides a good background on anti-obesity therapy, and the authors provide commentary on the future of this field of research.
Websites
- 201.TransTech Pharma obesity product pipeline. www.ttpharma.com/TherapeuticAreas/MetabolicDiseases/Obesity/TTP435/tabid/126/Default.aspx.
- 202.Bristol-Myers Squibb metabolics clinical trial registry. http://ctr.bms.com/OneBmsCtd/InitTrialAction.do?linkname=Metabolics&type=pharma&sortby=default.
- 203.Novo Nordisk: significant weight loss sustained in obese people treated with liraglutide for one year. www.novonordisk.com/science/Pipeline/liraglutide-press-releases.asp.
- 204.Amylin Pharmaceuticals, Inc. and Eli Lilly and Company: exenatide once weekly provided sustained improvements in glycemic control with weight loss over two years: DURATION-1 interim long-term data presented at ADA. 2009 www.amylin.com/assets/001/5097.pdf.
- 205.Amylin Pharmaceuticals, Inc. research pipeline. www.amylin.com/research/pipeline/exenatide-once-weekly.htm.
- 206.Novo Nordisk starts Phase I trial with long-acting oral GLP-1 analog. 2010 Jan 13; www.novonordisk.com/press/news/news.asp?sNewsTypeGUID=&lMonth=&lYear=&sLanguageCode=&sSearchText=NN9924&sShowNewsItemGUID=acc555cc-2124-4ebf-ad6d-7f923e2e2a78&sShowLanguageCode=en-GB.
- 207.Questions and answers – safety requirements for Victoza (liraglutide) www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm198543.htm.
- 208.Wyeth Pharmaceuticals acquires Thiakis Limited. www.drugs.com/news/wyeth-pharmaceuticals-acquires-thiakis-limited-15289.html.
- 209.Phase I/IIa clinical trial with obese individuals shows no effect of CYT009-GhrQb on weight loss. www.cytos.com/doc/Cytos_Press_E_061107.pdf.
- 210.Noxxon Pharma AG. Noxxon Pharma AG announces global strategic alliance with Pfizer NOX-B11 Spiegelmer® for obesity treatment licensed. http://145.253.103.53/noxxon/downloads/032306PRNOXXONPfizerAgreements.pdf.
- 211.Elixir Pharmaceuticals product pipeline. www.elixirpharm.com/product/index.html.
- 212.7TM Pharma: obinepitide. www.7tm.com/R-D/Metabolic_Disorders/Obinepitide.aspx.
- 213.7TM Pharma: TM30339. www.7tm.com/R-D/Metabolic_Disorders/TM30339.aspx.
- 214.Amylin Pharmaceuticals announces positive results from dose-ranging clinical study of pramlintide/metreleptin combination treatment for obesity. http://investors.amylin.com/phoenix.zhtml?c=101911&p=irol-newsArticle_pf&ID=1305954.
- 215.Amylin and Takeda announce decision to advance development of pramlintide/metreleptin combination treatment for obesity. www.takeda.com/press/article_35851.html.
- 216.Manhattan Pharmaceuticals announces results of Phase IIa studies for oral oleoyl-estrone. http://ir.manhattanpharma.com/releasedetail.cfm?ReleaseID=252872.
- 217.FDA: Guidance for industry developing products for weight management. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071612.pdf.